Abstract
We have previously demonstrated that nitric oxide (NO) exerts a greater modulatory influence on renal cortical blood flow in ANG II-infused hypertensive rats compared with normotensive rats. In the present study, we determined nitric oxide synthase (NOS) activities and protein levels in the renal cortex and medulla of normotensive and ANG II-infused hypertensive rats. Enzyme activity was determined by measuring the rate of formation of l-[14C]citrulline from l-[14C]arginine. Western blot analysis was performed to determine the regional expression of endothelial (eNOS), neuronal (nNOS), and inducible (iNOS) isoforms in the renal cortex and medulla of control and ANG II-infused rats. Male Sprague-Dawley rats were prepared by the infusion of ANG II at a rate of 65 ng/min via osmotic minipumps implanted subcutaneously for 13 days and compared with sham-operated rats. Systolic arterial pressures were 127 ± 2 and 182 ± 3 mmHg in control (n = 13) and ANG II-infused rats (n = 13), respectively. The Ca2+-dependent NOS activity, expressed as picomoles of citrulline formed per minute per gram wet weight, was higher in the renal cortex of ANG II-infused rats (91 ± 11) than in control rats (42 ± 12). Likewise, both eNOS and nNOS were markedly elevated in the renal cortex of the ANG II-treated rats. In both groups of rats, Ca2+-dependent NOS activity was higher in the renal medulla than in the cortex; however, no differences in medullary NOS activity were observed between the groups. Also, no differences in medullary eNOS levels were observed between the groups; however, medullary nNOS was decreased by 45% in the ANG II-infused rats. For the Ca2+-independent NOS activities, the renal cortex exhibited a greater activity in the control rats (174 ± 23) than in ANG II-infused rats (101 ± 10). Similarly, cortical iNOS was greater by 47% in the control rats than in ANG II-treated rats. No differences in the activity were found for the renal medulla between the groups. There was no detectable signal for iNOS in the renal medulla for both groups. These data indicate that there is a differential distribution of NOS activity, with the Ca2+-dependent activity and protein expression higher in the renal cortex of ANG II-infused rats compared with control rats, and support the hypothesis that increased constitutive NOS activity exerts a protective effect in ANG II-induced hypertension to maintain adequate renal cortical blood flow.
Keywords: endothelial nitric oxide synthase, neuronal nitric oxide synthase, inducible nitric oxide synthase, osmotic minipump, angiotensin II-induced hypertension, Western blot
LONG-TERM ADMINISTRATION of initially subpressor doses of angiotensin II (ANG II) elicits a progressive increase in arterial pressure after several days with a pressure profile and degree of hypertension similar to that observed in the two-kidney, one-clip (2K1C) Goldblatt hypertensive rat (7, 24, 25, 29). In these experimental models of hypertension, the renal vasoconstriction elicited by ANG II is partially attenuated by counteracting vasodilator factors (7, 21, 24). In support, we have previously demonstrated an enhanced influence of nitric oxide (NO) on the cortical circulation to maintain adequate renal blood flow (RBF) and glomerular filtration rate (GFR) in ANG II-induced hypertension (7). These observations indicate that NO serves as a major endogenous compensatory vasodilator system by its counteracting influence on the heightened ANG II-specific vasoconstrictor actions mediating the hypertensive state.
The differences in the regional blood flow responses to NO synthase (NOS) inhibition in control and hypertensive rats suggested that a differential regulation of intrarenal NOS activity accounts for differences between the cortical and medullary blood flow responses in the ANG II-infused hypertensive rats and the normotensive controls. In the ANG II-infused hypertensive rats, the progressive increases in arterial pressure and/or ANG II levels could lead to a greater endothelial shear stress to induce elevations in intracellular calcium and activate the Ca2+-dependent NOS activity (6, 9, 10, 18, 19, 28). Consequently, increased NO synthesis could partially counteract the ANG II-mediated renal cortical vasoconstrictor actions and allow preservation of renal hemodynamics. In line with this postulate, recent studies demonstrated that total kidney endothelial NOS protein, a calcium-dependent isoform, was significantly increased by 90% during chronic infusion of subpressor doses of ANG II for 10 days after pretreatment with converting enzyme inhibitors (9). These data suggest that ANG II may chronically increase NO synthesis by upregulating endothelial NOS protein and activity, but it was not determined whether this effect occurred primarily in the cortex or the medulla. Other studies (16, 22) have recently demonstrated that NO production is upregulated during the early phase (age 8−12 wk) in the spontaneously hypertensive rat (SHR), a genetic model of hypertension. The SHR showed significant increases in renal tissue endothelial and inducible NOS proteins compared with that in the Wistar-Kyoto (WKY) rats, thereby excluding the possibility of an impairment in the NO pathway contributing to the early phase of hypertension in SHR (22). Similarly, Nava et al. (16) reported that the Ca2+- independent NOS activity was greater in the renal cortex of SHR compared with the WKY rats.
