Abstract
Solid tumors such as mesothelioma exhibit a stubborn resistance to apoptosis that may derive from survival pathways, such as PI3K/Akt/mTOR, that are activated in many tumors, including mesothelioma. To address the role of PI3K/Akt/mTOR, we used a novel approach to study mesothelioma ex vivo as tumor fragment spheroids. Freshly resected mesothelioma tissue from 15 different patients was grown in vitro as 1- to 2-mm-diameter fragments, exposed to apoptotic agents for 48 hours with or without PI3K/Akt/mTOR inhibitors, and doubly stained for cytokeratin and cleaved caspase 3 to identify apoptotic mesothelioma cells. Mesothelioma cells within the tumor spheroids exhibited striking resistance to apoptotic agents such as TRAIL plus gemcitabine that were highly effective against monolayers. In a majority of tumors (67%; 10 of 15), apoptotic resistance could be reduced by more than 50% by rapamycin, an mTOR inhibitor, but not by LY294002, a PI3K inhibitor. Responsiveness to rapamycin correlated with staining for the mTOR target, p-S6K, in the original tumor, but not for p-Akt. As confirmation of the role of mTOR, siRNA knockdown of S6K reproduced the effect of rapamycin in three rapamycin-responsive tumors. Finally, in 37 mesotheliomas on tissue microarray, p-S6K correlated only weakly with p-Akt, suggesting the existence of Akt-independent regulation of mTOR. We propose that mTOR mediates survival signals in many mesothelioma tumors. Inhibition of mTOR may provide a nontoxic adjunct to therapy directed against malignant mesothelioma, especially in those with high baseline expression of p-S6K.
Keywords: apoptosis, biomarker, ex vivo, TRAIL, S6K
CLINICAL RELEVANCE
Using a three-dimensional ex vivo mesothelioma model, we have identified a role for mTOR in cell survival and identified p-S6K as a potentially useful biomarker. These findings are potentially valuable for treatment of mesothelioma and perhaps other solid tumors.
Chemoresistance of tumors may derive from an underlying resistance to apoptosis (1). Bypassing this apoptotic resistance may reveal new strategies for targeted, nontoxic approaches to effective cancer therapy. However, tumor cells in monolayer culture may not manifest the resistance seen in solid tumors, which can acquire a multicellular resistance via cell–cell contacts, cell–matrix interactions, or other factors within the tumor microenvironment (2). Currently, the NIH estimates that, of the anti-cancer agents that appear promising in the laboratory, fewer than 10% reach the clinic (3). More complex models that can represent the types of apoptotic resistance seen in vivo may provide more clinically useful information (4). Three-dimensional models, including tumor fragments grown from actual tumor, may manifest an apoptotic resistance analogous to that in vivo and thus may provide a useful model for pre-clinical studies (3, 4).
The PI3K/Akt/mTOR pathway has been shown to play a role in many functions critical to tumor generation and maintenance, including apoptotic resistance (5, 6). In animal studies, the ability of Akt to interfere with apoptosis was seen as central to its ability to produce tumors; in some studies, this anti-apoptotic/pro-malignant function could be localized to a major Akt downstream target, mTOR (7, 8), suggesting that blockade of mTOR could be an effective anti-cancer strategy. However, blockade of mTOR can enhance Akt activity by feedback mechanisms downstream of mTOR and thus may induce undesirable compensatory resistance mechanisms (9). Nonetheless, blockade of mTOR in certain animal models and in patients with renal cell carcinoma has shown promise (10). Questions about the role of the Akt pathway, the contribution of mTOR, and the possible negative effects of mTOR blockade are currently under debate.
Mesothelioma is an aggressive, highly lethal, drug-resistant tumor (11). Using mesothelioma cell lines grown as monolayers, we have explored effective combinations of agents including TRAIL plus sensitizers that can induce a synergistic, robust apoptosis in these cells (12–14). Nonetheless, using the human tumor fragment spheroid model we developed, we learned that mesothelioma cells in their tumor microenvironment exhibit a high degree of apoptotic resistance, some of which appeared to be related to the PI3K/Akt/mTOR pathway (15). Indeed, the Akt pathway is known to be active in mesothelioma (16) and, with inhibitors available for different members of the Akt pathway, understanding the participation of Akt or other members of its family in apoptotic resistance could lead to the use of these inhibitors for patients with mesothelioma.
In this study, using human mesothelioma tissue grown ex vivo as tumor fragment spheroids, we directly addressed the role of the PI3K/Akt/mTOR pathway in survival of mesothelioma cells in their tumor microenvironment. In so doing, we asked whether blockade of the PI3K/Akt/mTOR pathway would reverse apoptotic resistance in mesothelioma cells and, if so, whether any biomarkers correlated with this response. We also addressed whether inhibition of mTOR alone would be as effective as a broader inhibition of the PI3K/Akt/mTOR signaling pathway. Such preclinical information could support the use of PI3K/Akt/mTOR inhibitors in the treatment of this tumor.
MATERIALS AND METHODS
Tumor Procurement
We obtained human pleural malignant mesothelioma tumor tissue from two institutions, University of California San Francisco (D.M.J.) and the Brigham and Women's Hospital (D.J.S. and R.B.). Tumor tissue resected at surgery was placed sterilely in a conical tube with Dulbecco's modified Eagle's medium (DMEM) with 5% FBS and transported to our laboratory. From UCSF, tissue was processed within 4 to 10 hours of surgery; from Boston, tissue was sent by overnight carrier at room temperature and was processed within 24 to 36 hours of surgery. To confirm that overnight transport did not harm the tissue for these experiments, in three cases, we compared the handling of tumor tissue obtained from UCSF by processing the same day or by keeping overnight in media at room temperature before processing the next morning. There was no difference in the tumors processed at different times in their ability to form spheroids, in p-Akt or p-S6K staining intensity or in apoptotic responses. Thus, we used both local and distant sources for tumor (15 tumors total; 9 from UCSF, 6 from Brigham and Women's). The histopathology was determined on formalin-fixed, hematoxylin and eosin–stained sections of the tumor by a pathologist (S.L.N.). Of the 15 tumors, there were 5 epithelioid, 2 sarcomatous, and 8 mixed histopathologic subtypes.
Normal lungs were obtained from pathology specimens, or freshly obtained via lungs declined for transplantation. The peripheral lung containing pleura was excised and fixed overnight in 10% formalin before embedding in paraffin. All human tissues were used without identifiers under an approved CHR protocol.
