Abstract
Cancer prevention strategies utilizing selenium-containing compounds have demonstrated reduced cancer mortality and efficacy for some cancer types but considerable differences in cellular effects exist among the selenocompounds employed. The variability of the effects on cell viability, redox modulation, and disruption of subcellular compartments by the conventional selenium-containing amino acid, selenomethionine, the oxidized selenosugar metabolite, methylseleninic acid, and selenazolidines was investigated in A549 and BEAS-2B human lung cell lines. Selenomethionine had little effect whereas methylseleninic acid increased cellular thiols and stress in the endoplasmic reticulum. The cyclohexylselenazolidine increased mild oxidative stress in the adenocarcinoma cell line, A549, but the effects were attenuated in the normal, but virally transformed cell line, BEAS-2B. These data demonstrate that all selenocompounds are not equal and that the form of the organic selenocompound is a major determinant in the expected cellular response.
1. Introduction
Selenium plays a role in human health, with deficiencies in the diet predisposing individuals to certain rare diseases such as Keshan disease (Ge and Yang, 1993), as well as increasing susceptibility to cancer (Taylor et al., 1994). Several human studies have demonstrated that selenium supplementation attenuates cancer mortality and disease (Blot et al., 1993; Mark et al., 2000) and historically, in the United States, dietary selenium levels in forage crops inversely correlated with cancer mortality (Clark et al., 1991; Shamberger and Willis, 1971). The Nutritional Prevention of Cancer trial is the seminal study to date that identified a selenium cancer prevention benefit by demonstrating an overall decrease in cancer mortality with particular benefits to colon, lung, and prostate cancer incidence (Clark et al., 1996). However, selenium is toxic at high levels (Vinceti et al., 2001) and displays a bimodal response for cancer risk in certain forms (Novoselov et al., 2005). The mechanisms for selenium effects in cancer must be compound dependent since they can vary within animal models and are not solely based on the selenium content of the selenocompound (el-Bayoumy et al., 1993; Franklin et al., 2007; Li et al., 2005).
The cancer prevention activities and toxicities of selenocompounds vary considerably in lung tumor model systems. Organic selenocompounds like 1,4-phenylenebis(methylene)selenocyanate (p-XSC)†, and certain selenium containing amino acids, including selenocystine and many selenazolidines (selenocysteine prodrugs) have demonstrated cancer prevention activity (el-Bayoumy et al., 1993; el-Bayoumy et al., 1996; Franklin et al., 2007; Li et al., 2005; Prokopczyk et al., 1996), while the inorganic selenium compound sodium selenite has shown minimal activity. It is of interest that the form of selenium most frequently utilized in cancer prevention trials, selenomethionine, as well as a methylated form of selenocysteine, Se-methylselenocysteine, also show minimal cancer prevention activity in lung cancer model systems (el-Bayoumy et al., 1993; Li et al., 2005).
Some selenocompounds may have anticancer activities in addition to cancer prevention activities. In human lung cell lines various selenocompounds have demonstrated potential antiproliferative activities. For example, p-XSC and methylseleninic acid (MSA) induced cell cycle arrest and apoptosis (El-Bayoumy et al., 2006; Swede et al., 2003). Another selenocompound, selenomethionine, has demonstrated enhancement of cell killing when lung cancer cell lines, but not normal lung cancer cells, were subject to ionizing radiated (Shin et al., 2007).
The unexplained variability in chemopreventive efficacy, a variability that does not correlate with selenium levels alone, suggests that the mechanisms of selenium-mediated cancer prevention may vary among the various selenocompounds. Several selenazolidines have demonstrated cancer prevention activity in vivo (Franklin et al., 2007; Li et al., 2005) with low toxicity (Li et al., 2004); however, mechanistic information at the target organ (i.e. lung cells) of this class of selenocompounds is limited. In this comparative study, we examined the effects of several selenazolidines together with p-XSC, methylseleninic acid, and selenoamino acids on cellular viability, thiol status, generation of reactive oxygen species, and mechanisms of toxicity in two human lung cell lines, A549 and BEAS-2B. These cell lines were used to determine if there were different responses to the selenocompounds for a tumorigenic and non-tumorigenic line, respectively.
