Abstract
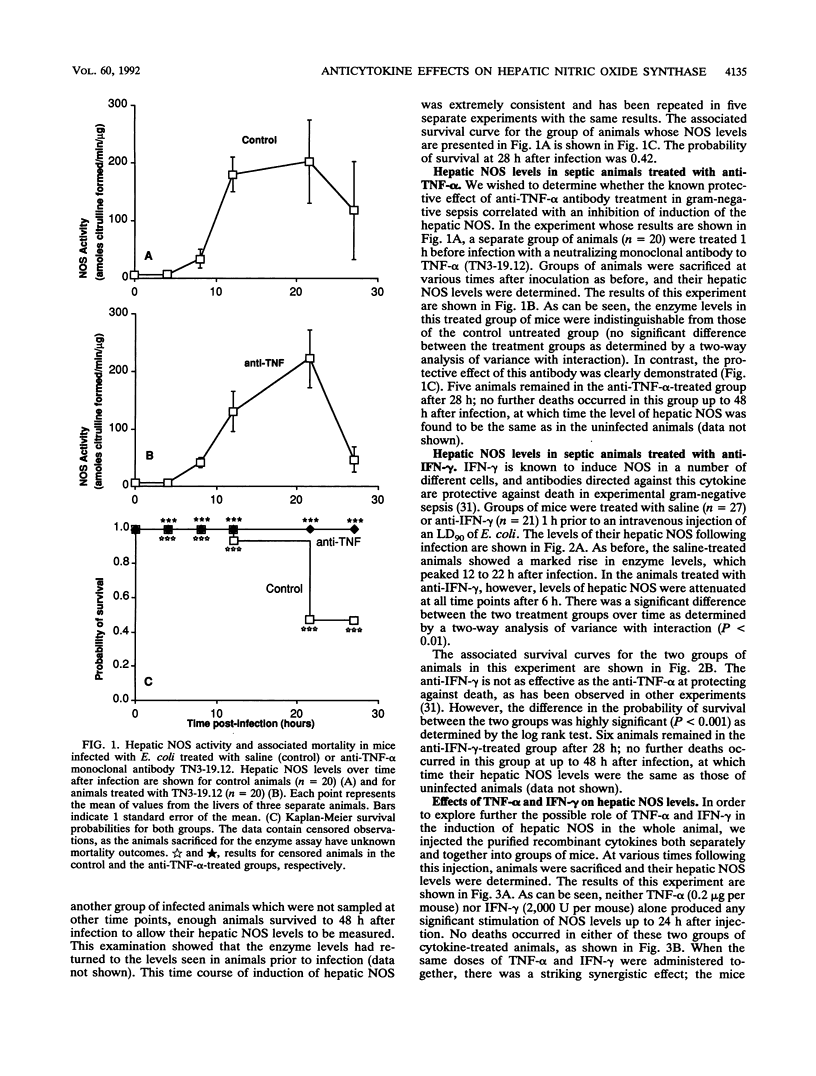
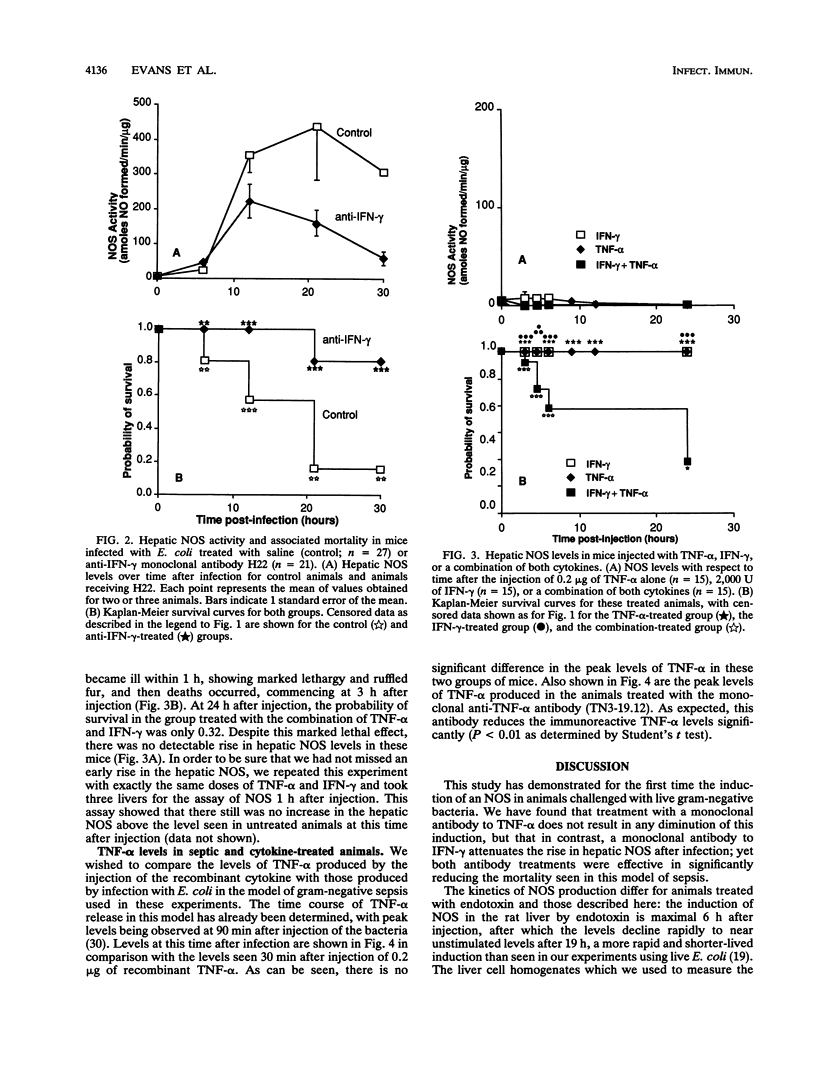
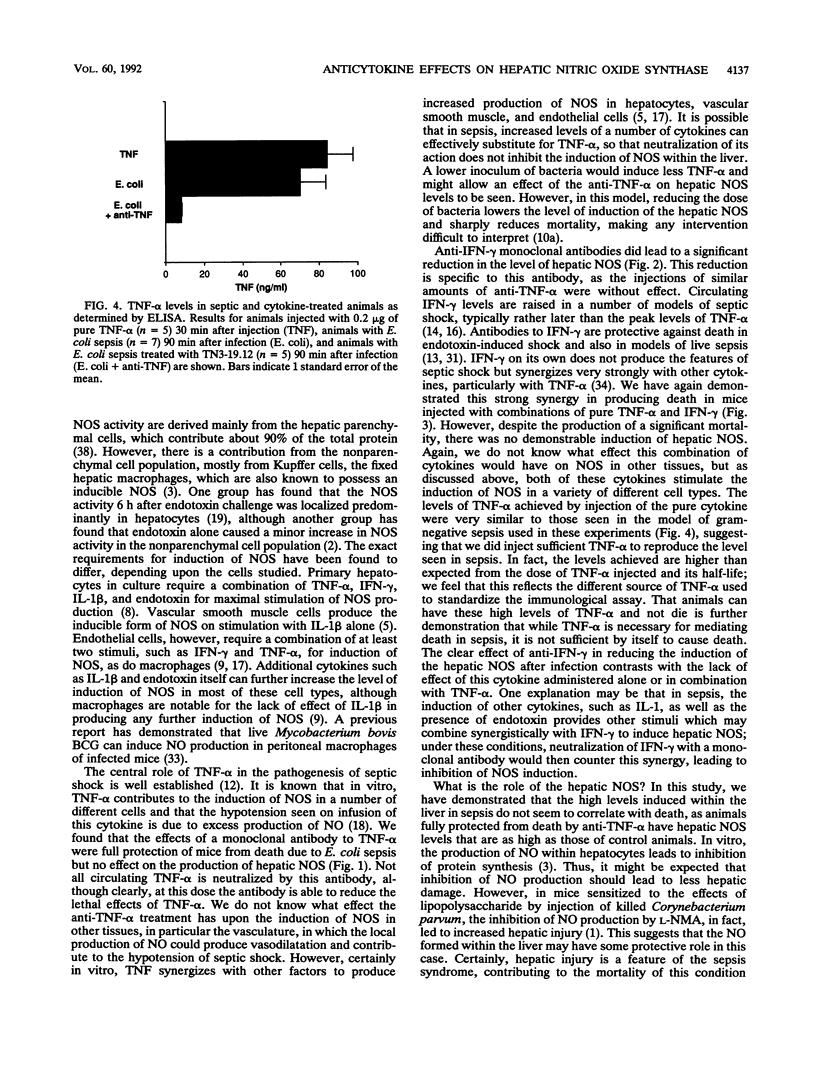
To investigate the stimuli required for the induction of nitric oxide synthase (NOS) in sepsis, we have analyzed the levels of this enzyme in the livers of mice infected with a 90% lethal dose of Escherichia coli in a model of gram-negative sepsis. Hepatic NOS levels are markedly induced in this model, with peak values occurring 12 to 22 h following infection. Treatment with TN3-19.12, a neutralizing monoclonal antibody to tumor necrosis factor alpha (TNF-alpha), resulted in complete protection from death in this model of sepsis but had no significant effect on the level of induction of hepatic NOS. Treatment with H22, a monoclonal antibody to gamma interferon (IFN-gamma), also gave significant protection against death and, in addition, did lead to a decrease in the level of induction of the hepatic NOS. Treatment of mice with pure TNF-alpha (0.2 microgram), IFN-gamma (2,000 U), or a combination of the two did not induce the hepatic NOS, but treatment with the combination led to significant mortality (probability of survival at 22 h, 0.32). Thus, the level of induction of NOS within the liver either in sepsis or by the coadministration of TNF-alpha and IFN-gamma does not correlate with death.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billiar T. R., Curran R. D., Harbrecht B. G., Stuehr D. J., Demetris A. J., Simmons R. L. Modulation of nitrogen oxide synthesis in vivo: NG-monomethyl-L-arginine inhibits endotoxin-induced nitrate/nitrate biosynthesis while promoting hepatic damage. J Leukoc Biol. 1990 Dec;48(6):565–569. doi: 10.1002/jlb.48.6.565. [DOI] [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Stuehr D. J., Stadler J., Simmons R. L., Murray S. A. Inducible cytosolic enzyme activity for the production of nitrogen oxides from L-arginine in hepatocytes. Biochem Biophys Res Commun. 1990 May 16;168(3):1034–1040. doi: 10.1016/0006-291x(90)91133-d. [DOI] [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Stuehr D. J., West M. A., Bentz B. G., Simmons R. L. An L-arginine-dependent mechanism mediates Kupffer cell inhibition of hepatocyte protein synthesis in vitro. J Exp Med. 1989 Apr 1;169(4):1467–1472. doi: 10.1084/jem.169.4.