Abstract
Introduction
Elevated zidovudine- and lamivudine-triphosphates have been observed in peripheral blood mononuclear cells (PBMCs) from females versus males and in patients with high inflammatory states versus lower inflammatory states. Consistent with high triphosphate exposures, these same patient groups also experience elevated rates of toxicity, including lipoatrophy. The purpose of this study was to evaluate the effects of oestradiol, progesterone and testosterone as well as tumour necrosis factor (TNF)-α and interferon (IFN)-α on zidovudine- and lamivudine-triphosphates in PBMCs and, for the cytokines, in 3T3-L1 adipocytes.
Methods
Multiple replicates of adipocytes and human PBMCs were incubated with experimental versus control conditions using several cytokine and sex hormone doses. Zidovudine- and lamivudine-triphosphate concentrations were determined with validated LC-MS-MS assays. A mixed effects, cell means model that accounted for experiment number was used to evaluate the effects of experimental conditions relative to control.
Results
In adipocytes, TNF-α doses below 2 ng/mL increased zidovudine-triphosphate by 18% (5–31%). Lamivudine-triphosphate was not detectable in adipocytes. In PBMCs, pooled IFN-α doses (0.1–10 U/mL) decreased zidovudine-triphosphate 26% (10–42%); 100 and 1000 ng/mL of progesterone decreased lamivudine-triphosphate by 22% (1–43%) and 47% (25–68%), respectively. Pooled testosterone doses (10–1000 ng/mL) decreased lamivudine-triphosphate by 24% (7–41%). No other statistically significant effects were observed.
Conclusions
We found evidence that sex hormones and cytokines influence zidovudine-triphosphate and lamivudine-triphosphate slightly in PBMCs and adipocytes in vitro. These findings provide insight and scientific direction to address inflammation-dependent and sex-dependent phosphorylation and responses in patients.
Keywords: nucleoside analogues, pharmacology, intracellular, toxicity, phosphorylation
Introduction
Nucleoside analogue reverse transcriptase inhibitors (NRTIs) require stepwise phosphorylation to the intracellular 5′-triphosphate for pharmacological activity. The eventual active triphosphate blocks viral replication through inhibition of the HIV reverse transcriptase enzyme and may also cause toxicity through inhibition of mitochondrial DNA polymerase γ or disruption of other mitochondrial/cellular functions.1–4 By pharmacological principle, the probability of both efficacy and toxicity is associated with the intensity of exposure to the active drug (the intracellular NRTI-triphosphate).5
NRTIs are phosphorylated by human enzymes and once phosphorylated the NRTI-phosphate cannot freely diffuse out of the cell given the polar phosphate moiety. This creates two important sets of pharmacokinetics for NRTIs: one for the parent NRTI in plasma and one for the intracellular NRTI-triphosphate.6
Little is known about what cellular factors in patients govern NRTI phosphorylation and intracellular NRTI-triphosphate pharmacokinetics.5,7 In a previous pharmacological study of zidovudine, lamivudine and indinavir in antiretroviral-naive HIV- infected adults, we found that women had 2.3 and 1.6-fold higher zidovudine-triphosphate and lamivudine-triphosphate, respectively, in peripheral blood mononuclear cells (PBMCs) compared with men.8 One possible explanation for these results is that male or female sex hormones influence zidovudine- and lamivudine-triphosphate in vivo. In the same study, we also found that, early in therapy, advanced HIV disease (CD4 <100 cells/mm3) was associated with elevated triphosphates in PBMCs compared with less advanced disease. We hypothesized that this later finding was due to the higher cellular activation state via elevated inflammation associated with advanced compared with less advanced HIV disease.9 This hypothesis was based on in vitro studies that showed dramatically increased zidovudine-triphosphates (>100-fold) and lamivudine-triphosphates (∼4-fold) in phytohaemagglutinin (PHA)-stimulated PBMCs versus resting PBMCs.10 We hypothesized that an elevated inflammatory state in patients (e.g. high cytokine concentrations and activation markers in advanced HIV) may be analogous to the effects of PHA on PBMCs in vitro and that these effects of inflammation may extend to other tissues besides PBMCs. Given that fat toxicity (lipoatrophy) is a common NRTI toxicity and that inflammation in fat is a risk factor for lipoatrophy,11,12 we hypothesize that activated cells in fat release cytokines that stimulate NRTI phosphorylation in adipocytes, which increases lipoatrophy risk.
The goal of the present study was to evaluate the effects of sex hormones and pro-inflammatory cytokines on zidovudine- and lamivudine-triphosphate concentrations in PBMCs and 3T3-L1 adipocytes.
Materials and methods
PBMC experiments
Blood was purchased from Bonfils Blood Centre under an IRB-approved contract. Blood was discarded therapeutic draws from haemochromatosis patients or was removed from discarded leucocyte depletion filters. No demographic information about the subjects was available.