2. Materials and Methods
2.1 Materials
Materials used included L-selenomethionine and L-selenocystine from Acros Organics (Morris Plains, NJ). 2-oxoselenazolidine-4(R)-carboxylic acid (OSCA) and 2-cyclohexylselenazolidine-4-(R)-carboxylic acid (ChSCA) were synthesized as described (Short et al., 2003; Xie et al., 2001) (supported by USPHS Grant No. GM058913). p-XSC was from LKT Laboratories, Inc (St. Paul, MN) and methylseleninic acid was from PharmaSe, Inc. (Lubbock, TX). The A549 cell line was purchased from American Tissue Type Culture Collection (Manassas, VA). BEAS-2B cells were a gift from Dr. Christopher Reilly (Univeristy of Utah). Monoclonal antibodies directed against BiP/GRP78 and α-tubulin were purchased from Becton Dickinson and Company (Franklin Lakes, NJ) and Zymed Laboratories, Inc. (South San Francisco, CA), respectively; donkey polyclonal anti-mouse antibodies conjugated with horseradish peroxidase were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Bovine serum albumin standard and Coomassie Plus Protein Reagent were from Pierce Biotechnology (Rockford, IL). Human fibronectin and bovine collagen were purchased from Fisher Scientific (Houston, TX). Lechner and LaVeck (LHC-8) medium, Advanced DMEM, monobromobimane (mBBr), 2,7-dichlorofluroescein diacetate (DCFH-DA), propidium iodide (PI), 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide (JC-1), NuPAGE 10% Bis-Tris gels and bovine serum albumin were purchased from Invitrogen (Carlsbad, CA). Protease inhibitor cocktail tablets (complete) were purchased from Roche (Indianapolis, IN). PVDF membrane was purchased from Millipore (Burlington, MA). Western Lighting chemiluminescence reagents were from PerkinElmer Life Sciences (Boston, MA). Dimethylsulfoxide (DMSO), retinoic acid, and epinephrine were purchased from Sigma-Aldrich (St. Louis, MO). Cell Counting Kit-8 (CCK-8) was from Dojindo (Gaithersburg, MD). Common buffers and salts were purchased from Sigma-Aldrich, Fisher Scientific or VWR scientific.
2.2 Cell Lines and Culture Conditions
The A549 cell line was cultured in Advanced Dulbecco’s modified essential medium supplemented with 2% fetal bovine serum and glutamine. BEAS-2B cells were cultured in Lechner and LaVeck (LHC-8) medium supplemented with 0.33 nM retinoic acid and 2.75 μM epinephrine. To facilitate adhesion, a plate coat of LHC medium supplemented with 1 mg/100 ml human fibronectin, 1 mg/100ml bovine collagen, and 0.75 g/100ml bovine serum albumin was applied to BEAS-2B cell culture flasks and plates prior to seeding cells. Both cell lines were maintained at 37°C in an atmosphere of 5% CO2 and 95% air, with passages made once cells reached ~ 80–90% confluence.
Selenocystine and selenomethionine were dissolved in the appropriate cell culture medium for the cell type. All other selenocompounds were dissolved in DMSO. Final concentrations of DMSO for selenocompounds treatments where DMSO served as vehicle were less than or equal to 0.1%.
2.3 Viability Assay
Cellular viability was determined using a Cell Counting Kit-8 (CCK-8) which relies on tetrazolium salt reduction by NADH in viable cells (Berridge et al., 2005). Briefly, cells were seeded into 48 well plates at 2–4 × 104 cells/well and allowed to recover overnight. Cells were then treated with selenocompounds at concentrations between 0–60 or 0–600 μM for 24 hours. Following the treatment period, medium was aspirated and replaced with 4% CCK-8 in the appropriate cell culture medium. Absorbance at 460 nm and 650 nm was measured after incubation at 37°C until the reagent developed sufficiently for maximal reading using a Perkin-Elmer VictorV3 Multimode Microplate Reader. The time for development was distinct for the two cell lines, the A549 cells were developed for 45 min. while the BEAS-2B cells were developed for 2.5 hr. Sample absorbance measured at 650 nm was subtracted from the 460 nm absorbance to ensure that the measurements were not affected by sample turbidity.
