Abstract
Lipopolysaccharide (LPS) is ubiquitous in the environment. Recent epidemiologic data suggest that occupational exposure to inhaled LPS can contribute to the progression of chronic obstructive pulmonary disease. To address the hypothesis that inhaled LPS can cause emphysema-like changes in mouse pulmonary parenchyma, we exposed C57BL/6 mice to aerosolized LPS daily for 4 weeks. By 3 days after the end of the 4-week exposure, LPS-exposed mice developed enlarged airspaces that persisted in the 4-week recovered mice. These architectural alterations in the lung are associated with enhanced type I, III, and IV procollagen mRNA as well as elevated levels of matrix metalloproteinase (MMP)-9 mRNA, all of which have been previously associated with human emphysema. Interestingly, MMP-9–deficient mice were not protected from the development of LPS-induced emphysema. However, we demonstrate that LPS-induced airspace enlargement was associated with apoptosis within the lung parenchyma, as shown by prominent TUNEL staining and elevated cleaved caspase 3 immunoreactivity. Antineutrophil antiserum-treated mice were partially protected from the lung destruction caused by chronic inhalation of LPS. Taken together, these findings demonstrate that inhaled LPS can cause neutrophil-dependent emphysematous changes in lung architecture that are associated with apoptosis and that these changes may be occurring through mechanisms different than those induced by cigarette smoke.
CLINICAL RELEVANCE
These findings provide further experimental support for the observation that not all chronic obstructive pulmonary disease is induced by cigarette smoke and that other environmental exposures are likely important in the etiology of this disease.
Chronic obstructive pulmonary disease (COPD) represents the fourth leading cause of death in the United States, and is the only common cause of death with an increased proportion of age-adjusted mortality in recent years (1). Environmental or occupational exposure to inhaled lipopolysaccharide (LPS) has been associated with the development of COPD (2) and byssinosis (3, 4), a persistent occupational airways disease with features in common with COPD (2, 5). Although smoking is the best-characterized risk factor for COPD, there is increasing evidence that occupational exposures independent of cigarette smoking can be risk factors as well (6, 7). There are now well-established animal models of environmental exposures other than cigarette smoke that can cause emphysema (8) and for which there is epidemiologic data in human disease including coal (8–10), silica (11), cadmium (12), and textiles (13–15) in which the active component is now known to be endotoxin (3, 4).
We have previously demonstrated that chronic LPS inhalation causes reversible airways inflammation (16), associated airflow obstruction (17, 18), and airway remodeling (19, 20) in mice. Previous studies have also shown that chronic treatment of rabbits and hamsters with intratracheal LPS instillation causes parenchymal architectural changes in the lung that are consistent with emphysema (21–23).
To address whether biologically relevant low-dose LPS inhalation causes the architectural changes seen in other animal models over time, we performed histologic and morphometric analyses on lungs from mice exposed to LPS aerosol inhalation daily for 1 month. Consistent with previous observations in hamsters, we observed emphysema-like lesions and demonstrate that low-dose daily LPS inhalation can cause ongoing emphysema-like histologic changes in mouse lung that are partially neutrophil (PMN)-dependent. We further show increased and persistent extracellular matrix (ECM) and protease gene expression in the lung, suggesting that this process is self sustaining. Finally, we show that these emphysematous changes are associated with both expression of caspase 3 and apoptosis of cells within the lung parenchyma.
MATERIALS AND METHODS
Experimental Animals
Twelve male C57BL/6 mice or matrix metalloproteinase (MMP)-9–deficient mice (Jackson Laboratories, Bar Harbor, ME) at 8 weeks of age were exposed to aerosolized LPS for 4 hours per day for 4 weeks followed by either a 3-day (Post) or a 4-week (Recovered) recovery period. Six additional age-matched control male C57BL/6 or MMP-9–deficient mice were killed at each time point (Post and Recovered).
Details of the exposure of C3H/HeBFeJ mice treated with anti-PMN antiserum have been published elsewhere (20). Briefly, 11 male C3H/HeBFeJ mice (Jackson Laboratories) 8 weeks of age were exposed to aerosolized LPS for 4 hours per day for 4 weeks followed by a 4-week (Recovered) recovery period. Eight mice received rabbit anti-mouse PMN antiserum, and three mice received normal rabbit serum (Accurate Chemical and Scientific, Westbury, NY). An additional 11 age-matched air controls were given either anti-PMN antiserum (n = 8) or normal rabbit serum (n = 3). To reduce the likelihood of infection in the neutropenic animals, all mice in this experiment were housed under pathogen-free conditions. The study protocol was conducted in accordance with guidelines set forth by the Duke University Animal Care and Use Committee.
Mice received 0.5 ml of diluted antiserum (1:10 dilution in sterile saline) by intraperitoneal injection. Initial injections were administered 72 hours before the inhalation exposure. The second dose was administered in the morning before the exposure started. The neutrophil antiserum was then administered three times a week for 5 weeks, which included an additional three doses after the exposure was completed. Concurrently, the control group of mice followed the same schedule and received intraperitoneal injections of 0.5 ml of diluted normal rabbit serum.
LPS Preparation and Aerosol Exposures
LPS was purchased as purified lyophilized powder (25 mg; 1–3,000,000 EU/mg), prepared by phenol extraction from Escherichia coli serotype 0111:B4 from Sigma (St. Louis, MO). LPS was reconstituted with 10-ml sterile HBSS, and stock aliquots (2.5 mg/ml) were stored at −20°C. Immediately before use, 160 ml LPS stock (4.7 mg; 7,000,000 EU) was diluted in 75 ml HBSS for nebulization. The target dose of aerosolized LPS (5 μg/m3 of air) has been shown to induce a response in mice similar to that which a grain elevator operator or hog farmer might experience in a workshift (24).