PBMCs were isolated using the standard density centrifugation protocol with lymphocyte separation media. Cells were counted with a haemocytometer and assessed for viability with Trypan Blue dye exclusion. Cells were resuspended in an incubation solution containing 10% fetal bovine serum, 1% penicillin and streptomycin in RPMI complete media so that the final cell concentration was ∼1 million cells/mL. Equal numbers of cells (∼10 million) were aliquotted into separate 10 mL incubation vessels. Zidovudine (Sigma-Aldrich) or lamivudine (GlaxoWellcome) were added to all the incubation vessels. Experimental conditions (sex hormones or cytokines) were added to a subset of vessels (not including controls). No cell activators (PHA or IL-2) were used in any of the PBMC experiments. Two doses of zidovudine were tested: 10 and 1 mg/L; the higher zidovudine dose was used to mimic previous in vitro studies of zidovudine that have been published and the lower dose was added to reflect typical peak concentration attained with standard zidovudine dosing in patients.13,14 Lamivudine was tested at one dose, 1 mg/L, which approximated both previous in vitro work and typical concentrations in patients.10,14 The sex hormones studied were oestradiol, progesterone and testosterone (Sigma-Aldrich). All were studied at three dose levels: 10, 100 and 1000 ng/mL. The testosterone was mixed in 100% DMSO to enable solubility, and DMSO controls were added for testosterone experiments. The cytokine experiments included tumour necrosis factor (TNF)-α and interferon (IFN)-α at various dose intervals (0.002–20 ng/mL for TNF-α and 0.1–100 U/mL for IFN-α). The doses chosen for the sex hormones and cytokines were designed to include physiologically relevant concentrations. Incubation times were studied up to 36 h and triphosphates were found to decline starting after 24 h. Thus, controls and experiments were incubated for up to a maximum of 16 h. Incubations were conducted at 37°C in 5% carbon dioxide. Cells were harvested simultaneously, pelleted, lysed with 0.5 mL 70% methanol/water solution and stored at −70°C until analysis.
Adipocyte experiments
3T3-L1 fibroblasts were a gift from Dr Dwight Klemm (VA Medical Center, Denver, CO, USA). The original stock of 3T3-L1 fibroblasts was stored at −70°C. Cells were removed for experiments. A homogeneous cell suspension was created, and an equal volume was split among multiple equal-sized flasks. The cells were allowed to become confluent in 10% FBS and 1% penicillin/streptomycin in Dulbecco's modified Eagle's medium (DMEM) incubated at 37°C for ∼48 h. The media was then changed to 10% FBS, 1% l-glutamine, 1% penicillin/streptomycin, 1 µM dexamethasone, 300 µM isobutylmethylxanthine and 10 mg/L insulin in DMEM for ∼48 h. The cells were then maintained in 10% FBS, 1% l-glutamine, 1% penicillin/streptomycin and 10 mg/L insulin in DMEM for 10 days. At the end of the 10 days, cells were inspected for lipid accumulation to confirm the differentiation to adipocytes. Individual flasks were then incubated with zidovudine or lamivudine alone (controls), or zidovudine or lamivudine plus cytokines (TNF-α or IFN-α) at the same dose levels as for the PBMCs above. Lamivudine was studied at 1 mg/L, and only the 10 mg/L dose was used for zidovudine. After 24 h incubation, the cells were treated with trypsin (15% to 30%) and sonicated to release them from the bottom of the flask. The cells were immediately washed, pelleted and lysed with 70% methanol solution and stored at −70°C until analysis. Cells were not counted due to significant clumping; the amount of triphosphate was normalized per flask, which all had the same starting volume of cell suspension.
Analytical methods
Zidovudine- and lamivudine-triphosphate concentrations were quantified in all cell fractions with separate validated liquid chromatography-tandem mass spectroscopy (LC-MS-MS) methods. The complete method for zidovudine-triphosphate has been published previously.15 The linear range of assay was 50–6400 fmol zidovudine with a minimum quantifiable limit of 50 fmol (5 fmol per million cells with 10 million cells analysed). Both the between-day and within-day coefficients of variation (CVs) for the quality controls during validation were <20% at all concentrations. A direct LC-MS-MS method was developed and validated for lamivudine-triphosphate quantification.16 An internal standard, dideoxycytidine-triphosphate, was added to cell lysates, and samples were dried under nitrogen and then reconstituted in HPLC-grade water. Chromatographic separation was performed on a Phenomenex Luna Phenyl-Hexyl, 2.0×150 mm column. The mobile phase consisted of 90% mobile phase A [20 mM triethylamine (TEA), 0.1% formic acid pH 9.0] and 10% mobile phase B (50:50 acetonitrile:40 mM TEA, 0.2% formic acid pH 9.0). Detection and quantification of lamivudine-triphosphate and the internal standard were achieved by MS-MS detection in negative ion mode with electro-spray ionization. The assay was linear in the range of 5–500 pmol with a minimum quantifiable limit of 5 pmol (1 pmol/million cells with 5 million cells analysed). Both the between-day and within-day CVs and percent deviation for the quality controls were <15%.