Materials
Combinations of human recombinant TRAIL (375-TEC; R&D Systems, Minneapolis, MN) together with either cycloheximide (Sigma, St. Louis, MO) or gemcitabine (Eli Lilly, Indianapolis, IN) were used to induce apoptosis. Other agents were rapamycin, a specific inhibitor of mTOR as the TOR complex 1 (TORC1) (Sigma); LY294002 (Sigma), a nonspecific inhibitor of PI3K and related kinases; and PI-103, a specific inhibitor for PI3K and mTOR (in both its complexes, TORC1 and TORC2) (generous gift of Drs. Kevan Shokat and Zachary Knight, UCSF) (17).
Cell Culture and Monolayer Studies
M28 epithelial-type human mesothelioma cells were used to test apoptotic responses in monolayers. Cells were plated the night before and used at 70 to 80% confluence. The cells were exposed to various agents for 24 hours, harvested by combining floating cells with trypsinized adherent cells, and assayed for apoptosis by counting for condensed nuclei stained with Hoescht (Sigma) as described (12).
Spheroid Culture and Treatment
Tumor fragment spheroids were prepared, as we have previously described, to maintain viable tumor cells in their three-dimensional environment (18). Essentially, fresh tumor tissue was diced with scalpels to pieces of approximately 1 to 2 mm diameter and suspended in DMEM supplemented with 5% FBS and penicillin/streptomycin in 10-cm plates coated with 0.8% agar. The cultures were maintained at 37°C in 5% CO2 with 100% relative humidity. Medium was changed twice weekly.
Approximately 2-week-old spheroids were studied by taking rounded 1- to 2-mm fragments and moving them to wells of 12-well plates. The next day, they were treated twice over 48 hours, with reagents added once at 0 hours and again at 24 hours. After treatment, spheroids were rinsed once with PBS, fixed with formalin (10%) at 4°C overnight, and transferred to mesh tissue bags (Shandon, Southern Instruments, Sewickley, PA) that were then placed in tissue cassettes (TissueTek, Torrance, CA). The tissue was processed and embedded in paraffin by the UCSF Department of Pathology.
In the experiments using tumor fragments spheroids, apoptosis was induced with either TRAIL plus cycloheximide, as we have previously reported, or with TRAIL plus gemcitabine, a clinically useful chemotherapeutic agent. Inhibitors of the PI3K/Akt/mTOR pathway (rapamycin, LY294002, PI-103) were used either singly or in combination (rapamycin plus LY294002) to learn whether a multitargeted inhibition was better than inhibition of mTOR alone. In the first study using four tumors, tumor fragments were exposed to vehicle or to TRAIL (5 ng/ml) plus cycloheximide (10 μg/ml) to induce apoptosis for 24 or 48 hours. In the next study, using 11 additional tumors, tumor fragments were exposed to vehicle or to TRAIL (5 ng/ml) plus gemcitabine (40 μM) for 48 hours. In each study, inhibitors of the PI3K/Akt/mTOR pathway (rapamycin 10 nM, LY294002 100 μM, or PI-103 2 μM; or rapamycin 10 nM plus LY294002 100 μM) were added at 24 hours and at 48 hours. Each tumor was studied in triplicate and the results averaged.
RNA Interference for S6K
To confirm a role for mTOR, we transfected spheroids from three different mesotheliomas with siRNA duplexes against S6K, a major downstream target of mTOR. We first tested different transfection methods using a GFP plasmid (pMaxGFP; Amaxa Biosystems, Gaithersburg, MD) to optimize transfection of cells in the spheroids. We focused on S6K instead of the other major target 4E-BP1 because S6K is the mTOR target fully inhibited by rapamycin (19). The siRNA sequences (Ambion, Austin, TX) were: S6K, CUG UUA GUU UCA CAU GAC CdTdT; random, ACG UGA CAC GUU CGG AGA AdTdT. The smallest spheroids (< 1 mm diameter, n = 10–40) were placed in each of two wells of 6-well plates in serum-containing media, and exposed dropwise to X-tremeGENE siRNA Transfection Reagent (Roche, Mannheim, Germany) combined in 5:1 ratio with siRNA (2 μg) for either S6K or random sequences. After 24 hours, spheroids were either fixed and stained for p-S6K, processed for immunoblot (see below) or transferred to 12-well plates for exposure to TRAIL plus gemcitabine for 48 hours with or without rapamycin. Treated spheroids were then fixed and stained for apoptotic mesothelioma cells, as described below.
Antibodies and Staining
Antibodies were used at the indicated dilutions to detect cytokeratin (1:200, AE1/AE3; DakoCytomation, Carpinteria, CA), cleaved caspase 3 (1:100; Chemicon, Temecula, CA), p-Akt (1:50, Ser473, rabbit #3787; Cell Signaling, Beverly, MA), and p-S6K (1:500, Thr389, mouse, #9206; Cell Signaling) and DR5 (1:100; DakoCytomation). The antibody to Akt detects Akt1 phosphorylated on serine 473, considered to represent full activation, and Akt2 and Akt3 when phosphorylated on equivalent sites; the p-S6K antibody detects S6K1 phosphorylated on threonine 389 and likely also S6K2 with phosphorylation at an analogous site. For each antibody, a range of dilutions was used before selecting the best dilution for specific staining over background. In each staining, a negative control was included, either omission of the primary antibody or a blocking peptide, such as for p-Akt (Ser473, #1140; Cell Signaling) if available.
Single immunohistochemical staining for p-Akt, p-S6K, and DR5 was performed with the EnVision plus kit with peroxidase detection (DakoCytomation). Antigen retrieval was performed in a pressure cooker in citrate buffer, pH 6.0, with time of retrieval between 5 and 20 minutes, depending on the antibody.
Double immunohistochemical staining was performed to detect apoptosis specifically in the mesothelioma cells, with staining for cytokeratin to identify the tumor cells and staining for cleaved caspase 3 to identify apoptosis, as described (18). The slides were first incubated with primary murine antibody to cytokeratin followed by rabbit polyclonal antibody to cleaved caspase 3. Cytokeratin was detected with a secondary biotinylated sheep anti-mouse antibody (1:200; Amersham Biosciences Corp., Piscataway, NJ) and a streptavidin-conjugated Oregon Green 488 (Molecular Probes, Eugene, OR). Cleaved caspase 3 was separately detected using a goat anti-rabbit IgG conjugated with AlexaFluor 546 (1:100; Molecular Probes).
Quantifying p-Akt and p-S6K Expression and Apoptosis
For determining the expression of p-Akt and p-S6K in the spheroids, the intensity of staining of individual cells was assessed by two observers blinded to the experimental conditions on a scale of 0 to 3, with the grade reached by agreement. An average staining intensity for each condition was calculated for at least three different spheroids, from three separately stained slides. For grading staining intensity, 0 = no staining greater than background, 1 = weak staining, 2 = moderate staining, 3 = strong staining.