Mice were placed in stainless steel wire cage exposure racks in a 20-L chamber and exposed for 4 hours. LPS solution was aerosolized with a Collison 6-Jet Nebulizer (model CN-25; BGI Inc., Waltham, MA) with all output directed to the exposure chamber. Filtered and dehumidified air was supplied to the nebulizer at 20-psi gauge pressure. The exposure chamber was vented at a flow rate of 28.0 L/minute.
LPS Assay
The airborne concentration of LPS was assessed by sampling 0.30 to 0.40 m3 of air drawn from the exposure chamber through 25-mm binder-free glass fiber filters (Gelman Sciences, Ann Arbor, MI) held within a 25-mm polypropylene in-line air-sampling filter holder (Gelman). Filters were placed in pyrogen-free petri dishes with 2 ml sterile PBS containing 0.05% Tween-20 (Sigma) and then placed on a rotating shaker at room temperature for 1 hour. Aliquots of the wash solution were serially diluted in pyrogen-free water and tested for endotoxin using a chromogenic Limulus amoebocyte lysate assay (QCL-1000; BioWhittaker, Walkersville, MD) according to the manufacturer's instructions.
Tissue Preparation
Mice were killed by CO2 inhalation, the chest was opened, and bilateral thoracotomy was performed. The trachea was cannulated and the lungs were perfused with saline through the pulmonary artery. The whole right lung was removed and snap-frozen in liquid nitrogen and stored at −70°C. Freshly prepared ice-cold 4% paraformaldehyde (Fisher Scientific, Pittsburgh, PA) in PBS (pH 7.4) was instilled through the tracheal cannula into the right lung at a constant pressure of 20 cm H2O. The trachea was clamped and the lung was removed and fixed overnight at 4°C in 4% paraformaldehyde. This tissue was embedded in paraffin; 5- to 6-μm-thick sections were cut and placed on positively charged slides (Super Frost Plus; Fisher Scientific, Pittsburgh, PA).
Morphometry
Morphometry was performed on ten random digital images taken of Masson's Trichrome stained 5-μm sagittal sections of the left lung. Minimum, maximum, and mean alveolar diameter and intersects/horizontal were measured using Image Pro Plus 5.0 (Media Cybernetics; Silver Spring, MD).
Quantitative PCR
Total RNA (50 ng/rxn) was reversed-transcribed into cDNA and amplified in a buffer containing SYBR Green (Applied Biosystems, Foster City, CA). PCR primers were specific for mouse MMP-9, pro-α 1 type I, pro-α 3 type I, or pro-α IV type III collagen. TFIID primers were used as an internal control. Samples were run in triplicate, and the mean value was normalized to the internal control.
TUNEL Staining
The DeadEnd Colorimetric TUNEL System assay kit was used according to the manufacturer's instructions (Promega, Madison, WI). Briefly, paraffin-embedded sections were deparaffinized by immersing slides in xylene and then through graded ethanol washes followed by 1× PBS. Tissue sections were then fixed in 4% paraformaldehyde for 15 minutes and digested with proteinase K (20 μg/ml) for 22 minutes to permeableize the tissue. The slides were then washed with 1× PBS and re-fixed in 4% paraformaldehyde for 5 minutes and washed again. In situ nick end labeling of nuclear DNA fragmentation was performed in a humid chamber for 1 hour in the dark at 37°C. A positive control slide was prepared by treating the tissue sections with RNase-free DNase for 10 minutes before the above labeling. A negative control slide was prepared by omitting the recombinant terminal deoxynucleotidyl transferase (rTdT) from the above labeling step. The labeling reaction was stopped by immersing the slides in 2× SSC for 15 minutes followed by a 1× PBS wash. Endogenous peroxidases were blocked by immersing the slides in 0.3% hydrogen peroxide in PBS for 5 minutes and then washing with 1× PBS. Color was developed by DAB. The slides were then mounted with Permount Mouting Medium (Fisher Scientific, Barrington, IL) and observed under a light microscope.
Cleaved Caspase 3 Western
Lung tissues were homogenized and lysed in ice-cold lysis buffer containing 20 mM Tris (pH 7.4),137 mM NaCl, 25 mM β-glycerolphosphate (pH 7.4), 2 mM PPiNa, 2 mM EDTA (pH 7.4),1% Triton X-100,10%Glycerol, 1 mM PMSF, 5 μg/ml leupeptin, 5 μg/ml pepstatin, 5 μg/ml aprotinin,1 mM Na3VO4, and 1 mM DTT. After centrifugation, tissue extracts were resolved by SDS-PAGE and were analyzed by immunoblot. The membranes were probed with antibodies to cleaved caspase-3 and tubulin as a loading control (Cell Signaling Technology, Danvers, MA). Blots were developed with ECL plus (Amersham Pharmacia Biotech, Piscataway, NJ).
Statistical Analyses
All data are expressed as mean ± SEM. mRNA analyses compared TGF-β, MMP-9, pro-α 1 type I, pro-α 3 type I, pro-α IV type III collagen from LPS-exposed mice to that from air-exposed mice. The differences between variables in each comparison were analyzed by Student's t test. Probability values of P < 0.05 were considered as statistically significant.
