Abstract
Mammary gland involution is a period of intensive tissue remodeling. Over the course of a relatively brief period, a large proportion of the mammary gland epithelium undergoes programmed cell death and is removed by phagocytes. In addition, the gland is cleared of residual milk fat globules as well as milk and adipocytes become the predominant cell type. The role of the immune system in this process has not been clearly defined. Professional phagocytes derived from the immune system can participate in the clearance of apoptotic and autophagic cells, the removal of residual milk components, and the prevention of mastitis during mammary gland involution. However, many of these functions can also be performed by non-professional phagocytes (e.g. mammary epithelial cells). This review will discuss the evidence that supports a role for innate immune cells in mammary gland remodeling during involution.
Keywords: Involution, Mammary gland, Phagocytosis, Innate immunity, Remodeling
Introduction
Mammary gland involution involves extensive tissue remodeling characterized by massive programmed cell death of milk producing epithelial cells, removal of apoptotic cells, milk fat globules, and milk, and repopulation of the mammary gland stroma with differentiated adipocytes. Remarkably, the majority of remodeling is complete in mice 5 days after involution has commenced; leaving a gland that closely resembles the mature virgin state. The role of cells derived from the immune system in coordinating mammary gland ductal development prior to pregnancy is well established [1, 2]. The precise role of these cells in regulating mammary gland involution is less clear. The requirement for the removal of a large quantity of apoptotic cells and milk components during involution makes it tempting to assume that professional phagocytes of the innate immune system play a critical role in this process.
Background
The mature virgin mammary gland consists of a branching network of ductal epithelium that extends throughout a stromal compartment composed of adipocytes, fibroblasts, blood vessels, and leukocytes. Lymph nodes are also present within the normal mammary gland stroma. During pregnancy, numerous secondary and tertiary branches sprout from the ductal epithelium and develop into the milk-producing secretory epithelium. As late pregnancy transitions to lactation, the mammary gland consists almost completely of secretory epithelium forming alveolar structures with lumens full of milk fat globules and milk. One dramatic indication of the magnitude of this change in mammary gland architecture is the difference in mammary gland weight. The fully developed lactating mammary gland in a mouse is seven–ten times heavier than the mature virgin gland.
Involution of the mammary gland in the mouse begins when pups stop suckling. In natural involution, pups will continue to suckle intermittently as they transition to a solid diet. Therefore, in natural involution, mammary gland remodeling proceeds in an unsynchronized fashion with different areas of the gland undergoing involution at different times. This scenario is further complicated by the fact that in many species the female may become pregnant again immediately after giving birth. In that case, the natural involution process is altered, as the lactating female will give birth to a new litter while in the transition phase from weaning her previous litter. In experimental models of forced involution, the male is removed from the cage of a pregnant female prior to parturition to simplify analysis. In addition, the numbers of pups are normalized at birth and all pups are removed simultaneously at mid-lactation, allowing for homogenous involution of the whole mammary gland and minimizing biological variability for experimental analysis. With forced involution in mice (Fig. 1), milk production continues during the first 24 h after weaning and the gland reaches its maximal weight. As the alveolar lumens distend with milk, the milk-producing epithelium begins to undergo programmed cell death [3, 4]. Biochemical and histological evidence of apoptosis (Type I PCD) is apparent within the first 24 h after involution [5–7].
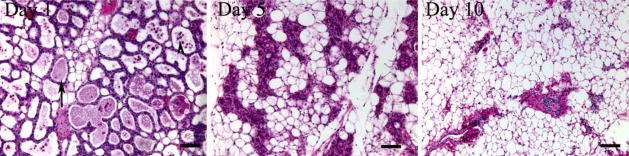
Figure 1.
Forced mammary gland involution in mice. Hematoxylin and Eosin stained sections taken from the mammary glands of mifce that have undergone forced involution. On day 1, residual milk components (arrow) can be seen within the alveolar lumens which still resemble the lactating gland. Apoptotic cells can also be seen shed into the alveolar lumens (arrowhead). By day 5, the alveolar structures have collapsed and adipocytes appear as a more prominent component of the gland. By day 10, there is scant evidence of the mammary gland epithelium and the gland is primarily made up of fat, resembling the mature virgin gland. (Bar=0.2 mm).
As MEC undergo PCD they express classic markers of apoptosis including redistribution of phosphatidylserine from the inner leaflet to the outer leaflet of the cell membrane [8]. Apoptotic cells that are not phagocytized while they remain in the epithelium lose contact with the basement membrane and are extruded into the alveolar and ductal lumens where they appear as late apoptotic cells or apoptotic bodies and are available for clearance by phagocytes (Fig. 1, day 1) [9]. In mice, the mammary gland can return to the lactating state if suckling by pups is resumed within the first 48 h after weaning. This interval has been described as the first stage of involution [4]. The importance of milk stasis in regulating the first 48 h involution has been experimentally demonstrated in models of unilateral teat sealing in which the sealed gland undergoes involution while the unsealed gland continues to sustain suckling pups [10]. These experiments also suggest that hormonal influences are not critical in triggering the early phase of involution. At some point the process of involution becomes irreversible and the release of proteases triggers the onset of massive PCD of the remaining secretory epithelium followed by differentiation of and repopulation of the mammary gland stroma by adipocytes [11]. In mice this latter stage of involution occurs after 48 h and is initially characterized by the highest proportion of apoptotic nuclei within the mammary gland [7, 12]. The massive burden of apoptotic cells in involution necessitates an orderly and efficient mechanism for clearance of these cells. In addition to physically removing these now redundant cells, apoptotic cell clearance prevents the inflammation associated with cell lysis and necrosis and stimulates phagocytes to secrete anti-inflammatory cytokines [13, 14]. For normal mammary gland remodeling to take place, milk fat globules and residual milk must also be efficiently and rapidly removed.