To obtain more direct evidence for an altered differential regulation of NOS activity in the renal circulation, we performed further studies measuring the NOS enzyme activities and protein levels of the different NOS isoforms in the cortical and medullary regions in control and ANG II-infused hypertensive rats. Therefore, the objective of this study was to determine the NOS activities in the renal cortex and medulla of normotensive and ANG II-infused hypertensive rats by measuring the rate of formation of radiolabeled l-[14C]citrulline from l-[14C]arginine in renal tissues harvested from these rats. Additional experiments were performed to characterize the regional expression of endothelial (eNOS), neuronal (nNOS) and inducible (iNOS) NOS isoforms by Western blot analysis.
METHODS
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were acclimatized in a temperature- and light-controlled room and allowed access to standard rat chow (Ralston-Purina, St. Louis, MO) and water ad libitum. The rats were randomly divided into control (n = 8; n = 5) and ANG II-infused (n = 8; n = 5) groups for the determination of NOS enzyme activity and protein levels by Western blot analysis, respectively. Preliminary surgery was performed in rats (200−220 g) anesthetized with pentobarbital sodium (50 mg/kg ip) to implant minipumps. In one group of rats, synthetic ANG II (Sigma Chemical, St. Louis, MO) was delivered continuously at a rate of 65 ng/min via osmotic minipumps (model 2002; Alza, Palo Alto, CA) implanted subcutaneously at the dorsum of the neck. A sham operation was performed on the normotensive control rats. Systolic arterial pressures were measured by tail-cuff plethysmography (Harvard Apparatus, South Natick, MA) before and on the 12th day after implantation of minipumps.
Experiments were performed on the 13th day after implantation of minipumps or sham operation to determine the enzyme activity and protein levels in the cortical and medullary regions of the kidney. Rats were anesthetized with pentobarbital sodium (50 mg/kg ip) and placed on a thermo-regulated surgical table to maintain body temperature at 37°C. The abdominal cavity was exposed via a midline incision. Both kidneys were removed and sectioned sagittally, facilitating separation of the medulla from the cortex for analysis of NOS activity and protein levels by Western blot analysis. The dissected samples were placed on a weighing paper over dry ice. Frozen tissues were directly homogenized manually in a homogenization buffer in volumes three times the weight of the tissues. The homogenization buffer containing 320 mM sucrose, 50 mM Tris base, 0.1 mM EDTA, 1 mM dithiothreitol, 10 μg/ml leupeptin, 10 μg/ml soybean trypsin inhibitor, and 2 μg/ml aprotinin was adjusted to pH 7.0 with HCl before addition of 10 mg/ml of phenylmethylsulfonyl fluoride (PMSF) (15, 16, 20). The crude homogenates were then centrifuged at 14,000 g for 40 min at 4°C. The supernatants were analyzed for determination of NOS activity.
NOS activity assay
NOS activity was determined by measuring the rate of formation of radiolabeled l-[14C]citrulline from l-[14C]arginine (50 μCi/ml with specific activity of 303 mCi/mmol, Amersham Life Science) as modified from previously described methods (15, 16, 20). Tissue extracts contained in 20 μl of supernatant were incubated in a total volume of 100 μl of assay buffer containing 50 mM potassium dihydrogen phosphate, 1 mM magnesium chloride, 2 mM calcium chloride, and 1 mM NADPH at pH 7.0, with 22 μM l-arginine and 3 μM radiolabeled l-[14C]arginine. Duplicate samples were incubated for 1 h at 37°C without any inhibitors, with addition of EGTA (4 mM), and with addition of EGTA plus Nω-nitro-l-arginine methyl ester (l-NAME, 5 mM) to determine the levels of Ca2+-dependent and Ca2+-independent NOS activities, respectively. The reactions were terminated with the addition of 1.5 ml of a 1:1 suspension of Dowex 50W (Na+ form, Sigma Chemical) in water to bind and remove the remaining l-arginine. The Na+ form of Dowex 50W was prepared by washing the H+ form of the resin with 1 N NaOH in approximately equal proportions for four washes and then washing with distilled water until the pH was below 7.4. The efficiency of the resin in capturing l-arginine was greater than 99% of the total activity in the assay buffer. The above mixture was then centrifuged before and after the extraction procedure. Five milliliters of distilled water was added to all samples for extraction, and the suspension was allowed to precipitate for 20 min at room temperature. A 4-ml aliquot of the supernatant containing radiolabeled l-[14C]citrulline was mixed with 10 ml of scintillation fluid to quantitate the radioactivity by a Beckman LS-9000 counter. The Ca2+-dependent NOS activity was calculated as the difference between the amount of l-[14C]citrulline formed in control tubes and the amount formed in the tubes incubated with EGTA. The Ca2+-independent NOS activity was calculated as the difference between the amount of l-[14C]citrulline formed in tubes incubated with EGTA alone and the amount formed in the tubes incubated with EGTA plus l-NAME (20). NOS enzyme activity level was expressed as picomoles of l-[14C]citrulline formed per minute per gram wet kidney weight.
Measurement of NOS activity based on the conversion of l-arginine to l-citrulline is currently the standard assay to characterize NO formation in both crude and purified enzyme preparations. The specificity of this assay for the NOS enzymatic pathway is due mainly to the direct enzymatic conversion of l-arginine to l-citrulline, which is stoichiometric with the formation of NO in eukaryotic cells. However, biological tissues contain variable amounts of endogenous levels of l-arginine substrate as well as additional metabolic pathways that can compete for the substrate and independently form the l-citrulline product (15). It is therefore important, as presented in this study, to define the EGTA + l-NAME-inhibitable formation of l-citrulline to characterize the NOS-specific activity. In addition, the presence of endogenous levels of l-arginine in the tissue homogenates could lead to the dilution of the radiolabeled substrate with reduced formation of l-[14C]citrulline, resulting in an underestimate of NOS activity as previously suggested (15). In the present study, nonlabeled l-arginine was added to buffer the influence of regional differences in the amount of endogenous levels of l-arginine.