RESULTS
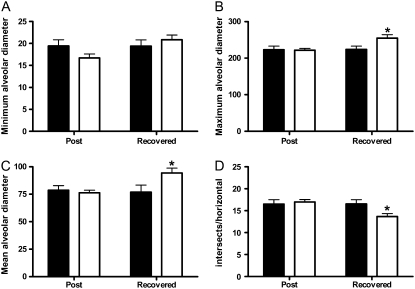
Chronic LPS Inhalation Challenge Causes Changes in Parenchymal Architecture Consistent with Emphysema in C57BL/6 Mouse Lung
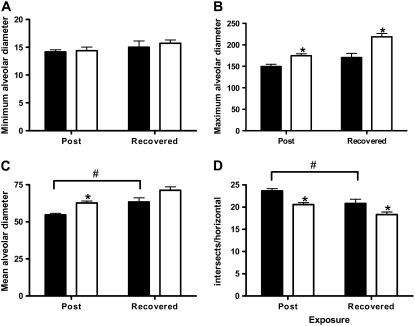
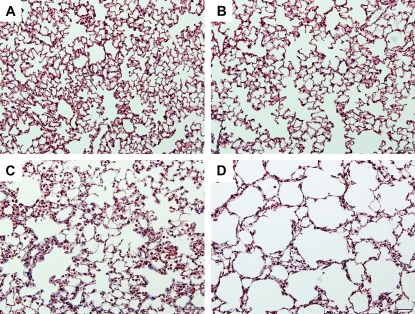
Previous studies have shown that chronic treatment of rabbits and hamsters with intratracheal LPS instillation causes parenchymal architectural changes in the lung that are consistent with emphysema (21–23). To more closely reflect the biological processes associated with environmental exposure to a bioaerosol containing LPS, we measured the minimum, maximum, and mean alveolar diameter as well as the number of linear intercepts per field in lung parenchyma at 3 days and at 4 weeks after the end of the LPS exposure (Figure 1). We show that, independent of exposure, as mice age mean alveolar diameter increases over time in unexposed mice (Figure 1C). We also show that LPS inhalation challenge increases these changes significantly (Figures 1B and 1D), and that this exposure significantly increases maximum alveolar diameter over unexposed mice at both time points examined (Figure 1B). These changes can be appreciated histopathologically in Figure 2.
Figure 1.
Chronic lipopolysaccharide (LPS) inhalation challenge causes changes in parenchymal architecture consistent with emphysema in C57BL/6 mouse lung. Quantitative morphometric analysis of C57BL/6 mouse lung histology measuring (A) minimum, (B) maximum, and (C) mean alveolar diameter, and (D) intersects per horizontal. Data are mean ± SEM. *P < 0.05 versus air; #P < 0.05 Post versus Recovered. Solid bars, air; open bars, LPS.
Figure 2.
Chronic LPS inhalation challenge causes changes in parenchymal architecture consistent with emphysema in C57BL/6 mouse lung. Representative histopathology of C57BL/6 mouse lung exposed to (A and B) filtered air or (C and D) daily LPS inhalation for (A and C) 4 weeks or (B and D) 4 weeks followed by 4 weeks of recovery. Images are ×200 original magnification. Bar = 200 μM.
Emphysematous Changes in C57BL/6 Mouse Lung Are Associated with Elevated and Persistent Procollagen Gene Expression and Are Independent of MMP-9
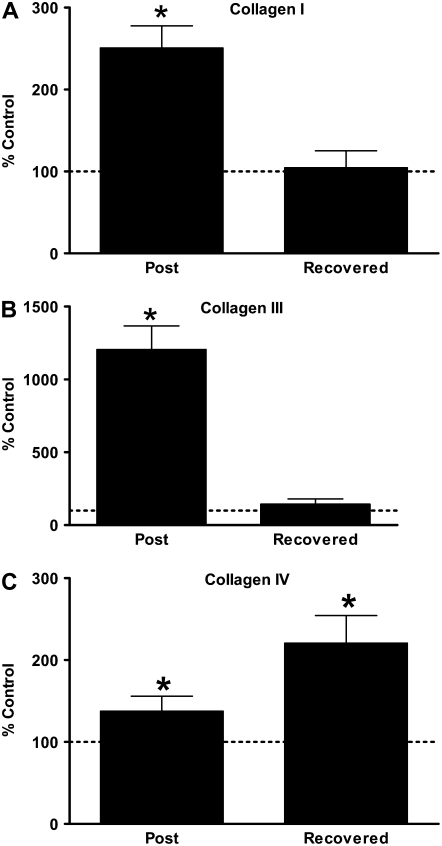
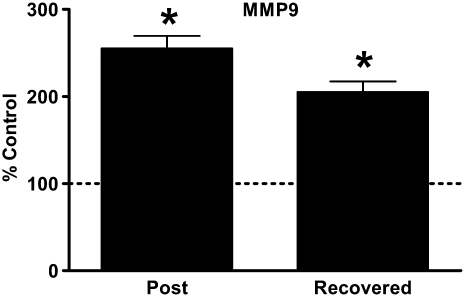
Emphysematous changes in both human lung (25) and in an elastin-induced rat emphysema model are accompanied by excess collagen deposition and thickening of the remaining alveolar structures (26). Consistent with these observations, we show in Figure 3 that mRNAs for procollagen types I, III, and IV are significantly elevated in mouse lung 3 days after the end of chronic LPS exposure. One month after the end of the exposure, procollagen type IV gene expression is still significantly elevated over baseline (Figure 3C). Previous studies have demonstrated that patients with emphysema have elevated MMP-9 levels (27), and we have previously shown that elevated MMP-9 protein levels are associated with chronic LPS-induced airway remodeling (19). We show in Figure 4 that persistently elevated MMP-9 is associated with LPS-induced emphysematous changes in mouse lung.
