Abstract
“For me, the realization that I am a freak was not the result of a childhood accumulation of unkind remarks about my appearance. Nor, for that matter, was it the consequence of an inadvertently-placed mirror, a job-offer in a circus show, a horrified plastic surgeon, or a callously disinterested schoolgirl. Rather the dawning was the outcome of an esoterically designed medical test for which I volunteered…. One minute I was, to all intents and purposes normal. Fifteen minutes later I was a medical curiosity….” 1
In Phillip Kerr’s 1994 spellbinding novel A Philosophical Investigation, the medical test to which the protagonist refers is a functional brain scan based on positron emission tomography (PET). It is used to run large studies of male and female brains and, following a lead suggested by animal studies, has been used to identify rare cases of human male subjects who lack the ventral medial nucleus (VMN). This nucleus, in the experiment (and the story), is hypothesized to inhibit the activity of the sexually dimorphic nucleus (SDN), a preoptic area of the male brain believed to be a repository of male aggressive response. Prior research, we learn from the plot, shows that 3 in 100,000 human males are VMN-negative. Thirty percent of those affected are believed to be in prison or to have a criminal record; 70% are believed to stabilize levels of aggression by producing increased levels of estrogen. Identities of subjects participating in the brain scan studies are protected through the conversion of real names to those of famous figures of the past — Bertrand Russell, Charles Dickens. Those for whom results are unfavorable are recontacted, and they are offered counseling.
The protagonist’s codename is Ludwig Wittgenstein. Upon discovering the result of his test, he falls victim to latent criminal tendencies. This previously quiet, although socially quirky, pharmacy technician turns serial killer. His prey: other VMN-negative males whom he perceives to be a threat to society.
How does this fiction inform our work on incidental findings (IFs) in the brain and in other research? While not an “incidental finding” of the experiment in the strictest sense defined by this special issue of JLME — the study was designed to detect the remote instances of VMN-negativity — the account nonetheless provides a cautionary tale about professional responsibility, health risk and benefit, and privacy and disclosure.
Clinical brain imaging has certainly not been immune to unexpected findings, but the occurrence of such findings in research was not formally documented until the late 1990s.2 The reasons for the delay are unknown, but the many different imaging modalities under development and in use, their varying scanning resolutions, the many brain regions of interest, and the nonmedical setting for many research studies may have been contributing factors. Nonetheless, it is clear that IFs have been present in imaging research since Hans Berger first demonstrated the possibility of recording brain signals in the 1920s. Now, with advanced methods such as magnetic resonance imaging (MRI), and greater attention to ethical issues in research, the Pandora’s Box of incidental findings has been opened and the need for guidance and resolution has become clear.3 The integrity of the research itself demands as much; using a brain scan from a volunteer who is supposed to be healthy but who is discovered to have a tumor or arteriovenous malformation is, at the very least, problematic. Certainly such affected data would not be accepted from an animal model. The ethics of the scientist-volunteer relationship also demands a resolution to the incidental findings problem, as trust and reciprocity in the research process are vital.4 The implications are both practical and philosophical. How should a finding of potential clinical significance be handled in the research setting, subject welfare be protected, and privacy be safeguarded? What duties belong to basic research scientists who do not have medical training? Whose responsibility is it to communicate the finding to a subject or surrogate, to follow up, and to treat if needed?
Now, ten years after the first empirical investigation of IFs in the brain, the answers to the associated challenges are becoming clearer. That is not to say, however, that the answers are easy ones, or that one solution fits all.
The State of the Art (and Science)
Incidence Studies
Establishing the incidence of incidental findings is challenging. In our own data, incidental abnormalities on MRIs were found to be present in brain images of 47 (21%) pediatric subjects recruited to studies as healthy controls.5 This study, and all those on IFs by our group at Stanford University, were conducted with IRB approvals. Seventeen of these (36%) were determined to require routine referral for further evaluation; a single case was categorized as an urgent referral. Of 151 studies of adults we studied separately in a similarly retrospective way, we found that incidental findings requiring referral occurred in 6.6% of subjects.6 These data are consistent with reports by Gregory Katzman7 and Alex Marmourian,8 for example, but are far greater estimates than those of Frank Weber and Heinz Knopf.9 The debate over incidence is occurring in the context of MRI and other brain scanning methods that are being refined continuously and used for increasing diverse applications, both within the medical research setting and in the commercial marketplace.10
In an effort to shed further light on the question of incidence for the present discussion, we performed a literature search on IFs using the Pubmed.gov search engine. We used the simple search string “incidental finding and brain” and categorized each relevant, nonduplicative article retrieved into one of three types of publications: (1) empirical study of IFs, (2) case report of an IF found in the human brain using a brain imaging technique, or (3) reviews, news articles, correspondence, and commentaries on ethical implications of IFs in neuroimaging research. A fourth category was set aside for articles that were returned in the search but that did not focus on IFs found in human brains using brain imaging, that used the term “incidental finding” in the abstract or summary but did not focus on IFs (e.g., “it may also be an incidental finding” or “whether it is an incidental finding is unknown”), and that reported IFs in animal studies. We further tracked the percentages of empirical studies and case reports on children, those reporting IFs found at autopsy, and studies reporting multiple types of incidental findings.