Data analyses
Each experimental condition and control condition were run in multiple replicates for each experiment. The ratio of experimental to control condition was calculated for each replicate. Thus, a ratio above 1 indicated enhanced NRTI phosphorylation for the experimental conditions.
All tests assumed a two-sided test of hypothesis with a significance level of 0.05, and analyses were considered to be hypothesis-generating with no corrections for multiple comparisons. For each stimulation, separate models assuming linear, log-linear, categorical and combined stimulation doses were considered, with the final choice based on Akaike's information criterion.17 In all cases, the best stimulation dose classification was categorical, using either all or combined doses. A mixed effects, cell means model was then utilized to provide a mean ratio and 95% confidence interval for each observed combination of drug, dose, stimulation, stimulation dose and cell type. The model was programmed using SAS's MIXED procedure (SAS Institute, Inc., Cary, NC, USA) with a random intercept to account for the source (experiment number). A mean ratio that was significantly greater or less than 1.0 indicated that NRTI phosphorylation was enhanced or decreased, respectively.
Results
Adipocyte experiments
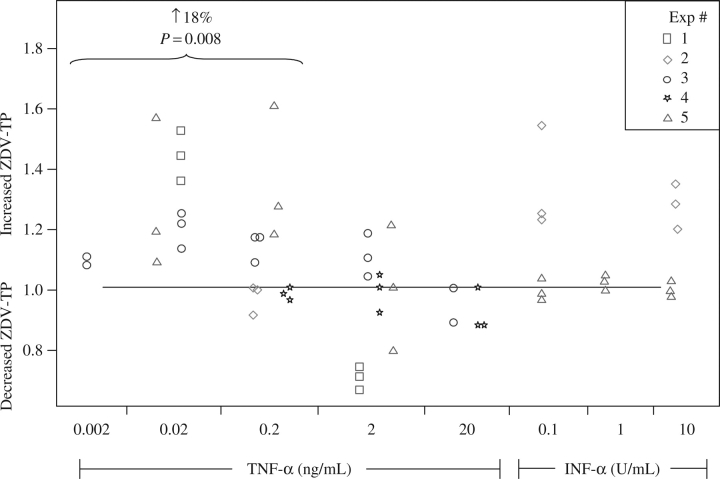
Lamivudine-triphosphate was not detectable in the 3T3-L1 adipocytes under any of the conditions studied (neither with controls nor with three doses of TNF-α or two doses of IFN-α). Zidovudine-triphosphate concentrations were influenced by TNF-α in a dose-dependent manner. At TNF-α doses below 2 ng/mL (0.02 and 0.2 ng/mL), zidovudine-triphosphate was increased by 18% (5–31%), but no effect was observed at higher TNF-α doses (2 and 20 ng/mL). No other significant effects were found; sex hormones were not tested in the 3T3-L1 adipocytes. The experimental data are summarized and displayed in Figure 1.
Figure 1.
Effects of cytokines on zidovudine (ZDV)-triphosphate (TP) in adipocytes. The y-axis indicates the ratio of experimental to control conditions (a ratio above 1 indicates increased ZDV-TP relative to control). Each experiment (distinguished by separate shapes) was run with two to three replicates. Experimental conditions are shown along the x-axis.