Figure 3.
Emphysematous changes in C57BL/6 mouse lung are associated with elevated and persistent procollagen gene expression. Quantitative RT-PCR for (A) pro-α I type I collagen, (B) pro-α III type IV collagen, and (C) pro-α IV type III collagen from mouse whole lung total RNA. Data presented are mean percent of age-matched air control ± SEM. Horizontal line represents 100% of age-matched air control. *P < 0.05 versus age-matched air controls.
Figure 4.
Emphysematous changes in C57BL/6 mouse lung are associated with elevated and persistent matrix metalloproteinase (MMP)-9 gene expression. Quantitative RT-PCR for MMP-9 from mouse whole lung RNA. Data presented are mean percent of age-matched air control ± SEM. Horizontal line represents 100% of age-matched air control. *P < 0.05 versus age-matched air controls.
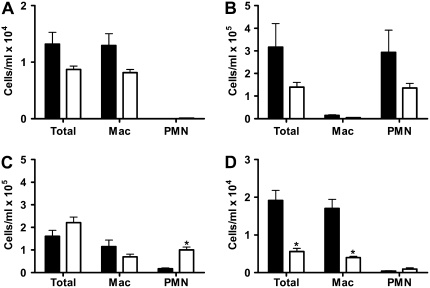
To address the role of MMP-9 in LPS-induced alveolar remodeling, we exposed MMP-9–deficient mice to chronic LPS inhalation challenge for 4 weeks and assessed pulmonary inflammation by lavage and differential cell counting and the histophathologic changes morphometrically at 3 days and 4 weeks after the end of the exposure. An additional group of animals was exposed acutely to a single LPS inhalation challenge to evaluate the acute inflammatory response. We show in Figure 5 that at baseline (Figure 5A) and after a single LPS inhalation challenge (Figure 5B) there is a trend toward a difference in the number of inflammatory cells in the lungs between C57BL/6 and MMP-9–deficient mice. However, there is no significant difference between C57BL/6 and MMP-9–deficient mice in the numbers of inflammatory cells in the lung at any time point except 1 month after the end of the chronic LPS exposure period (Figure 5D). We also show that compared with age-matched controls, MMP-9–deficient mice were not protected from the chronic effects of LPS inhalation challenge 1 month after the end of the chronic LPS exposure period (Figure 6). However, there was no difference between exposed and control MMP-9–deficient mice 3 days after the end of the last exposure in any of the parameters measured (Figure 6).
Figure 5.
Acute and chronic LPS inhalation challenge causes substantial neutrophilic inflammation in both C57BL/6 (solid bars) and MMP-9–deficient (open bars) mice. Total cells, macrophages, and neutrophils (PMN) in C57BL/6 and MMP-9–deficient mice (A) at baseline or (B) after a single acute LPS inhalation challenge, or (C) 3 days or (D) 1 month after the end of chronic LPS inhalation challenge. *P < 0.05 versus C57BL/6.
Figure 6.
Chronic LPS inhalation challenge causes changes in parenchymal architecture independent of MMP-9 in MMP-9–deficient mice. Quantitative morphometric analysis of MMP-9–deficient mouse lung histology measuring (A) minimum, (B) maximum, and (C) mean alveolar diameter, and (D) intersects per horizontal. Data are mean ± SEM. *P < 0.05 versus air. Solid bars, air; open bars, LPS.
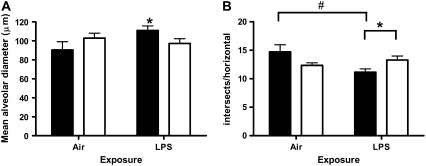
Emphysematous Changes in C3HeB/FeJ Mouse Lung Are Partially Dependent on the Presence of Neutrophils
We have previously shown that chronic LPS-induced airway remodeling is dependent on the presence of neutrophils in mice exposed to chronic LPS inhalation challenge (20) and that treatment of mice with anti-neutrophil antiserum attenuates both the total number of circulating neutrophils in peripheral blood and the total number of lavageable neutrophils both 3 days and 4 weeks after the end of chronic LPS exposure (20). Previous reports have also shown that neutrophil elastase may be involved in both loss of alveolar architecture as well as in ECM deposition (28). We show in Figure 7 that mice treated with pre-immune serum have a significant increase in mean alveolar diameter (Figure 7A) and a significant decrease in the number of horizontal intersects per histologic section (Figure 7B) 1 month after the end of chronic LPS exposure that is consistent with emphysematous changes. Mice treated with anti-PMN serum do not show an increase in mean alveolar diameter and are protected from the decrease in number of intersects per horizontal (Figure 7B).
Figure 7.
Emphysematous changes in C3HeB/FeJ mouse lung are partially dependent on the presence of neutrophils. Quantitative morphometric analysis of C3HeB/FeJ mouse lung histology from 1 month recovered animals measuring (A) mean alveolar diameter and (B) intersects per horizontal. Data are mean ± SEM. *P < 0.05 pre-immune serum (solid bars) versus anti-PMN serum (open bars). #P < 0.05 air versus LPS.