For each empirical study and case report, respectively, we recorded the type of IF and computed overall frequency using the sum of all articles as the denominator. Note that the full range of literature was not captured by our method, since we chose to use only one search string rather than running a comprehensive search using all possible relevant strings such as “accidental findings” and “unexpected findings” and related Medical Subject Heading (MeSH) terms.
We retrieved 157 papers using the method described above. The earliest article we accepted into the database was published in June 1969, the most recent in July 2007. The majority of papers retrieved were case reports, and more than 80% of reported findings in the categories of empirical studies and case reports were in living adults as shown in Table 1. Findings found at autopsy and reported in the literature are shown in Table 2. Cysts, tumors, infection, inflammation, and vascular malformations were the most commonly reported indicental findings in emprirical research and case reports (Tables 3 and 4).
Table 1.
Total Number of Reports (N=157) Retrieved from PubMed.gov in which the Search String “Incidental Finding and Brain” was Identified(June 1969–July 2007)
| NUMBER OF REPORTS | PERCENTAGE OF REPORTS PERTAINING TO ADULT OR PEDIATRIC IFs | |
|---|---|---|
| EMPIRICAL STUDIES | 27 | 83% adult
17% pediatric (13% found at autopsy; 13% multiple IFs) |
| CASE REPORTS | 72 | 80% adult
20% pediatric (13% found at autopsy; 0 multiple IFs [brain and other]) |
| REVIEWS, NEWS ARTICLES, CORRESPONDENCE AND COMMENTARIES | 22 | |
| ARTICLES NOT FOCUSING ON IFs IN THE HUMAN BRAIN | 56 |
Table 2.
Incidental Findings in the Brain Discovered at Autopsy as Reported in the Literature
| INCIDENTAL FINDINGS AT AUTOPSY | |
|---|---|
| EMPIRICAL STUDIES | Neurocysticerosis
Meningioma Lewy Bodies Alzheimer’s Disease |
| CASE REPORTS | Pituicytoma
Alzheimer’s Disease Persistent Hypoglossal Artery Lipomatous Hamartoma Ganglion Cells in Posterior Pituitary Intracranial Lipoma Myeloid Metaplasia Marchiafava-Bignami’s Disease Sarcocystis Infection |
Table 3.
Types of Incidental Findings Reported in Empirical Research Studies on Living Subjects and at Autopsy
EMPIRICAL STUDIES
|
SINGULAR OCCURRENCES OF:
|
Table 4.
Incidental Findings Reported in Case Reports, Combining Reports on Living Subjects and at Autopsy
CASE REPORTS
|
SINGULAR OCCURRENCES OF:
|
Research Protocols
In follow-up to our initial study of incidence, we examined how researchers handle incidental findings in brain research using MRI.11 Seventy-four investigators who conduct MRI studies in the United States and in six other countries responded to a Web-based survey. Of the investigators who responded to the question of whether they had knowledge of incidental findings in their studies, 82% (54/66) responded affirmatively. In this small sample, discoveries were arteriovenous malformations, brain tumors, and developmental abnormalities. We found substantial variability in which personnel were permitted to operate the MRI equipment, procedures for handling and communicating IFs to subjects, whether a neuroradiologist was involved in evaluating evidence of an incidental finding.
A much larger study is needed to fully comprehend how incidental findings are managed. Nonetheless, since 2004 a trend toward some form of management of IFs has emerged within the neuroimaging community.12
Subject Expectations
Healthy control subjects (N=105) who had previously participated in brain scan studies with MRI in medical and nonmedical settings were surveyed by our group about their expectations and attitudes toward unexpected clinical findings on their research brain scans.13 We hypothesized that, although participants consent to a scanning procedure for research purposes alone, they still expect pathology, if present, to be detected and reported to them. Responding to a Web-based survey, 54% of participants reported that they expect that research scans will detect any abnormalities that exist. Nearly all subjects (>90%) reported that they would want findings communicated. No significant differences were found between participants scanned in medical and nonmedical settings.