The Role of Non-professional Phagocytes in Mammary Gland Involution
As mentioned above, apoptotic cells, cellular debris, and milk components must be cleared for normal involution to proceed. The removal of apoptotic cells has been historically attributed to phagocytosis by tissue macrophages [15], the classic “professional phagocyte”. However, it has become increasingly evident that all cell types have the ability to phagocytize apoptotic cells and that they utilize many of the same receptors used by professional phagocytes. Therefore “nonprofessional phagocytes” such as epithelial cells, endothelial cells, and fibroblasts all have the capability to participate in the removal of neighboring cells that have undergone apoptosis [16–18]. The important contribution of epithelial-mediated phagocytosis in maintaining tissue homeostasis has been demonstrated in vivo in the retinal epithelium [19, 20]. Retinal epithelial cells use the αvβ5 integrin to phagocytize spent rod photoreceptor outer segments that are shed daily in a cyclical fashion. Mice deficient in the β5 integrin subunit have impaired epithelial-mediated phagocytosis of rod outer segments and develop age-dependent blindness. Several other types of epithelial cells have been shown to be capable of phagocytosis in vitro. Human alveolar and airway epithelial cells can engulf apoptotic eosinophils [21–23], suggesting that epithelial-mediated phagocytosis may help to accelerate the resolution of inflammation in the lung.
While it has been convincingly demonstrated that most, if not all, cell types can participate in phagocytosis, the relative efficiency of apoptotic cell clearance by nonprofessional phagocytes has not been experimentally resolved. One recent study used time-lapse in vitro microscopy to compare the efficacy of phagocytosis by professional and nonprofessional phagocytes. Microglia (the professional phagocytes in brain) engulfed apoptotic prey within minutes of encountering them by extending lamellipodia around them. Digestion of ingested cells by microglia was also rapid and complete within 35–95 min of ingestion. The BHK fibroblast cell line was studied in vitro as a non-professional phagocyte. As opposed to the motile microglia, BHK cells were sessile in vitro and, when encountering apoptotic prey, intermittently produced membrane ruffles at areas of contact with the apoptotic cells. BHK cells, however, did not ingest the apoptotic cells for several hours, did not extend lamellipodia for engulfment, and after ingestion took longer than their professional counterparts to digest apoptotic cells [24]. When the authors fed the BHK cells apoptotic prey that had been allowed to remain in culture for 2 or 9 h prior to coculture, the BHK cells were much more efficient at ingesting the cells suspended for 9 h than 2 h. The authors concluded that cells that have recently undergone apoptosis are recognized only by professional phagocytes and that further unknown changes in the cell surface of the apoptotic cell are required for recognition and engulfment by nonprofessional phagocytes. Other in vitro data suggest that non-professional phagocytes are less efficient at phagocytosis [8, 24]. Though non-professional phagocytes may take longer to ingest apoptotic cells, they can be an important source of cytokines, much like their professional counterparts. For example, cultured bovine mammary gland epithelial cells can secrete Il-1α, Il-8, and TNFα in response to stimulation with E. Coli and lipopolysaccharide [25, 26]. Non-professional phagocytes can also influence the resolution of inflammation through phagocytosis of apoptotic inflammatory cells [27].
In the mammary gland, epithelial cells mediate a significant proportion of phagocytosis of apoptotic cells during involution (Fig. 2) [7, 28–30]. One recent report showed that in mice, by day 3 of forced involution, the majority of viable epithelial cells contained ingested apoptotic cells within their cytoplasm [8]. MEC utilize many of the same molecules for apoptotic cell clearance as professional phagocytes. Mammary gland cell lines express calreticulin, CD91, CD36, and the αvβ3 integrin, and uptake of apoptotic cells in vitro by MEC can be attenuated by the addition of inhibitors to C1q and MBP, two collectins that target apoptotic cells for phagocytosis [8]. Furthermore, the lipopolysaccharide receptor CD14, which serves as a scavenger receptor for apoptotic cell clearance [31], is strongly expressed on murine MEC [32]. Two recent reports have shown that MEC use the bridging molecule Mfge8 for clearance of apoptotic primary mammary gland epithelial cells and thymocytes in vitro [7, 12]. Mice deficient in Mfge8 have impaired in vivo epithelial mediated phagocytosis of epithelial cells [7]. Lactadherin (the human orthologue of Mfge8), CD36, and thrombospondin-1, a molecule with an established in vitro role in apoptotic cell clearance, are all expressed in the human mammary gland [33–36]. Like professional phagocytes, MEC can produce key cytokines and growth factors in response to engulfment of their apoptotic neighbors. MEC produce the anti-inflammatory cytokine TGFβ in vitro in response to apoptotic cell uptake [37], and a mammary gland cell line produces VEGF after apoptotic cell engulfment [38].
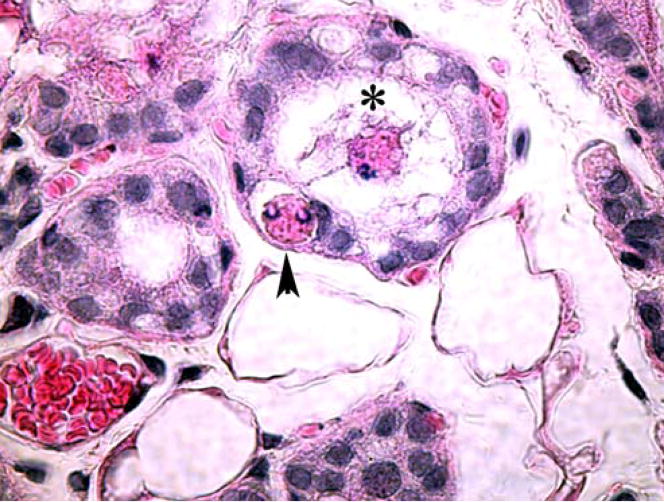
Figure 2.
Mammary gland epithelial cell-mediated apoptotic cell clearance in vivo. Late apoptotic cells (asterisk) can be seen in the alveolar lumen. An apoptotic cell can be seen within the cytoplasm of a viable epithelial cell (arrowhead) with a rim of clear-appearing extracellular fluid surrounding the apoptotic cell (the spacious phagosome).
MEC also participate in the removal of milk fat globules, as demonstrated by extensive histological evidence of milk fat globules within the cytoplasm of viable epithelial cells during early involution. In the involuting bovine mammary gland, the non-secretory epithelial cells extend pseudopodia to phagocytize casein micelles and milk fat globules that can then be found within cytoplasmic vacuoles [32, 39] Histological evidence of milk fat globules within the viable epithelial cells during forced involution in mice is easily discernible. Like apoptotic cells, milk fat globules express phosphatidylserine on their external surface and are cleared by an Mfge8-dependent mechanism [12]. The ability of MEC to remove apoptotic cells and residual milk components and to produce anti-inflammatory cytokines and growth factors provides a strong rationale for an important role for these non-professional phagocytes in mammary gland remodeling.