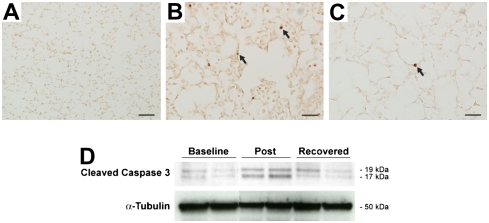
Emphysematous Changes in C57BL/6 Mice Exposed to Chronic LPS Inhalation Are Associated with Apoptosis
We hypothesized that loss of alveolar architecture could be due to loss of structural cells in the lung parenchyma. To evaluate this hypothesis we performed TUNEL staining on histologic sections from C57BL/6 mouse lung exposed to chronic LPS inhalation challenge. We show prominent TUNEL positivity in inflammatory cells and in structural cells in mice allowed to recover for 3 days (Figure 8B). There is persistent TUNEL positivity in structural cells in the mice allowed to recover for 4 weeks after the end of the LPS exposure (Figure 8C). We also show prominently increased cleaved caspase immunoreactivity by Western analysis both in mice allowed to recover for 3 days and in those allowed to recover for 4 weeks after the end of LPS exposure (Figure 8D).
Figure 8.
Emphysematous changes in C57BL/6 mice exposed to chronic LPS inhalation are associated with apoptosis. TUNEL staining in C57BL/6 mouse lung and cleaved caspase 3 Western blot from C57BL/6 mouse lung total protein. (A) Air-exposed at ×100 original magnification; bar = 200 μM. (B) Post at ×400 original magnification; bar = 50 μM. (C) Recovered at ×400 original magnification; bar = 50 μM. (D) Representative cleaved caspase-3 Western blot at baseline and in Post and Recovered groups with α-tubulin as a loading control.
DISCUSSION
We show here that daily LPS inhalation causes persistent and progressive changes in mouse lung parenchyma that are consistent with emphysema. We show that these changes are partially neutrophil dependent and are associated with significantly elevated persistent ECM and protease gene expression and with hallmarks of apoptosis, including TUNEL positivity in the pulmonary parenchyma and increased caspase 3 immunoreactivity. Interestingly, we observed that MMP-9–deficient mice were not protected from the long-term effects of chronic LPS inhalation despite the persistent increase in MMP-9 mRNA expression. Interestingly, we observed no difference between groups in the Post group (3 d after the end of the exposure), suggesting that MMP-9 may have an indirect role in the emphysematous changes observed in C57BL/6 mice in the Post group but that it is not essential for the ongoing alveolar remodeling we observe at the later time point. We have previously shown that chronic LPS inhalation leads to airway wall thickening and persistent airways hyperreactivity (17, 18, 20). Taken together, these data support the hypothesis that occupational exposure to inhaled LPS is a risk factor for COPD and emphysema in humans independent of cigarette smoke exposure. Finally, although polymorphisms in MMP-9 have been identified in association with increased risk for cigarette smoke–induced COPD (29), and although MMP-9 has been associated with development of COPD (30), our observations suggest that LPS-induced architectural changes occur independently of direct effects of MMP-9.
Tobacco smoke is by far the greatest risk factor for COPD and emphysema. However, it is becoming increasingly clear that other environmental exposures likely contribute to disease etiology as well (31). Classically, COPD results from repeated episodes of neutrophilic inflammation in the lung resulting from ongoing tobacco smoke exposure leading to enlarged airspaces due to loss of alveolar architecture (32), enhanced ECM gene expression, and small airway narrowing (33, 34). In addition to inflammation, an imbalance of proteases and antiproteases in the lungs, and oxidative stress, have been implicated in disease pathogenesis (35). We have previously shown that daily LPS inhalation challenge in mice causes repeated episodes of neutrophilic inflammation, enhanced collagen deposition, and thickening of the airways (17, 20), similar to cigarette smoke–induced COPD. Emphysematous changes in human lung (25) and a rat model of emphysema are also associated with collagen deposition and thickening of the remaining alveolar structures (26), suggesting that enhanced ECM deposition affects not only the airway submucosa but also the lung parenchyma.
Similar to tobacco smoke, LPS can contribute to many of the pathologic mechanisms in the lung previously implicated in the pathogenesis of emphysema, including oxidant stress (36), protease/antiprotease imbalance (37, 38), and apoptosis (39–42). It has been shown, for example, that LPS can increase the production of ceramide in an acid sphingomyelinase–dependent manner. This phospholipase has been shown to be induced by TNF-α, which is known to be strongly induced by LPS both in vitro and in vivo. Ceramide has been shown to cause apoptosis of endothelial cells (39), and more recently it has been shown that pulmonary ceramide can cause apoptosis in the lung and emphysematous lesions in mice (43). It has also been shown that there are elevated ceramide levels in the lungs of patients with COPD (43). Finally, it has been shown recently that ceramide can induce and activate caspase 3 in pulmonary endothelial (44) and epithelial cells (45) in vitro. Thus, it is likely that a major commonality between the smoke and the LPS model of emphysema is the induction of TNF-α and the downstream consequences of this induction.