The source of subject expectations — which may be language in consent forms or the therapeutic misconception — is a focus of continuing research for us. This idea that subjects want to be told of clinically significant findings is also featured prominently in the personal story of a medical student who recounted her pre- and post-operative experience after an arteriovenous malformation was detected by one of her classmates during a basic research functional MRI memory study.14
Recommendations
To begin to address concerns about brain incidental findings in a pragmatic way, representatives from different Institutes of the National Institutes of Health (NIH) (the National Institute on Neurologic Disorders and Stroke, the National Institute on Drug Abuse, the National Institute on Biomedical Imaging and Bioengineering, the National Institute on Aging, and the National Institute on Mental Health) and Stanford University held a 2005 workshop that focused on five key areas: (1) detection of incidental findings; (2) IRB involvement; (3) communicating with subjects; (4) research protocols, the scanning environment, and training of personnel; and (5) subject selection. The goal was to generate initial recommendations for minimal, if not optimal, standards that could be adopted by universities, laboratories, IRBs, and research sponsors and could inform future policymaking.15
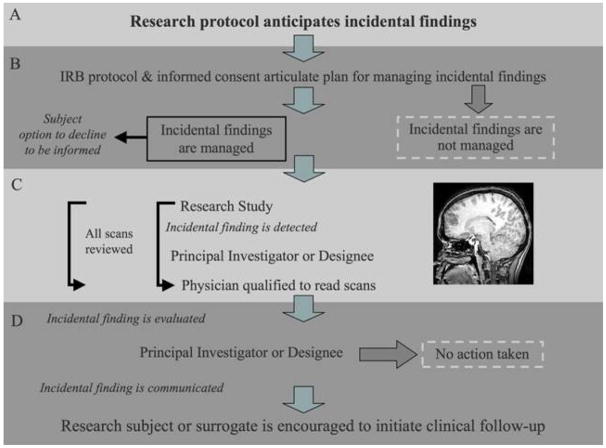
The group’s recommendations are illustrated in the pathway in Figure 1 and summarized below.
Figure 1.
The group reached consensus that investigators engaged in brain imaging research should anticipate incidental findings in their experimental protocols and establish pathway for handling them. Principal Investigators (PIs) bear primary responsibility for handling all findings in their research, whether those findings are expected or not, and for managing them appropriately. Training of personnel should address procedures for managing such findings, without trying to create amateur or “para” neuroradiologists. The task of reading scans for clinical diagnosis is appropriately left to trained experts.
The group saw no ethical requirement for collecting additional screening or clinical scans beyond those required for the research. Although the group noted that the NIH Clinical Center, for example, obtains an annual clinical scan screened by a neuroradiologist for each subject in an imaging study, the majority of the group felt that requiring clinical screening for each participant would be overly costly and impractical. This is a particular consideration for the growing number of research settings in which imaging studies are performed that are not based within medical centers.
The management pathway should be stated in the research protocol so that the IRB may evaluate it, and in the written and verbal informed consent process for prospective subjects. The pathway should address who will evaluate a suspected incidental finding, what further review process will occur, and to whom the incidental finding will be communicated. If statistics about the incidence of unexpected findings and the proportion with potential clinical significance are included, the sources of the data should be cited. Subjects should have the option to decline being informed of an incidental finding.
Researchers formulating informed consent documents should be aware of the Common Rule (45 C.F.R. 46), which states that informed consent cannot include exculpatory language. Although researchers should not be responsible for failure to detect an abnormality, they should be wary of disclaiming responsibility for establishing an IF management plan.
The group recognized that some PIs might elect a pathway that opts out of incidental findings evaluation. This approach should be communicated to the IRB in the protocol submitted for review and to research subjects during the process of obtaining informed consent. The option to opt-out was not generally favored, however, in part because of concerns that subjects may suffer harm if an incidental finding requiring urgent assessment is not recognized and clinical evaluation is never triggered.
The involvement of a physician in all research imaging studies and the specific involvement of neuroradiologists, to permit verification and assessment of incidental findings, is an ongoing source of debate. Figure 1 (Panel C), therefore, illustrates two options: one in which the involvement of a physician is a matter of routine, the other in which involvement occurs on a need-only basis. Concerns about the sheer numbers of scans that might require reading by a physician associated with a research team, and the cost burden of routine readings, are central to the debate. Compounding the potential burden of either routine or ad hoc physician involvement are issues of access to such physicians not readily available outside of medical centers, as well as possible deficiencies in the research scans for clinical purposes.