For quantifying mesothelioma cell apoptosis, images of stained spheroids were captured using a fluorescent microscope (Zeiss, Gottingen, Germany) and image acquisition software (Spot Advanced, Chantilly, VA). The images were later overlaid with a grid and examined by independent observers blinded to the experimental conditions. Cytokeratin-positive cells, identified by cell-specific green staining above background, from between 10 and 20 spheroids were counted for each experimental condition for each tumor. All cytokeratin-positive cells were counted as either nonapoptotic (green only) or as apoptotic (green plus red staining for cleaved caspase 3 above background levels). Mesothelioma cell apoptosis was calculated as the percentage of all cytokeratin-positive cells with staining for cleaved caspase 3.
Immunoblotting
Twenty to thirty spheroids were used for each condition; three tumors were studied. After 48 hours, spheroids were ground with a dounce pestle on ice, transferred to an Eppendorf tube and lysed with the addition of boiling lysis buffer (2.5% SDS, Tris-HCl 250 mM, pH 7.4). Whole cell lysates were sonicated, centrifuged, and the supernatant analyzed for protein concentration by the Bradford method (Bio-Rad, Hercules, CA). Equal protein (50 μg) was resolved by SDS-PAGE (7.5% acrylamide) and transferred to PVDF membranes (Immobilon; Millipore, Billerica, MA). Membranes were blocked with a protein-free TBS blocking buffer (Pierce, Rockford, IL) and probed with primary antibodies at 4°C overnight including those to p-Akt (1:1,000, Ser473, rabbit mAb #4058), to p-S6K (1:1,000, Thr389, mouse mAb # 9206), to S6K (1:1,000, rabbit polyclonal Ab #9202) from Cell Signaling, and α-tubulin antibody (1:10,000) from Sigma. The signal was detected by the enhanced SuperSignal West Pico Chemiluminescent Substrate (Pierce).
Statistics
For nonparametric staining intensity data, differences were analyzed by the Friedman statistic (for repeated measures) or Kruskal-Wallis test, and correlations by the Spearman rank test. For parametric apoptotic data, differences were analyzed by ANOVA with repeated measures with Tukey's test to define where the differences lay. Statistical analysis was performed with the GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA). A P value less than 0.05 was considered statistically significant.
RESULTS
Rapamycin and LY294002 Differentially Inhibit the Akt Pathway in Tumor Fragment Spheroids
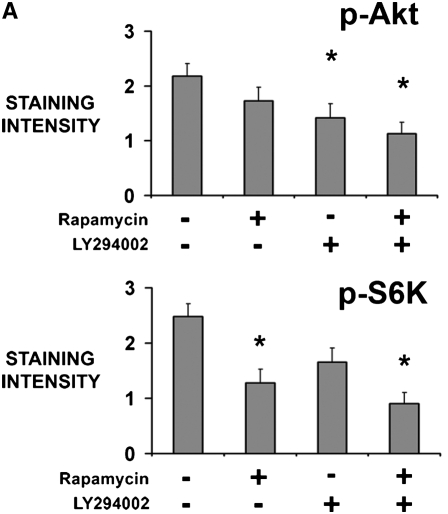
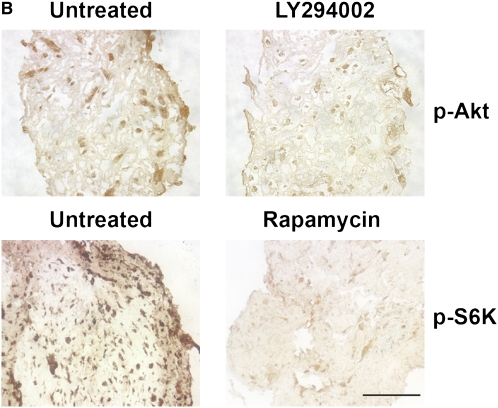
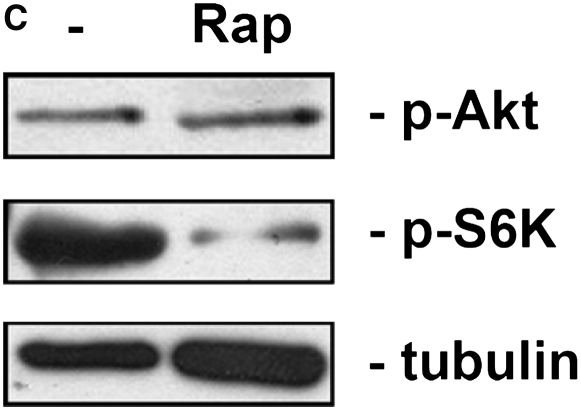
To confirm activity of the inhibitors, tumor fragment spheroids from four tumors were exposed to rapamycin, LY294002, or both for 48 hours and stained for p-Akt (Ser473) or p-S6K (Thr389), a downstream target of mTOR. Rapamycin (10 nM) and LY294002 (100 μM) were used at concentrations shown to be effective previously (15). LY294002 decreased p-Akt without significantly decreasing p-S6K (P = 0.045); rapamycin reduced p-S6K without evidence of a compensatory up-regulation of p-Akt (P = 0.30)(Figures 1A and 1B). The combination of inhibitors decreased both p-Akt and p-S6K (P = 0.025). Staining for p-Akt and p-S6K was homogeneous throughout the spheroid, with similar staining at the edge and center, suggesting an adequate diffusion of inhibitors (Figure 1B). By immunoblot of three separate tumors, rapamycin was confirmed to inhibit p-S6K, again without evidence of a feedback up-regulation of Akt (Figure 1C). The inhibitors were used at these concentrations for further studies.
Figure 1.
Inhibitors of the PI3K/Akt/mTOR pathway show activity in tumor fragment spheroids. Tumor fragments were exposed to rapamycin, LY294002, or the combination for 48 hours and analyzed for expression of p-Akt and p-S6K by (A, B) immunohistochemistry or (C) immunoblot. (A) By immunohistochemistry, LY294002 was shown to decrease p-Akt, whereas rapamycin was shown to decrease p-S6K. The combination of rapamycin with LY294002 was effective at both. No apparent feedback activation of p-Akt after rapamycin was seen (* P < 0.05, different from no inhibitor; n = 4 tumor fragment spheroids from different tumors, mean ± SEM). (B) Representative images show that the inhibition of signaling molecules was found throughout the spheroid (bar = 200 μm). (C) By immunoblot of tumor fragments, rapamycin was shown to inhibit p-S6K without increasing p-Akt (blot representative of blots of tumor fragment spheroids from 3 separate tumors).