Evidence Supporting Roles for Professional Phagocytes in Mammary Gland Remodeling
Infiltration of the Mammary Gland with Cells of the Immune System During Involution
One important issue relevant to the role professional phagocytes might play in mammary gland involution is the temporal and spatial distribution of these cells during this time. Though earlier studies suggested that the macrophage population did not increase during involution [30, 40], several recent studies using immunostaining with cell type specific antibodies during forced involution in mice have shown an increase in neutrophils, macrophages and lymphocytes [4, 7, 12, 32]. Hanayama et al. reported that cells expressing CD68 (a cell surface protein specific for monocytes and macrophages) were completely absent in the mammary gland during lactation, first appeared on day 2 of involution and subsequently increased in number until at least day 4 [12]. Using morphological analysis coupled with immunohistochemistry staining for F4/80 antigen (another macrophage-specific marker) and B220 antigen (a marker found on B-cells), Stein et al., showed very few neutrophils, macrophages, or eosinophils on lactation day 7, with an increase in neutrophils beginning on day 1 and macrophages, eosinophils, plasma cells, and B-lymphocytes beginning on day 4 of involution [32]. Based on immunostaining with antibody to CD45 (a marker present on the surface of all leukoctyes), we found very few leukocytes on lactation day 10, with a progressive increase in number during involution [7]. Others have found a similar increase in leukocytes in the involuting mammary glands of sheep [41]. Investigators studying dairy cows and sheep have extensively characterized leukocytes present in milk obtained during involution. Numerous studies have identified macrophages, neutrophils, lymphocytes, and a small proportion of dendritic cells in secretions obtained from involuting glands [37, 41, 42]. Taken together, these data suggest that involution is associated with an early influx of neutrophils followed closely by macrophages and lymphocytes. It is important to note that since the mammary gland decreases tremendously in size during early involution and milk production stops, any increase in the number of inflammatory cells per tissue section or microliter of ductal secretions may not reflect an increase in the total number of inflammatory cells in the gland, but rather an increase in the concentration of the cell types [37, 43]. Even with this caveat in mind, the histological and cytological data suggest that at least during the later stages of involution, inflammatory cells are present within the mammary gland and can contribute to the removal of apoptotic cells and residual milk, thereby facilitating mammary gland remodeling.
The anatomical location of professional phagocytes within the mammary gland can also provide some insight into their potential roles in remodeling (Fig. 3). The presence of macrophages in the mammary gland interstitium is well described. However, it is not as clear whether these cells are present within the ductal lumens, where epithelial apoptotic cells are shed and milk fat globules and milk proteins reside. One study in sheep identified CD45 positive cells within ductal lumens adjacent to apoptotic epithelial cells, suggesting that these immune cells are actively involved in uptake of shed epithelial cells [41]. In our experience of forced involution in mice, CD45 positive cells are found predominantly in the interstitial spaces but can also be seen immediately adjacent to the alveolar and ductal lumens (Fig. 3). While we have not observed immune cells within the ductal lumens in mice, the preponderance of data identifying immune cells in milk secretions taken from sheep and cows strongly suggests that these cells traverse into the ductal lumens.
Figure 3.
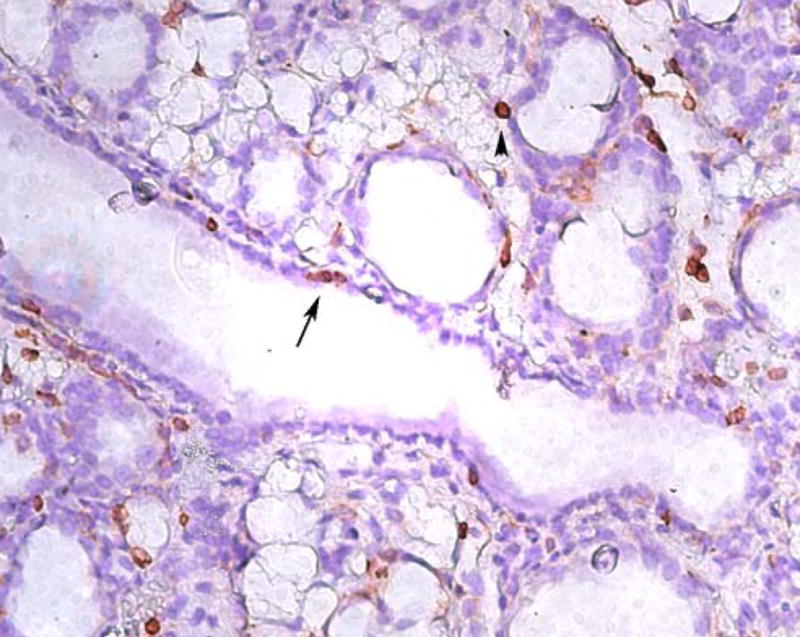
Distribution of immune cells in the mammary gland during involution. Immunolocalization of cells from the immune system using an anti-CD45 antibody on day 4 of forced involution. Note that immune cells can be found scattered throughout the interstitium as well as adjacent to alveolar lumens (arrowhead) and mammary gland ducts (arrow).
Functional Roles of Cells of the Immune System During Mammary Gland Involution
Accepting that immune cells increase in number during involution, the next important question is whether they are involved in clearance of apoptotic cells and milk components. Tatarczuch et al. [41] used electron microscopy to examine the cytoplasmic contents of neutrophils and macrophages recovered from sheep mammary gland involution secretions. Both cell types contained evidence of apoptotic bodies, lipid vacuoles, and casein. Other studies have also implicated professional phagocytes in clearance of milk components and epithelial cells in vivo [44–46]. Interestingly, several investigators have suggested that the phagocytic capacity of neutrophils and macrophages during involution is impaired because they are occupied with clearance of milk components [47, 48]. Tatarczuch et al. evaluated the phagocytic capability of neutrophils and macrophages isolated from mammary secretions at different time points during involution [42]. Using an in vitro assay of uptake of latex beads, they found that the phagocytic capacity of neutrophils and macrophages obtained from sheep was maximal later in involution, coinciding with when these cells had less cytoplasmic evidence of ingested milk components. Similarly, others have shown that neutrophils and macrophages are less competent at phagocytosis of bacteria in vitro when they are obtained from early involution compared with late involution [49]. Sordillo et al. [50] reported that the clearance capacity of professional phagocytes isolated from milk is lower than of those isolated from blood. It is important to note that the literature generated from studies of sheep and cows comes primarily from the dairy industry and is geared towards evaluating optimal strategies for milk production [51]. When evaluating phagocytic capacity, these investigators are primarily interested in evaluating the ability of professional phagocytes to ingest bacteria. Whether the ability of professional phagocytes to engulf apoptotic epithelial cells is impaired by clearance of milk components in vivo is unclear. In models of forced involution in mice, where infection does not appear to be a significant concern, the primary cell that needs to be cleared by phagocytes is the apoptotic epithelial cell. In mice, it is likely that interstitial macrophages participate in phagocytosis of milk components and apoptotic epithelial cells. Macrophages isolated from mouse mammary glands in early involution maintain efficient uptake capacity when engulfing apoptotic thymocytes in vitro [12].
