Abstract
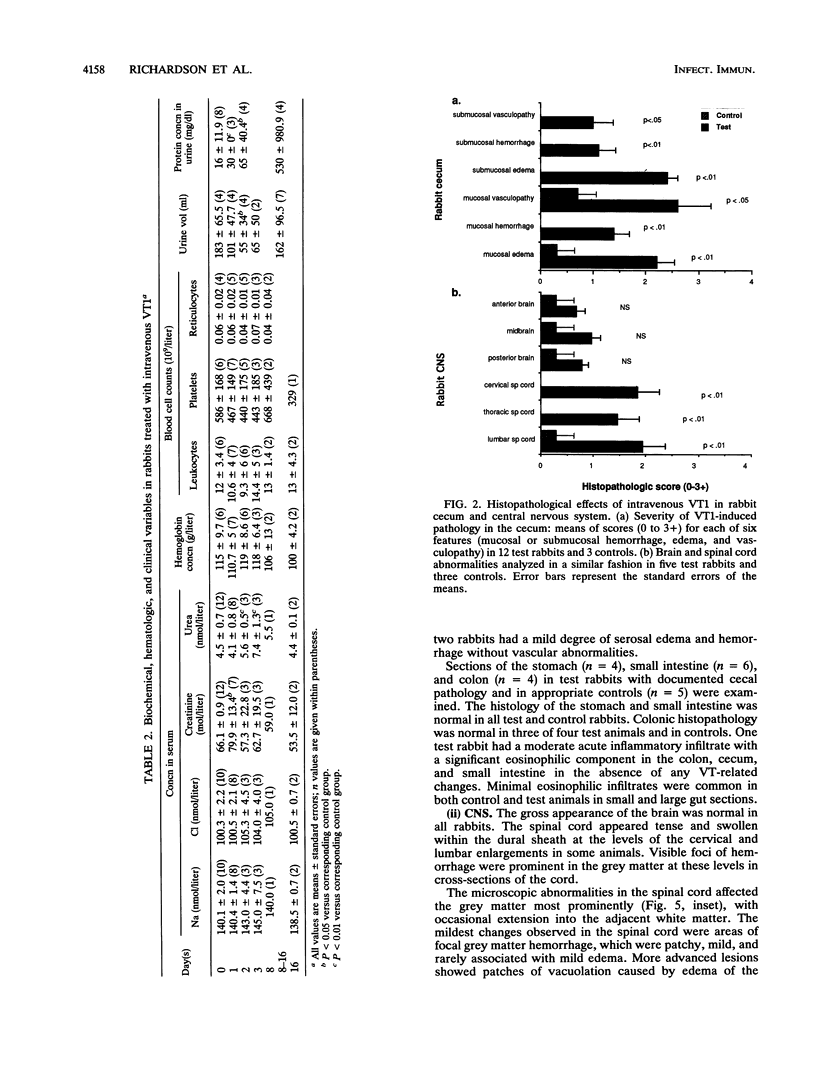
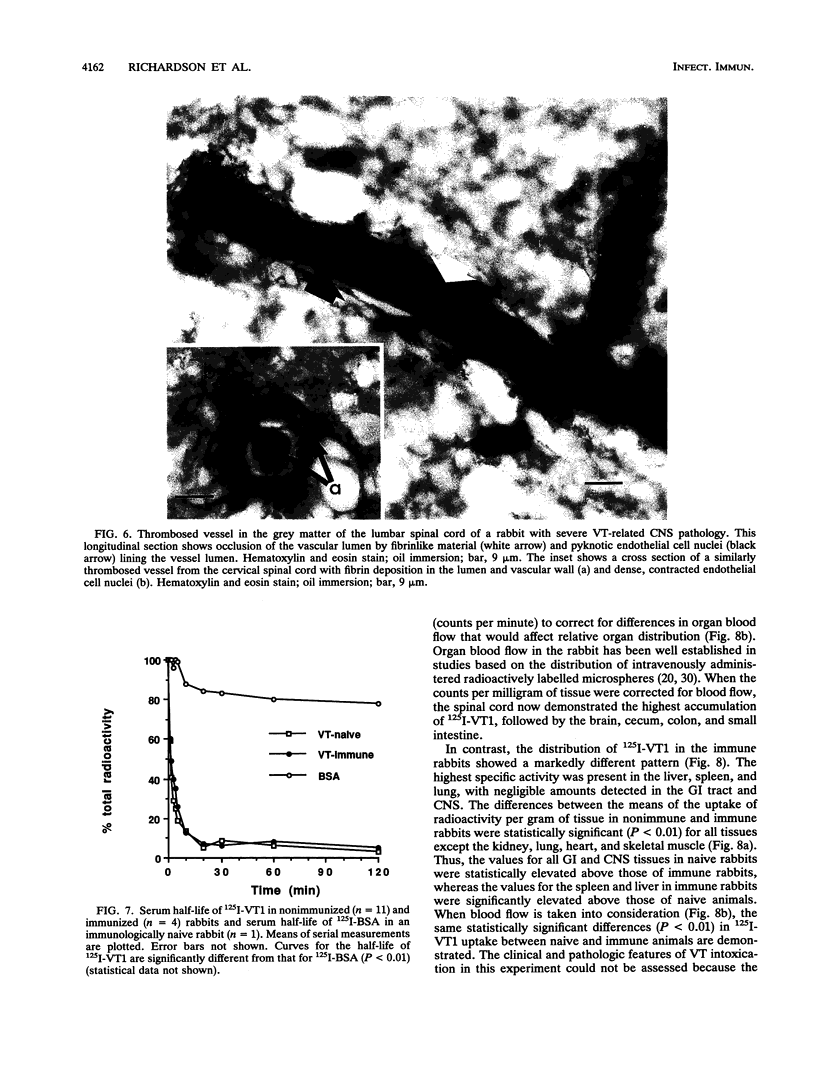
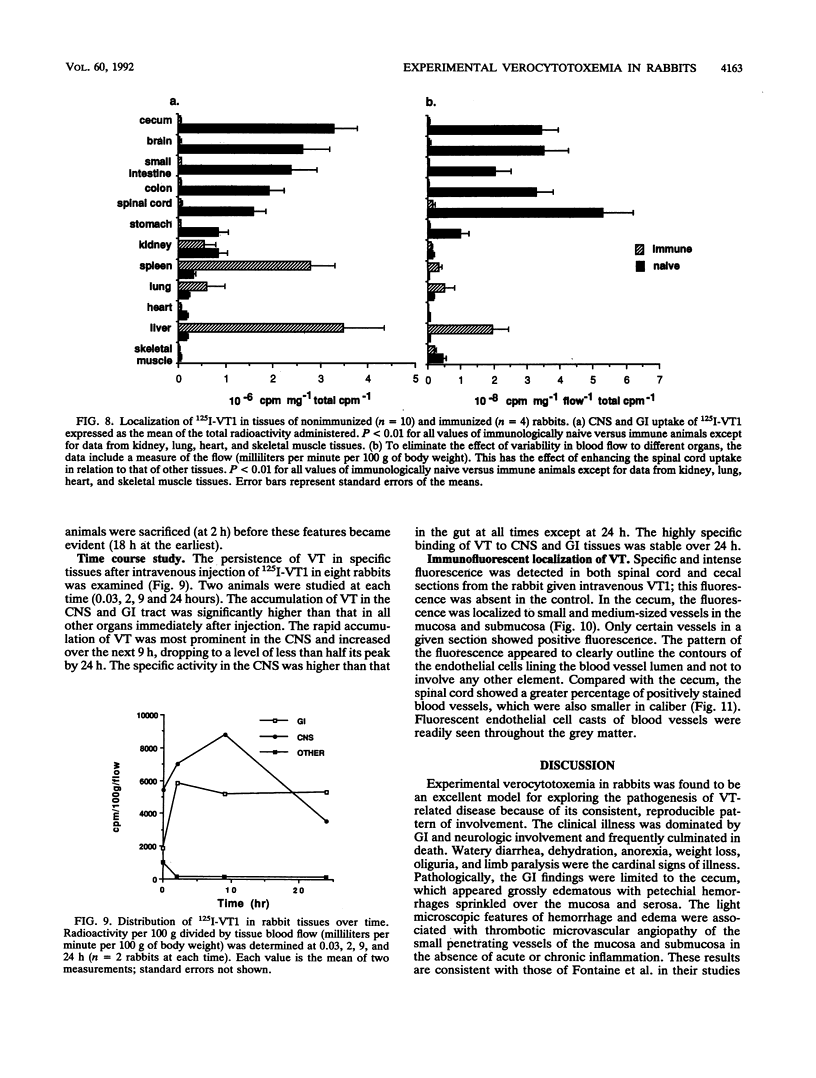
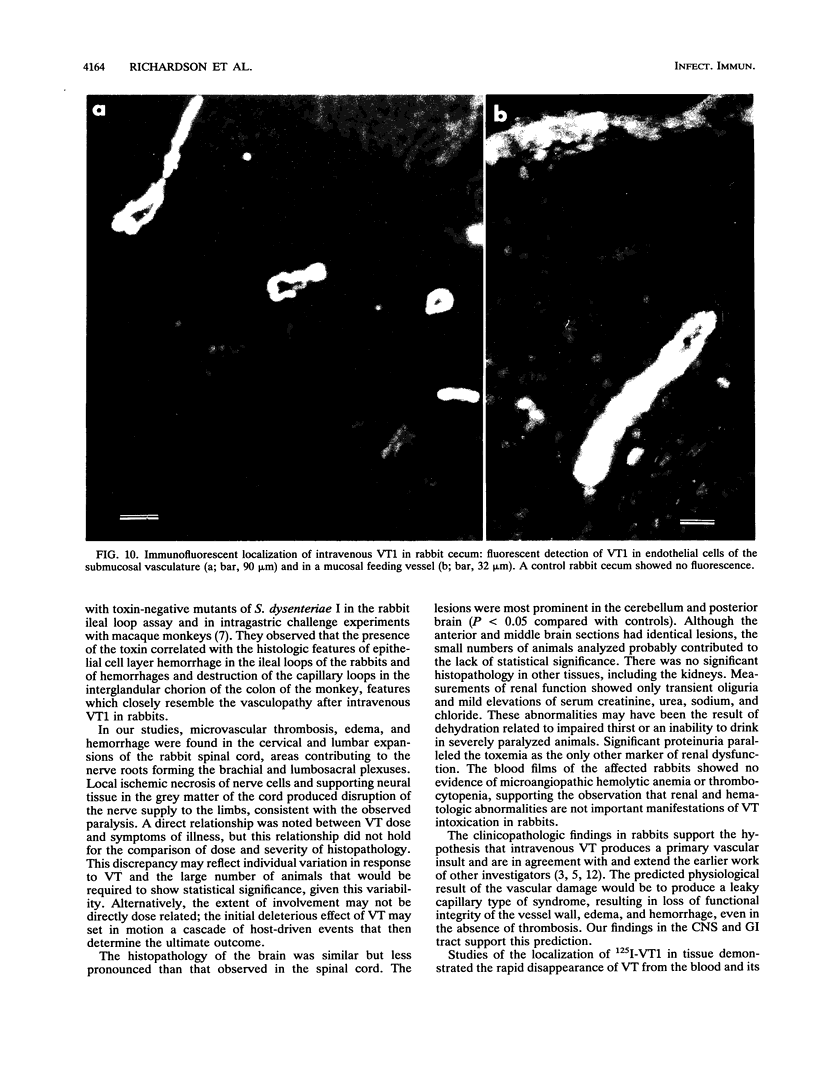
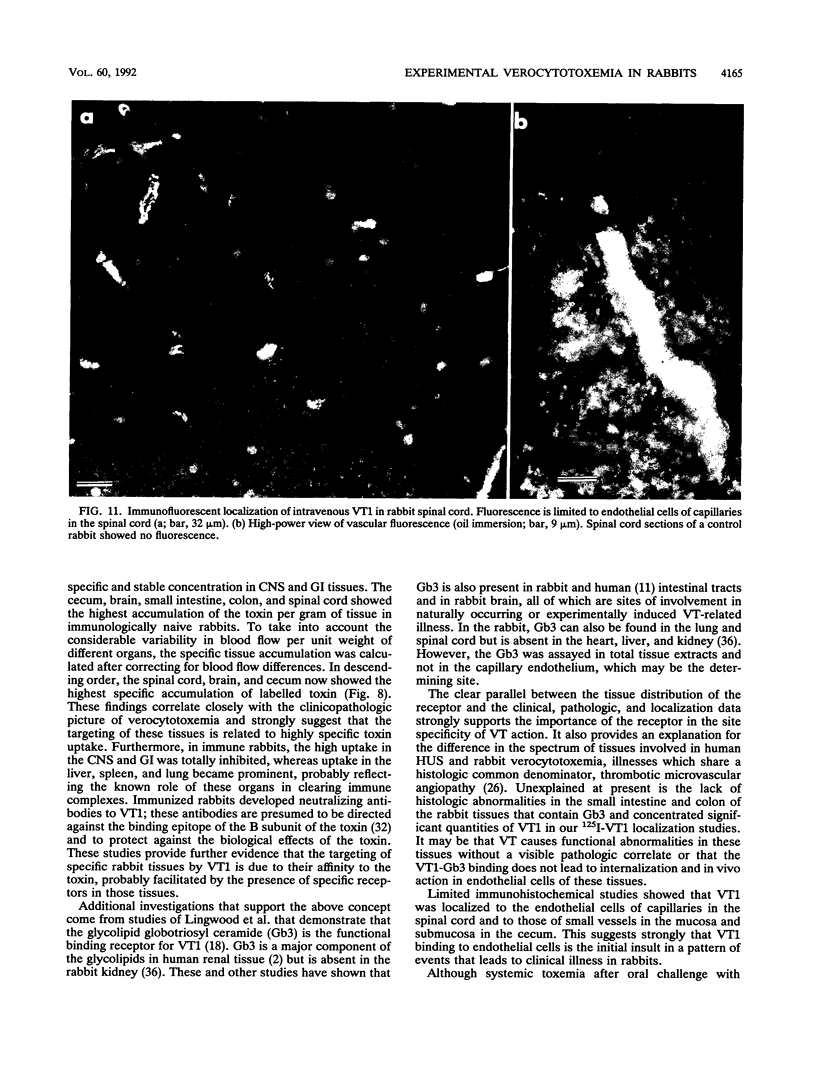
The clinicopathologic effects of intravenously administered purified verocytotoxin 1 (VT1; Shiga-like toxin 1) in 2-kg male rabbits was studied. The 50% lethal dose was 0.2 micrograms of protein per kg of body weight (2 x 10(4) 50% cytotoxic doses per kg). The clinical features included nonbloody diarrhea and a progressive flaccid paresis, usually culminating in death. The histopathology was characterized by edema and hemorrhage in the mucosa and submucosa of the cecum and edema, hemorrhage, and neuronal necrosis in the brain and gray matter of the spinal cord. Thrombotic microangiopathy, the characteristic histopathologic renal lesion in the hemolytic-uremic syndrome, was also found to be the underlying lesion in verocytotoxemic rabbits. To determine the specific distribution of VT1 in rabbit tissues, purified 125I-labelled VT1 was administered intravenously to 20 rabbits (both immunologically naive and VT1-immune rabbits). The highest specific uptake of 125I-VT1 was in the spinal cord, brain, cecum, colon, and small bowel in unimmunized animals but in the liver, spleen, and lungs in immune animals. Immunofluorescent staining of cecal and spinal cord tissues after intravenous administration of VT1 showed evidence of specific vascular endothelial cell binding of the toxin. The striking correlation of the central nervous system and gastrointestinal localization of 125I-VT1 with the sites of known histopathology is