Interestingly, the recently developed compound PI-103, a high-affinity inhibitor of mTOR (TORC1 and 2) and PI3K, did not reduce expression of either p-Akt or p-S6K in the tumor fragment spheroids, whereas it effectively reduced both in mesothelioma cells in culture (17, 20). We considered that the agent was not active in the conditions of these experiments and did not use it further. For broad inhibition, we relied instead on the combination of rapamycin plus LY294002.
Rapamycin Enhances Apoptosis When Used with TRAIL Plus Cycloheximide
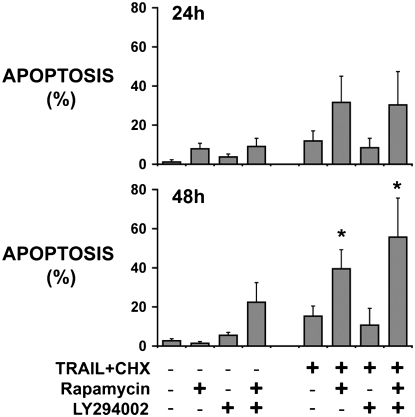
Tumor fragments from four tumors were exposed to TRAIL plus cycloheximide with and without inhibitors and analyzed for mesothelioma cell apoptosis. Despite inducing nearly total apoptosis in M28 mesothelioma cells in monolayer (96 ± 5%, n = 3) at 24 hours, TRAIL plus cycloheximide had no significant effect on mesothelioma cells in the tumor fragment spheroids at either 24 hours, even when given at twice the concentration, or at 48 hours, after two dosings at twice the concentration (Figure 2). Rapamycin, although it had no effect by itself, significantly enhanced the apoptotic response to TRAIL plus cycloheximide at 48 hours (Figure 2). Interestingly, LY294002 had no apparent effect, either alone or when used in combination with rapamycin. These results suggested that the target of rapamycin, mTOR, was the main effector of survival. The results further suggested that mTOR inhibition alone was effective at reducing apoptotic resistance, without requiring inhibition of a possible feedback activation of upstream PI3K/Akt.
Figure 2.
Rapamycin sensitizes human mesothelioma cells in tumor fragment spheroids to TRAIL plus cycloheximide. Tumor fragment spheroids were exposed for either 24 or 48 hours to TRAIL plus cycloheximide together with inhibitors of the PI3K/Akt/mTOR pathway. Apoptosis in the mesothelioma cells was counted after double labeling for cytokeratin and for cleaved caspase 3. Mesothelioma cell apoptosis was enhanced by rapamycin alone or together with LY294002 at 48 hours (*P < 0.05, different from control untreated at 48 h; n = 4, mean ± SEM).
In Monolayers, Mesothelioma Cells Undergo Apoptosis after TRAIL Plus Gemcitabine
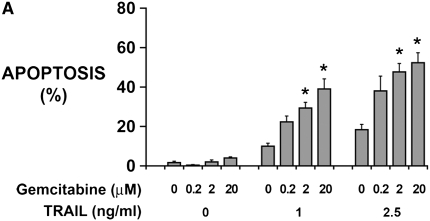
Cycloheximide, while a valuable agent for sensitizing to TRAIL, is not a clinically useful agent. We thus replaced cycloheximide with gemcitabine, a nucleoside analog used in mesothelioma treatment regimens (21), to test PI3K/Akt/mTOR inhibition in a more clinically relevant setting. First, using monolayer cultures of M28 human mesothelioma cells, TRAIL was used with and without gemcitabine up to the concentration of 20 μM reported to saturate the enzymatic activation step (22). Neither TRAIL (1, 2.5 ng/ml) nor gemcitabine (0.2–20 μM) alone induced significant apoptosis; nonetheless, the combination induced synergistic apoptosis (Figure 3A). To aim for adequate concentrations in tumor fragment spheroids, we doubled the maximum concentration and used TRAIL (5 ng/ml) plus gemcitabine (40 μM) in further studies.
Figure 3.
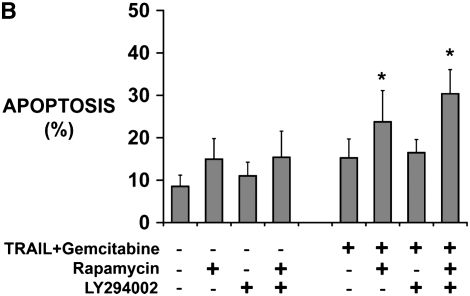
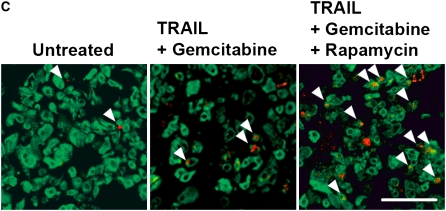
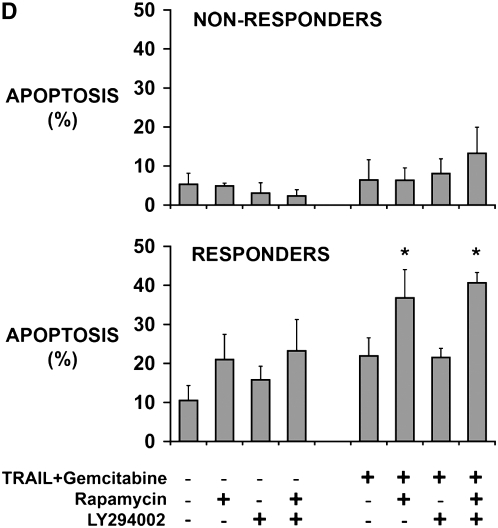
Rapamycin sensitizes human mesothelioma cells in tumor fragment spheroids to TRAIL plus gemcitabine. (A) Mesothelioma cells (M28) in monolayer culture were exposed to varying concentrations of TRAIL (1 and 2.5 ng/ml) and gemcitabine (0.2–20 μM) and harvested at 24 hours. Whereas neither TRAIL nor gemcitabine alone increased apoptosis, the combination did (*P < 0.05, greater than sum of individual responses; n = 3, mean ± SEM). (B) Tumor fragment spheroids exposed to TRAIL plus gemcitabine together with inhibitors for 48 hours were double-stained for cytokeratin and cleaved caspase 3 and evaluated for apoptosis in the mesothelioma cells. Rapamycin, whether alone or with LY294002, was effective in increasing the response to TRAIL and gemcitabine (*P < 0.05, greater than to TRAIL + gemcitabine alone; n = 11, mean ± SEM) (C) Immunofluorescent images of tumor fragments show mesothelioma cells (green, stained for cytokeratin) and apoptotic cells (red, staining for cleaved caspase 3) (see arrows). There are some apoptotic mesothelioma cells in the control and in the spheroid exposed to TRAIL plus gemcitabine. In this tumor, the addition of rapamycin to the treatment with TRAIL plus gemcitabine led to a significant increase in apoptosis (bar = 100 μM). (D) Tumors responded differently to rapamycin. Tumors shown in B are separated here into those that responded to rapamycin with an increase in apoptosis of more than 50% (n = 7) and those that did not (n = 4) (*P < 0.05, greater than to TRAIL plus gemcitabine alone; n = 11, mean ± SEM).