Gene Expression Array Data Supporting an Inflammatory Response During Forced Involution
The most compelling recent evidence highlighting a prominent role for inflammatory cells and inflammation in mammary gland remodeling comes from two studies of differential gene expression during forced involution in mice [32, 52, 53]. Clarkson et al. [53] compared gene expression at 12 time points, including the virgin gland, several time points during pregnancy and lactation, and days 1 through 4 of involution. The authors observed that 6,796 transcripts (54% of the transcripts assessed and approximately half of the protein-encoding content of the mouse genome) changed across the 12 time points evaluated, underscoring the complexity of the changes seen during the pregnancy/lactation/involution cycle in the mammary gland. Significantly, 28% of all transcripts analyzed were either up or down-regulated during involution. Further analysis comparing five involution time points (12, 24, 48, 72, and 96 h) with two lactation time points (day 5 and day 10) revealed an initial transient increase in pro-inflammatory genes followed by a delayed increase in expression of genes involved in inflammation, innate immunity, and antimicrobial defense. In the first 12 h of forced involution, there was an increase in the pro-inflammatory cytokines IL-1α, IL-1β, and IL-13, the neutrophil chemoattractant Cxcl1, and the acute phase mediator Lifr. There was no increase in neutrophil markers, such as Fut4, Tnfrsf5, or Caecam, perhaps because of the relatively low concentrations of RNA present in neutrophils. In addition, genes that attenuate the inflammatory response of neutrophils, such as clusterin and utercalin, were upregulated. The authors concluded that the early acute phase response seen in involution is tightly regulated, with expression of both pro and anti-inflammatory molecules. As involution progressed, there was an increase in monocyte, macrophage, and lymphocyte chemoattractants, and in monocyte/macrophage markers, such as Lrp, CD14, and Csf1r, as well as in immunoglobin gene expression. Genes encoding soluble defense factors important in innate immunity, such as complement components and lactoferrin, were also upregulated. A simultaneously published study by Stein et al. [32] compared gene expression during the first 4 days of involution with lactation day 7. Although the specific genes induced in this study were somewhat different from those in the study by Clarkson, et al., the overall pattern was quite similar. Of the 145 genes that were upregulated during the first 4 days of involution, 49 were immunoglobin genes and several were genes involved in the acute phase response, such as CD14, STAT3 and LPS-binding protein (LBP). The neutrophil-attracting chemokine CXCL1/KC was increased on day 1 of involution and supported an early neutrophilic response. Genes encoding macrophage and eosinophil chemokines and differentiation markers, such as CXCL14, CD68, and cathepsin S, increased on days 3 and 4 of involution, supporting a role for macrophages and eosinophils at later time points. An increase in genes expressed by plasma cells and B lymphocytes was also seen on day 3 and 4 of involution.
The Role of Autophagy in Mammary Gland Involution
The process of apoptosis, or Type I PCD, has not been extensively studied in the involuting mammary gland, yet it is clear that evidence of apoptosis wanes just a few days into forced involution in mice, when large-scale clearance of the secretory epithelial cells is still ongoing. The process of autophagy, or Type II PCD is understudied, though it is a phylogenetically old process that is conserved across species [54]. Like apoptosis, autophagy can occur in both normal tissue homeostasis and in disease and is important in tissues with high cell turnover. Autophagy is morphologically characterized by degradation of cytoplasmic components through formation of autophagic vacuoles prior to nuclear collapse. Like apoptosis, cells that undergo autophagic death are removed by phagocytosis [54, 55]. The relative importance and triggers of autophagic cell death in the involuting mammary gland have not been well established. Several studies have identified MEC autophagy during involution [9, 56]. A role in mammary gland tumors has also been suggested [57, 58]. One recent report suggests that TGFβ1 induces both autophagy and apoptosis in a bovine mammary epithelial cell line [59]. The consequences to mammary gland remodeling from cell death due to autophagy are unknown. Apoptotic cell death is associated with expression of phosphatidylserine on the cell surface, and binding of phosphatidylserine has been proposed to elicit a potent anti-inflammatory effect through the release of TGFβ [60, 61]. Whether phagocytosis of autophagic cells elicits the same anti-inflammatory responses from phagocytes is unknown.
Summary/Conclusions
The experimental data of forced involution in mice suggests that at early stages epithelial cells play a dominant role in the remodeling process, with a progressively increasing role for professional phagocytes from the immune system as involution progresses and epithelial cells regress (Fig. 4). A primary role for epithelial cells in the first stage of forced involution in mice is supported by several observations. In the first 24–48 h, there are very few professional phagocytes within the mammary gland. At this time, a large number of epithelial cells have undergone apoptosis and require clearance, and there is the maximal accumulation of milk and milk fat globules that also require clearance. The histological evidence of milk fat globules and apoptotic epithelial cells within the viable mammary gland epithelium at this time demonstrate that MEC are actively involved in the remodeling process. Though professional phagocytes are present, in absolute numbers they represent less than 4% of the total population of viable cells in the gland. Therefore, it appears unlikely that these cells are present in sufficient numbers to mediate the majority of clearance of apoptotic cells and milk components at this time. This reasoning, however, does not exclude an important contribution of professional phagocytes at this stage. The gene expression array data during the first 24 h after forced involution provides evidence for an acute inflammatory response. Neutrophils constitute the first wave of professional phagocytes recruited to the involuting gland. At this stage, neutrophils may be critical in preventing mastitis in the vulnerable period of milk stasis through phagocytosis and eradication of bacteria. The co-expression of anti-inflammatory genes may serve to limit the potential deleterious effects of neutrophil activation and/or apoptosis, especially if no pathogens are encountered.
