Abstract
Two histidines are known to be essential for zinc potentiation of rat P2X2 receptors, but the chemistry of zinc coordination would suggest that other residues also participate in this zinc binding site. There is also a second lower affinity zinc binding site in P2X2 receptors whose constituents are unknown. To assess whether extracellular acidic residues of the P2X2 receptor contribute to zinc potentiation or inhibition, site-directed mutagenesis was used to produce alanine substitutions at each extracellular glutamate or aspartate. Two electrode voltage clamp recordings from Xenopus oocytes indicated that 7 of the 34 mutants (D82A, E85A, E91A, E115A, D136A, D209A, and D281A) were deficient in zinc potentiation and one mutant (E84A) was deficient in zinc inhibition. Additional tests on cysteine mutants at these 8 positions indicated that D136 is the only residue that is a strong candidate to be at the potentiating zinc binding site, and that E84 is unlikely to be at the inhibitory zinc binding site.
Keywords: P2X receptors, zinc modulation, MTSET, MTSES, substituted-cysteine accessibility
Introduction
The divalent cation zinc is found in the mammalian central nervous system either tightly bound to proteins or as a smaller pool of free, chelatable zinc (Cuajungco and Lees., 1997; Zatta et al. 2003). Genetic deletion of the zinc transporter ZnT3 in mice results in a dramatic decrease in the amount of zinc that can be detected in synaptic terminals but produces no obvious change in the behavior of mice (Palmiter et al. 1996; Cole et al. 1999; Salazar et al. 2005). Despite this observation, there is considerable evidence in support of the idea that synaptically released zinc plays a role as a regulator of synaptic transmission in the brain (Li et al. 2003). The most direct in vivo evidence for zinc as a physiological modulator in the brain is the observation that mice that express a mutant form of the glycine receptor α1 subunit gene (Glra1) that is zinc insensitive, but fully responsive to glycine, show a dramatic startle-prone phenotype, hyperekplexia (Hirzel et al. 2006).
In addition to modulating the activity of glycine receptors in vivo, in vitro studies indicate that extracellular zinc can potentiate or inhibit the current responses of nicotinic acetylcholine receptors (Hsiao et al. 2001; Hsiao et al. 2006), NMDA receptors (Forsythe et al. 1988; Rassendren et al. 1990) and GABA type A receptors (Gibbs et al. 2000). It has also been shown that zinc potentiates ATP-activated currents in many cell types including rat sympathetic neurons (Cloues et al. 1993), rat nodose ganglion neurons (Wright and Li., 1995), and acutely isolated rat hypothalamic neurons (Vorobjev et al. 2003). ATP released from cells can function as a synaptic neurotransmitter, an autocrine signal, or a paracrine signal in a wide range of mammalian tissues (Ralevic and Burnstock., 1998; Stojilkovic and Koshimizu., 2001). This signaling is mediated by ionotropic P2X receptors (P2XRs) and G-protein-coupled P2Y receptors. Because ATP and zinc are both released from some neurons in an activity-dependent manner (Wright and Li., 1995), it is plausible that neurotransmission at P2X utilizing synapses is modulated in vivo by zinc.
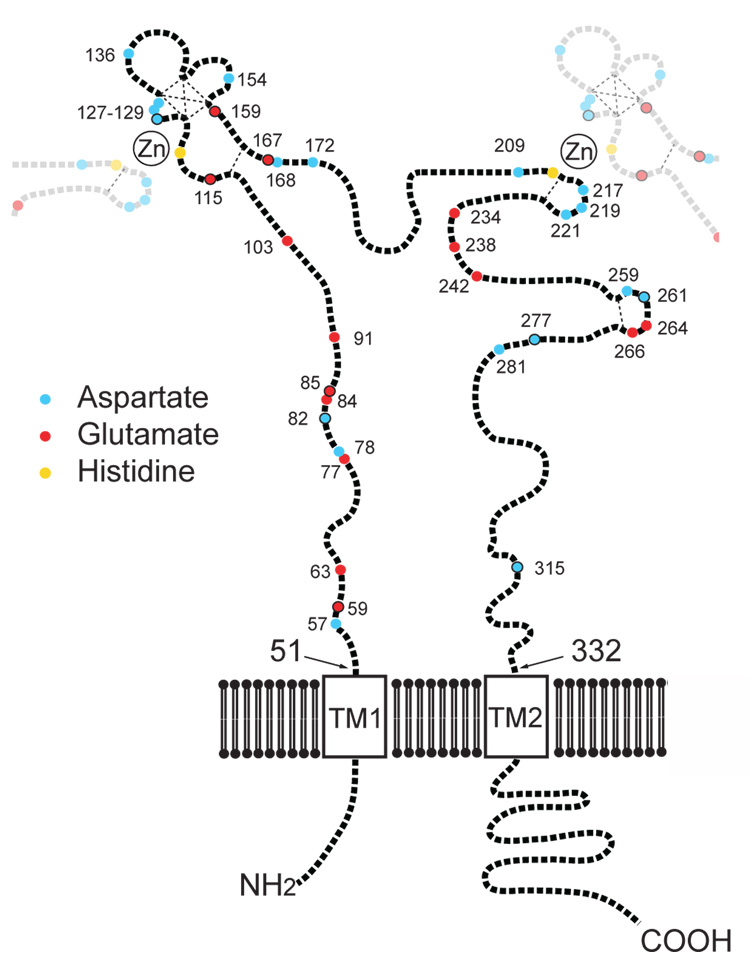
The P2X receptor family consists of seven genes (P2X1-X7) and the proteins they encode function as homomeric or heteromeric oligomers of three subunits (North, 2002). Each P2X subunit has a short intracellular amino terminus, a first transmembrane domain, a large extracellular domain, a second transmembrane domain and a carboxy terminal domain of diverse length among subunits (Fig. 1). With respect to P2X2 receptors, zinc exerts a concentration-dependent biphasic modulation that is thought to result from an increase in ATP affinity at low concentrations of zinc, and a voltage-independent inhibition that reduces the efficacy of channel opening at high concentrations of zinc (Wildman et al. 1998; Clyne et al. 2002a; Vorobjev et al. 2003). It has been shown previously by alanine-scanning mutagenesis that two extracellular histidines, H120 and H213, are required for zinc potentiation of P2X2 (Clyne et al. 2002a). Furthermore, the coordination of zinc by P2X2 has been shown to occur at the interface between adjacent subunits (Nagaya et al. 2005). Prior studies on zinc finger transcription factors and metalloenzymes have shown that zinc is typically coordinated in a tetrahedral configuration that requires nitrogen, oxygen, and sulfur ligands from the side chains of histidine, aspartate, glutamate, or cysteine residues (Maret, 2005). All of the extracellular histidines (Clyne et al. 2002a) and cysteines (Clyne et al. 2002b) of P2X2 have already been tested for their potential role in zinc potentiation. Therefore, in the current study we tested whether negatively charged residues of the P2X2 extracellular domain are structural determinants of the excitatory and/ or inhibitory zinc binding sites.
Figure 1. Residues of the extracellular domain of P2X2 tested in this study.
P2X2 receptors function as homotrimers. The full length of one of the subunits (black) is shown with pieces of the two neighboring subunits (gray) that contribute to the intersubunit coordination of zinc at the potentiating site. All of the negative residues in the extracellular region are numbered in accord with the rat P2X2 sequence. Within the extracellular domain aspartate residues are shown in cyan, and glutamate residues are shown in red. D or E residues with black outlines are conserved in at least 6 of the 7 rat P2X receptors. The two extracellular histidines (H120 and H213) known to coordinate zinc between subunits at the site for potentiation are shown in yellow. The seven other extracellular histidines are not shown. With the exception of the histidines that bind zinc and the 10 conserved extracellular cysteines that are believed to form 5 disulfide bonds (dashed lines), the relative positions of residues is unknown, so residues quite far apart in this schematic diagram might be physically quite close.
Experimental Procedures
Mutagenesis
Rat P2X2 cDNA (encoding a 472-amino acid protein) in pcDNA1 was obtained from Dr. D. Julius (University of California, San Francisco, CA). The mutations were generated using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). The sequences of mutant subunits were confirmed by DNA sequencing (University of Michigan DNA Sequencing Core). Each mutant is referred to by the original amino acid (one-letter code) followed by the residue number and the substituted amino acid (one-letter code).