In Tumor Fragment Spheroids, Mesothelioma Cells Are Resistant to TRAIL Plus Gemcitabine, a Resistance Inhibited by Rapamycin
Tumor fragment spheroids from 11 additional tumors were then treated with TRAIL plus gemcitabine, with and without the inhibitors. As seen with TRAIL plus cycloheximide, TRAIL plus gemcitabine alone did not significantly increase apoptosis. Rapamycin, although it had no effect alone, significantly enhanced the apoptotic response to the TRAIL combination (Figures 3B and 3C). Again, LY294002 had no effect, either alone or in combination with rapamycin.
The tumor response to rapamycin was noted to be variable. To investigate possible explanations for this variation, we first categorized the tumors on the basis of their response to the use of rapamycin. Responders were defined as those tumors in which the addition of rapamycin (alone or with LY294002) increased apoptosis by at least 50% over the matched control without rapamycin. By this criteria, of the 11 tumors treated with TRAIL plus gemcitabine, there were 7 responders and 4 nonresponders (Figure 3D). The response to rapamycin was reproducible: tumor fragment spheroids from each tumor were treated in at least one other experiment and, in each case, the response category was reproduced. In addition, spheroids from three tumors were exposed once to TRAIL and cycloheximide and later to TRAIL plus gemcitabine; the response to rapamycin did not vary depending on the treatment.
Knockdown of S6K Enhances Apoptosis
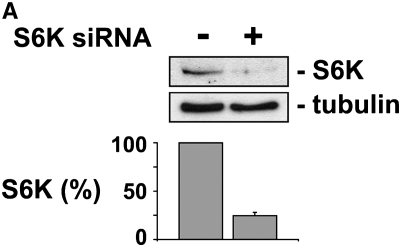
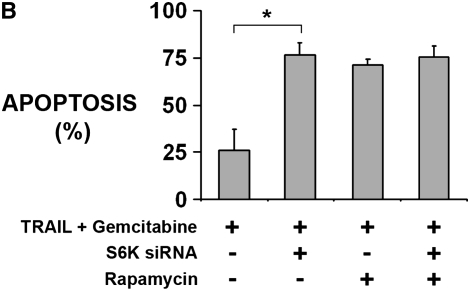
To confirm that the effect of rapamycin was due to inhibition of its known target, mTOR, we attempted to reproduce the effect of rapamycin with the knockdown of one of the major downstream targets of mTOR, S6K. In tumor fragments from three different rapamycin-responsive tumors, we transfected siRNA duplexes to S6K or to a random, nontargeting sequence. After 24 hours, spheroids with siRNA to S6K showed a decrease in p-S6K staining compared with spheroids with siRNA to a random, nontargeting sequence (S6K siRNA, 1.6 ± 0.6; random siRNA, 2.5 ± 0.8; P < 0.05). Knockdown of S6K was confirmed by immunoblot of transfected tumor fragment spheroids (Figure 4A). After 48 hours of exposure to TRAIL plus gemcitabine, spheroids with S6K siRNA showed a significant increase in apoptosis compared with the control spheroids, similar to the effect of rapamycin (Figure 4B). The addition of rapamycin to spheroids with S6K knockdown did not add to that of S6K knockdown alone.
Figure 4.
S6K knockdown sensitizes human mesothelioma cells in tumor fragment spheroids to TRAIL plus gemcitabine. Tumor fragment spheroids were transfected with siRNA against S6K or a random, nontargeting sequence and then exposed for 48 hours to TRAIL plus gemcitabine. (A) Knockdown of S6K was confirmed by immunoblotting transfected tumor fragment spheroids after 48 hours. Immunoblot is representative of three different studies. Bar graph shows relative intensity of bands normalized to tubulin (n = 3, P < 0.05). (B) Knockdown of S6K enhanced the apoptosis of mesothelioma cells to TRAIL plus gemcitabine to the same extent as did rapamycin alone. Addition of rapamycin to tumor fragment spheroids with knockdown of S6K had no additional effect over S6K knockdown alone (*P < 0.05, n = 3 different tumors).
Response to Rapamycin Correlates with Tumor Expression of p-S6K but Not of p-Akt
To explore tumor characteristics that correlated with responsiveness to rapamycin, the original tumors preserved in paraffin were used to generate a tumor tissue microarray with normal pleura as a control. The slides were then stained for p-Akt (Ser473) and p-S6K (Thr389) as well as for the death receptor 5 (DR5), a major receptor for TRAIL. For these studies, we included all tumors that had been exposed to TRAIL either with cycloheximide (n = 4) or with gemcitabine (n = 11).
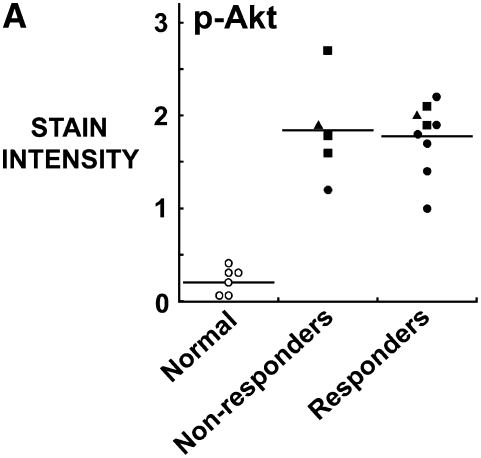
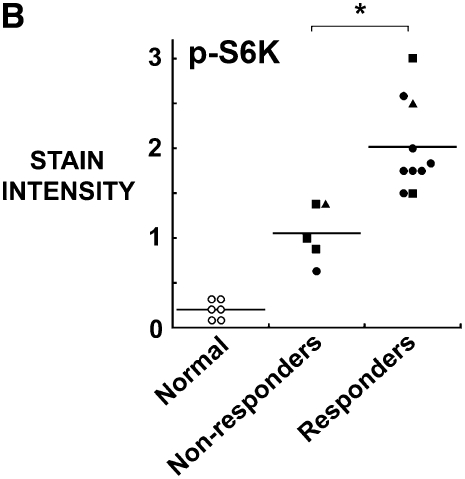
Compared with normal pleural mesothelium, all mesothelioma tumors expressed an elevated p-Akt and p-S6K (Figures 5A and 5B). Interestingly, the responsiveness of the tumors to rapamycin did not show a correlation with the intensity of staining for p-Akt (Figure 5A), but did correlate with the intensity of staining for p-S6K (Figure 5B).