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci U S A. 1989 Nov;86(22):9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R., Mülsch A. Induction of nitric oxide synthase by cytokines in vascular smooth muscle cells. FEBS Lett. 1990 Nov 26;275(1-2):87–90. doi: 10.1016/0014-5793(90)81445-t. [DOI] [PubMed] [Google Scholar]
- Cohen J., McConnell J. S. Observations on the measurement and evaluation of endotoxemia by a quantitative limulus lysate microassay. J Infect Dis. 1984 Dec;150(6):916–924. doi: 10.1093/infdis/150.6.916. [DOI] [PubMed] [Google Scholar]
- Curran R. D., Billiar T. R., Stuehr D. J., Hofmann K., Simmons R. L. Hepatocytes produce nitrogen oxides from L-arginine in response to inflammatory products of Kupffer cells. J Exp Med. 1989 Nov 1;170(5):1769–1774. doi: 10.1084/jem.170.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran R. D., Billiar T. R., Stuehr D. J., Ochoa J. B., Harbrecht B. G., Flint S. G., Simmons R. L. Multiple cytokines are required to induce hepatocyte nitric oxide production and inhibit total protein synthesis. Ann Surg. 1990 Oct;212(4):462–471. doi: 10.1097/00000658-199010000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Evans T., Carpenter A., Cohen J. Purification of a distinctive form of endotoxin-induced nitric oxide synthase from rat liver. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5361–5365. doi: 10.1073/pnas.89.12.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley A. R., Cohen J., Buurman W., Owen R., Hanson G., Lumley J., Aulakh J. M., Bodmer M., Riddell A., Stephens S. Monoclonal antibody to TNF in severe septic shock. Lancet. 1990 May 26;335(8700):1275–1277. doi: 10.1016/0140-6736(90)91337-a. [DOI] [PubMed] [Google Scholar]
- Glauser M. P., Zanetti G., Baumgartner J. D., Cohen J. Septic shock: pathogenesis. Lancet. 1991 Sep 21;338(8769):732–736. doi: 10.1016/0140-6736(91)91452-z. [DOI] [PubMed] [Google Scholar]
- Heinzel F. P. The role of IFN-gamma in the pathology of experimental endotoxemia. J Immunol. 1990 Nov 1;145(9):2920–2924. [PubMed] [Google Scholar]