Expression of P2X2 Receptors
P2X2 receptors were expressed in defolliculated stage V–VI Xenopus laevis oocytes. Oocytes were harvested using procedures approved by the University of Michigan Committee on the Use and Care of Vertebrate Animals and have been described in detail previously (Zhou and Hume, 1998). RNAs encoding wild type and mutant P2X2 receptors were synthesized using the mMessage mMachine T7 kit (Ambion, Austin, TX). Each oocyte was injected with 50 nl of RNA at 100–150 ng/µl, concentrations that produce a maximal response in wild type P2X2 (Clyne et al. 2003).
Electrophysiological Recordings
Two-electrode voltage clamp experiments were performed 2–5 days after RNA injection. All of the recordings were made at a holding potential of −50 mV. Recording electrodes were pulled from thin-walled borosilicate glass on a model P-87 Flaming Brown puller (Sutter Instrument Company, Novato, CA) and had resistances of 0.5–1 MΩ. The currents were recorded with a Turbo TEC-03 voltage clamp amplifier (npi electronic GmBH, Tamm, Germany). Data acquisition was performed using a Digidata 1322A interface controlled by pCLAMP 9 (Molecular Devices, Union City, CA).
Materials
[2-(trimethylammonium) ethyl] methanethiosulfonate bromide (MTSET) and sodium (2-sulfonatoethyl) methane thiosulfonate (MTSES) were obtained from Toronto Research Chemicals. All other chemicals were obtained from Sigma.
Solutions
The external recording solution contained (in mM): 90 NaCl, 1 KCl, 1.3 MgCl2, and 10 HEPES, pH 7.5. Electrodes were filled with an internal solution of 3 M KCl. Disodium ATP was prepared as a 100 mM stock in double-distilled H2O and stored at −20 °C. For recording, ATP solutions were made by diluting the stock in external recording solution. ATP solutions with concentrations of 200 µM and above were supplemented with MgCl2 to account for chelation of Mg2+ by ATP. The amount to add to each concentration of ATP was calculated using Bound and Determined (Brooks and Storey., 1992) so that the free magnesium concentration was 1 mM. The ATP concentrations of recording solutions were verified by spectroscopic measurement at 259 nm (using an extinction coefficient of 15.4*103 M−1cm−1). Zinc chloride was prepared as a 100 mM stock in double-distilled H2O that was acidified with 0.01 M HCl to prevent precipitation. The pH of ATP solutions with and without zinc was adjusted to 7.5 prior to recording. For experiments assaying pH potentiation, the pH of ATP solutions to be tested was adjusted to 6.5 prior to recording. All ATP recording solutions were used within 48 hours.
The sulfhydryl-reactive reagents MTSET and MTSES were prepared as 1 M stocks in dimethyl sulfoxide and stored in 10-µl aliquots at −20 °C. For recording, the stocks were diluted in the standard HEPES-buffered external recording solution to a working concentration of 1 or 10 mM. For each incubation, 50 µl of the working solution was added to the recording chamber with flow through the chamber stopped. After incubation for 1–2 minutes, the oocytes were washed in external recording solution for one minute, and then assayed with the same protocol used before treatment. This was repeated until there was no further change in response.
Quantification of Zinc Modulation and Selection of Zinc and ATP Concentrations
The response of P2X2 receptors to zinc is biphasic; at low levels, zinc causes potentiation, but at high levels it results in inhibition of current (Wildman et al. 1998). In a previous study, we used the non-potentiating mutants H120A and H213A to demonstrate that these two effects of zinc are separate processes. At a zinc concentration of 20 µM or below, the inhibition is negligible, whereas at all higher zinc concentrations both processes are occurring (Clyne et al. 2002a). We therefore used 20̣ µM zinc in all experiments testing only zinc potentiation, even though 50 and 100 µM zinc produce greater potentiation.
To compare the magnitude of zinc potentiation between groups of oocytes, it is essential that all of the oocytes be studied at similar points on the ATP concentration response relation, because as the concentration of ATP increases, potentiation decreases so that there is no zinc potentiation when a saturating concentration of ATP is present (Wildman et al. 1998). Furthermore, the EC50 for ATP of different oocytes expressing the same construct can vary significantly (Clyne et al. 2003). We dealt with these complications by testing each oocyte with both a low concentration of ATP which we expected would be close to the EC10 based on the average concentration response relation for each construct, and with 1,000 µM ATP, a maximal concentration for all constructs used in this study. Only data from oocytes for which it was verified that the low ATP concentration used was between the EC5 and the EC15 (so that at least a 6-fold increase in current was possible) were analyzed. All currents were measured after the responses had come to a steady state.
To test for zinc potentiation, oocytes were given low ATP followed by low ATP plus 20̣ µM zinc. We defined potentiation to 20 µM zinc as [(current in low ATP plus 20 µM zinc/current in low ATP) -1]. Thus, a cell that showed no increase in current in response to zinc had zinc potentiation equal to 0, and a cell inhibited by 20 µM zinc had negative zinc potentiation.
To test for zinc inhibition, oocytes were given low ATP plus 20 µM zinc followed by low ATP plus 1,000 µM zinc. We defined zinc inhibition as [1-(current in low ATP with 1000 µM zinc/current in low ATP with 20 µM zinc)]%. Thus, a construct in which high zinc completely inhibited the response would have an inhibition index of 100%, a construct unresponsive to high zinc would have 0% inhibition, and a construct in which 1,000 µM zinc potentiated more than 20 µM zinc would have negative inhibition.
Data Analysis
Data were analyzed using Clampfit and Microsoft Excel. Error bars shown in all figures are the standard error of the mean (SEM). The significance of differences between experimental conditions was tested using the student’s t test function of Excel. When the same oocyte was tested before and after some treatment, the paired t test was used, while when groups of oocytes were compared with each other the unpaired t test was used. To minimize the possibility that with so many mutants compared to the wild type apparent significance might arise based on chance alone, significance was taken to be p<0.01.
Concentration response relations for ATP were fit to the three parameter Hill equation using the nonlinear curve fitting program of Sigmaplot 9.0 (Systat Software Inc., San Jose, CA). For displaying average concentration response data, the points from each oocyte were normalized to between 0 and 100% based on the maximum value of the fitted curve. The scaled data were then averaged and plotted with error bars indicating the standard error of the mean. The lines fit to the data indicate the average parameters of the individual fits.
Results
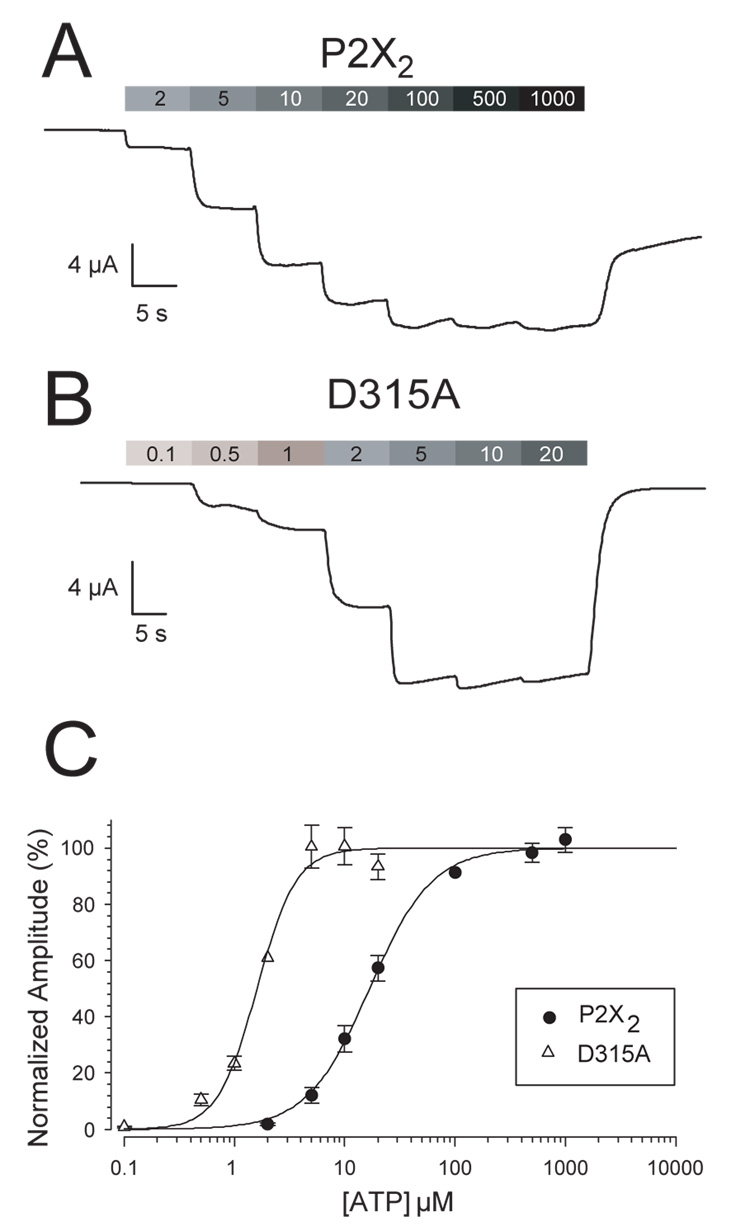
When voltage clamped at −50 mV, oocytes expressing all 34 of the P2X2 mutants tested (Fig. 1) responded to 100 µM ATP with readily detectable inward currents. Concentration response relations were assessed by giving an ascending series of ATP concentrations without intervening washes (Fig. 2A, B), and the data were fit to the Hill equation (Fig. 2C). All shifts in the EC50 were less than 10-fold (Table 1). The mutant receptor E85A showed the greatest rightward shift in EC50 (about 5-fold), and the mutant receptor D315A showed the greatest leftward shift in EC50 (about 6.5-fold, Fig. 2B). In addition to changes in EC50, some mutants showed Hill coefficients that were somewhat larger or smaller than wild type. We did not explore the mechanisms that caused these changes because there was no apparent correlation between having an altered Hill coefficient or EC50 and having a change in the ability to respond to zinc (Table 1). To determine the maximum response that each construct could produce when saturating levels of RNA were injected, we used 1,000 µM ATP, which is maximal for all mutants studied. All mutants had maximal currents of at least −3 µA (Table 1), indicating that several million functional receptors were successfully delivered to the cell surface.