consistent with direct toxin-mediated injury to these tissues, initiated by the specific binding of VT1 to the vascular endothelium. We conclude that the vascular damage induced by VT1 in affected rabbit tissues is similar to that seen in the kidneys and other tissues in patients with verocytotoxin-producing Escherichia coli-associated hemolytic-uremic syndrome. This suggests that although the rabbit model fails to replicate human hemolytic-uremic syndrome, it is useful for studying the pathogenesis of the vascular lesions in verocytotoxin-producing E. coli-associated diseases.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRIDGWATER F. A., MORGAN R. S., ROWSON K. E., WRIGHT G. P. The neurotoxin of Shigella shigae: morphological and functional lesions produced in the central nervous system of rabbits. Br J Exp Pathol. 1955 Oct;36(5):447–453. [PMC free article] [PubMed] [Google Scholar]
- Boulanger J., Petric M., Lingwood C., Law H., Roscoe M., Karmali M. Neutralization receptor-based immunoassay for detection of neutralizing antibodies to Escherichia coli verocytotoxin 1. J Clin Microbiol. 1990 Dec;28(12):2830–2833. doi: 10.1128/jcm.28.12.2830-2833.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd B., Lingwood C. Verotoxin receptor glycolipid in human renal tissue. Nephron. 1989;51(2):207–210. doi: 10.1159/000185286. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Griffin D. E., Rothman S. W., Doctor B. P. Purification and biological characterization of shiga toxin from Shigella dysenteriae 1. Infect Immun. 1982 Jun;36(3):996–1005. doi: 10.1128/iai.36.3.996-1005.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAVANAGH J. B., HOWARD J. G., WHITBY J. L. The neurotoxin of Shigella shigae; a comparative study of the effects produced in various laboratory animals. Br J Exp Pathol. 1956 Jun;37(3):272–278. [PMC free article] [PubMed] [Google Scholar]