Western blot analysis
For Western blot analysis, frozen tissues were homogenized with the Polytron in RIPA buffer containing 20 mM Tris · HCl, pH 7.4, 150 mM NaCl, 0.5% sodium deoxycholate, 1% Igepal, and 0.1% Triton X-100 in volumes two times the tissue weight with the additions of 30 μl/g of 10 mg/ml PMSF and 10 μl/ml of 100 mM sodium orthovanadate, according to Brugge and Erikson (from 1977) with modifications (5, 17). The homogenates were centrifuged at 100,000 g for 1 h at 4°C. The protein concentration of the supernatant was determined with the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA). Kidney tissue samples (160 μg of protein for the cortex and 100 μg for the medulla) were electrophoretically size-fractionated on 6% polyacrylamide Tris-glycine precast gel (Novex, San Diego, CA) at 125 V for 90−120 min. High-range molecular weight marker proteins were myosin (205 kDa), β-galactosidase (121 kDa), bovine serum albumin (74 kDa), and ovalbumin (47 kDa, Bio-Rad). After electrophoresis, the proteins were transferred to Immun-Blot polyvinylidene difluoride membrane (Bio-Rad) at 25 V for 2 h. The membranes were then incubated with polyclonal rabbit anti-NOS antibodies (Santa Cruz Biotechnology, San Diego, CA) in Tris-buffered saline containing 0.1% Tween-20 and 5% nonfat dry milk overnight at 4°C. Polyclonal antibodies for eNOS, nNOS, and iNOS were diluted to 1:200 for the renal cortical protein samples and 1:500 for the medullary samples. The membranes were washed for 30 min, with the wash buffer changed every 10 min before incubation with the horseradish peroxidase-labeled goat anti-rabbit IgG (1:1,000) and 5% nonfat dry milk in Tris-buffered saline for 2 h at 4°C. The washes were again repeated for 30 min before the membrane was detected for the bound antibody by chemiluminescence using the ECL detection reagents (Amersham, Piscataway, NJ) on X-ray film (Hyperfilm-ECL; Amersham). The films were scanned with the densitometer (Alpha Innotech, San Leandro, CA) to determine the integrated density values. When the control values were detectable, the data were normalized to the densitometric ratio of the average control value.
Statistical analysis
Data are expressed as means ± SE. Student's unpaired t-test was performed to determine statistical differences between two separate groups. Statistical significance was defined as P < 0.05 or exceeding the 95% critical value.
RESULTS
Before implantation of osmotic minipumps, systolic blood pressure (SBP) values in the conscious rats were not statistically different between the groups, averaging 125 ± 3 and 126 ± 4 mmHg in control (n = 13) and ANG II-infused rats (n = 13), respectively. In accordance with previous studies (7, 24, 25, 29), ANG II-infused rats exhibited progressive increases in SBP over the course of a 12-day period. As shown in Fig. 1, SBP in ANG II-infused rats increased from a control value of 126 ± 4 to 182 ± 3 mmHg by day 12. In control rats, SBP remained relatively unchanged at 127 ± 2 mmHg. The body weights of the rats averaged 295 ± 6 g in the ANG II-infused hypertensive rats and 305 ± 5 g in the normotensive controls.
Fig. 1.
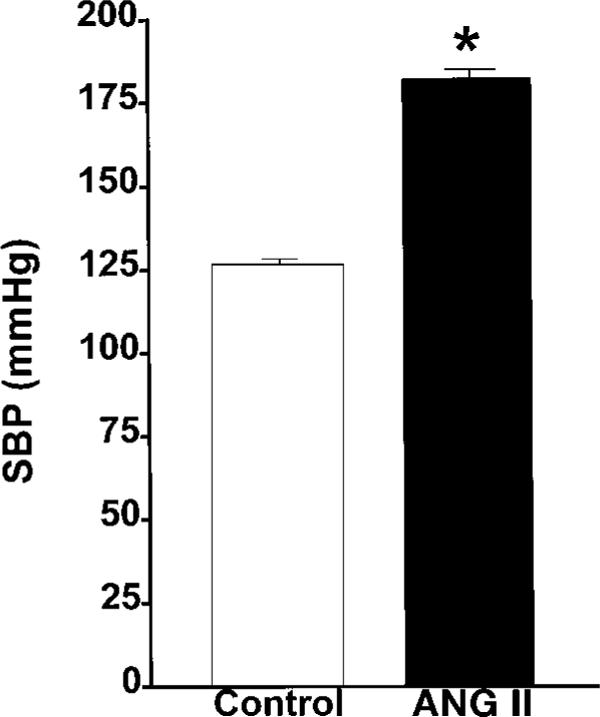
Comparison of systolic blood pressures (SBP) in normotensive control (n = 13) and ANG II-infused rats (n = 13). *P < 0.05 vs. control. Data are means ± SE.