PBMC experiments
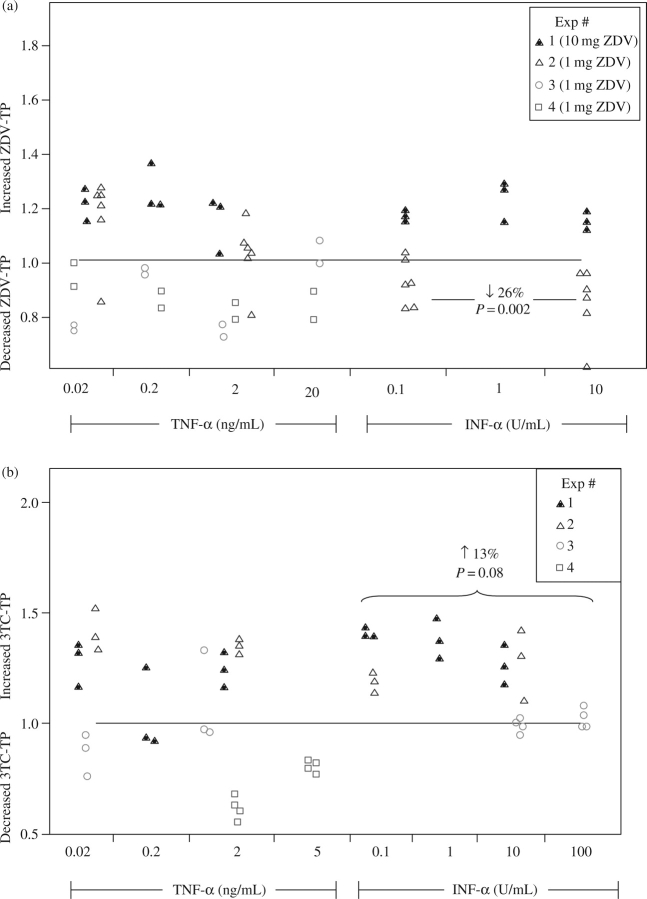
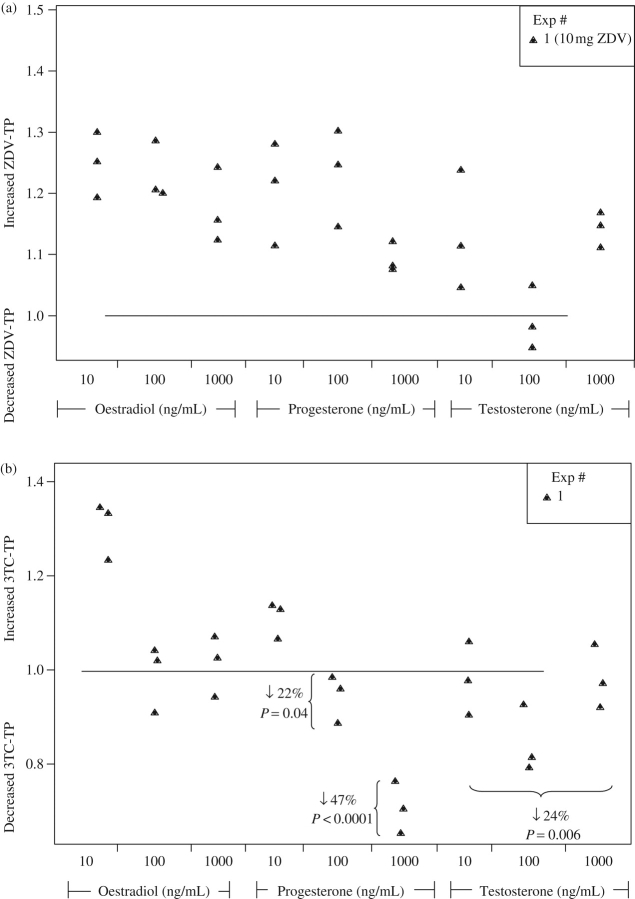
Zidovudine-triphosphate was decreased by 26% (10–42%) when the IFN-α dose levels were combined and the 1 mg/L zidovudine dose was studied. On the other hand, there were increased lamivudine-triphosphate concentrations by 13% (−2% to 27%; P = 0.08) in the combined IFN-α dose groups. The cytokine data are summarized and displayed in Figure 2. The middle and highest progesterone dose levels (100 and 1000 ng/mL) decreased lamivudine-triphosphate by 22% (1–43%) and 47% (25–68%), respectively. Pooled testosterone doses decreased lamivudine-triphosphate by ∼24% (7–41%). The sex hormone data are summarized and displayed in Figure 3. No other statistically significant relationships were found.
Figure 2.
Effects of cytokines on zidovudine (ZDV)- and lamivudine (3TC)-triphosphate (TP) in PBMCs. (a) ZDV-TP. (b) 3TC-TP. The y-axis indicates the ratio of experimental to control conditions (a ratio above 1 indicates increased ZDV- or 3TC-TP relative to control). Each experiment (distinguished by different shapes) was run with two to six replicates. The experimental conditions are shown along the x-axis.
Figure 3.
Effects of sex hormones on zidovudine (ZDV)- and lamivudine (3TC)-triphosphate (TP) in PBMCs. (a) ZDV-TP. (b) 3TC-TP. The y-axis indicates the ratio of experimental to control conditions (a ratio above 1 indicates increased ZDV- or 3TC-TP relative to control). Each experiment (distinguished by different shapes) was run with three replicates. The experimental conditions are shown along the x-axis.
Discussion
We found that TNF-α, at doses <2 ng/mL, increased zidovudine-triphosphate by 18% in 3T3-L1 adipocytes, but not in PBMCs. We also found that lamivudine-triphosphate was not detectable in adipocyte experiments. In PBMCs, pooled IFN-α doses lowered zidovudine-triphosphate by 26%, but lamivudine-triphosphate tended to be higher by 13% under the same conditions. Finally, we found that pooled doses of testosterone and middle and high doses of progesterone decreased lamivudine-triphosphate by 24%, 22% and 47%, respectively.