Figure 5.
Response to rapamycin correlates with staining of original tumor for p-S6K but not for p-Akt. Paraffin-embedded mesothelioma tumors were used to generate a tissue microarray, stained for (A) p-Akt and (B) p-S6K and correlated with the response of the tumor fragment spheroids generated from these tumors to rapamycin. (A) Staining for p-Akt was elevated in all tumors compared with normal tissue, although there was no difference seen between tumors that responded to rapamycin and those that did not (n = 5 non-responders; n = 9 responders with one sample missing from study). (B) Staining for p-S6K was also elevated in all tumors compared with normal pleura and p-S6K, unlike p-Akt, correlated with the response to rapamycin (*P < 0.05, different from non-responders; n = 5 nonresponders, n = 10 responders). Open circles, normal; solid circles, mixed; squares, epithelial; triangles, sarcomatous.
Responsiveness was not associated with any one histopathologic subtype. Responders (n = 10) included epithelial (2), sarcomatous (1), and mixed (7). Nonresponders (n = 5) included epithelial (3), sarcomatous (1), and mixed (1). The responsiveness did not correlate with the city of origin of the tumor. Tumors all contained moderate to high staining for the TRAIL receptor, DR5; on a 0 to 3 scale, staining in mesothelioma was 2.5 ± 0.3 compared with normal pleura at 1.0 ± 0.2 (n = 15 mesothelioma; n = 6 pleura; P < 0.02; mean ± SEM). The responsiveness to rapamycin did not correlate with the level of DR5 (responders 2.6 ± 0.2, nonresponders 2.4 ± 0.3; mean ± SEM).
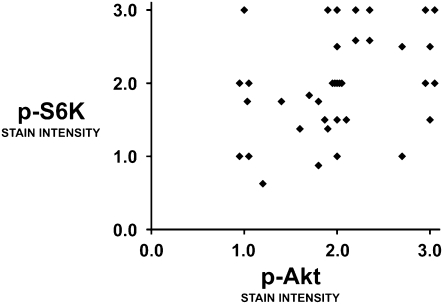
In the examination of the 37 mesotheliomas on the tissue microarray, all tumors had elevated staining of p-Akt, p-S6K, and DR5 compared with that of normal pleural mesothelium. There was no significant difference in expression of p-Akt, p-S6K, or DR5 among the histopathologic subtypes. The expression of p-Akt correlated with that of p-S6K in a significant but weak to moderate degree (Spearman rank correlation of 0.41 [P < 0.013]) (Figure 6).
Figure 6.
p-S6K correlates weakly with p-Akt in mesothelioma tumors. A tumor microarray containing tissue from 37 mesotheliomas was stained for p-Akt and p-S6K and assessed for intensity of staining. Normal pleural samples (n = 5) had staining intensity below 0.5 (not shown). Correlation by Spearman rank was 0.41 (P < 0.013), suggesting a significant but weak correlation between p-Akt and p-S6K.
DISCUSSION
Understanding the mechanisms by which tumors resist apoptosis may open new doors for bypassing chemoresistance and designing more effective therapy (1). Nonetheless, investigating apoptosis using monolayer cultures may be limited because tumor cells in a two-dimensional environment may not exhibit the multicellular resistance seen in human solid tumors (3). Compared with cells grown on two-dimensional plastic dishes, tumor cells in three-dimensional structures acquire a high level of resistance that may relate better to the clinical setting (23). One putative survival factor that may contribute to acquired resistance in solid tumors is the Akt/mTOR pathway (7, 24). In a recent study, we have also shown that mesothelioma cell lines allowed to form into three-dimensional aggregates called multicellular spheroids acquired a multicellular resistance in part due to mTOR (20). Thus, in this and in other studies (20, 25, 26), the survival function of mTOR may be exhibited best in the three-dimensional setting, suggesting that findings in three dimensions may offer novel insights not necessarily predicted from their two-dimensional counterparts.
To study the role of the Akt/mTOR pathway in a clinically relevant three-dimensional setting, we used tumor fragment spheroids generated from mesothelioma tumor tissue. Malignant mesothelioma, a tumor extremely resistant to chemotherapy, seems well suited for this approach: surgical resection generally provides a large amount of tumor that can be studied ex vivo, and mesothelioma is known to form spheroidal aggregates in vivo (27). Novel approaches in preclinical studies are also attractive for mesothelioma because it is a relatively uncommon tumor, thereby slowing accrual into clinical trials. Thus, we turned to an ex vivo model of mesothelioma to explore its defenses against apoptosis. We chose to stimulate apoptosis with the strategy that we have found to be most effective in resistant mesothelioma cells in monolayer culture, the activation of the extrinsic death receptor apoptotic pathway using TRAIL combined with the activation of the intrinsic damage pathway (12–14).
In striking contrast to cell lines in monolayer culture, mesothelioma cells in the tumor fragment spheroids are resistant to the combination of TRAIL together with either of its sensitizers, cycloheximide or gemcitabine, even when used at higher concentrations and at multiple dosing over longer times. In this setting of high resistance, rapamycin-induced blockade of mTOR was able to sensitize the cells to apoptosis from TRAIL combinatorial therapy. Interestingly, LY294002, a broad PI3K inhibitor, had no significant effect on apoptosis, despite suggestions of benefit in a small number of tumors in our previous study (15) and a high concentration (100 μM), perhaps because LY294002 did not fully inhibit mTOR (see Figure 1A). This speculation is supported in our recent study of multicellular spheroids (20), in which LY294002 alone did inhibit mTOR more effectively than it did in the tumor fragment spheroids used in this study, and was able to inhibit apoptotic resistance. However, in both studies, whether using multicellular spheroids or tumor fragment spheroids, the combination of LY294002 with rapamycin was no more effective than rapamycin alone, suggesting that mTOR, not Akt, was the important effector of survival in mesothelioma cells. Indeed, in some studies of the tumorigenic function of Akt, both the survival and the oncogenic effects of Akt have been attributed to mTOR (7, 8). Thus, great interest has arisen in the potential benefits of mTOR inhibition in tumors (6, 10). Our study supports a therapeutic role for mTOR inhibition in mesothelioma. Indeed, one potential drawback of mTOR inhibition, a possible feedback activation of Akt (9), was not seen in our study, at least during short-term inhibition.
mTOR sits at an intersection of inputs to the cell, integrating signals from both growth factors and nutrients and mediating its effects via two known effectors with roles in protein translation, S6K and 4E-BP1/eIF4E (5, 6, 10). The mTOR pathway has several known effects on survival, including phosphorylation and sequestration of the pro-apoptotic Bad (28), up-regulation of FLIPs (29), translation of anti-apoptotic proteins, or sustaining the energy of the cell and the mitochondrial potential difference (5, 30). Hyperactive mTOR signaling may also signal the presence of dependency or “addiction” to this oncogenic kinase pathway (31), so that inhibition of mTOR could promote cell death by interrupting this lifeline or by attenuating survival signals (32). In many cases, however, the specific mechanism by which mTOR mediates survival has been elusive (1). It is worth noting that the inhibition of mTOR did not itself induce apoptosis, but sensitized to the apoptotic effect of other agents. Thus, for the rapamycin-responsive tumors, it appears that mTOR is acting as a brake on apoptosis, and its inhibition could serve as an adjunct to other apoptotic therapies. The nonresponsive tumors, with a lower mTOR activity, may instead rely on other mechanisms for survival.