Comparison of the average data for the activity representing absolute amount of l-citrulline formed revealed that in the presence of EGTA plus l-NAME when presumably all of the NOS-dependent l-citrulline formation was blocked, there was much higher NOS-independent formation of l-citrulline in the cortex than in the medulla for both groups, averaging 142 ± 14 vs. 5 ± 2 pmol · min−1 · g−1, respectively, in the control rats and 90 ± 8 vs. 1 ± 0.9 pmol · min−1 · g−1 in the ANG II-infused rats. The conversion of l-arginine to l-citrul-line in the presence of EGTA plus l-NAME provides evidence of additional metabolic pathways other than the NOS enzymatic pathway for the synthesis of l-citrulline from l-arginine in renal cortical tissue.
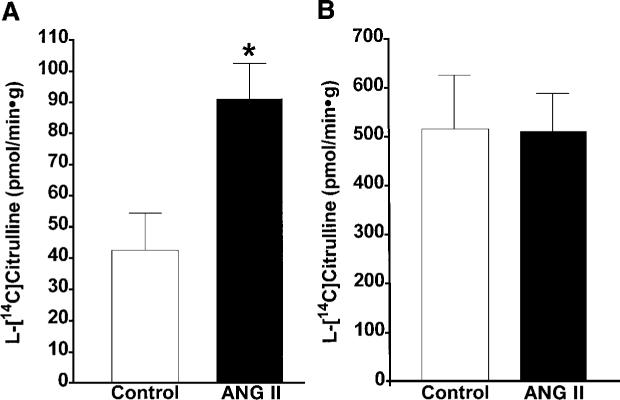
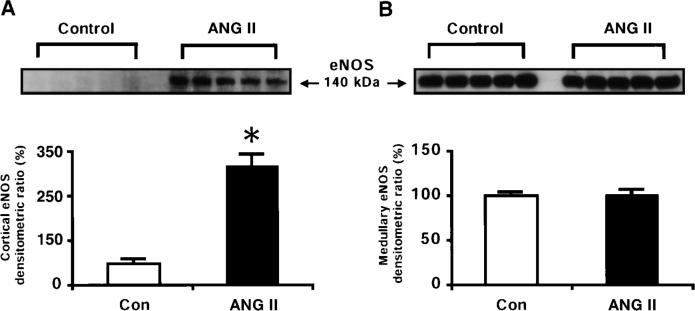
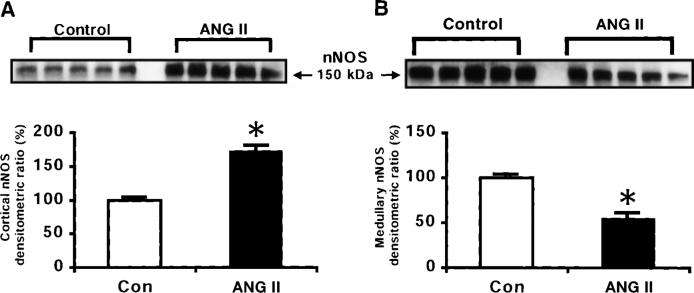
The NOS activity was expressed as picomoles of radiolabeled l-[14C]citrulline formation per minute per gram wet kidney weight. The Ca2+-dependent NOS activity was increased by about twofold in the renal cortex of ANG II-infused rats (91 ± 11 pmol · min−1 · g−1) compared with normotensive controls, which averaged 42 ± 12 pmol · min−1 · g−1 (Fig. 2). This was accompanied by a marked increase in the renal cortical eNOS protein as well as in the nNOS protein levels in the ANG II-infused rats compared with normotensive controls (Figs. 3 and 4). Cortical nNOS protein level was substantially increased by 73% in the ANG II-infused hypertensive rats compared with the control rats (Fig. 4). Representative Western blots of eNOS (140 kDa) and nNOS (150 kDa) are shown in the top of Figs. 3 and 4, respectively. Barely detectable signals for the eNOS protein were observed in the renal cortex of normotensive controls, but marked induction was observed in the ANG II-infused rats.
Fig. 2.
Comparison of Ca2+-dependent nitric oxide synthase (NOS) activity in the renal cortex (A) and medulla (B) of normotensive control (n = 8) and ANG II-infused hypertensive rats (n = 8). *P < 0.05 vs. control. Data are means ± SE.
Fig. 3.
Representative Western blots of endothelial NOS (eNOS) protein (top) and densitometric analyses of eNOS protein (bottom) in renal cortex (A) and renal medulla (B) of normotensive control (n = 5) and ANG II-infused hypertensive rats (n = 5). *P < 0.05 vs. control. Data are means ± SE.
Fig. 4.
Representative Western blots of neuronal (nNOS) protein (top) and densitometric analyses of nNOS protein (bottom) in renal cortex (A) and renal medulla (B) of normotensive control (n = 5) and ANG II-infused hypertensive rats (n = 5). *P < 0.05 vs. control. Data are means ± SE.