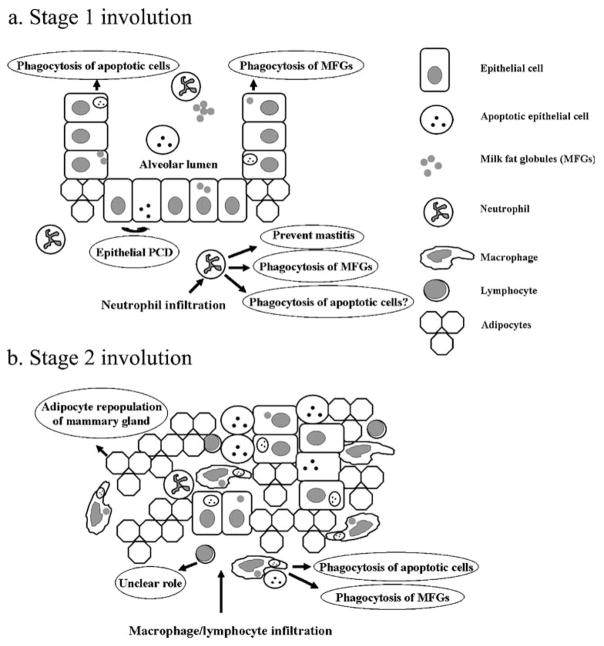
Figure 4.
Schematic diagram of mammary gland remodeling during involution. a Stage 1 of forced involution in mice (initial 48 h) is characterized by programmed cell death of epithelial cells and infiltration of the gland with neutrophils. At this stage the alveolar luminal structure of lactation remains intact, and viable epithelial cells are the predominant nonprofessional phagocytes responsible for removing apoptotic cells and milk fat globules. Note the relative paucity of adipocytes. b Stage 2 involution is characterized by massive epithelial cell apoptosis and alveolar collapse with infiltration of the gland by macrophages and lymphocytes. Macrophages likely mediate a proportion of apoptotic cell and milk fat globule phagocytosis at this time. Evidence of ingested apoptotic cells and milk fat globules within the cytoplasm of the remaining viable epithelial cells supports their ongoing participation in engulfment at this stage. The role of B-lymphocytes and plasma cells that infiltrate the gland is unclear. As Stage 2 involution progresses, adipocytes differentiate and repopulate the mammary gland stroma and with the loss of epithelial cells become the predominant cell type.
As forced involution proceeds to the protease-dependent stage, there is further massive apoptosis and autophagic cell death of the remaining secretory epithelium, followed by accumulation in the gland of more professional phagocytes. By this time the removal of milk components and apoptotic cells have resulted in a near sevenfold decrease in the size of the murine gland. This decrease in size of the gland over the first 48 h highlights the significant contribution that nonprofessional phagocytes make to the remodeling process. By day 3 and day 4, a large proportion of the secretory epithelium has either been cleared or has undergone programmed cell death with the remaining viable epithelium containing abundant evidence of ingested cells and debris. Days 3 and 4 are also the times at which the number of infiltrating macrophages increases. Though the remaining mammary gland epithelium participates in phagocytosis, the now abundant interstitial macrophages likely contribute to the removal of apoptotic cells and milk components.
By day 5 of forced involution in mice, the majority of apoptotic cells and milk components have been cleared and the gland is well on its way to resembling the mature quiescent gland. Although there is a progressive decrease in cellular debris and milk fat globules requiring removal, increased numbers of immune cells remain within the gland. These cells are likely to play important roles that extend beyond clearance of apoptotic cells and milk. Of particular interest is the histological evidence for an increase in the number of lymphocytes and gene expression array data for an increase in expression of immunoglobin genes. It is unclear whether this response is directed against bacterial pathogens or intracellular antigens released by dying cells. It has been suggested that bovine macrophages function as antigen-presenting cells when encountering bacteria during involution [62], and macrophages are often found attached to lymphocytes in sheep mammary gland involution secretions [42] and in human colostrum [47].
The extensive literature from the dairy industry focuses on the role of professional phagocytes in preventing bacterial infections, mastitis, and subsequent impaired milk production. Though these studies do not examine forced involution, their observations are consistent with the more scientifically precise mouse models of forced involution. Specifically, they provide evidence for infiltration of the mammary gland during involution with professional phagocytes that engulf milk components and bacteria. These studies have shown that aberrant mammary gland remodeling due to mastitis leads to mammary gland dysfunction and impaired lactation [51, 63]. Interestingly, delayed involution and non-infectious mastitis in mice are also associated with aberrant mammary gland remodeling and impaired lactation with subsequent pregnancies [7, 12, 64].
Future Directions
The mammary gland provides a unique experimental model for the study of the process of tissue regression. With each cycle of pregnancy and lactation, the gland undergoes massive expansion of the milk-producing secretory epithelium coupled with regression of adipocytes followed by reversal of this process during involution. Much has been learned over the past decade about the molecular mechanisms that regulate the epithelial cell apoptosis that is at the center of this process. However, much remains to be learned about how these cells are removed and how the process of recruitment, retention and elimination of immune cells is coordinated in the remodeling gland. For example, it is unknown whether epithelial cell death is itself responsible for initiating the recruitment of immune cells, and if it is, how this process is regulated. Nonprofessional phagocytes are clearly important in removing apoptotic cells early in involution. Like professional phagocytes, these cells have been shown to secrete inhibitors of tissue inflammation, such as TGFβ, during the process of ingesting apoptotic cells. Are these same cells also the source of the chemoattractants responsible for inflammatory cell recruitment? A related question is the primary roles of immune cells that are recruited to the involuting gland. The roles of plasma cells and B-lymphocytes, which are recruited during the late stages of involution, remain especially obscure. Although removal of apoptotic cells and debris is likely to be one important function of neutrophils and macrophages, it seems reasonable that evolution might have favored recruitment of these cells for defense against microbial pathogens that contribute to potentially fatal infections and to impairment of subsequent milk production and thus impairment of the growth and survival of offspring.