- Fong J. S., de Chadarevian J. P., Kaplan B. S. Hemolytic-uremic syndrome. Current concepts and management. Pediatr Clin North Am. 1982 Aug;29(4):835–856. doi: 10.1016/s0031-3955(16)34216-x. [DOI] [PubMed] [Google Scholar]
- Fontaine A., Arondel J., Sansonetti P. J. Role of Shiga toxin in the pathogenesis of bacillary dysentery, studied by using a Tox- mutant of Shigella dysenteriae 1. Infect Immun. 1988 Dec;56(12):3099–3109. doi: 10.1128/iai.56.12.3099-3109.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D. H., Moxley R. A., Andraos C. Y. Edema disease-like brain lesions in gnotobiotic piglets infected with Escherichia coli serotype O157:H7. Infect Immun. 1989 Apr;57(4):1339–1342. doi: 10.1128/iai.57.4.1339-1342.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianantonio C. A., Vitacco M., Mendilaharzu F., Gallo G. E., Sojo E. T. The hemolytic-uremic syndrome. Nephron. 1973;11(2):174–192. doi: 10.1159/000180229. [DOI] [PubMed] [Google Scholar]
- HOWARD J. G. Observations on the intoxication produced in mice and rabbits by the neurotoxin of Shigella shigae. Br J Exp Pathol. 1955 Oct;36(5):439–446. [PMC free article] [PubMed] [Google Scholar]