This study is the first to evaluate the perspective that TNF-α and IFN-α may influence zidovudine-triphosphate and lamivudine-triphosphate concentrations in adipocytes, although others have quantified NRTI-triphosphate in adipocytes in vitro.18 The rationale for this perspective is that pro-inflammatory cytokines such as TNF-α are over-expressed in adipocyte tissue in those with lipoatrophy.12,19 Infiltrated activated macrophages and the adipocytes themselves are the source of pro-inflammatory cytokine release in fat.19 Furthermore, the risk severe lipoatrophy is much greater in those with the most advanced HIV, a condition associated with very high production of pro-inflammatory cytokines.20,21 We hypothesize that over-production of inflammatory mediators in fat leads to over-production of zidovudine-triphosphate in adipocytes, which leads to increased mitochondrial (or other cellular) toxicity and lipoatrophy seen clinically. However, in this study, we could not find major changes in zidovudine-triphosphate when 3T3-L1 adipocytes were exposed to TNF-α. Zidovudine-triphosphate was increased slightly (by 18%) at TNF-α doses below 2 ng/mL. These findings provide a starting point for additional research to evaluate the interaction between inflammation and NRTI-triphosphate in fat.
The rationale for including PBMCs in the cytokine studies stems from an observation that zidovudine-triphosphate in PBMCs was 3-fold higher in patients with advanced HIV compared with less advanced HIV when measured early in therapy.8 Also, others have found elevated zidovudine-phosphates in HIV-infected patients versus HIV-negative volunteers.22,23 In line with this finding, a recent study found that thymidine kinase activity (phosphorylates zidovudine and stavudine) in PBMCs was higher in people with HIV infection versus without.24 We believe that these in vivo studies are providing evidence that elevated inflammation associated with HIV causes elevated NRTI-triphosphate in PBMCs, particularly for zidovudine. Evidence also comes from the in vitro setting, which showed that zidovudine-triphosphates were over 100-fold higher and lamivudine-triphosphates were ∼4-fold higher in ‘activated’ (PHA-stimulated) versus ‘resting’ PBMCs.10,25 Examples from the clinical setting provide high human health relevance to this area as NRTI responses are exaggerated in advanced HIV infection, consistent with the idea that NRTI-triphosphates are higher in this setting. As one example, patients with advanced HIV experience the highest rate of zidovudine-associated blood cell toxicities (anaemia and neutropenia) and lipoatrophy.5,8,22,26
However, there is at least one alternative hypothesis to explain the elevated zidovudine- and lamivudine-triphosphate concentrations in PBMCs from advanced HIV patients versus HIV-negative volunteers. Zidovudine- and lamivudine-triphosphate concentrations in PBMCs may be influenced by the CD4 content in the PBMCs, which is severely depressed in HIV, especially in advanced HIV. To address this alternative hypothesis, we evaluated zidovudine- and lamivudine-triphosphate in PBMCs, CD4-depleted PBMCs and CD4-enriched cells of HIV-negative volunteers taking standard zidovudine and lamivudine doses (to remove the possible effects from HIV immune ‘activation’). We found that zidovudine-triphosphate and lamivudine-triphosphate were slightly elevated in CD4-depleted PBMCs (and lower in CD4 purified cells), but our findings could not explain the observed 3-fold higher zidovudine-triphosphate concentrations that we observed in advanced HIV-infected patients.8,16
Thus, in this study, we hypothesized that pro-inflammatory cytokines, TNF-α and IFN-α, would increase zidovudine- and lamivudine-triphosphate in PBMCs. However, we did not observe this to be the case. In fact, zidovudine-triphosphate was decreased by 26% in the pooled IFN-α dose conditions (with the low zidovudine dose), whereas lamivudine-triphosphate tended to be higher in the pooled IFN-α dose conditions. We attempted to use physiological cytokine and NRTI concentrations to create the most physiologically relevant conditions. Our in vitro results are in line with the new in vivo data that evaluated zidovudine-triphosphate, stavudine-triphosphate and lamivudine-triphosphate in HCV–HIV co-infection before and after pegylated IFN-α 2a/ribavirin therapy was added.27 There was an arm in the study of pegylated IFN-α 2a-only (without ribavirin). No evidence was found that either ribavirin or pegylated IFN-α 2a influenced zidovudine-triphosphate, stavudine-triphosphate or lamivudine-triphosphate (or the corresponding endogenous deoxynucleotide-triphosphates) in PBMCs. Inter-subject variability was pronounced in this study,27 which limited the statistical power to detect small to moderate differences. Overall, we cannot find evidence that TNF-α and IFN-α cause large changes in zidovudine- and lamivudine-triphosphate in PBMCs.