Figure 2. Method for obtaining ATP concentration response relations.
A and B. Representative traces illustrating concentration response relationship of wild type P2X2 receptors and the mutant D315A (the most left-shifted mutant studied). The numbers in the boxes above each trace indicate the ATP concentration in µM and the length of the boxes indicates the duration of each application. All traces were obtained from Xenopus oocytes voltage clamped at −50 mV. C. Concentration response relationships of oocytes expressing wild type P2X2 and D315A. The error bars represent the standard error of the mean (SEM).
Table 1.
Characteristics of rat P2X2 receptors and mutant receptors with an alanine substitution at one negatively charged extracellular residue. Values reported are the mean and the SEM for each construct tested. N indicates the number of oocytes tested to determine the concentration response relation. Data from additional oocytes were sometimes included in estimates of the maximal current. The seven mutants with significantly decreased zinc potentiation (five with p<0.01 plus two additional mutants that differed from wild type at p<0.015) are indicated with light gray shading, and the one mutant with significantly decreased zinc inhibition is indicated with dark gray shading.
| Mutant | EC50 (µM) |
Hill Coefficient | Peak inward current at −50 mV (µA) |
N | Number of rat P2X receptors with a negative residue at this position |
||
|---|---|---|---|---|---|---|---|
| wild type | 15 ± 1.3 | 1.9 ± 0.05 | 12.8 ± 1.6 | 20 | |||
| D57A | 43 ± 9.9 * | 1.3 ± 0.21 * | 12.0 ± 1.4 | 6 | 4 | ||
| E59A | 10 ± 0.6 | 1.9 ± 0.08 | 9.8 ± 1.5 | 4 | 6 | ||
| E63A | 20 ± 6.9 | 1.0 ± 0.08 * | 3.1 ± 0.3 | 9 | 2 | ||
| E77A | 22 ± 1.0 | 1.7 ± 0.03 | 6.3 ± 1.4 | 5 | 1 | ||
| D78A | 33 ± 5.5 * | 1.6 ± 0.12 | 6.1 ± 1.0 | 5 | 2 | ||
| D82A | 55 ± 8.0 * | 1.1 ± 0.07 * | 9.6 ± 1.2 | 11 | 7 | ||
| E84A | 3 ± 0.2 * | 2.1 ± 0.09 | 16.7 ± 1.4 | 5 | 1 | ||
| E85A | 66 ± 5.2 * | 1.8 ± 0.09 | 7.3 ± 1.9 | 8 | 7 | ||
| E91A | 48 ± 6.6 * | 1.4 ± 0.03 * | 7.3 ± 0.4 | 5 | 1 | ||
| E103A | 7 ± 1.0 * | 1.9 ± 0.09 | 12.5 ± 1.5 | 6 | 1 | ||
| E115A | 42 ± 2.7 * | 1.5 ± 0.04 | 8.0 ± 0.7 | 5 | 7 | ||
| D127A | 12 ± 1.4 | 3.0 ± 0.55 * | 25.5 ± 5.3 | 9 | 7 | ||
| D128A | 35 ± 2.9 | 1.3 ± 0.06 * | 18.1 ± 1.4 | 6 | 3 | ||
| D129A | 20 ± 1.5 | 1.9 ± 0.07 | 23.0 ± 4.6 | 5 | 4 | ||
| D136A | 17 ± 3.6 | 2.0 ± 0.10 | 9.3 ± 1.5 | 6 | 4 | ||
| D154A | 9 ± 0.6 | 3.2 ± 0.64 * | 16.3 ± 1.6 | 5 | 1 | ||
| E159A | 9 ± 0.3 * | 4.1 ± 0.52 * | 16.1 ± 1.7 | 8 | 7 | ||
| E167A | 7 ± 0.3 * | 1.3 ± 0.16 * | 16.0 ± 3.2 | 5 | 7 | ||
| D168A | 20 ± 2.5 | 1.5 ± 0.11 | 24.8 ± 2.8 | 5 | 4 | ||
| D172A | 11 ± 0.6 | 1.7 ± 0.04 | 17.1 ± 2.4 | 5 | 2 | ||
| D209A | 43 ± 4.5 * | 1.5 ± 0.05 * | 9.1 ± 0.5 | 6 | 2 | ||
| D217A | 10 ± 0.5 | 2.1 ± 0.09 | 18.6 ± 2.2 | 5 | 2 | ||
| D219A | 10 ± 0.3 | 1.8 ± 0.06 | 15.1 ± 1.0 | 5 | 2 | ||
| D221A | 5 ± 0.7 * | 2.3 ± 0.10 * | 16.4 ± 3.2 | 6 | 2 | ||
| E234A | 15 ± 1.0 | 1.8 ± 0.13 | 15.3 ± 0.8 | 6 | 1 | ||
| E238A | 12 ± 1.4 | 1.5 ± 0.04 * | 20.8 ± 2.8 | 6 | 2 | ||
| E242A | 2 ± 0.2 * | 1.9 ± 0.05 | 18.5 ± 2.4 | 5 | 5 | ||
| D259A | 19 ± 3.0 | 1.7 ± 0.17 | 11.0 ± 1.8 | 5 | 4 | ||
| D261A | 9 ± 0.5 * | 2.0 ± 0.09 | 7.7 ± 0.8 | 8 | 7 | ||
| E264A | 8 ± 0.4 * | 2.3 ± 0.20 * | 17.9 ± 3.7 | 7 | 1 | ||
| E266A | 7 ± 0.4 * | 1.7 ± 0.09 | 18.2 ± 2.1 | 5 | 2 | ||
| D277A | 22 ± 4.2 | 1.2 ± 0.10 * | 11.6 ± 2.2 | 5 | 6 | ||
| D281A | 4 ± 0.3 * | 1.9 ± 0.04 | 12.7 ± 2.0 | 6 | 2 | ||
| D315A | 2 ± 0.4 * | 2.5 ± 0.45 | 26.6 ± 8.1 | 6 | 7 |
Asterisks indicate EC50s or Hill coefficients that were significantly different from wild type P2X2 (p<0.01, two tailed t test assuming equal variance).