- Hii J. H., Gyles C., Morooka T., Karmali M. A., Clarke R., De Grandis S., Brunton J. L. Development of verotoxin 2- and verotoxin 2 variant (VT2v)-specific oligonucleotide probes on the basis of the nucleotide sequence of the B cistron of VT2v from Escherichia coli E32511 and B2F1. J Clin Microbiol. 1991 Dec;29(12):2704–2709. doi: 10.1128/jcm.29.12.2704-2709.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgersson J., Strömberg N., Breimer M. E. Glycolipids of human large intestine: difference in glycolipid expression related to anatomical localization, epithelial/non-epithelial tissue and the ABO, Le and Se phenotypes of the donors. Biochimie. 1988 Nov;70(11):1565–1574. doi: 10.1016/0300-9084(88)90292-1. [DOI] [PubMed] [Google Scholar]
- Huang A., de Grandis S., Friesen J., Karmali M., Petric M., Congi R., Brunton J. L. Cloning and expression of the genes specifying Shiga-like toxin production in Escherichia coli H19. J Bacteriol. 1986 May;166(2):375–379. doi: 10.1128/jb.166.2.375-379.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali M. A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989 Jan;2(1):15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali M. A., Petric M., Lim C., Fleming P. C., Arbus G. S., Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985 May;151(5):775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- Konowalchuk J., Speirs J. I., Stavric S. Vero response to a cytotoxin of Escherichia coli. Infect Immun. 1977 Dec;18(3):775–779. doi: 10.1128/iai.18.3.775-779.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood C. A., Law H., Richardson S., Petric M., Brunton J. L., De Grandis S., Karmali M. Glycolipid binding of purified and recombinant Escherichia coli produced verotoxin in vitro. J Biol Chem. 1987 Jun 25;262(18):8834–8839. [PubMed] [Google Scholar]