In both groups, the Ca2+-dependent NOS activity was significantly greater in the renal medulla than in the cortex, averaging 516 ± 110 and 510 ± 78 pmol · min−1 · g−1 in control and ANG II-infused rats, respectively (Fig. 2). No differences in the Ca2+-dependent NOS activity were observed in the renal medulla between the groups. Likewise, eNOS protein levels in the renal medulla did not differ between the groups (Fig. 3). However, medullary nNOS protein levels were decreased by 45% in the ANG II-treated rats compared with the controls (Fig. 4). These data are in accordance with previous studies in which the medullary tissue has been reported to have about a 10-fold higher NOS activity compared with that in the cortex in normotensive controls (15).
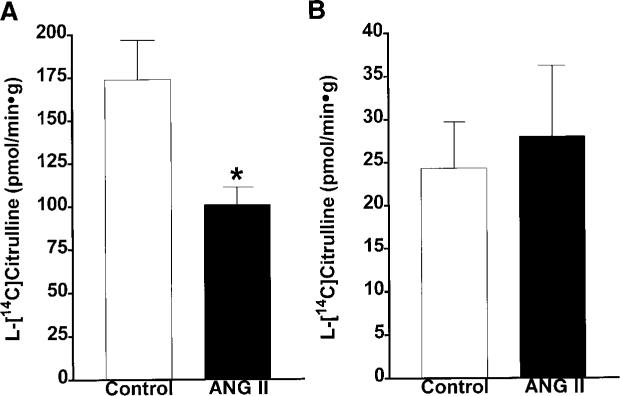
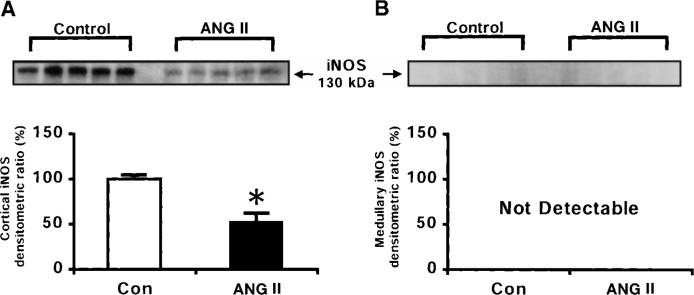
For the Ca2+-independent NOS activities (Fig. 5), the trend was reversed, with the renal cortex exhibiting a greater activity in the control rats (174 ± 23 pmol · min−1 · g−1) than in ANG II-infused rats (101 ± 10 pmol · min−1 · g−1). Similarly, iNOS protein levels were diminished by 47% in the renal cortex of the ANG II-infused rats compared with control rats (Fig. 6). A representative Western blot of the iNOS (130 kDa) is shown in the top of Fig. 6. In the medulla, Ca2+-independent NOS activity was much lower than the Ca2+-dependent NOS activity; and no differences in the Ca2+-independent NOS activity were observed between the control rats (24 ± 5 pmol · min−1 · g−1) and the ANG II-infused rats (28 ± 8 pmol · min−1 · g−1). In agreement, we did not detect iNOS protein in the renal medulla in either the control or the ANG II-treated rats.
Fig. 5.
Comparison of Ca2+-independent NOS activity in renal cortex (A) and medulla (B) of normotensive control (n = 8) and ANG II-infused hypertensive rats (n = 8). *P < 0.05 vs. control. Data are means ± SE.
Fig. 6.
Representative Western blots of inducible NOS (iNOS) protein (top) and densitometric analyses of iNOS protein (bottom) in renal cortex (A) and renal medulla (B) of normotensive control (n = 5) and ANG II-infused hypertensive rats (n = 5). *P < 0.05 vs. control. Data are means ± SE.
DISCUSSION
The present study provides evidence of an altered differential regulation of NOS activity and protein levels in the kidneys of ANG II-infused hypertensive rats compared with normotensive controls. In normotensive rats, the Ca2+-dependent NOS activity level was considerably higher in the renal medulla than in the cortex. Likewise, the relative level of eNOS protein was higher in the renal medulla than in the cortex. These results indicate that the renal medulla has substantially greater capacity to generate NO via a Ca2+-dependent NOS mechanism. In accordance with our results, Moridani and Kline (15) recently demonstrated that the renal medulla had about a 10-fold higher NOS activity compared with that in the cortex. Moreover, Nava et al. (16) showed that the renal medulla had a much higher Ca2+-dependent NOS activity than the cortex in control rats. Similarly, biochemical studies by Biondi and Romero (4) indicate that the renal medulla has a greater potential for the NO-dependent formation of cGMP than does the renal cortex in basal and stimulated conditions with acetylcholine. Furthermore, these observations are consistent with our whole kidney studies in which acute systemic inhibition of NO synthesis led to a greater relative decrease in medullary blood flow than in cortical blood flow in control rats (7). Collectively, these data indicate that NO exerts a greater modulatory influence on the medullary than on the cortical circulation in normotensive control rats.