It is also unclear how the eventual clearance of immune cells is accomplished in the mammary gland. Our recent description of progressively abnormal mammary gland remodeling in association with persistent inflammation in mice lacking Mfge8 suggests that failure of this process could itself lead to mammary gland dysfunction [7]. It is becoming increasingly clear that immune cells also play important roles in the biology of epithelial tumors, with the potential to either enhance or inhibit tumor initiation, growth and metastasis [65–67]. Tumors of the mammary gland are usually associated with increased numbers of cells of both the innate and cognate immune system [68–70]. A better understanding of the factors that regulate recruitment, retention and elimination of immune cells from the involuting mammary gland could also provide insight into the mechanisms that regulate these processes in the context of mammary carcinogenesis.
Acknowledgments
The authors were supported by funds HL64353, HL56385, and HL53949 from the National Heart, Lung, and Blood Institute (D.S.), CA57621 from the National Cancer Institute and ES12801 from the National Institute of Environmental Health Sciences and the National Cancer Institute (Z.W.), and a National Institutes of Health fellowship 1F32 HL073530-01 (K.A.).
Abbreviations
- MEC
mammary epithelial cells
- PCD
programmed cell death
References
- 1.Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127(11):2269–82. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- 2.Pollard JW, Hennighausen L. Colony stimulating factor 1 is required for mammary gland development during pregnancy. Proc Natl Acad Sci USA. 1994;91(20):9312–6. doi: 10.1073/pnas.91.20.9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marti A, Feng Z, Altermatt HJ, Jaggi R. Milk accumulation triggers apoptosis of mammary epithelial cells. Eur J Cell Biol. 1997;73(2):158–65. [PubMed] [Google Scholar]
- 4.Lund LR, Romer J, Thomasset N, Solberg H, Pyke C, Bissell MJ, et al. Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development. 1996;122(1):181–93. doi: 10.1242/dev.122.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quarrie LH, Addey CV, Wilde CJ. Programmed cell death during mammary tissue involution induced by weaning, litter removal, and milk stasis. J Cell Physiol. 1996;168(3):559–69. doi: 10.1002/(SICI)1097-4652(199609)168:3<559::AID-JCP8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 6.Quarrie LH, Addey CV, Wilde CJ. Apoptosis in lactating and involuting mouse mammary tissue demonstrated by nick-end DNA labelling. Cell Tissue Res. 1995;281(3):413–9. doi: 10.1007/BF00417859. [DOI] [PubMed] [Google Scholar]
- 7.Atabai K, Fernandez R, Huang X, Ueki I, Kline A, Li Y, et al. Mfge8 is critical for mammary gland remodeling during involution. Mol Biol Cell. 2005;16(12):5528–37. doi: 10.1091/mbc.E05-02-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monks J, Rosner D, Geske FJ, Lehman L, Hanson L, Neville MC, et al. Epithelial cells as phagocytes: apoptotic epithelial cells are engulfed by mammary alveolar epithelial cells and repress inflammatory mediator release. Cell Death Differ. 2005;12(2):107–14. doi: 10.1038/sj.cdd.4401517. [DOI] [PubMed] [Google Scholar]
- 9.Walker NI, Bennett RE, Kerr JF. Cell death by apoptosis during involution of the lactating breast in mice and rats. Am J Anat. 1989;185(1):19–32. doi: 10.1002/aja.1001850104. [DOI] [PubMed] [Google Scholar]
- 10.Li M, Liu X, Robinson G, Bar-Peled U, Wagner KU, Young WS, et al. Mammary-derived signals activate programmed cell death during the first stage of mammary gland involution. Proc Natl Acad Sci USA. 1997;94(7):3425–30. doi: 10.1073/pnas.94.7.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander CM, Selvarajan S, Mudgett J, Werb Z. Stromelysin-1 regulates adipogenesis during mammary gland involution. J Cell Biol. 2001;152(4):693–703. doi: 10.1083/jcb.152.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanayama R, Nagata S. Impaired involution of mammary glands in the absence of milk fat globule EGF factor 8. Proc Natl Acad Sci USA. 2005;102(46):16886–91. doi: 10.1073/pnas.0508599102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fadok VA. Clearance: the last and often forgotten stage of apoptosis. J Mammary Gland Biol Neoplasia. 1999;4(2):203–11. doi: 10.1023/a:1011384009787. [DOI] [PubMed] [Google Scholar]
- 14.Henson PM, Bratton DL, Fadok VA. Apoptotic cell removal. Curr Biol. 2001;11(19):R795–805. doi: 10.1016/s0960-9822(01)00474-2. [DOI] [PubMed] [Google Scholar]
- 15.Geske FJ, Monks J, Lehman L, Fadok VA. The role of the macrophage in apoptosis: hunter, gatherer, and regulator. Int J Hematol. 2002;76(1):16–26. doi: 10.1007/BF02982714. [DOI] [PubMed] [Google Scholar]
- 16.Bennett MR, Gibson DF, Schwartz SM, Tait JF. Binding and phagocytosis of apoptotic vascular smooth muscle cells is mediated in part by exposure of phosphatidylserine. Circ Res. 1995;77(6):1136–42. doi: 10.1161/01.res.77.6.1136. [DOI] [PubMed] [Google Scholar]
- 17.Dini L, Lentini A, Diez GD, Rocha M, Falasca L, Serafino L, et al. Phagocytosis of apoptotic bodies by liver endothelial cells. J Cell Sci. 1995;108(Pt 3):967–73. doi: 10.1242/jcs.108.3.967. [DOI] [PubMed] [Google Scholar]
- 18.Hughes J, Liu Y, Van Damme J, Savill J. Human glomerular mesangial cell phagocytosis of apoptotic neutrophils: mediation by a novel CD36-independent vitronectin receptor/thrombospondin recognition mechanism that is uncoupled from chemokine secretion. J Immunol. 1997;158(9):4389–97. [PubMed] [Google Scholar]