- Neutze J. M., Wyler F., Rudolph A. M. Use of radioactive microspheres to assess distribution of cardiac output in rabbits. Am J Physiol. 1968 Aug;215(2):486–495. doi: 10.1152/ajplegacy.1968.215.2.486. [DOI] [PubMed] [Google Scholar]
- O'Brien A. D., Holmes R. K. Shiga and Shiga-like toxins. Microbiol Rev. 1987 Jun;51(2):206–220. doi: 10.1128/mr.51.2.206-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrig T. G., Del Vecchio P. J., Brown J. E., Moran T. P., Rowland B. M., Judge T. K., Rothman S. W. Direct cytotoxic action of Shiga toxin on human vascular endothelial cells. Infect Immun. 1988 Sep;56(9):2373–2378. doi: 10.1128/iai.56.9.2373-2378.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai C. H., Kelly J. K., Meyers G. L. Experimental infection of infant rabbits with verotoxin-producing Escherichia coli. Infect Immun. 1986 Jan;51(1):16–23. doi: 10.1128/iai.51.1.16-23.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
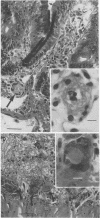
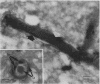
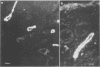
- Richardson S. E., Karmali M. A., Becker L. E., Smith C. R. The histopathology of the hemolytic uremic syndrome associated with verocytotoxin-producing Escherichia coli infections. Hum Pathol. 1988 Sep;19(9):1102–1108. doi: 10.1016/s0046-8177(88)80093-5. [DOI] [PubMed] [Google Scholar]
- Schmitt C. K., McKee M. L., O'Brien A. D. Two copies of Shiga-like toxin II-related genes common in enterohemorrhagic Escherichia coli strains are responsible for the antigenic heterogeneity of the O157:H- strain E32511. Infect Immun. 1991 Mar;59(3):1065–1073. doi: 10.1128/iai.59.3.1065-1073.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Shadduck J. A., Pakes S. P. Encephalitozoonosis (nosematosis) and toxoplasmosis. Am J Pathol. 1971 Sep;64(3):657–672. [PMC free article] [PubMed] [Google Scholar]