The predominant cell type recruited to the lung after exposure to LPS either systemically or by inhalation is the neutrophil. Intratracheal instillation of high concentrations of neutrophil elastase by itself can lead to emphysema in mice (46). Corbel and coworkers (47) have shown that 8 months of thrice-weekly aerosolized LPS exposure significantly increases the hydroxyproline content of the lung concomitant with significantly elevated expression of both MMP-2 and MMP-9 mRNA. We show here that there is significant ECM and MMP-9 gene expression associated with repeated LPS exposure. Elevated expression of these metalloproteases has also been demonstrated in alveolar macrophages from patients with emphysema (27). However, we show that MMP-9–deficient mice were not protected from the effects of chronic LPS exposure despite an acute and chronic inflammatory response that was not different from that of C57BL/6 mice during LPS exposure. It has also been observed that emphysematous changes induced by intratracheally instilled LPS can be inhibited by recombinant secretory leukocyte proteinase inhibitor (48), supporting the hypothesis that neutrophil derived proteases are important to the development of emphysema in this model system. Thus, despite strong similarities between emphysema induced by cigarette smoke and the LPS model we present here, there are some key differences as well.
Mice deficient in TLR4, the primary receptor for bacterial LPS, develop spontaneous emphysema later in life (49). This has been attributed to the ability of TLR4 to regulate the oxidant/antioxidant balance in normal lung through inhibition of Nox3 (49). The role of Nox3 in generating oxygen radicals in the lung in response to LPS has not been examined but it has been shown that TLR4 interacts directly with other Nox family members in the generation of oxygen radicals in response to LPS (50). This suggests a dual role for TLR4 in both maintaining integrity of lung tissue by helping maintain the oxidant/antioxidant balance in normal lung and in host defense by stimulating the production of oxygen radicals in response to pathogens.
The clinical manifestations of exposure to organic dusts containing LPS such as cotton or grain dust has received some attention in the occupational health literature recently (51). There is evidence in the literature and we also show in a mouse model that exposure to organic dusts containing LPS over time may share some common mechanisms of COPD development. Historically, however, the study of organic dust-related disease has focused on acute airway responses, including chest tightness, cross-shift drops in forced expiratory volume in 1 s (FEV1), and airway hyperresponsiveness. These acute airway responses may even be observed in healthy volunteers exposed for a short time in model cardrooms (52), or when exposed to extracts of grain dust (53). However, manifestations of a COPD-like presentation can appear after several years of exposure (5). For example, a 15-year longitudinal cohort study (54) observing both cotton and silk workers showed significant annual declines in FEV1 and forced vital capacity (FVC) in the cotton workers only, suggesting that in human subjects, long-term exposure to organic dusts containing LPS can lead to a clinical presentation very similar to that of COPD. We now present evidence from a mouse model that long-term exposure to low-dose biologically relevant inhaled LPS can result in pathopysiologic changes consistent with both emphysema and COPD but that may occur through other mechanisms than those resulting from cigarette smoke. These observations support the use of this model for the further study of the biology of occupational lung disease.
This work was funded by the intramural research programs at NIEHS and NHLBI and by the Department of Veterans Affairs (Merit Review), extramural grants (ES11375 [D.A.S.], ES7498 [D.A.S.], ES9607 [D.A.S.], ES11961 [J.W.H., W.M.F.], AI58161 [J.W.H.], and HL91335 [J.W.H.]).
Originally Published in Press as DOI: 10.1165/rcmb.2007-0448OC on June 6, 2008
Conflict of Interest Statement: D.A.S. has a patent on TLR4 polymorphisms that are associated with decreased responsiveness to LPS. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global burden of disease study. Lancet 1997;349:1498–1504. [DOI] [PubMed] [Google Scholar]
- 2.Khan AJ, Nanchal R. Cotton dust lung diseases. Curr Opin Pulm Med 2007;13:137–141. [DOI] [PubMed] [Google Scholar]
- 3.Haglind P, Rylander R. Exposure to cotton dust in an experimental cardroom. Br J Ind Med 1984;41:340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castellan RM, Olenchock SA, Kinsley KB, Hankinson JL. Inhaled endotoxin and decreased spirometric values: an exposure-response relation for cotton dust. N Engl J Med 1987;317:605–610. [DOI] [PubMed] [Google Scholar]
- 5.Wang XR, Zhang HX, Sun BX, Dai HL, Hang JQ, Eisen EA, Wegman DH, Olenchock SA, Christiani DC. A 20-year follow-up study on chronic respiratory effects of exposure to cotton dust. Eur Respir J 2005;26:881–886. [DOI] [PubMed] [Google Scholar]
- 6.Mayer AS, Stoller JK, Bucher Bartelson B, James Ruttenber A, Sandhaus RA, Newman LS. Occupational exposure risks in individuals with PI*Z α1-antitrypsin deficiency. Am J Respir Crit Care Med 2000;162:553–558. [DOI] [PubMed] [Google Scholar]