- 19.Finnemann SC. Role of alphavbeta5 integrin in regulating phagocytosis by the retinal pigment epithelium. Adv Exp Med Biol. 2003;533:337–42. doi: 10.1007/978-1-4615-0067-4_42. [DOI] [PubMed] [Google Scholar]
- 20.Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D, Finnemann SC. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin. J Exp Med. 2004;200(12):1539–45. doi: 10.1084/jem.20041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sexton DW, Blaylock MG, Walsh GM. Human alveolar epithelial cells engulf apoptotic eosinophils by means of integrin- and phosphatidyl-serine receptor-dependent mechanisms: a process upregulated by dexamethasone. J Allergy Clin Immunol. 2001;108(6):962–9. doi: 10.1067/mai.2001.119414. [DOI] [PubMed] [Google Scholar]
- 22.Sexton DW, Al-Rabia M, Blaylock MG, Walsh GM. Phagocytosis of apoptotic eosinophils but not neutrophils by bronchial epithelial cells. Clin Exp Allergy. 2004;34(10):1514–24. doi: 10.1111/j.1365-2222.2004.02054.x. [DOI] [PubMed] [Google Scholar]
- 23.Walsh GM, Sexton DW, Blaylock MG, Convery CM. Resting and cytokine-stimulated human small airway epithelial cells recognize and engulf apoptotic eosinophils. Blood. 1999;94(8):2827–35. [PubMed] [Google Scholar]
- 24.Parnaik R, Raff MC, Scholes J. Differences between the clearance of apoptotic cells by professional and non-professional phagocytes. Curr Biol. 2000;10(14):857–60. doi: 10.1016/s0960-9822(00)00598-4. [DOI] [PubMed] [Google Scholar]
- 25.Boudjellab N, Chan-Tang HS, Li X, Zhao X. Interleukin 8 response by bovine mammary epithelial cells to lipopolysaccharide stimulation. Am J Vet Res. 1998;59(12):1563–7. [PubMed] [Google Scholar]
- 26.McClenahan DJ, Sotos JP, Czuprynski CJ. Cytokine response of bovine mammary gland epithelial cells to Escherichia coli, coliform culture filtrate, or lipopolysaccharide. Am J Vet Res. 2005;66(9):1590–7. doi: 10.2460/ajvr.2005.66.1590. [DOI] [PubMed] [Google Scholar]
- 27.Savill J, Smith J, Sarraf C, Ren Y, Abbott F, Rees A. Glomerular mesangial cells and inflammatory macrophages ingest neutrophils undergoing apoptosis. Kidney Int. 1992;42(4):924–36. doi: 10.1038/ki.1992.369. [DOI] [PubMed] [Google Scholar]
- 28.Sekhri KK, Pitelka DR, DeOme KB. Studies of mouse mammary glands. I. Cytomorphology of the normal mammary gland. J Natl Cancer Inst. 1967;39(3):459–90. [PubMed] [Google Scholar]
- 29.Richards RC, Benson GK. Involvement of the macrophage system in the involution of the mammary gland in the albino rat. J Endocrinol. 1971;51(1):149–56. doi: 10.1677/joe.0.0510149. [DOI] [PubMed] [Google Scholar]
- 30.Richards RC, Benson GK. Ultrastructural changes accompanying involution of the mammary gland in the albino rat. J Endocrinol. 1971;51(1):127–35. doi: 10.1677/joe.0.0510127. [DOI] [PubMed] [Google Scholar]
- 31.Devitt A, Parker KG, Ogden CA, Oldreive C, Clay MF, Melville LA, et al. Persistence of apoptotic cells without autoimmune disease or inflammation in CD14−/− mice. J Cell Biol. 2004;167(6):1161–70. doi: 10.1083/jcb.200410057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stein T, Morris JS, Davies CR, Weber-Hall SJ, Duffy MA, Heath VJ, et al. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res. 2004;6(2):R75–91. doi: 10.1186/bcr753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lonnerdal B. Human milk proteins: key components for the biological activity of human milk. Adv Exp Med Biol. 2004;554:11–25. [PubMed] [Google Scholar]
- 34.Moodley Y, Rigby P, Bundell C, Bunt S, Hayashi H, Misso N, et al. Macrophage recognition and phagocytosis of apoptotic fibroblasts is critically dependent on fibroblast-derived thrombospondin 1 and CD36. Am J Pathol. 2003;162(3):771–9. doi: 10.1016/S0002-9440(10)63874-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pechoux C, Clezardin P, Dante R, Serre CM, Clerget M, Bertin N, et al. Localization of thrombospondin, CD36 and CD51 during prenatal development of the human mammary gland. Differentiation. 1994;57(2):133–41. doi: 10.1046/j.1432-0436.1994.5720133.x. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe ML. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci USA. 2001;98(22):12485–90. doi: 10.1073/pnas.171460498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monks J, Geske FJ, Lehman L, Fadok VA. Do inflammatory cells participate in mammary gland involution? J Mammary Gland Biol Neoplasia. 2002;7(2):163–76. doi: 10.1023/a:1020351919634. [DOI] [PubMed] [Google Scholar]
- 38.Golpon HA, Fadok VA, Taraseviciene-Stewart L, Scerbavicius R, Sauer C, Welte T, et al. Life after corpse engulfment: phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. FASEB J. 2004;18(14):1716–8. doi: 10.1096/fj.04-1853fje. [DOI] [PubMed] [Google Scholar]
- 39.Brooker BE. Pseudopod formation and phagocytosis of milk components by epithelial cells of the bovine mammary gland. Cell Tissue Res. 1983;229(3):639–50. doi: 10.1007/BF00207703. [DOI] [PubMed] [Google Scholar]
- 40.Mayberry HE. Macrophages in post-secretory mammary involution in mice. Anat Rec. 1964;149:99–111. doi: 10.1002/ar.1091490110. [DOI] [PubMed] [Google Scholar]
- 41.Tatarczuch L, Philip C, Bischof R, Lee CS. Leucocyte phenotypes in involuting and fully involuted mammary glandular tissues and secretions of sheep. J Anat. 2000;196(Pt 3):313–26. doi: 10.1046/j.1469-7580.2000.19630313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tatarczuch L, Bischof RJ, Philip CJ, Lee CS. Phagocytic capacity of leucocytes in sheep mammary secretions following weaning. J Anat. 2002;201(5):351–61. doi: 10.1046/j.0021-8782.2002.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nickerson SC. Immunological aspects of mammary involution. J Dairy Sci. 1989;72(6):1665–78. doi: 10.3168/jds.S0022-0302(89)79278-X. [DOI] [PubMed] [Google Scholar]