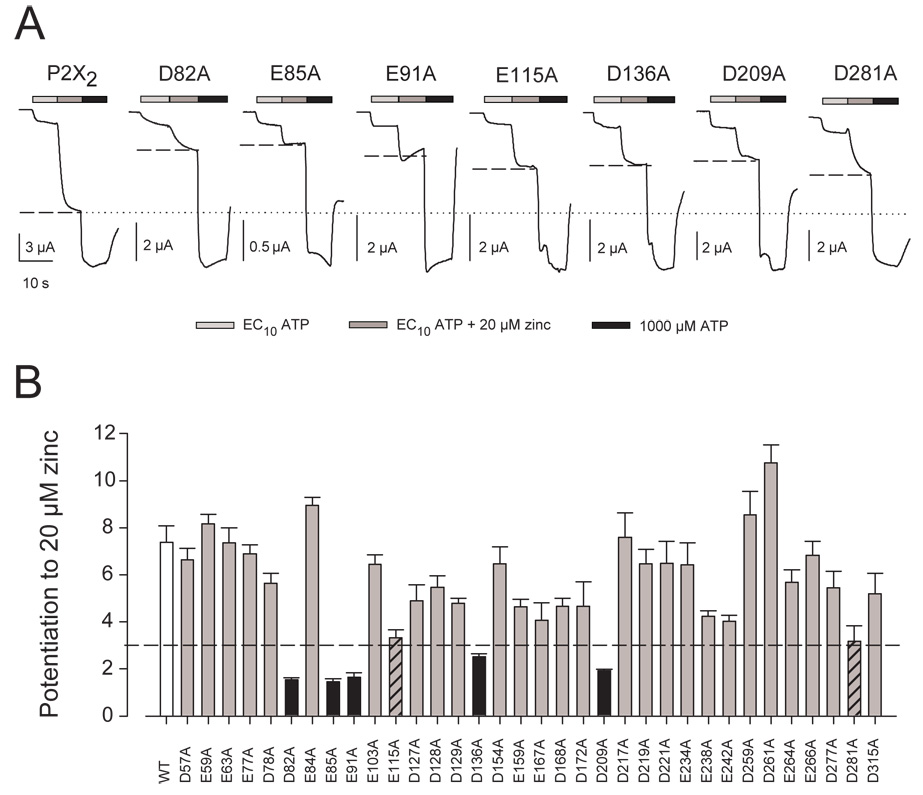
Zinc potentiation was assayed (Fig. 3A) and quantified as described in Methods. Wild type P2X2 exhibited mean zinc potentiation of about 7 while the zinc insensitive mutations previously studied (H120A and H213A) gave potentiation near 0. All 34 mutants tested in this study gave detectable zinc potentiation (Fig. 3B). None of the mutants showed a significant increase in zinc potentiation. Five mutants (D82A, E85A, E91A, D136A, and D209A), all with an average zinc potentiation below 3, potentiated significantly less (p<0.01) than wild type P2X2. Two other mutants (E115A and D281A) had error bars that extended below 3 and just missed passing our test of significance (with p<0.015 but not <0.01). We focused the rest of our studies of zinc potentiation on these seven mutants.
Figure 3. Potentiation of wild type and mutant P2X2 receptors by 20 µM zinc.
A. Representative traces from wild type P2X2 and each mutant receptor that was deficient in zinc potentiation. Each construct was first tested with an amount of ATP that produced approximately 10% of the maximal response (EC10 ATP), then with EC10 ATP plus 20 µM zinc, and finally with 1,000 µM ATP. All traces were scaled so that the response to 1,000 µM ATP was of equal height. The current in response to this concentration of ATP produced a maximal response for all constructs. The dotted line across all the traces represents the predicted amplitude of the current if zinc potentiation was unchanged. The dashed line intersecting each trace indicates the actual amount of zinc potentiation. B. Potentiation in response to 20 µM zinc for all mutants studied. Zinc potentiation was calculated as defined in Methods. The dashed line indicates a potentiation ratio of 3.0. For each construct, data from 5–10 oocytes were averaged. Error bars represent the SEM. Black bars indicate mutants that showed significantly reduced zinc potentiation (p<0.01) compared to wild type P2X2. Two additional mutants, E115A and D281A differed from wild type at p<0.015 and are indicated with diagonal lines.
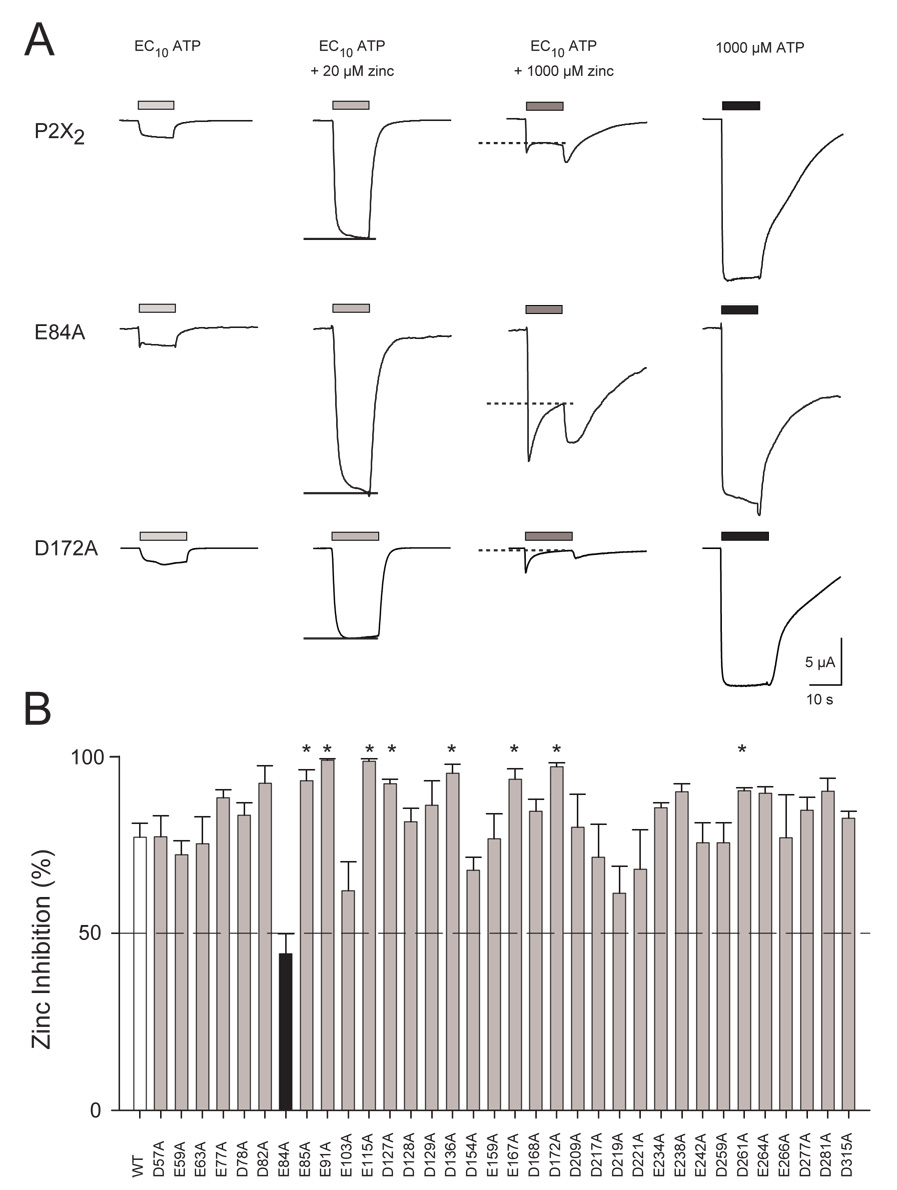
In wild type P2X2, the potentiation to zinc declines as zinc concentration rises above 100 µM. This is due to the action of a low affinity inhibitory site with an estimated IC50 of about 120 µM (Clyne et al. 2002a). As a screen for whether any of these mutations might have altered the inhibitory zinc binding site, we compared the amplitude of responses to the EC10 concentration of ATP alone, the EC10 concentration of ATP plus 20 µM zinc, the EC10 concentration of ATP plus 1,000 µM zinc, and 1,000 µM ATP (a saturating concentration) alone (Fig. 4A). The quantitative index of zinc inhibition (Fig. 4B) was calculated as defined in Methods. For wild type P2X2, the current declined about 5-fold as zinc was increased from 20 µM to 1,000 µM, such that the amplitude of responses to low ATP alone and low ATP plus 1,000 µM zinc were similar. Of the 34 alanine mutants assayed, 25 exhibited no significant difference from the ~75% inhibition by 1,000 µM zinc measured in wild type P2X2. Eight mutants, (E85A, E91A, E115A, D127A, D136A, E167A, D172A and D261) showed significantly enhanced zinc inhibition (Fig 4B; p<0.01), and one mutant (E84A) showed significantly decreased zinc inhibition. An increase in zinc inhibition is opposite to the result expected if these residues were part of the inhibitory zinc binding site. It should be noted that when the combination of low ATP and high zinc was applied or removed, currents of wild type P2X2 receptors showed transients at both the onset and the offset (see Fig 4A). These transients are a manifestation of the slower on-kinetics and faster off-kinetics of the low affinity zinc inhibition as compared to the higher affinity zinc potentiation process (Clyne et al. 2002a). The on and off transients were much larger in the currents of the E84A mutant (Fig. 4A).