To obtain further insight regarding the regulation of NOS activity in the early phase of hypertension, we examined the differential regulation of Ca2+-dependent and Ca2+-independent NOS activities and protein levels of the different NOS isoforms in the kidneys of ANG II-infused hypertensive rats. As previously reported, chronic infusion of initially subpressor doses of ANG II over the course of 13 days leads to a slowly developing hypertension resembling the early phase of 2K1C Goldblatt renovascular hypertension (7, 24, 25, 29). In these models of hypertension, RBF and GFR are either within the normal range or only slightly diminished, despite elevated blood pressure and circulating ANG II levels (21, 24, 25). Previous data have suggested that the renal vasoconstriction elicited by elevated intrarenal ANG II levels is partially counteracted by enhanced activity of vasodilator factors. We have previously demonstrated that one of these factors is an increased NO synthesis during the early phase of ANG II-induced hypertension (7). Acute systemic inhibition of NO synthesis led to greater increases in arterial pressure in the ANG II-infused hypertensive rats compared with the normotensive controls. Despite the greater increases in blood pressure, cortical blood flow and total RBF were concomitantly reduced to a greater extent in the hypertensive rats than in the controls. These results are consistent with the hypothesis that a compensatory increase in NOS activity counteracts the vasoconstrictor influences of elevated circulating and intrarenal ANG II levels to sustain renal hemodynamics. In accordance with these functional observations, the increased Ca2+-dependent NOS enzyme activity as well as elevated protein expression of eNOS and nNOS in the cortical tissue provide further evidence of an upregulation of renal Ca2+-dependent NOS activity in the renal cortex of ANG II-infused hypertensive rats.
Although the Ca2+-dependent NOS activities were higher in the renal medulla than in the cortex, there were no differences between the control and hypertensive rats. Likewise, medullary eNOS protein levels were not different and nNOS protein levels were actually reduced in the ANG II-infused rats compared with control rats. Furthermore, the renal medulla has substantial potential to synthesize NO, but its overall contribution to the total amount of NO formation is limited due to the much greater cortical mass. An enhanced production of NO in the cortical region of the kidney could explain the greater renal vasoconstrictor responses observed with pharmacological inhibitors of NOS. These observations are in accordance with our previous study (7) in which Nω-nitro-l-arginine (NLA), an inhibitor of NO synthesis, led to a greater decrease in cortical blood flow, which represents the major fraction of total RBF, in the ANG II-infused hypertensive rats compared with normotensive controls.
The present results support previous findings that the medullary blood flow responses to NLA were less in the ANG II-infused hypertensive rats than in the normotensive controls. The mechanisms responsible for a differential regulation of NOS activity in the medullary circulation remain unclear. It is possible that the greater increases in arterial pressure concomitantly blunted the NLA-induced decreases in medullary blood flow to a greater extent in the ANG II-infused rats, thereby masking the actual level of NOS activity. In support, eNOS protein levels in the medulla did not differ between the groups. The present finding of the decreased expression of nNOS in the renal medulla of ANG II-infused rats is consistent with recent studies performed by Ichihara et al. (11) regarding the differential effect of nNOS inhibition in the juxtamedullary microvasculature of control and ANG II-infused hyper-tensive rats. Superfusion with the specific nNOS inhibitor, S-methyl-l-thiocitrulline, under conditions of enhanced flow to the loop of Henle, led to greater afferent arteriolar constriction in controls than in ANG II-infused hypertensive rats (11). Previous studies have demonstrated that the most abundant NOS identified in the kidney is the nNOS specifically found in the macula densa cells, glomeruli, renal vasculature, collecting duct, and inner medullary thin limb and that the detection of eNOS has been more difficult (2, 3). It is possible that our failure to detect the low-level constitutive eNOS expression in the renal cortex of control rats is due to the limited sensitivity of the Western blotting technique.
With regard to the Ca2+-independent NOS activities, the trend was reversed, with the renal cortex exhibiting a greater activity in the control rats than in ANG II-infused hypertensive rats. Likewise, the iNOS protein expression was greater in the renal cortex of normotensive controls than in the ANG II-treated group. Moreover, no differences were observed in the renal medulla between the groups. We were not able to detect even low-level iNOS expression in the renal medulla. Our results for the Ca2+-independent NOS activity in the renal medulla are similar to previous studies performed in SHR and WKY rats with values averaging 40 ± 13 and 40 ± 12 pmol · min−1 · g−1, respectively (16). Using reverse transcription-polymer-ase chain reaction, the presence of inducible NOS mRNA expression was demonstrated in the arcuate and interlobular arteries, glomeruli, and proximal tubules, which are primarily localized in the renal cortex, as well as in the thick ascending limbs and collecting ducts (1, 14). The constitutive expression of this particular NOS isoform indicates its contributory role in the normal homeostatic regulation of the kidney apart from its role induced during pathological states (12). Mattson et al. (13) demonstrated that acute systemic infusion of aminoguanidine, a selective inhibitor of iNOS enzyme, to anesthetized rats significantly decreased urine flow in the absence of any alterations in blood pressure or renal cortical blood flow, suggesting that iNOS may participate in the homeostatic regulation of the kidney possibly via its renal tubular effects.