- 44.Lee CS, McDowell GH, Lascelles AK. The importance of macrophages in the removal of fat from the involuting mammary gland. Res Vet Sci. 1969;10(1):34–8. [PubMed] [Google Scholar]
- 45.Lee CS, Outteridge PM. Leucocytes of sheep colostrum, milk and involution secretion, with particular reference to ultrastructure and lymphocyte sub-populations. J Dairy Res. 1981;48(2):225–37. doi: 10.1017/s0022029900021646. [DOI] [PubMed] [Google Scholar]
- 46.Nickerson SC, Sordillo LM. Role of macrophages and multinucleate giant cells in the resorption of corpora amylacea in the involuting bovine mammary gland. Cell Tissue Res. 1985;240(2):397–401. doi: 10.1007/BF00222352. [DOI] [PubMed] [Google Scholar]
- 47.Crago SS, Prince SJ, Pretlow TG, McGhee JR, Mestecky J. Human colostral cells. I. Separation and characterization. Clin Exp Immunol. 1979;38(3):585–97. [PMC free article] [PubMed] [Google Scholar]
- 48.Dulin AM, Paape MJ, Nickerson SC. Comparison of phagocytosis and chemiluminescence by blood and mammary gland neutrophils from multiparous and nulliparous cows. Am J Vet Res. 1988;49(2):172–7. [PubMed] [Google Scholar]
- 49.Fox LK, McDonald JS, Hillers JK, Corbeil LB. Function of phagocytes obtained from lacteal secretions of lactating and nonlactating cows. Am J Vet Res. 1988;49(5):678–81. [PubMed] [Google Scholar]
- 50.Sordillo LM, Shafer-Weaver K, DeRosa D. Immunobiology of the mammary gland. J Dairy Sci. 1997;80(8):1851–65. doi: 10.3168/jds.S0022-0302(97)76121-6. [DOI] [PubMed] [Google Scholar]
- 51.Paape MJ, Shafer-Weaver K, Capuco AV, Van Oostveldt K, Burvenich C. Immune surveillance of mammary tissue by phagocytic cells. Adv Exp Med Biol. 2000;480:259–77. doi: 10.1007/0-306-46832-8_31. [DOI] [PubMed] [Google Scholar]
- 52.Clarkson RW, Watson CJ. Microarray analysis of the involution switch. J Mammary Gland Biol Neoplasia. 2003;8(3):309–19. doi: 10.1023/b:jomg.0000010031.53310.92. [DOI] [PubMed] [Google Scholar]
- 53.Clarkson RW, Wayland MT, Lee J, Freeman T, Watson CJ. Gene expression profiling of mammary gland development reveals putative roles for death receptors and immune mediators in post-lactational regression. Breast Cancer Res. 2004;6(2):R92–109. doi: 10.1186/bcr754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bursch W. The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ. 2001;8(6):569–81. doi: 10.1038/sj.cdd.4400852. [DOI] [PubMed] [Google Scholar]
- 55.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15(3):1101–11. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Helminen HJ, Ericsson JL. Effects of enforced milk stasis on mammary gland epithelium, with special reference to changes in lysosomes and lysosomal enzymes. Exp Cell Res. 1971;68(2):411–27. doi: 10.1016/0014-4827(71)90167-4. [DOI] [PubMed] [Google Scholar]
- 57.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402(6762):672–6. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 58.Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61(2):439–44. [PubMed] [Google Scholar]
- 59.Gajewska M, Gajkowska B, Motyl T. Apoptosis and autophagy induced by TGF-B1 in bovine mammary epithelial BME-UV1 cells. J Physiol Pharmacol. 2005;56(Suppl 3):143–57. [PubMed] [Google Scholar]
- 60.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148(7):2207–16. [PubMed] [Google Scholar]
- 61.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109(1):41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Politis I, Zhao X, McBride BW, Burton JH. Function of bovine mammary macrophages as antigen-presenting cells. Vet Immunol Immunopathol. 1992;30(4):399–410. doi: 10.1016/0165-2427(92)90108-3. [DOI] [PubMed] [Google Scholar]
- 63.Paape M, Mehrzad J, Zhao X, Detilleux J, Burvenich C. Defense of the bovine mammary gland by polymorphonuclear neutrophil leukocytes. J Mammary Gland Biol Neoplasia. 2002;7(2):109–21. doi: 10.1023/a:1020343717817. [DOI] [PubMed] [Google Scholar]
- 64.Lund LR, Bjorn SF, Sternlicht MD, Nielsen BS, Solberg H, Usher PA, et al. Lactational competence and involution of the mouse mammary gland require plasminogen. Development. 2000;127(20):4481–92. doi: 10.1242/dev.127.20.4481. [DOI] [PubMed] [Google Scholar]
- 65.Jakobisiak M, Lasek W, Golab J. Natural mechanisms protecting against cancer. Immunol Lett. 2003;90(2–3):103–22. doi: 10.1016/j.imlet.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 66.Fioretti F, Fradelizi D, Stoppacciaro A, Ramponi S, Ruco L, Minty A, et al. Reduced tumorigenicity and augmented leukocyte infiltration after monocyte chemotactic protein-3 (MCP-3) gene transfer: perivascular accumulation of dendritic cells in peritumoral tissue and neutrophil recruitment within the tumor. J Immunol. 1998;161(1):342–6. [PubMed] [Google Scholar]
- 67.Zhang L, Yoshimura T, Graves DT. Antibody to Mac-1 or monocyte chemoattractant protein-1 inhibits monocyte recruitment and promotes tumor growth. J Immunol. 1997;158(10):4855–61. [PubMed] [Google Scholar]
- 68.Bhan AK, DesMarais CL. Immunohistologic characterization of major histocompatibility antigens and inflammatory cellular infiltrate in human breast cancer. J Natl Cancer Inst. 1983;71(3):507–16. [PubMed] [Google Scholar]
- 69.Hurlimann J, Saraga P. Mononuclear cells infiltrating human mammary carcinomas: immunohistochemical analysis with monoclonal antibodies. Int J Cancer. 1985;35(6):753–70. doi: 10.1002/ijc.2910350610. [DOI] [PubMed] [Google Scholar]
- 70.Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65(19):8896–904. doi: 10.1158/0008-5472.CAN-05-1734. [DOI] [PubMed] [Google Scholar]