- Smith D. R., Smith H. J., Rajjoub R. K. Measurement of spinal cord blood flow by the microsphere technique. Neurosurgery. 1978 Jan-Feb;2(1):27–30. doi: 10.1227/00006123-197801000-00005. [DOI] [PubMed] [Google Scholar]
- Strockbine N. A., Jackson M. P., Sung L. M., Holmes R. K., O'Brien A. D. Cloning and sequencing of the genes for Shiga toxin from Shigella dysenteriae type 1. J Bacteriol. 1988 Mar;170(3):1116–1122. doi: 10.1128/jb.170.3.1116-1122.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strockbine N. A., Marques L. R., Holmes R. K., O'Brien A. D. Characterization of monoclonal antibodies against Shiga-like toxin from Escherichia coli. Infect Immun. 1985 Dec;50(3):695–700. doi: 10.1128/iai.50.3.695-700.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S., Chow C. W., Powell H. R. Cerebral infection with Escherichia coli O157:H7 in humans and gnotobiotic piglets. J Clin Pathol. 1988 Oct;41(10):1099–1103. doi: 10.1136/jcp.41.10.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S., Wachsmuth K. I., Smithers J., Jackson C. Studies in gnotobiotic piglets on non-O157:H7 Escherichia coli serotypes isolated from patients with hemorrhagic colitis. Gastroenterology. 1988 Mar;94(3):590–597. doi: 10.1016/0016-5085(88)90228-4. [DOI] [PubMed] [Google Scholar]
- Upadhyaya K., Barwick K., Fishaut M., Kashgarian M., Siegel N. J. The importance of nonrenal involvement in hemolytic-uremic syndrome. Pediatrics. 1980 Jan;65(1):115–120. [PubMed] [Google Scholar]