It is not clear why the Ca2+-independent NOS activity was reduced in the renal cortex of ANG II-induced hypertensive rats. Previous studies have demonstrated that the vascular smooth muscle iNOS activity is depressed in SHR compared with cultured cells derived from WKY rats (8). Presumably, the downregulation of iNOS expression under such conditions of elevated ANG II levels could result from the stimulated production of cytokines, growth factors, or hormones. In line with this observation, studies have demonstrated that ANG II stimulates the expression of transforming growth factor-β (TGF-β) in cultured renal cells (27). Moreover, glomerular TGF-β1 mRNA expression was shown to be upregulated in the nonclipped kidneys of 2K1C hypertensive rats compared with control kidneys (26). In terms of its role in inducing iNOS expression, TGF-β1 appears to negatively modulate renal iNOS expression in vivo. These findings are supported by recent studies in the TGF-β1 knockout mice that exhibit increased levels of iNOS expression in the kidney (23). Accordingly, it is possible that ANG II may facilitate the ability of TGF-β to counteract the vascular trophic effects of ANG II by downregulating the Ca2+-independent iNOS expression in the early phase of ANG II-induced hypertension. Thus the nonimmune regulation of the constitutive Ca2+-independent iNOS could be one mechanism to counteract the vascular growth-promoting action of elevated intrarenal ANG II levels in the renal circulation.
In conclusion, these data indicate that there is a differential distribution of NOS activity in the kidney, with the Ca2+-dependent activity and protein expression higher in the renal cortex of ANG II-infused rats compared with control rats. These findings exclude the possibility of NO deficiency as a primary contributor to ANG II-induced hypertension and further support the hypothesis that enhanced constitutive NOS activity exerts a protective effect during the early phases of ANG II-induced hypertension to maintain adequate renal cortical blood flow and partially compensate for the hypertensinogenic and vasoconstrictive effects of elevated ANG II levels.
Acknowledgments
We thank Dr. Edwin Rabon, Natalia Louneva, Sam Rulli, Hector Licea Vargas, Ginny Primrose, Dr. Su-Teh Li, and Dr. Lisa Harrison-Bernard for technical assistance.
This study was supported by National Heart, Lung, and Blood Institute Grant HL-26371. S. Y. Chin is a predoctoral fellow supported by the Louisiana Board of Regents Educational Quality Support Fund. C. Moreno is a predoctoral fellow in the Department of Physiology of the University of Murcia, Spain, who was a visiting graduate student at Tulane.
REFERENCES
- 1.Ahn KY, Mohaupt MG, Madsen KM, Kone BC. In situ hybridization localization of mRNA encoding inducible nitric oxide synthase in rat kidney. Am. J. Physiol. 1994;267:F748–F757. doi: 10.1152/ajprenal.1994.267.5.F748. (Renal Fluid Electrolyte Physiol. 36)
- 2.Bachmann S, Mundel P. NO in the kidney: synthesis, localization and function. Am. J. Kidney Dis. 1994;24:112–129. doi: 10.1016/s0272-6386(12)80170-3. [DOI] [PubMed] [Google Scholar]
- 3.Baylis C, Qiu C. Importance of nitric oxide in the control of renal hemodynamics. Kidney Int. 1996;49:1727–1731. doi: 10.1038/ki.1996.256. [DOI] [PubMed] [Google Scholar]
- 4.Biondi ML, Romero JC. Nitric oxide-mediated reactions stimulate cyclic GMP in the dog kidney. J. Vasc. Med. Biol. 1990;2:294–298. [Google Scholar]
- 5.Brugge JS, Erikson RL. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977;269:346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- 6.Busse R, Fleming I. Pulsatile stretch and shear stress: physical stimuli determining the production of endothelium-derived relaxing factors. J. Vasc. Res. 1998;35:73–84. doi: 10.1159/000025568. [DOI] [PubMed] [Google Scholar]
- 7.Chin SY, Wang CT, Majid DSA, Navar LG. Renoprotective effects of nitric oxide in angiotensin II-induced hypertension in the rat. Am. J. Physiol. 1998;274:F876–F882. doi: 10.1152/ajprenal.1998.274.5.F876. (Renal Physiol. 43)
- 8.Dubois G. Decreased l-arginine-nitric oxide pathway in cultured myoblasts from spontaneously hypertensive versus normotensive Wistar-Kyoto rats. FEBS Lett. 1996;392:242–244. doi: 10.1016/0014-5793(96)00817-4. [DOI] [PubMed] [Google Scholar]
- 9.Hennington BS, Zhang H, Miller MT, Granger JP, Reckelhoff JF. Angiotensin II stimulates synthesis of endothelial nitric oxide synthase. Hypertension. 1998;31:283–288. doi: 10.1161/01.hyp.31.1.283. [DOI] [PubMed] [Google Scholar]