In support of mTOR as a mediator of survival, its downstream target S6K appeared to play an important role. S6K knockdown in the tumor fragments enhanced sensitivity to apoptosis in the same way as did rapamycin. In addition, the staining for p-S6K, and not p-Akt, in the original tumors correlated with the responsiveness of the spheroids to rapamycin. Indeed, when analyzed in 37 mesotheliomas, the two phosphorylated kinases did not strongly correlate with each other, suggesting that mTOR has inputs and regulation in addition to that of Akt in this tumor. It is now known, for example, that mTOR, in its role as a sensor of nutrients as well as of growth factors, can be activated by factors other than Akt, such as by elevated levels of amino acids or ATP (33) and by the ERK pathway (34). mTOR may also be inhibited in ways that do not involve Akt signals, such as by heat shock or osmotic stress (35). Thus, compared with p-Akt, it should not be surprising that p-S6K would be a better marker for mTOR pathway activation and for those tumors that could respond to mTOR inhibition.
mTOR activity in the tumors, as evidenced by p-S6K staining, correlated with the response to rapamycin but did not correlate with histologic subtype. We do not have access to clinical information to know whether p-S6K staining or the responsiveness to rapamycin correlated with patient outcome or other clinical variables. In recent studies of other tumors, however, mTOR activation has been associated with a poor prognosis (36, 37). Thus, activation of the mTOR pathway in mesothelioma may portend a poor prognosis even as it signals a possible therapeutic intervention. If confirmed in further studies to be a useful biomarker, p-S6K has the potential for serving as a window into tumor biology and providing an independent assessment of mesothelioma prognosis and anticipated response to therapies, separate from pathologic appearance.
The tumor fragment spheroid model attempts to retain the microenvironment of the actual human tumor. We had previously shown that the spheroids grown from mesothelioma contained viable tumor cells for weeks to months, whereas the cells disaggregated from these same tumors failed to proliferate (15). In this study, we also attempted to grow mesothelioma cells disaggregated from these tumors but found that the cells showed an unacceptably high background level of apoptosis. Thus, tumor fragments can be a useful way to maintain human tumor cells in culture. Although diffusion may be a factor in any three-dimensional model, we did not see evidence of a limitation of diffusion in our study; the homogeneous reduction of p-S6K and p-Akt with inhibitors and the homogeneous location of apoptotic cells after treatment suggested that diffusion was adequate. Moreover, diffusion is a factor that is relevant to actual tumors, where the avascular unit that relies on diffusion is thought to be between 1 and 2 mm in diameter, similar to the size of our fragments (38). Thus, the issues of diffusion will be relevant to treatment of tumors clinically (39).
mTOR inhibitors are now under investigation in the treatment of many tumors (10), including mesothelioma. This ex vivo study suggests that mTOR inhibition would be a useful adjunct for enhancing apoptotic strategies in mesothelioma. Furthermore, p-S6K could be an appropriate biomarker for identifying those who would respond to this approach. The mesothelioma tumors also expressed high levels of DR5, the TRAIL receptor, suggesting that they have the capacity to respond to TRAIL therapy. Indeed, clinical trials with TRAIL or with agonist antibodies to the TRAIL receptor have begun for many tumors, including mesothelioma. It is our hope that our studies will help identify ways to enhance the efficacy of TRAIL cancer therapy.
In summary, we have used an ex vivo model to investigate the apoptotic resistance of actual human tumor cells. The findings suggest a role for the mTOR pathway in a majority of mesothelioma tumors and propose a biomarker for identifying those who might benefit. Such findings will need to be confirmed in patients with this disease. The ex vivo approach of using tumor fragment spheroids may lend itself to studies of other novel therapies considered for mesothelioma or other solid tumors.
Acknowledgments
The authors acknowledge Dr. Michael Matthay and Dr. Walt Finkbeiner for normal lung specimens and Drs. Kevan Shokat and Zachary Knight for PI-103.
Research was supported by National Institutes of Health grant RO1 CA95671 (V.C.B.), the Buzzi-INICEM Foundation (D.B.), and the Karen and Jeffrey Peterson Family Foundation (V.C.B.).