Figure 4. Inhibition of wild type and mutant P2X2 receptors by 1,000 µM zinc.
A. Examples of inhibition by high zinc in wild type P2X2 and in mutants that showed less inhibition (E84A) and enhanced inhibition (D172A). Each trace is the response as the bathing solution was switched from external solution alone to the indicated solutions for the duration indicated by the bar. Five minutes of recovery was allowed between adjacent traces. Zinc inhibition was determined based on the amplitude of the current with EC10 ATP + 1000 µM zinc (dashed line) and the current with EC10 ATP + 20 µM zinc (solid line). B. Inhibition in response to 1,000 µM zinc for all mutants studied. Zinc inhibition was calculated as defined in Methods, with 100% representing complete inhibition, and 0% representing no inhibition. The dashed line indicates 50% inhibition. For each construct, data from 3–10 oocytes were averaged. Error bars represent the SEM. The black bar indicates the one mutant that showed significantly reduced zinc inhibition (p<0.01) compared to wild type P2X2. Asterisks indicate mutants that showed significantly increased zinc inhibition (p<0.01) compared to wild type P2X2.
The decrease in either potentiation to low zinc (20 µM) or inhibition to high zinc (1,000 µM) might reflect an impeded ability to bind zinc at the modulatory sites, a diminution of communication between the occupied zinc binding site and the gating machinery, enhanced opposing modulation, or a general defect in channel gating. In the latter case, these candidate mutants might have been expected to also be deficient in potentiation to protons, an allosteric modulator (Nakazawa et al. 1997) that acts at a different site (Clyne et al. 2002a). In wild type receptors, application of an ATP concentration that produced an EC10 response at pH 7.5 produced a large increase in current when the pH was dropped to 6.5. Each of the mutants that were highly deficient in zinc potentiation or inhibition had pH potentiation that was indistinguishable from wild type P2X2 (wild type 6.3±1.1, D82A 6.2±0.7, E84A 8.4±1.3, D85A 7.0±0.8, E91A 6.1±0.5, E115A 6.2±0.8, D136A 5.4±1.0, D209A 6.9±0.7, D281A 7.2±1.5), so none of these mutations produced a general defect in channel gating.
Cysteines support greater zinc potentiation than alanines at several candidate sites
To test the role of amino acid residues identified in the alanine screen, we generated individual cysteine substitutions at the candidate positions and determined their effects on zinc potentiation and inhibition. If a candidate residue participates in zinc binding, it would be expected that the response to zinc would be enhanced in the “C” version over the “A” version, because it is known that cysteine residues perform better than non-polar alanine residues as ligands for zinc (Maret, 2005). Responses to ATP were recorded from all the cysteine substituted candidates. Cysteine substitution increased zinc potentiation over alanine substitution at positions 82, 136, and 209. There was no significant difference between zinc potentiation for A and C mutants at positions 84, 85, 91 and 281, and a cysteine at position 115 virtually eliminated zinc potentiation (Fig. 5A).
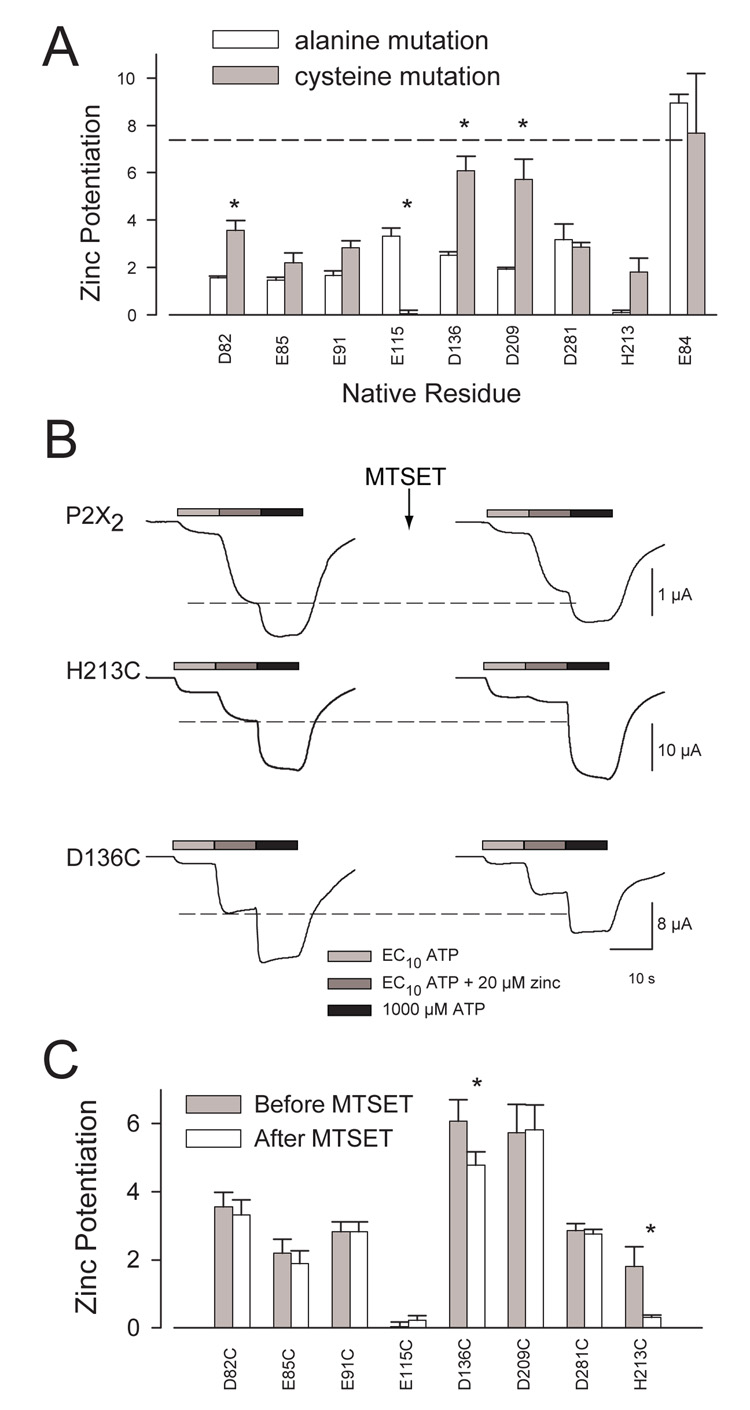
Figure 5. Zinc potentiation in mutants with cysteines at candidate zinc binding site locations.
A. Potentiation to 20 µM zinc when an alanine or a cysteine was substituted at each indicated position. Each bar represents the average zinc potentiation (as defined in Methods) of 4–9 oocytes for each construct assayed. White bars represent alanine mutants, gray bars represent cysteine mutants, and error bars represent SEM. The dashed line represents the mean potentiation for wild type. The data for the alanine mutants are the same as shown in Figure 3. Asterisks indicate when the A and C mutants at the same position were significantly different (p<0.01). B. Representative traces for wild type, H213C, and D136C expressing oocytes before and after MTSET treatment. Zinc potentiation was present in the untreated H213C mutant, but was greatly reduced following a 1 minute exposure to 1 mM MTSET. The dashed lines illustrate the expected amplitude of the current if MTSET did not interfere with zinc responses. C. Results from the MTSET experiments done on potentiation candidates shown as the mean potentiation before MTSET treatment (gray bars) and the mean potentiation after treatment (white bars). Only the potentiated responses of oocytes expressing D136C and the H213C positive controls were significantly (p<0.01) reduced by MTSET treatment (asterisks). Error bars represent SEM for 3–7 oocytes per construct.
To test whether any of the cysteine substituted zinc modulation candidates were accessible to the positively charged, thiol-reactive compound MTSET (Javitch et al. 1994), oocytes expressing the cysteine substituted candidates were assayed for zinc potentiation or inhibition before and after treatment with MTSET. For a positive control to verify that the MTSET had not been degraded, we used H213C, which had previously been shown to be accessible to MTSET. For H213C, MTSET treatment produced a significant decrease in potentiation as expected. Of the cysteine substituted potentiation candidates, the zinc potentiation before and after MTSET treatment was significantly different for only D136C (Fig. 5B, C). The other cysteine substituted mutants showed potentiation ratios similar to levels observed before MTSET treatment (Fig. 5C). These results show that position 136 is accessible to MTSET, and therefore meets an essential criterion for participation in a site coordinating extracellular zinc.