- 10.Hutcheson IR, Griffith TM. Central role of intracellular calcium stores in acute flow- and agonist-evoked endothelial nitric oxide release. Br. J. Pharmacol. 1997;122:117–125. doi: 10.1038/sj.bjp.0701340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ichihara A, Imig JD, Navar LG. Neuronal nitric oxide synthase-dependent afferent arteriolar function in angiotensin II-induced hypertension. Hypertension. 1999;33:462–466. doi: 10.1161/01.hyp.33.1.462. [DOI] [PubMed] [Google Scholar]
- 12.Kone BC, Baylis C. Biosynthesis and homeostatic roles of nitric oxide in the normal kidney. Am. J. Physiol. 1997;272:F561–F578. doi: 10.1152/ajprenal.1997.272.5.F561. (Renal Physiol. 41)
- 13.Mattson DL, Maeda CY, Bachman TD, Cowley AW., Jr. Inducible nitric oxide synthase and blood pressure. Hypertension. 1998;31:15–20. doi: 10.1161/01.hyp.31.1.15. [DOI] [PubMed] [Google Scholar]
- 14.Mohaupt MG, Elzie JL, Ahn KY, Clapp WL, Wilcox CS, Kone BC. Differential expression and induction of RNAs encoding two inducible nitric oxide synthases in rat kidney. Kidney Int. 1994;46:653–665. doi: 10.1038/ki.1994.318. [DOI] [PubMed] [Google Scholar]
- 15.Moridani BA, Kline RL. Effect of endogenous l-arginine on the measurement of nitric oxide synthase activity in the rat kidney. Can. J. Physiol. Pharmacol. 1996;74:1210–1214. [PubMed] [Google Scholar]
- 16.Nava E, Llinas MT, Gonzalez JD, Salazar FJ. Nitric oxide synthase activity in renal cortex and medulla of normotensive and spontaneously hypertensive rats. Am. J. Hypertens. 1996;9:1236–1239. doi: 10.1016/s0895-7061(96)00325-1. [DOI] [PubMed] [Google Scholar]
- 17.Pandey KN. Atrial natriuretic factor inhibits autophosphorylation of protein kinase C and a 240-kDa protein in plasma membranes of bovine adrenal glomerulosa cells: involvement of cGMP-dependent and independent signal transduction mechanisms. Mol. Cell. Biochem. 1994;141:103–111. doi: 10.1007/BF00926173. [DOI] [PubMed] [Google Scholar]
- 18.Pueyo ME, Arnal JF, Rami J, Michel JB. Angiotensin II stimulates the production of NO and peroxynitrite in endothelial cells. Am. J. Physiol. 1998;274:C214–C220. doi: 10.1152/ajpcell.1998.274.1.C214. (Cell Physiol. 43)
- 19.Ranjan V, Xiao Z, Diamond SL. Constitutive NOS expression in cultured endothelial cells is elevated by fluid shear stress. Am. J. Physiol. 1995;269:H550–H555. doi: 10.1152/ajpheart.1995.269.2.H550. (Heart Circ. Physiol. 38)
- 20.Salter M, Knowles RG, Moncada S. Widespread tissue distribution and changes in activity of Ca2+-dependent and Ca2+-independent nitric oxide synthases. FEBS Lett. 1991;291:145–149. doi: 10.1016/0014-5793(91)81123-p. [DOI] [PubMed] [Google Scholar]
- 21.Sigmon DH, Beierwaltes WH. Renal nitric oxide and angiotensin II interaction in renovascular hypertension. Hypertension. 1993;22:237–242. doi: 10.1161/01.hyp.22.2.237. [DOI] [PubMed] [Google Scholar]
- 22.Vaziri ND, Zhenmin N, Oveisi F. Upregulation of renal and vascular nitric oxide synthase in young spontaneously hypertensive rats. Hypertension. 1998;31:1248–1254. doi: 10.1161/01.hyp.31.6.1248. [DOI] [PubMed] [Google Scholar]
- 23.Vodovotz Y, Geiser AG, Chesler L, Letterio JJ, Campbell A, Lucia MS, Sporn MB, Roberts AB. Spontaneously increased production of nitric oxide and aberrant expression of the inducible nitric oxide synthase in vivo in the transforming growth factor beta 1 null mouse. J. Exp. Med. 1996;183:2337–2342. doi: 10.1084/jem.183.5.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Von Thun AM, Vari RC, El-Dahr SS, Navar LG. Augmentation of intrarenal angiotensin II levels by chronic angiotensin II infusion. Am. J. Physiol. 1994;266:F120–F128. doi: 10.1152/ajprenal.1994.266.1.F120. (Renal Fluid Electrolyte Physiol. 35)
- 25.Wang CT, Zou L, Navar LG. Renal responses to AT1 blockade in angiotensin II-induced hypertensive rats. J. Am. Soc. Nephrol. 1997;8:535–542. [PubMed] [Google Scholar]
- 26.Wolf G, Schneider A, Wenzel U, Helmchen U, Stahl RA. Regulation of glomerular TGF-β expression in the contralateral kidney of two-kidney, one-clip hypertensive rats. J. Am. Soc. Nephrol. 1998;9:763–772. doi: 10.1681/ASN.V95763. [DOI] [PubMed] [Google Scholar]
- 27.Wolf G, Ziyadeh FN, Zahner G, Stahl RA. Angiotensin II-stimulated expression of transforming growth factor beta in renal proximal tubular cells: attenuation after stable transfection with the c-mas oncogene. Kidney Int. 1995;48:1818–1827. doi: 10.1038/ki.1995.480. [DOI] [PubMed] [Google Scholar]
- 28.Ziegler T, Silacci P, Harrison VJ, Hayoz D. Nitric oxide synthase expression in endothelial cells exposed to mechanical forces. Hypertension. 1998;32:351–355. doi: 10.1161/01.hyp.32.2.351. [DOI] [PubMed] [Google Scholar]
- 29.Zou LX, Imig JD, Von Thun AM, Hymel A, Ono H, Navar LG. Receptor-mediated intrarenal angiotensin II augmentation in angiotensin II-infused rats. Hypertension. 1996;28:669–677. doi: 10.1161/01.hyp.28.4.669. [DOI] [PubMed] [Google Scholar]