- 7.Jaen A, Zock JP, Kogevinas M, Ferrer A, Marin A. Occupation, smoking, and chronic obstructive respiratory disorders: a cross sectional study in an industrial area of catalonia, spain. Environ Health 2006;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coggon D, Newman Taylor A. Coal mining and chronic obstructive pulmonary disease: a review of the evidence. Thorax 1998;53:398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Love RG, Miller BG. Longitudinal study of lung function in coal-miners. Thorax 1982;37:193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Attfield MD. Longitudinal decline in FEV1 in united states coalminers. Thorax 1985;40:132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hnizdo E, Baskind E, Sluis-Cremer GK. Combined effect of silica dust exposure and tobacco smoking on the prevalence of respiratory impairments among gold miners. Scand J Work Environ Health 1990;16:411–422. [DOI] [PubMed] [Google Scholar]
- 12.Davison AG, Fayers PM, Taylor AJ, Venables KM, Darbyshire J, Pickering CA, Chettle DR, Franklin D, Guthrie CJ, Scott MC, et al. Cadmium fume inhalation and emphysema. Lancet 1988;1:663–667. [DOI] [PubMed] [Google Scholar]
- 13.Niven RM, Fletcher AM, Pickering CA, Fishwick D, Warburton CJ, Simpson JC, Francis H, Oldham LA. Chronic bronchitis in textile workers. Thorax 1997;52:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glindmeyer HW, Lefante JJ, Jones RN, Rando RJ, Abdel Kader HM, Weill H. Exposure-related declines in the lung function of cotton textile workers: relationship to current workplace standards. Am Rev Respir Dis 1991;144:675–683. [DOI] [PubMed] [Google Scholar]
- 15.Glindmeyer HW, Lefante JJ, Jones RN, Rando RJ, Weill H. Cotton dust and across-shift change in FEV1 as predictors of annual change in FEV1. Am J Respir Crit Care Med 1994;149:584–590. [DOI] [PubMed] [Google Scholar]
- 16.Wohlford-Lenane CL, Deetz DC, Schwartz DA. Cytokine gene expression after inhalation of corn dust. Am J Physiol 1999;276:L736–L743. [DOI] [PubMed] [Google Scholar]
- 17.Brass DM, Savov JD, Gavett SH, Haykal-Coates N, Schwartz DA. Subchronic endotoxin inhalation causes persistent airway disease. Am J Physiol Lung Cell Mol Physiol 2003;6:6. [DOI] [PubMed] [Google Scholar]
- 18.Brass DM, Savov JD, Whitehead GS, Maxwell AB, Schwartz DA. LPS binding protein is important in the airway response to inhaled endotoxin. J Allergy Clin Immunol 2004;114:586–592. [DOI] [PubMed] [Google Scholar]
- 19.Savov JD, Brass DM, Berman KG, McElvania E, Schwartz DA. Fibrinolysis in LPS-induced chronic airway disease. Am J Physiol Lung Cell Mol Physiol 2003;285:L940–L948. [DOI] [PubMed] [Google Scholar]
- 20.Savov JD, Gavett SH, Brass DM, Costa DL, Schwartz DA. Neutrophils play a critical role in development of LPS-induced airway disease. Am J Physiol Lung Cell Mol Physiol 2002;283:L952–L962. [DOI] [PubMed] [Google Scholar]
- 21.Klut ME, van Eeden SF, Whalen BA, Verburgt LM, English D, Hogg JC. Neutrophil activation and lung injury associated with chronic endotoxemia in rabbits. Exp Lung Res 1996;22:449–465. [DOI] [PubMed] [Google Scholar]
- 22.Stolk J, Rudolphus A, Davies P, Osinga D, Dijkman JH, Agarwal L, Keenan KP, Fletcher D, Kramps JA. Induction of emphysema and bronchial mucus cell hyperplasia by intratracheal instillation of lipopolysaccharide in the hamster. J Pathol 1992;167:349–356. [DOI] [PubMed] [Google Scholar]
- 23.Rudolphus A, Stolk J, van Twisk C, van Noorden CJ, Dijkman JH, Kramps JA. Detection of extracellular neutrophil elastase in hamster lungs after intratracheal instillation of E. coli lipopolysaccharide using a fluorogenic, elastase-specific, synthetic substrate. Am J Pathol 1992;141:153–160. [PMC free article] [PubMed] [Google Scholar]
- 24.Clapp WD, Thorne PS, Frees KL, Zhang X, Lux CR, Schwartz DA. The effects of inhalation of grain dust extract and endotoxin on upper and lower airways. Chest 1993;104:825–830. [DOI] [PubMed] [Google Scholar]
- 25.Martin-Mosquero C, Peces-Barba G, Rubio ML, Ortega M, Rodriguez-Nieto MJ, Martinez Galan L, Gonzalez-Mangado N. Increased collagen deposition correlated with lung destruction in human emphysema. Histol Histopathol 2006;21:823–828. [DOI] [PubMed] [Google Scholar]
- 26.Finlay GA, O'Donnell MD, O'Connor CM, Hayes JP, FitzGerald MX. Elastin and collagen remodeling in emphysema: a scanning electron microscopy study. Am J Pathol 1996;149:1405–1415. [PMC free article] [PubMed] [Google Scholar]
- 27.Finlay GA, O'Driscoll LR, Russell KJ, D'Arcy EM, Masterson JB, FitzGerald MX, O'Connor CM. Matrix metalloproteinase expression and production by alveolar macrophages in emphysema. Am J Respir Crit Care Med 1997;156:240–247. [DOI] [PubMed] [Google Scholar]
- 28.Chua F, Laurent GJ. Neutrophil elastase: mediator of extracellular matrix destruction and accumulation. Proc Am Thorac Soc 2006;3:424–427. [DOI] [PubMed] [Google Scholar]