- Hesse D. G., Tracey K. J., Fong Y., Manogue K. R., Palladino M. A., Jr, Cerami A., Shires G. T., Lowry S. F. Cytokine appearance in human endotoxemia and primate bacteremia. Surg Gynecol Obstet. 1988 Feb;166(2):147–153. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- Kiener P. A., Marek F., Rodgers G., Lin P. F., Warr G., Desiderio J. Induction of tumor necrosis factor, IFN-gamma, and acute lethality in mice by toxic and non-toxic forms of lipid A. J Immunol. 1988 Aug 1;141(3):870–874. [PubMed] [Google Scholar]
- Kilbourn R. G., Belloni P. Endothelial cell production of nitrogen oxides in response to interferon gamma in combination with tumor necrosis factor, interleukin-1, or endotoxin. J Natl Cancer Inst. 1990 May 2;82(9):772–776. doi: 10.1093/jnci/82.9.772. [DOI] [PubMed] [Google Scholar]
- Kilbourn R. G., Gross S. S., Jubran A., Adams J., Griffith O. W., Levi R., Lodato R. F. NG-methyl-L-arginine inhibits tumor necrosis factor-induced hypotension: implications for the involvement of nitric oxide. Proc Natl Acad Sci U S A. 1990 May;87(9):3629–3632. doi: 10.1073/pnas.87.9.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. G., Merrett M., Salter M., Moncada S. Differential induction of brain, lung and liver nitric oxide synthase by endotoxin in the rat. Biochem J. 1990 Sep 15;270(3):833–836. doi: 10.1042/bj2700833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew F. Y., Millott S., Parkinson C., Palmer R. M., Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. J Immunol. 1990 Jun 15;144(12):4794–4797. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Nava E., Palmer R. M., Moncada S. Inhibition of nitric oxide synthesis in septic shock: how much is beneficial? Lancet. 1991 Dec 21;338(8782-8783):1555–1557. doi: 10.1016/0140-6736(91)92375-c. [DOI] [PubMed] [Google Scholar]
- Ohlsson K., Björk P., Bergenfeldt M., Hageman R., Thompson R. C. Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature. 1990 Dec 6;348(6301):550–552. doi: 10.1038/348550a0. [DOI] [PubMed] [Google Scholar]
- Petros A., Bennett D., Vallance P. Effect of nitric oxide synthase inhibitors on hypotension in patients with septic shock. Lancet. 1991 Dec 21;338(8782-8783):1557–1558. doi: 10.1016/0140-6736(91)92376-d. [DOI] [PubMed] [Google Scholar]