The impetus to study the effects of sex hormones on NRTI-triphosphate in PBMCs arose from a previous study in which we observed 1.6-fold higher lamivudine-triphosphate and 2.3-fold higher zidovudine-triphosphate in HIV-infected women versus men.8 Another previous study had also observed ∼2-fold higher zidovudine-phosphates in women versus men.28 These data align with clinical experiences, as women experience exaggerated NRTI effects compared with men. As an example, a recent study found that the incidence of life-threatening lactic acidosis in African persons taking NRTIs was 13.4 higher in women than in men.29 However, some controversy exists for zidovudine-phosphate differences between the sexes, as shown in a recent study that investigated intracellular zidovudine and zidovudine-phosphates in 16 men and 18 women and according to hormonal birth control therapy in the women.30 The study found that men had elevated intracellular zidovudine (∼3-fold), zidovudine-monophosphate (∼8-fold) and zidovudine-triphosphate (∼2-fold) AUCs compared with women. Furthermore, in 14 women who initiated hormonal contraception (8 intramuscular depo-medroxyprogesterone and 6 oral norethindrone/ethinyl oestradiol), there was a trend for elevated intracellular zidovudine-phosphates after hormonal contraception, but none of the parameters reached statistical significance. In our study, lamivudine-triphosphate concentrations were lower with middle and high progesterone doses and pooled testosterone doses. No significant effects were observed with zidovudine. The effects observed for lamivudine-triphosphate, particularly the larger effects with progesterone, warrant follow-up research in patients receiving exogenous hormone therapy.
There were some limitations to this in vitro study. First, our study was not designed to elucidate mechanisms for our findings. This was a pilot study to probe whether sex hormones and/or cytokines caused large NRTI-triphosphate changes under our experimental conditions, and given the exploratory nature of the study, all findings should be considered to be hypothesis generating. Further confirmatory research is needed to understand the mechanisms for our findings. Second, we felt it was important to evaluate multivariate models that considered the effects of different blood donors and different zidovudine doses on the experimental results. The model was conservative in that the observed effects had to be consistent enough to persist after adjusting for these effects. Conversely, we did not have a great deal of statistical power under this model to detect small experimental effects, and thus some small effects may have been missed. Third, we did not identify major effects on zidovudine- and lamivudine-triphosphate (e.g. several-fold). We do not know what the clinical significance is of the smaller effects that were observed. We would point out that it is important that we ruled out large effects (under our study conditions), and taken together, our findings provide scientific direction for future research in this area. Lastly, as in all in vitro research, we cannot predict how our findings translate to patients. The in vivo system is many times more complex than in vitro conditions, and we cannot rule out that sex hormones and TNF-α and IFN-α may have different effects in vivo.
Finally, we wish to highlight the potential clinical relevance of this line of research. For example, one hypothesis for the surprisingly poor efficacy of the triple NRTI regimens that included tenofovir plus lamivudine and either didanosine or abacavir is that low NRTI-triphosphates in the activated cell compartment may have allowed virological breakthrough in activated cells.31,32 This is because none of the triphosphates of these drugs are preferentially phosphorylated or virologically active in activated cells (PHA-stimulated in vitro).33,34 Other studies have shown better virological outcomes with triple NRTI regimens including zidovudine, which theoretically targets the activated cell compartment.35,36 Another example already mentioned above is that women experience exaggerated responses to NRTIs and the mechanism for this difference has not been elucidated. More research is needed to elucidate the pharmacological basis for sex differences in response to NRTIs and to provide a better understanding of how the cellular activation state in patients affects NRTI-triphosphate pharmacology and responses.
Funding
This research was supported by Grants R01 AI64029 (PLA) and P30-AI054907 (Junior Faculty Award from the Colorado Center for AIDS Research to PLA) from NIAID, NIH.
Transparency declarations
None to declare.
Acknowledgements
We wish to thank Drs Omar Janneh and Dwight Klemm for guidance with the in vitro conditions.