- 29.Tesfaigzi Y, Myers OB, Stidley CA, Schwalm K, Picchi M, Crowell RE, Gilliland FD, Belinsky SA. Genotypes in matrix metalloproteinase 9 are a risk factor for COPD. Int J Chron Obstruct Pulmon Dis 2006;1:267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mercer PF, Shute JK, Bhowmik A, Donaldson GC, Wedzicha JA, Warner JA. MMP-9, TIMP-1 and inflammatory cells in sputum from COPD patients during exacerbation. Respir Res 2005;6:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23:932–946. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell RS, Stanford RE, Johnson JM, Silvers GW, Dart G, George MS. The morphologic features of the bronchi, bronchioles, and alveoli in chronic airway obstruction: a clinicopathologic study. Am Rev Respir Dis 1976;114:137–145. [DOI] [PubMed] [Google Scholar]
- 33.Bosken CH, Wiggs BR, Pare PD, Hogg JC. Small airway dimensions in smokers with obstruction to airflow. Am Rev Respir Dis 1990;142:563–570. [DOI] [PubMed] [Google Scholar]
- 34.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med 1968;278:1355–1360. [DOI] [PubMed] [Google Scholar]
- 35.Repine JE, Bast A, Lankhorst I. Oxidative stress in chronic obstructive pulmonary disease: oxidative stress study group. Am J Respir Crit Care Med 1997;156:341–357. [DOI] [PubMed] [Google Scholar]
- 36.Nowak D, Pietras T, Antczak A, Krol M, Piasecka G. Effect of bacterial lipopolysaccharide on the content of lipid peroxidation products in lungs and other organs of mice. Antonie Van Leeuwenhoek 1993;63:77–83. [DOI] [PubMed] [Google Scholar]
- 37.Barbey-Morel C, Pierce JA, Campbell EJ, Perlmutter DH. Lipopolysaccharide modulates the expression of alpha 1 proteinase inhibitor and other serine proteinase inhibitors in human monocytes and macrophages. J Exp Med 1987;166:1041–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cury JD, Campbell EJ, Lazarus CJ, Albin RJ, Welgus HG. Selective up-regulation of human alveolar macrophage collagenase production by lipopolysaccharide and comparison to collagenase production by fibroblasts. J Immunol 1988;141:4306–4312. [PubMed] [Google Scholar]
- 39.Haimovitz-Friedman A, Cordon-Cardo C, Bayoumy S, Garzotto M, McLoughlin M, Gallily R, Edwards CK III, Schuchman EH, Fuks Z, Kolesnick R. Lipopolysaccharide induces disseminated endothelial apoptosis requiring ceramide generation. J Exp Med 1997;186:1831–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Otterbein LE, Chin BY, Mantell LL, Stansberry L, Horowitz S, Choi AM. Pulmonary apoptosis in aged and oxygen-tolerant rats exposed to hyperoxia. Am J Physiol 1998;275:L14–L20. [DOI] [PubMed] [Google Scholar]
- 41.Aoshiba K, Yokohori N, Nagai A. Alveolar wall apoptosis causes lung destruction and emphysematous changes. Am J Respir Cell Mol Biol 2003;28:555–562. [DOI] [PubMed] [Google Scholar]
- 42.Petit C, Monsigny M, Roche AC. Macrophage activation by muramyl dipeptide bound to neoglycoproteins and glycosylated polymers: cytotoxic factor production. J Biol Response Mod 1990;9:33–43. [PubMed] [Google Scholar]
- 43.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med 2005;11:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medler TR, Petrusca DN, Lee PJ, Hubbard WC, Berdyshev EV, Skirball J, Kamocki K, Schuchman E, Tuder RM, Petrache I. Apoptotic sphingolipid signaling by ceramides in lung endothelial cells. Am J Respir Cell Mol Biol 2008;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ravid T, Tsaba A, Gee P, Rasooly R, Medina EA, Goldkorn T. Ceramide accumulation precedes caspase-3 activation during apoptosis of A549 human lung adenocarcinoma cells. Am J Physiol Lung Cell Mol Physiol 2003;284:L1082–L1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Houghton AM, Quintero PA, Perkins DL, Kobayashi DK, Kelley DG, Marconcini LA, Mecham RP, Senior RM, Shapiro SD. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest 2006;116:753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corbel M, Theret N, Caulet-Maugendre S, Germain N, Lagente V, Clement B, Boichot E. Repeated endotoxin exposure induces interstitial fibrosis associated with enhanced gelatinase (MMP-2 and MMP-9) activity. Inflamm Res 2001;50:129–135. [DOI] [PubMed] [Google Scholar]
- 48.Rudolphus A, Stolk J, Dijkman JH, Kramps JA. Inhibition of lipopolysaccharide-induced pulmonary emphysema by intratracheally instilled recombinant secretory leukocyte proteinase inhibitor. Am Rev Respir Dis 1993;147:442–447. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X, Shan P, Jiang G, Cohn L, Lee PJ. Toll-like receptor 4 deficiency causes pulmonary emphysema. J Clin Invest 2006;116:3050–3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: Direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol 2004;173:3589–3593. [DOI] [PubMed] [Google Scholar]
- 51.Christiani DC, Wang XR. Respiratory effects of long-term exposure to cotton dust. Curr Opin Pulm Med 2003;9:151–155. [DOI] [PubMed] [Google Scholar]
- 52.Rylander R, Haglind P. Exposure of cotton workers in an experimental cardroom with reference to airborne endotoxins. Environ Health Perspect 1986;66:83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clapp WD, Becker S, Quay J, Watt JL, Thorne PS, Frees KL, Zhang X, Koren HS, Lux CR, Schwartz DA. Grain dust-induced airflow obstruction and inflammation of the lower respiratory tract. Am J Respir Crit Care Med 1994;150:611–617. [DOI] [PubMed] [Google Scholar]
- 54.Christiani DC, Ye TT, Wegman DH, Eisen EA, Dai HL, Lu PL. Cotton dust exposure, across-shift drop in FEV1, and five-year change in lung function. Am J Respir Crit Care Med 1994;150:1250–1255. [DOI] [PubMed] [Google Scholar]