Originally Published in Press as DOI: 10.1165/rcmb.2007-0460OC on May 29, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Wendel HG, Lowe SW. Reversing drug resistance in vivo. Cell Cycle 2004;3:847–849. [PubMed] [Google Scholar]
- 2.Smalley KSM, Lioni M, Herlyn M. Life isn't flat: taking cancer biology to the next dimension. In Vitro Cell Dev Biol 2006;42:242–247. [DOI] [PubMed] [Google Scholar]
- 3.Pampaloni F, Reynoud EG, Stelzer EHK. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 2007;8:839–845. [DOI] [PubMed] [Google Scholar]
- 4.Kunz-Schughart LA, Freyer JP, Hofstaedter F, Ebner R. The use of 3-D cultures for high-throughput screening: the multicellular spheroid model. J Biomol Screen 2004;9:273–285. [DOI] [PubMed] [Google Scholar]
- 5.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell 2007;12:9–22. [DOI] [PubMed] [Google Scholar]
- 6.Chiang GG, Abraham RT. Targeting the mTOR signaling network in cancer. Trends Mol Med 2007;13:433–442. [DOI] [PubMed] [Google Scholar]
- 7.Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature 2004;428:332–337. [DOI] [PubMed] [Google Scholar]
- 8.Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Z, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med 2004;10:594–601. [DOI] [PubMed] [Google Scholar]
- 9.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 2006;66:1500–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abraham RT, Gibbons JJ. The mammalian target of rapamycin signaling pathway: twists and turns in the road to cancer therapy. Clin Cancer Res 2007;13:3109–3114. [DOI] [PubMed] [Google Scholar]
- 11.Tsiouris A, Walesby RK. Malignant pleural mesothelioma: current concepts in treatment. Nat Clin Pract Oncol 2007;4:344–352. [DOI] [PubMed] [Google Scholar]
- 12.Liu W, Bodle E, Chen JY, Gao M, Rosen GD, Broaddus VC. Tumor necrosis factor-related apoptosis-inducing ligand and chemotherapy cooperate to induce apoptosis in mesothelioma cell lines. Am J Respir Cell Mol Biol 2001;25:111–118. [DOI] [PubMed] [Google Scholar]
- 13.Vivo C, Liu W, Broaddus VC. JNK contributes to apoptotic synergy induced by TRAIL plus DNA damage in chemoresistant, p53 inactive mesothelioma cells. J Biol Chem 2003;278:25461–25467. [DOI] [PubMed] [Google Scholar]
- 14.Abayasiriwardana KS, Barbone D, Kim KU, Vivo C, Lee KK, Dansen TB, Hunt AE, Evan GI, Broaddus VC. Malignant mesothelioma cells are rapidly sensitized to TRAIL-induced apoptosis by low dose anisomycin via Bim. Mol Cancer Ther 2007; (In press). [DOI] [PubMed]
- 15.Kim KU, Wilson SM, Abayasiriwardana KS, Collins R, Fjellbirkeland L, Xu Z, Jablons DM, Nishimura SL, Broaddus VC. A novel in vitro model of human mesothelioma for studying tumor biology and apoptotic resistance. Am J Respir Cell Mol Biol 2005;33:541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altomare DA, You H, Xiao GH, Ramos-Nino ME, Skele KL, De Rienzo A, Jhanwar SC, Mossman BT, Kane AB, Testa JR. Human and mouse mesotheliomas exhibit elevated AKT/PKB activity, which can be targeted pharmacologically to inhibit tumor cell growth. Oncogene 2005;24:6080–6089. [DOI] [PubMed] [Google Scholar]
- 17.Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B, et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell 2006;125:733–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim BK. Three-dimensional tissue culture models in cancer biology. Semin Cancer Biol 2005;15:365–377. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Beugnet A, Murakami M, Yamanaka S, Proud CG. Distinct signaling events downstream of mTOR cooperate to mediate the effects of amino acids and insulin on initiation factor 4E-binding proteins. Mol Cell Biol 2005;25:2558–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbone D, Yang TM, Morgan JR, Gaudino G, Broaddus VC. mTOR contributes to the acquired apoptotic resistance of human mesothelioma multicellular spheroids. J Biol Chem 2008;283:13021–13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Carbonero R, Paz-Ares L. Systemic chemotherapy in the management of malignant peritoneal mesothelioma. Eur J Surg Oncol 2007;32:676–681. [DOI] [PubMed] [Google Scholar]
- 22.Abbuzzese JL, Grunewald R, Weeks EA, Gravel D, Adams T, Nowak B, Mineishi S, Tarassoff P, Watterlee W, Raber MN. A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J Clin Oncol 1991;9:491–498. [DOI] [PubMed] [Google Scholar]
- 23.Desoize B, Jardillier JC. Multicellular resistance: a paradigm for clinical resistance? Crit Rev Oncol Hematol 2000;36:193–207. [DOI] [PubMed] [Google Scholar]
- 24.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene 2005;24:7455–7464. [DOI] [PubMed] [Google Scholar]
- 25.Liu M, Howes A, Lesperance J, Stallcup WB, Hauser CA, Kadoya K, Oshima RG, Abraham RT. Antitumor activity of rapamycin in a transgenic mouse model of ErbB2-dependent human breast cancer. Cancer Res 2005;65:5325–5336. [DOI] [PubMed] [Google Scholar]
- 26.Howes AL, Chiang GG, Lang ES, Ho CB, Powis G, Vuori K, Abraham RT. The phosphatidylinositol 3-kinase inhibitor, PX-866, is a potent inhibitor of cancer cell motility and growth in three-dimensional cultures. Mol Cancer Ther 2007;6:2505–2514. [DOI] [PubMed] [Google Scholar]
- 27.Delahaye M, de John AA, Versnel MA, Hoogsteden HC, Teeling P, van der Kwast TH. Cytopathology of malignant mesothelioma: reappraisal of the diagnostic value of collagen cores. Cytopathology 1990;1:137–145. [DOI] [PubMed] [Google Scholar]
- 28.Harada H, Andersen JS, Mann M, Terada N, Korsmeyer SJ. p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proc Natl Acad Sci USA 2001;98:9666–9670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panner A, James CD, Berger MS, Pieper RO. mTOR controls FLIPs translation and TRAIL sensitivity in glioblastoma multiforme cells. Mol Cell Biol 2005;25:8809–8823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edinger AL, Thompson CB. An activated mTOR mutant supports growth factor-independent, nutrient-dependent cell survival. Oncogene 2004;23:5654–5663. [DOI] [PubMed] [Google Scholar]
- 31.Weinstein IB, Joe AK. Mechanisms of disease: oncogene addiction—a rationale for molecular targeting in cancer therapy. Nat Clin Pract Oncol 2006;3:448–457. [DOI] [PubMed] [Google Scholar]
- 32.Sharma SV, Gajowniczek P, Way IP, Lee DY, Jiang J, Yuza Y, Classon M, Haber DA, Settlemen J. A common signaling cascade may underlie “addiction” to the Src, BCR-ABL, and EGF receptor oncogenes. Cancer Cell 2006;10:425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 2006;124:471–484. [DOI] [PubMed] [Google Scholar]
- 34.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by ERK: implications for tuberous sclerosis and cancer pathogenesis. Cell 2005;121:179–193. [DOI] [PubMed] [Google Scholar]
- 35.Reiling JH, Sabatini DM. Stress and mTORture signaling. Oncogene 2006;25:6373–6383. [DOI] [PubMed] [Google Scholar]
- 36.Pantuck AJ, Seligson DB, Klatte T, Yu H, Leppert JT, Moore L, O'Toole T, Gibbons JJ, Belldegrun AS, Figlin RA. Prognostic relevance of the mTOR pathway in renal cell carcinoma. Cancer 2007;109:2257–2267. [DOI] [PubMed] [Google Scholar]
- 37.Herberger B, Puhalla H, Lehnert M, Wrba F, Novak S, Brandstetter A, Gruenberger B, Gruenberger T, Pirker R, Filipits M. Activated mammalian target of rapamycin is an adverse prognostic factor in patients with biliary tract adenocarcinoma. Clin Cancer Res 2007;13:4795–4799. [DOI] [PubMed] [Google Scholar]
- 38.Naumov GN, Akslen LA, Folkman J. Role of angiogenesis in human tumor dormancy. Cell Cycle 2006;5:1779–1787. [DOI] [PubMed] [Google Scholar]
- 39.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer 2006;6:583–592. [DOI] [PubMed] [Google Scholar]