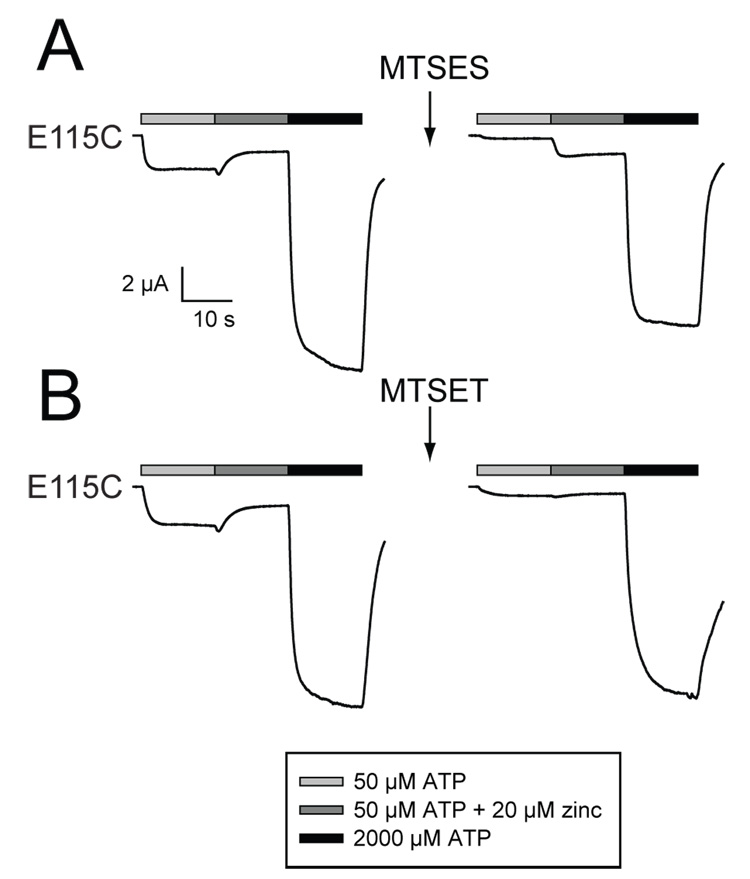
Because oocytes expressing the E115A mutant showed modest zinc potentiation, the failure to see any zinc potentiation in oocytes expressing E115C was unexpected. One possible explanation for this result is a cysteine at this site is unable to engage in zinc binding because it has been trapped in an unnatural disulfide bond with one of the 10 endogenous cysteines of the extracellular domain of P2X2. To test this possibility, we treated oocytes expressing E115C with the reducing agent dithiothreitol (DTT), and then retested for zinc potentiation. It had previously been demonstrated that DTT has no effect on zinc potentiation in wild type oocytes, but that receptors that carry cysteines replacing both of the histidines required for zinc potentiation (H120C/H213C) form disulfide bonds that make them zinc resistant until after DTT treatment (Nagaya et al. 2005). Treatment of oocytes expressing E115C with DTT (10 mM for 5 minutes) caused no significant increase in potentiation to 20 µM zinc (before −0.6 ± 0.1, after −0.4 ± 0.1; N=3), while DTT caused a robust and highly significant increase in the positive control oocytes expressing the H120C/H213C double mutant (before 0.1 ± 0.1, after 2.4 ± 0.4; N=3; p<0.001). As the failure of E115C to allow zinc potentiation did not appear to be caused by trapping the cysteine in a disulfide bond, we considered the possibility that the absence of a negative charge at position 115 forced the receptor into a new conformation that either indirectly eliminated the zinc binding site, or prevented bound zinc from modifying channel gating. To test these possibilities, we treated oocytes expressing E115C with MTSES, a negatively charged cysteine reactive reagent (Figure 6). In contrast to the slight zinc inhibition shown before treatment (which gave negative zinc potentiation of −0.4 ± 0.1), MTSES treated oocytes expressing E115C showed pronounced potentiation to 20 µM zinc (4.2 ± 0.5; N=6; p<0.0001). The zinc potentiation after MTSES treatment was a consequence of both a significant decrease in the response to low ATP alone (to 18% ± 3% of its previous amplitude) and an increase in the response to ATP plus 20 µM zinc (to 166% ± 28% of its previous amplitude) with relatively little change in the response to a high concentration (2 mM) of ATP (85% ± 3%; N=6 for each). The effect on E115C was specific for a negatively charged reagent, as treatment with positively charged MTSET had no effect on zinc potentiation on oocytes expressing E115C (potentiation before = −0.5 ± 0.1; potentiation after = −0.2 ± 0.3). However, MTSET treatment did decrease the amplitude of currents in response to low ATP (to 19% ± 5%) and the currents in response to 2 mM ATP (to 85% ± 8%; N=5), which were very similar to the effects of MTSES treatment on these parameters. There was no significant effect of MTSES treatment on the zinc potentiation of oocytes expressing wild type P2X2.
Figure 6. Effect of 20 µM zinc on oocytes expressing E115C before and after treatment with disulfide reactive reagents.
Following the initial test for responses to ATP and zinc, each oocyte was incubated for 2 minutes in 10 mM of the indicated reagent and then washed for one minute (arrow) before being retested. A. MTSES restored zinc potentiation. B. MTSET did not restore zinc potentiation.
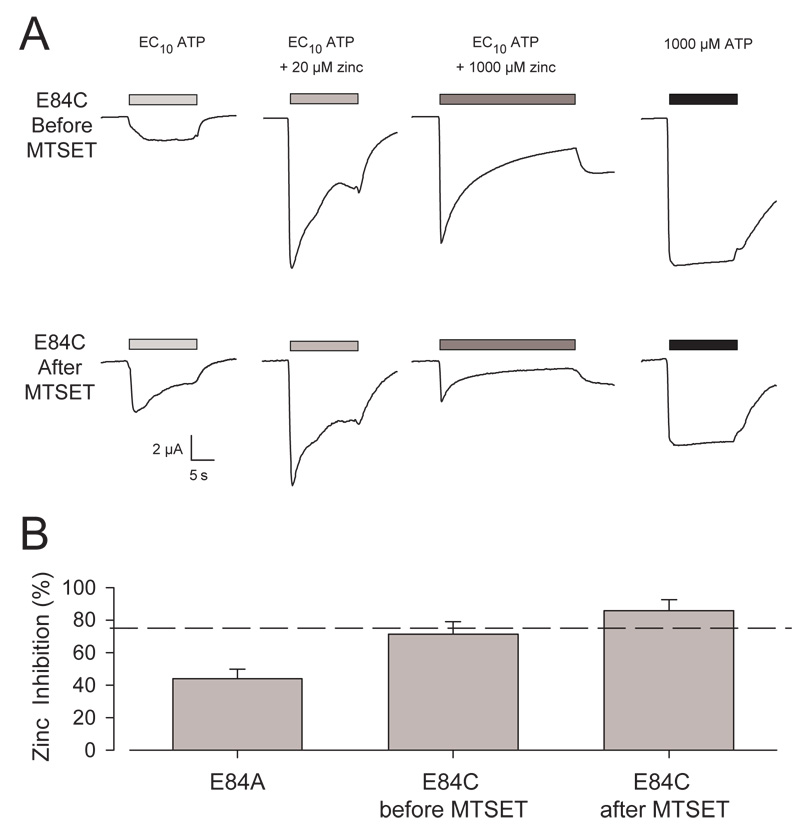
The currents from oocytes expressing the E84C mutant showed several unexpected features (Fig. 7A). Like wild type and the E84A mutant (Fig. 5A), the response of E84C to an EC10 concentration of ATP plus 20 µM zinc resulted in substantial zinc potentiation. However, a large component of this potentiation was only transient, while in wild type and E84A it was sustained. Furthermore, the steady state responses to low ATP plus 20 µM zinc or 1,000 µM zinc were quite similar in E84C, while in wild type and E84A the currents were much smaller in high zinc. MTSET treatment of individual oocytes expressing E84C resulted in little or no change in the responses to low ATP alone or to low ATP plus 20 µM zinc (Fig. 7A), but the steady state inhibition by 1,000 µM zinc was substantially enhanced (Fig. 7A, B). MTSET treatment of oocytes expressing E84C also attenuated the current evoked by 1,000 µM ATP alone (from 9,900 nA ± 1,100 to 6,800 nA ± 700, N=5).
Figure 7. MTSET sensitivity of zinc inhibition in oocytes expressing E84C.