References
- 1.Furman PA, Fyfe JA, St Clair MH, et al. Phosphorylation of 3′-azido-3′-deoxythymidine and selective interaction of the 5′-triphosphate with human immunodeficiency virus reverse transcriptase. Proc Natl Acad Sci USA. 1986;83:8333–7. doi: 10.1073/pnas.83.21.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson AA, Ray AS, Hanes J, et al. Toxicity of antiviral nucleoside analogs and the human mitochondrial DNA polymerase. J Biol Chem. 2001;276:40847–57. doi: 10.1074/jbc.M106743200. [DOI] [PubMed] [Google Scholar]
- 3.Mallon PW, Unemori P, Sedwell R, et al. In vivo nucleoside reverse-transcriptase inhibitors alter expression of both mitochondrial lipid metabolism genes in the absence of depletion of mitochondrial DNA. J Infect Dis. 2005;191:1686–96. doi: 10.1086/429697. [DOI] [PubMed] [Google Scholar]
- 4.Lund KC, Peterson LL, Wallace KB. Absence of a universal mechanism of mitochondrial toxicity by nucleoside analogs. Antimicrob Agents Chemother. 2007;51:2531–9. doi: 10.1128/AAC.00039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson PL, Kakuda TN, Lichtenstein KA. The cellular pharmacology of nucleoside- and nucleotide-analogue reverse-transcriptase inhibitors and its relationship to clinical toxicities. Clin Infect Dis. 2004;38:743–53. doi: 10.1086/381678. [DOI] [PubMed] [Google Scholar]
- 6.Back DJ, Burger DM, Flexner CW, et al. The pharmacology of antiretroviral nucleoside and nucleotide reverse transcriptase inhibitors: implications for once-daily dosing. J Acquir Immune Defic Syndr. 2005;39(Suppl. 1):S1–23. doi: 10.1097/01.qai.0000168882.67942.3f. [DOI] [PubMed] [Google Scholar]
- 7.Hoggard PG, Back DJ. Intracellular pharmacology of nucleoside analogues and protease inhibitors: role of transporter molecules. Curr Opin Infect Dis. 2002;15:3–8. doi: 10.1097/00001432-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Anderson PL, Kakuda TN, Kawle S, et al. Antiviral dynamics and sex differences of zidovudine and lamivudine triphosphate concentrations in HIV-infected individuals. AIDS. 2003;17:2159–68. doi: 10.1097/00002030-200310170-00003. [DOI] [PubMed] [Google Scholar]
- 9.Hestdal K, Aukrust P, Muller F, et al. Dysregulation of membrane-bound tumor necrosis factor-alpha and tumor necrosis factor receptors on mononuclear cells in human immunodeficiency virus type 1 infection: low percentage of p75-tumor necrosis factor receptor positive cells in patients with advanced disease and high viral load. Blood. 1997;90:2670–9. [PubMed] [Google Scholar]
- 10.Gao WY, Agbaria R, Driscoll JS, et al. Divergent anti-human immunodeficiency virus activity and anabolic phosphorylation of 2′,3′-dideoxynucleoside analogs in resting and activated human cells. J Biol Chem. 1994;269:12633–8. [PubMed] [Google Scholar]
- 11.Maher B, Alfirevic A, Vilar FJ, et al. TNF-alpha promoter region gene polymorphisms in HIV-positive patients with lipodystrophy. AIDS. 2002;16:2013–8. doi: 10.1097/00002030-200210180-00005. [DOI] [PubMed] [Google Scholar]
- 12.Bastard JP, Caron M, Vidal H, et al. Association between altered expression of adipogenic factor SREBP1 in lipoatrophic adipose tissue from HIV-1-infected patients and abnormal adipocyte differentiation and insulin resistance. Lancet. 2002;359:1026–31. doi: 10.1016/S0140-6736(02)08094-7. [DOI] [PubMed] [Google Scholar]
- 13.Arner ES, Valentin A, Eriksson S. Thymidine and 3′-azido-3′-deoxythymidine metabolism in human peripheral blood lymphocytes and monocyte-derived macrophages. A study of both anabolic and catabolic pathways. J Biol Chem. 1992;267:10968–75. [PubMed] [Google Scholar]
- 14.Kakuda TN, Page LM, Anderson PL, et al. Pharmacological basis for concentration-controlled therapy with zidovudine, lamivudine, and indinavir. Antimicrob Agents Chemother. 2001;45:236–42. doi: 10.1128/AAC.45.1.236-242.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King T, Bushman L, Anderson PL, et al. Quantitation of zidovudine triphosphate concentrations from human peripheral blood mononuclear cells by anion exchange solid phase extraction and liquid chromatography-tandem mass spectroscopy; an indirect quantitation methodology. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;831:248–57. doi: 10.1016/j.jchromb.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 16.Anderson PL, Zheng JH, King T, et al. Concentrations of zidovudine- and lamivudine-triphosphate according to cell type in HIV-seronegative adults. AIDS. 2007;21:1849–54. doi: 10.1097/QAD.0b013e3282741feb. [DOI] [PubMed] [Google Scholar]
- 17.Littell RC, Milliken GA, Stroup WW, et al. SAS System for Mixed Models. Cary, NC: SAS Institute Inc.; 1996. pp. 101–2. [Google Scholar]
- 18.Janneh O, Hoggard PG, Tjia JF, et al. Intracellular disposition and metabolic effects of zidovudine, stavudine and four protease inhibitors in cultured adipocytes. Antivir Ther. 2003;8:417–26. [PubMed] [Google Scholar]