- Rackow E. C., Astiz M. E. Pathophysiology and treatment of septic shock. JAMA. 1991 Jul 24;266(4):548–554. [PubMed] [Google Scholar]
- Schreiber R. D., Hicks L. J., Celada A., Buchmeier N. A., Gray P. W. Monoclonal antibodies to murine gamma-interferon which differentially modulate macrophage activation and antiviral activity. J Immunol. 1985 Mar;134(3):1609–1618. [PubMed] [Google Scholar]
- Sheehan K. C., Ruddle N. H., Schreiber R. D. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J Immunol. 1989 Jun 1;142(11):3884–3893. [PubMed] [Google Scholar]
- Silva A. T., Appelmelk B. J., Buurman W. A., Bayston K. F., Cohen J. Monoclonal antibody to endotoxin core protects mice from Escherichia coli sepsis by a mechanism independent of tumor necrosis factor and interleukin-6. J Infect Dis. 1990 Aug;162(2):454–459. doi: 10.1093/infdis/162.2.454. [DOI] [PubMed] [Google Scholar]
- Silva A. T., Bayston K. F., Cohen J. Prophylactic and therapeutic effects of a monoclonal antibody to tumor necrosis factor-alpha in experimental gram-negative shock. J Infect Dis. 1990 Aug;162(2):421–427. doi: 10.1093/infdis/162.2.421. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Induction of nitrite/nitrate synthesis in murine macrophages by BCG infection, lymphokines, or interferon-gamma. J Immunol. 1987 Jul 15;139(2):518–525. [PubMed] [Google Scholar]
- Talmadge J. E., Bowersox O., Tribble H., Lee S. H., Shepard H. M., Liggitt D. Toxicity of tumor necrosis factor is synergistic with gamma-interferon and can be reduced with cyclooxygenase inhibitors. Am J Pathol. 1987 Sep;128(3):410–425. [PMC free article] [PubMed] [Google Scholar]
- Thiemermann C., Vane J. Inhibition of nitric oxide synthesis reduces the hypotension induced by bacterial lipopolysaccharides in the rat in vivo. Eur J Pharmacol. 1990 Jul 17;182(3):591–595. doi: 10.1016/0014-2999(90)90062-b. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Waage A., Halstensen A., Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987 Feb 14;1(8529):355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- Wright C. E., Rees D. D., Moncada S. Protective and pathological roles of nitric oxide in endotoxin shock. Cardiovasc Res. 1992 Jan;26(1):48–57. doi: 10.1093/cvr/26.1.48. [DOI] [PubMed] [Google Scholar]