The pathway shows that if an anomaly is detected and verified by a physician, then the subject or the subject’s surrogate (as for a minor or adult without decisional capacity) should be the first to receive that information. Communication is the responsibility of the PI or his or her designate, and the finding should not be further disclosed (i.e., to the subject’s primary care physician or a neurologist) without the subject’s or surrogate’s authorization. A PI who is not a physician competent to evaluate the presence of an incidental finding should exercise considerable care when describing the finding to the subject. Subjects should be encouraged to pursue clinical follow-up, bearing in mind that the research scan may not be of clinical grade and that the initial finding is merely a suspicious anomaly requiring further assessment.
Implied in this pathway is the fact that researchers may ethically assign the subject or the subject’s surrogate the responsibility for seeking further medical evaluation. Subjects from populations traditionally considered vulnerable, and subjects without a primary care physician or without medical insurance, however, may need the assistance of the investigative team in initially pursuing avenues for follow-up.
Bridging the Practical and Philosophical
These recommendations and our broader survey of the incidental findings problem suggest several conclusions. First, as one of us (JI) has written before, no single approach will solve the vexing problem of managing IFs in brain imaging research. It is a multilayered challenge, and a range of morally acceptable options exists. The best approach for any particular research protocol lies in clear recognition of the possibility that an IF will occur, and in the thoughtful development of a response that is respectful of the institution and laboratory in which the study is conducted and subject in whom the discovery might be made. This kind of recognition is essential when planning the protocol, during the process of consent, and when disclosure and discussion are needed.
Second, ongoing experience with the challenge of IFs suggests that one of the most contentious issues is the risk to the research enterprise, that is, the protection of hypothesis-driven experiments from which clinical benefit cannot be expected. Co-mingling of clinical expectations in research raises the possibility of creating blurred goals and even blurrier professional responsibilities. The implications for the preservation of the research process are not trivial and cannot be dismissed lightly.
Subjects’ right to opt-out of being told about an incidental finding is a third substantial issue. Analysis of this problem can be guided by risk-benefit analyses in other types of medical research, such as clinical pharmacologic trials.16 Our position, as authors of the present paper, is that the cost of loss of a life or quality of life due to an undisclosed or unmanaged IF is greater than the psychological and financial cost of the occasional false positive.
A fourth and particularly difficult challenge concerns the financial responsibility for follow-up. Should the cost of initial clinical work-up be assumed by the laboratory in which the discovery was made? Research grants do not typically cover this eventuality. Should funds be allocated to this as a direct cost of research or from some other source? Insisting that researchers bear the burden might diminish future research funding for brain imaging. However, is it sufficient for the research team merely to communicate the need for follow-up and where care can be obtained? Referral to clinical care is a minimum requirement, though there remains the necessity to addresses the needs of subjects who are homeless, disenfranchised, or already suffering from a neurological or psychiatric disorder. Subject abandonment is not an acceptable trajectory.
It is now clear that we must cope with IFs in the current era of neuroimaging. Fortunately, identification of this problem has been met by scientific interest and a robust, interdisciplinary response. How to handle incidental findings in archived and shared data, and how to approach functional anomalies that may even one day become predictors of aggression or other socially deviant behavior represent topics for the next generation of work. The challenges are substantial.
Acknowledgments
The authors are grateful to Ellen Wright Clayton for bringing Phillip Kerr’s A Philosophical Investigation to their attention, and to Sofia Lombera for editorial assistance. Work on this paper was supported by The Greenwall Foundation (Judy Illes, PI), NIH/ NINDS grant #RO1 NS O45831 (Judy Illes, PI) and NHGRI grant #R01 HG003178 (PI, S. M. Wolf; Co-Is, J. P. Kahn, F. Lawrenz, C. A. Nelson). The views expressed are those of the authors and should not be attributed to the funders.
Contributor Information
Judy Illes, Judy Illes, Ph.D., is the Canada Research Chair in Neuroethics and a Professor of Neurology at the University of British Columbia. She directs the National Core for Neuroethics that is devoted to ethical, social, and policy challenges at the intersection of the neurosciences and biomedical ethics. Dr. Illes has written numerous books, edited volumes, and articles. Her latest book, Neuroethics: Defining the Issues in Theory, Practice and Policy, was published by Oxford University Press in 2006.