A. Representative traces for E84C before and after treatment with 1 mM MTSET. Shown are responses to low ATP, low ATP with 20 µM zinc, low ATP with 1,000 µM zinc, and 1,000 µM ATP. B. Histogram representing the average percent inhibition of responses of E84A (N = 4), and E84C before and after treatment with MTSET (N = 6). Error bars represent the SEM. The dashed line represents the average percent inhibition for wild type P2X2 (76% ± 3%, N=6) stimulated with low ATP containing 1,000 µM zinc.
Discussion
The goal of this study was to test whether any of the negatively charged residues in the extracellular domain of P2X2 might directly contribute to zinc binding. As an initial test of this idea, we made alanine mutations at all 34 extracellular positions at which either a glutamate or an aspartate was present, characterized the concentration response relation to ATP of each mutant and then carried out assays for zinc potentiation and zinc inhibition.
General effects of mutation of negatively charged residues of P2X2
When the sequences of the seven rat P2X receptors are compared, 7 of the 34 positions we tested have negatively charged residues only in P2X2. Of the remaining positions tested, 16 have a negatively charged residue in two to four subunits, 3 have a negatively charged residue in five or six subunits, and 8 have a negatively charged residue in all seven subunits (Table 1). When alanine was substituted at each of the 23 negatively charged positions that are not highly conserved, there were only modest changes in the concentration response relations for ATP. Similarly, although conservation between multiple members of a gene family often indicates that a residue plays an essential role, we found only modest changes in the concentration response relations for ATP when the 11 most highly conserved residues were replaced with alanines. This agrees with previous work on all of the homologous residues in human P2X1 (Ennion et al. 2001) and many of the same residues in rat P2X2 (Jiang et al. 2000). Similar to the previous work, the mutants with the most distinctive phenotypes in our study were E85A and D315A (which are equivalent to D89A and D316A in human P2X1). In oocytes, both the P2X1 D89A and the P2X2 E85A mutants produce a modest rightward shift in ATP potency. A previous attempt to express P2X2 E85A and D261A in HEK293 cells resulted in no detectable current (Jiang et al. 2000). Perhaps the ability to express very high levels of protein in oocytes accounted for our ability to observe currents from these mutants. In all three studies, the most left-shifted mutant of the 11 conserved extracellular acidic residues was produced by placing an alanine at the position equivalent to D315 of rat P2X2. All changes at this position are not equivalent, as mutation to valine by Nakazawa et al. (1998) resulted in a 60-fold decrease in ATP potency. In summary, neither the conserved nor the unconserved negative residues of the extracellular domain could be demonstrated to play an essential direct role in allowing P2X2 receptors to respond to ATP. This stands in stark contrast to the dramatic effect on the EC50 caused by mutating some of the conserved positive residues of P2X receptors (Ennion et al. 2000; Jiang et. al., 2000).
Are any negatively charged resides involved in binding zinc?
If a residue is required for binding zinc to the potentiating site, our expectation was that when the residue was replaced with an alanine the enhancement of ATP-evoked currents by 20 µM zinc would be substantially attenuated. Of the 34 positions tested in our alanine scan, seven (D82, E85, E91, E115, D136, D209, and D281) showed substantial attenuation of zinc potentiation, although all showed normal pH potentiation. Similarly, if a residue was required for binding to the inhibitory site, then the attenuation of ATP-evoked currents in the presence of 1,000 µM zinc would be significantly lessened. Only one mutant, E84A, passed this preliminary test. Each of these 8 candidate residues was subjected to a secondary screen which involved producing mutants that carried a cysteine at each site under study and then testing the effect of MTSET on zinc modulation. The rationale for this approach was that if the candidate residue is part of the zinc binding site, modification with a bulky compound like MTSET should occlude the site, and weaken zinc binding.
The candidate for participation in the inhibitory zinc binding site, E84, can be ruled out as a participant in zinc binding, because MTSET treatment of oocytes expressing E84C resulted in greater zinc inhibition. If position 84 contributed directly to zinc binding at the inhibitory site, less zinc inhibition would be the expected result. It was also noted that zinc inhibition was significantly enhanced when alanine was placed in 8 positions. Because the zinc concentration response relations for potentiation and inhibition overlap (Clyne et al, 2002a), it was not a surprise that some mutations that depressed zinc potentiation (E85A, E91A, E115A, and D136A) showed enhanced inhibition, as no change to the inhibitory process would be needed to obtain this result. It is unclear how mutations D127A, E167A, D172A and D261A enhanced inhibition while producing no significant reduction of potentiation. It was not surprising that the E84A mutation, which had a decreased ability to produce inhibition to high zinc did not result in enhanced potentiation to 20 µM zinc, because very little inhibition is produced in wild type oocytes by 20 µM zinc (Clyne et al. 2002a).
Of the seven candidates for participation in the potentiating site, only D136C showed significant attenuation of zinc potentiation following MTSET treatment. However, even when the MTSET effect had reached saturation (as determined by a failure to change when the duration of MTSET exposure was doubled), substantial zinc potentiation remained after MTSET treatment. Like the modification of D136C we report here, MTSET modification of H120C and H213C, residues known to be in the zinc binding site, produces only attenuation and not elimination of zinc potentiation. One potential explanation is that although P2X2 receptors are homotrimers with three zinc binding sites producing potentiation, it may not be possible to simultaneously modify all three zinc binding sites with MTSET. If this is so, residual zinc potentiation would be expected after MTSET treatment, because it was previously demonstrated that even a single zinc binding site is sufficient to produce substantial potentiation (Nagaya et al. 2005).
Of the other six candidates for involvement in zinc binding to the excitatory site, the cysteine mutant E115C did not potentiate to zinc. Additional experiments demonstrated that it is unlikely that E115 is in the potentiating zinc binding site, as MTSES treatment restored the ability to respond to zinc, while attaching such a large molecule to a cysteine in the zinc binding site would have been expected to occlude zinc binding. There are two possible explanations why the cysteine mutants of the other five candidates did not have altered responses to zinc following MTSET treatment. A simple one is that MTSET bound to the cysteine, but had no effect because the candidate residue was not near the zinc binding site. An alternative is that one or more of these residues are part of the excitatory zinc binding site, but MTSET was not able to access the cysteine replacing the endogenous residue. This second explanation seems unlikely because we know that MTSET can access cysteines replacing the two histidines (H120 or H213) that are known to be part of the zinc binding site (Nagaya et al. 2005). If these six candidates are not direct participants in zinc binding, what else might have caused alanine mutations at these sites to attenuate zinc potentiation? A plausible idea is that these negative charges are essential for the normal execution of the conformational changes that follow zinc binding, but not for opening the channel in response to ATP alone, or to potentiation by acidic pH, both of which are relatively normal in these mutants.
If D136 proves to be part of the excitatory zinc binding site, then one additional residue likely remains to be discovered, as most zinc sites are tetrahedral, and two histidines had previously been identified as participating in zinc binding. It is potentially of interest that the position equivalent to D136 is also negatively charged in P2X1, P2X4, and P2X7. P2X4 is able to potentiate in response to zinc (Wildman et al. 1999a; Xiong et al. 1999), while P2X1 and P2X7 are inhibited by zinc (Virginio et al. 1997; Wildman et al. 1999b). None of these subunits contain histidines at positions equivalent to H120 or H213 of P2X2, so if the aspartate equivalent to D136 plays a role in zinc potentiation in P2X4, one would have to look elsewhere in the sequence of this subunit for the rest of the excitatory binding site. Similarly, if E84 proves to be involved in binding zinc at the inhibitory site of P2X2, one would have to seek a different site to explain zinc inhibition of P2X1 and P2X7, as a negative residue is not found at the equivalent position in these subunits (nor in any subunit other than P2X2).
Acknowledgments
We thank Shlomo Dellal, Naomi Nagaya, Jamila Power, and Rachel Tittle for helpful discussions and for comments on the manuscript. This work was supported by PHS grant NS039196.