- 19.Villarroya F, Domingo P, Giralt M. Lipodystrophy in HIV 1-infected patients: lessons for obesity research. Int J Obes (Lond) 2007;31:1763–76. doi: 10.1038/sj.ijo.0803698. [DOI] [PubMed] [Google Scholar]
- 20.Lichtenstein KA, Ward DJ, Moorman AC, et al. Clinical assessment of HIV-associated lipodystrophy in an ambulatory population. AIDS. 2001;15:1389–98. doi: 10.1097/00002030-200107270-00008. [DOI] [PubMed] [Google Scholar]
- 21.Lichtenstein KA, Delaney KM, Armon C, et al. Incidence of and risk factors for lipoatrophy (abnormal fat loss) in ambulatory HIV-1-infected patients. J Acquir Immune Defic Syndr. 2003;32:48–56. doi: 10.1097/00126334-200301010-00007. [DOI] [PubMed] [Google Scholar]
- 22.Barry M, Wild M, Veal G, et al. Zidovudine phosphorylation in HIV-infected patients and seronegative volunteers. AIDS. 1994;8:F1–5. doi: 10.1097/00002030-199408000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Wattanagoon Y, Na Bangchang K, Hoggard PG, et al. Pharmacokinetics of zidovudine phosphorylation in human immunodeficiency virus-positive Thai patients and healthy volunteers. Antimicrob Agents Chemother. 2000;44:1986–9. doi: 10.1128/aac.44.7.1986-1989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turriziani O, Butera O, Gianotti N, et al. Thymidine kinase and deoxycytidine kinase activity in mononuclear cells from antiretroviral-naive HIV-infected patients. AIDS. 2005;19:473–9. doi: 10.1097/01.aids.0000162335.12815.12. [DOI] [PubMed] [Google Scholar]
- 25.Gao WY, Shirasaka T, Johns DG, et al. Differential phosphorylation of azidothymidine, dideoxycytidine, and dideoxyinosine in resting and activated peripheral blood mononuclear cells. J Clin Invest. 1993;91:2326–33. doi: 10.1172/JCI116463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richman DD, Fischl MA, Grieco MH, et al. The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987;317:192–7. doi: 10.1056/NEJM198707233170402. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Torres M, Torriani FJ, Soriano V, et al. Effect of ribavirin on intracellular and plasma pharmacokinetics of nucleoside reverse transcriptase inhibitors in patients with human immunodeficiency virus-hepatitis C virus coinfection: results of a randomized clinical study. Antimicrob Agents Chemother. 2005;49:3997–4008. doi: 10.1128/AAC.49.10.3997-4008.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stretcher BN, Pesce AJ, Frame PT, et al. Pharmacokinetics of zidovudine phosphorylation in peripheral blood mononuclear cells from patients infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1994;38:1541–7. doi: 10.1128/aac.38.7.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolhaar MG, Karstaedt AS. A high incidence of lactic acidosis and symptomatic hyperlactatemia in women receiving highly active antiretroviral therapy in Soweto, South Africa. Clin Infect Dis. 2007;45:254–60. doi: 10.1086/518976. [DOI] [PubMed] [Google Scholar]
- 30.Aweeka FT, Rosenkranz SL, Segal Y, et al. The impact of sex and contraceptive therapy on the plasma and intracellular pharmacokinetics of zidovudine. AIDS. 2006;20:1833–41. doi: 10.1097/01.aids.0000244202.18629.36. [DOI] [PubMed] [Google Scholar]
- 31.Gallant JE, Rodriguez AE, Weinberg WG, et al. Early virologic nonresponse to tenofovir, abacavir, and lamivudine in HIV-infected antiretroviral-naive subjects. J Infect Dis. 2005;192:1921–30. doi: 10.1086/498069. [DOI] [PubMed] [Google Scholar]
- 32.Jemsek J, Hutcherson P, Harper E. Poor virologic responses in early emergence of resistance in treatment naive, HIV-infected patients receiving a once daily triple nucleoside regimen of didanosine, lamivudine and tenofovir DF. Abstracts of the Eleventh Conference on Retroviruses and Opportunistic Infections; San Francisco, CA. 2004. Abstract 51. [Google Scholar]
- 33.Anderson PL, Kakuda TN. Comment on: Suboptimal CD4 gains in HIV-infected patients receiving didanosine plus tenofovir. J Antimicrob Chemother. 2006;58:220–1. doi: 10.1093/jac/dkl203. [DOI] [PubMed] [Google Scholar]
- 34.Kuritzkes DR. Less than the sum of its parts: failure of a tenofovir–abacavir–lamivudine triple-nucleoside regimen. J Infect Dis. 2005;192:1867–8. doi: 10.1086/498070. [DOI] [PubMed] [Google Scholar]
- 35.Gulick RM, Ribaudo HJ, Shikuma CM, et al. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med. 2004;350:1850–61. doi: 10.1056/NEJMoa031772. [DOI] [PubMed] [Google Scholar]
- 36.Rey D, Krebs M, Partisani M, et al. Virologic response of zidovudine, lamivudine, and tenofovir disoproxil fumarate combination in antiretroviral-naive HIV-1-infected patients. J Acquir Immune Defic Syndr. 2006;43:530–4. doi: 10.1097/01.qai.0000245885.74133.d9. [DOI] [PubMed] [Google Scholar]