Vivian Nora Chin, Vivian Nora Chin was the Program Manager for Neuroethics at the Stanford Center for Biomedical Ethics. Hoping to eventually specialize in neurology, she is currently a first-year medical student at Robert Wood Johnson Medical School in Piscataway, NJ.
References
- 1.Kerr P. A Philosophical Investigation. New York: Bantam Books; 1995. p. 34. [Google Scholar]
- 2.Katzman GL, et al. Incidental Findings on Brain Magnetic Resonance Imaging from 1000 Asymptomatic Volunteers. JAMA. 1999;282(1):36–39. doi: 10.1001/jama.282.1.36. [DOI] [PubMed] [Google Scholar]
- 3.Grossman RI, Bernat JL. Incidental Research Imaging Findings: Pandora’s Costly Box. Neurology. 2004;62(6):849–850. doi: 10.1212/01.wnl.0000118214.02495.41. [DOI] [PubMed] [Google Scholar]; Illes J. On the Contents of Pandora’s Box of Incidental Findings in Brain Imaging Research. Nature Clinical Practice Neurology. 2006;2(2):60–61. doi: 10.1038/ncpneuro0119. [DOI] [PubMed] [Google Scholar]
- 4.Illes J, Chin V. Trust and Reciprocity: Foundational-Principles in Human Subjects Imaging Research. Canadian Journal of Neurological Sciences. 2007;34(1):3–4. doi: 10.1017/s0317167100005710. [DOI] [PubMed] [Google Scholar]
- 5.Kim BS, et al. Incidental Findings on Pediatric MR Images of the Brain. American Journal of Neuroradiology. 2002;23(10):1674–1677. [PMC free article] [PubMed] [Google Scholar]
- 6.Illes J, et al. Practical Approaches to Incidental Findings on Brain Imaging Research. Neurology. 2008;70(5):384–390. doi: 10.1212/01.wnl.0000280469.17461.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Id., at 2.
- 8.Mamourian A. Incidental Findings on Research Functional MR Images: Should We Look? American Journal of Neuroradiology. 2004;25(4):520–522. [PMC free article] [PubMed] [Google Scholar]
- 9.Weber F, Knopf H. Incidental Findings in Magnetic Resonance Imaging of Healthy Young Men. Journal of Neurological Sciences. 2006;240(s 1–2):81–84. doi: 10.1016/j.jns.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Illes J, Kirschen M. New Prospects and Ethical Challenges for Neuroimaging Within and Outside the Health Care System. American Journal of Neuroradiology. 2003;24(10):1932–1934. [PMC free article] [PubMed] [Google Scholar]; Eaton ML, Illes J. Commercializing Cognitive Neurotechnology: The Ethical Terrain. Nature Biotechnology. 2007;25(4):393–397. doi: 10.1038/nbt0407-393. [DOI] [PubMed] [Google Scholar]
- 11.Illes J, et al. Discovery and Disclosure of Incidental Findings on Brain MRI in Research. Journal of Magnetic Resonance Imaging. 2004;20(5):743–747. doi: 10.1002/jmri.20180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.For more details, see Detection and Disclosure of Incidental Findings in Neuroimaging Research, available at <http://www.ninds.nih.gov/news_and_events/proceedings/ifexecsummary.htm> (last visited February 15, 2008) and MRI Research Safety and Ethics, available at <http://www.nimh.nih.gov/council/advis.cfm> (last visited February 15, 2008).
- 13.Kirschen MP, et al. Subjects’ Expectations in Neuroimaging Research. Journal of Magnetic Resonance Imaging. 2006;23(2):205–209. doi: 10.1002/jmri.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilgenberg S. Transformation: From Medical Student to Patient. Annals of Internal Medicine. 2006;144(10):779–780. doi: 10.7326/0003-4819-144-10-200605160-00015. [DOI] [PubMed] [Google Scholar]
- 15.Id., at 6.
- 16.Kass NE, et al. Balancing Justice and Autonomy in Clinical Research with Healthy Volunteers. Clinical Pharmacology and Therapeutics. 2007;82(2):219–227. doi: 10.1038/sj.clpt.6100192. [DOI] [PubMed] [Google Scholar]; Miller FG. Ethical Issues in Research with Healthy Volunteers: Risk-Benefit Assessment. Clinical Pharmacology and Therapeutics. 2003;74(6):513–515. doi: 10.1016/j.clpt.2003.08.006. [DOI] [PubMed] [Google Scholar]