Footnotes
Abbreviation used: MTSET, [2- (trimethylammonium) ethyl] methanethiosulfonate bromide, MTSES, (2-sulfonatoethyl) methane thiosulfonate, DTT, dithiothreitol
References
- Brooks SP, Storey KB. Bound and determined: a computer program for making buffers of defined ion concentrations. Anal Biochem. 1992;201:119–126. doi: 10.1016/0003-2697(92)90183-8. [DOI] [PubMed] [Google Scholar]
- Cloues R, Jones S, Brown DA. Zn2+ potentiates ATP-activated currents in rat sympathetic neurons. Pflugers Arch. 1993;424:152–158. doi: 10.1007/BF00374606. [DOI] [PubMed] [Google Scholar]
- Clyne JD, Brown TC, Hume RI. Expression level dependent changes in the properties of P2X2 receptors. Neuropharmacology. 2003;44:403–412. doi: 10.1016/s0028-3908(02)00406-9. [DOI] [PubMed] [Google Scholar]
- Clyne JD, LaPointe LD, Hume RI. The role of histidine residues in modulation of the rat P2X(2) purinoceptor by zinc and pH. J Physiol. 2002a;539:347–359. doi: 10.1113/jphysiol.2001.013244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne JD, Wang LF, Hume RI. Mutational analysis of the conserved cysteines of the rat P2X2 purinoceptor. J Neurosci. 2002b;22:3873–3880. doi: 10.1523/JNEUROSCI.22-10-03873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TB, Wenzel HJ, Kafer KE, Schwartzkroin PA, Palmiter RD. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc Natl Acad Sci U S A. 1999;96:1716–1721. doi: 10.1073/pnas.96.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuajungco MP, Lees GJ. Zinc metabolism in the brain: relevance to human neurodegenerative disorders. Neurobiol Dis. 1997;4:137–169. doi: 10.1006/nbdi.1997.0163. [DOI] [PubMed] [Google Scholar]
- Ennion S, Hagan S, Evans RJ. The role of positively charged amino acids in ATP recognition by human P2X1 receptors. J Biol Chem. 2000;275:29361–29367. doi: 10.1074/jbc.M003637200. [DOI] [PubMed] [Google Scholar]
- Ennion SJ, Ritson J, Evans RJ. Conserved negatively charged residues are not required for ATP action at P2X(1) receptors. Biochem Biophys Res Commun. 2001;289:700–704. doi: 10.1006/bbrc.2001.6034. [DOI] [PubMed] [Google Scholar]
- Forsythe ID, Westbrook GL, Mayer ML. Modulation of excitatory synaptic transmission by glycine and zinc in cultures of mouse hippocampal neurons. J Neurosci. 1988;8:3733–3741. doi: 10.1523/JNEUROSCI.08-10-03733.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs JW, 3rd, Zhang YF, Shumate MD, Coulter DA. Regionally selective blockade of GABAergic inhibition by zinc in the thalamocortical system: functional significance. J Neurophysiol. 2000;83:1510–1521. doi: 10.1152/jn.2000.83.3.1510. [DOI] [PubMed] [Google Scholar]
- Hirzel K, Muller U, Latal AT, Hulsmann S, Grudzinska J, Seeliger MW, Betz H, Laube B. Hyperekplexia phenotype of glycine receptor alpha1 subunit mutant mice identifies Zn(2+) as an essential endogenous modulator of glycinergic neurotransmission. Neuron. 2006;52:679–690. doi: 10.1016/j.neuron.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Hsiao B, Dweck D, Luetje CW. Subunit-dependent modulation of neuronal nicotinic receptors by zinc. J Neurosci. 2001;21:1848–1856. doi: 10.1523/JNEUROSCI.21-06-01848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao B, Mihalak KB, Repicky SE, Everhart D, Mederos AH, Malhotra A, Luetje CW. Determinants of zinc potentiation on the alpha4 subunit of neuronal nicotinic receptors. Mol Pharmacol. 2006;69:27–36. doi: 10.1124/mol.105.015164. [DOI] [PubMed] [Google Scholar]
- Jiang LH, Rassendren F, Surprenant A, North RA. Identification of amino acid residues contributing to the ATP-binding site of a purinergic P2X receptor. J Biol Chem. 2000;275:34190–34196. doi: 10.1074/jbc.M005481200. [DOI] [PubMed] [Google Scholar]
- Javitch JA, Li X, Kaback J, Karlin A. A cysteine residue in the third membrane-spanning segment of the human D2 dopamine receptor is exposed in the binding-site crevice. Proc Natl Acad Sci U S A. 1994;91:10355–10359. doi: 10.1073/pnas.91.22.10355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YV, Hough CJ, Sarvey JM. Do we need zinc to think? Sci STKE 2003. 2003;182:pe19. doi: 10.1126/stke.2003.182.pe19. [DOI] [PubMed] [Google Scholar]
- Maret W. Zinc coordination environments in proteins determine zinc functions. J Trace Elem Med Biol. 2005;19:7–12. doi: 10.1016/j.jtemb.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Nagaya N, Tittle RK, Saar N, Dellal SS, Hume RI. An intersubunit zinc binding site in rat P2X2 receptors. J Biol Chem. 2005;280:25982–25993. doi: 10.1074/jbc.M504545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Ohno Y, Inoue K. An aspartic acid residue near the second transmembrane segment of ATP receptor/channel regulates agonist sensitivity. Biochem Biophys Res Commun. 1998;244:599–603. doi: 10.1006/bbrc.1998.8312. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Liu M, Inoue K, Ohno Y. pH dependence of facilitation by neurotransmitters and divalent cations of P2X2 purinoceptor/channels. Eur J Pharmacol. 1997;337:309–314. doi: 10.1016/s0014-2999(97)01293-4. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, Cole TB, Quaife CJ, Findley SD. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc Natl Acad Sci U S A. 1996;93:14934–14939. doi: 10.1073/pnas.93.25.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Rassendren FA, Lory P, Pin JP, Nargeot J. Zinc has opposite effects on NMDA and non-NMDA receptors expressed in Xenopus oocytes. Neuron. 1990;4:733–740. doi: 10.1016/0896-6273(90)90199-p. [DOI] [PubMed] [Google Scholar]
- Salazar G, Craige B, Love R, Kalman D, Faundez V. Vglut1 and ZnT3 co-targeting mechanisms regulate vesicular zinc stores in PC12 cells. J Cell Sci. 2005;118:1911–1921. doi: 10.1242/jcs.02319. [DOI] [PubMed] [Google Scholar]
- Stojilkovic SS, Koshimizu T. Signaling by extracellular nucleotides in anterior pituitary cells. Trends Endocrinol Metab. 2001;12:218–225. doi: 10.1016/s1043-2760(01)00387-3. [DOI] [PubMed] [Google Scholar]
- Virginio C, Church D, North RA, Surprenant A. Effects of divalent cations, protons and calmidazolium at the rat P2X7 receptor. Neuropharmacology. 1997;36:1285–1294. doi: 10.1016/s0028-3908(97)00141-x. [DOI] [PubMed] [Google Scholar]
- Vorobjev VS, Sharonova IN, Sergeeva OA, Haas HL. Modulation of ATP-induced currents by zinc in acutely isolated hypothalamic neurons of the rat. Br J Pharmacol. 2003;139:919–926. doi: 10.1038/sj.bjp.0705321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman SS, King BF, Burnstock G. Zn2+ modulation of ATP-responses at recombinant P2X2 receptors and its dependence on extracellular pH. Br J Pharmacol. 1998;123:1214–1220. doi: 10.1038/sj.bjp.0701717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman SS, King BF, Burnstock G. Modulation of ATP-responses at recombinant rP2X4 receptors by extracellular pH and zinc. Br J Pharmacol. 1999a;126:762–768. doi: 10.1038/sj.bjp.0702325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman SS, King BF, Burnstock G. Modulatory activity of extracellular H+ and Zn2+ on ATP-responses at rP2X1 and rP2X3 receptors. Br J Pharmacol. 1999b;128:486–492. doi: 10.1038/sj.bjp.0702802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JM, Li C. Zn2+ potentiates steady-state ATP activated currents in rat nodose ganglion neurons by increasing the burst duration of a 35 pS channel. Neurosci Lett. 1995;193:177–180. doi: 10.1016/0304-3940(95)11694-r. [DOI] [PubMed] [Google Scholar]
- Xiong K, Peoples RW, Montgomery JP, Chiang Y, Stewart RR, Weight FF, Li C. Differential modulation by copper and zinc of P2X2 and P2X4 receptor function. J Neurophysiol. 1999;81:2088–2094. doi: 10.1152/jn.1999.81.5.2088. [DOI] [PubMed] [Google Scholar]
- Zatta P, Lucchini R, van Rensburg SJ, Taylor A. The role of metals in neurodegenerative processes: aluminum, manganese, and zinc. Brain Res Bull. 2003;62:15–28. doi: 10.1016/s0361-9230(03)00182-5. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Hume RI. Two mechanisms for inward rectification of current flow through the purinoceptor P2X2 class of ATP-gated channels. J. Physiol. 1998;507:353–364. doi: 10.1111/j.1469-7793.1998.353bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]