2.4 Cytometric Assays for Redox metrics
Cells were seeded into 6-well plates at a density of ~2 × 105 cells/well and allowed to grow overnight. Culture medium was refreshed at the time of treatment. Cellular fluorescence concentrations were determined 24 hrs after treatment using a Beckman Cell Lab Quanta SC flow cytometer by dividing the fluorescence for each cell by its measured electronic volume. For each assay, a minimum of 10,000 events per sample was recorded.
Free Thiols
Cells were trypsinized, centrifuged at 250×g for 5 minutes, and resuspended in 1 ml phosphate buffered saline (PBS). Cells were then centrifuged at 250×g for 5 minutes and resuspended in a fresh 1 ml of 1 × PBS. 40 μM monobromobimane (mBBr) was added and samples were incubated at room temperature in the dark for 5 minutes. mBBr fluorescence concentration was then determined. This method allows for measurement of cellular thiols, predominantly glutathione, and since fluorescence concentration is measured, the measurement is cell size independent (Hedley and Chow, 1994; Keij et al., 1999).
Reactive Oxygen Species
20 μM 2,7-dichlorofluroescein diacetate (DCFH-DA) was added to media of cells in 6 well plates following selenocompound treatment and incubated at 37°C and 5% CO2 for 30 minutes. Cells were trypsinized, centrifuged at 250×g for 5 minutes, and resuspended in 1 ml PBS. 2 μg/ml propidium iodide (PI) was then added to the cell suspension to distinguish between viable and compromised cell populations. Both DCF and PI fluorescence were measured for each sample (LeBel et al., 1992). DCF fluorescence concentrations from PI negative (viable) cells are the reported results.
2.5 Subcellular organelle targets
Cells were seeded into 6-well plates at a density of ~ 2 × 105 cells/well and allowed to grow overnight. Culture medium was refreshed at the time of treatment. Selenocompound effects were measured by evaluation of the mitochondrial potential and immunochemical hybridization to determine expression levels of the ER chaperonin BiP/GRP78 as a marker of the unfolded protein response (UPR) (Zu et al., 2006).
Mitochondrial potential
1 μM JC-1 was added to medium of cells attached to 6-well plates after selenocompound treatment. Cells were incubated at 37°C with 5% CO2 and 95% air for 20 minutes and then trypsinized, washed and resuspended as described above for cytometric analysis. JC-1 fluorescence at 525 nm (JC-1 “green”) and 575 nm (JC-1 “red” or “J-aggregates”) was determined for each sample. JC-1 fluoresces green when the mitochondrial potential has been depolarized and forms aggregates that fluoresce red when the mitochondrion is polarized (Salvioli et al., 1997). Carbonyl cyanide 3-chlorophenylhydrazone (CCCP) was used at 25 μM to disrupt the mitochondrial membrane potential as a positive control.
BiP/GRP78 Western blot
Cells in 6-well plates were placed on ice. Media was aspirated and cells were then washed with 1ml of cold 1× PBS and the PBS aspirated. 100 μl of a lyses buffer containing 50 μl of 25× complete protease inhibitor cocktail, 12.5 μl of 10% sodium dodecyl sulfate, 1187.5 μl of a buffer containing 50 mM Tris pH 7.4, 100 mM NaCl, and 2 mM EDTA was added to each well and cells scraped. Lysate was transferred to 1.5 ml microfuge tubes and sonicated 10× using a 40% duty cycle on an ultrasonic processor. Lysates were centrifuged 10,000×g for 10 min at 4°C and the supernatants transferred. Protein concentrations were determined using Bradford reagents. Absorbance at 595 nm was measured using a Perkin-Elmer VictorV3 Multimode Microplate Reader and sample concentrations were determined using a bovine serum albumin standard curve. The membrane was probed with primary anti-BiP/GRP78 antibody at a 1:1000 dilution overnight at 4°C. Membranes were then washed with washing buffer (5 mM Tris pH 8.0, 150 mM NaCl, 2.7 mM KCl, 0.1% Tween-20), probed with secondary donkey anti-mouse IgG horseradish peroxidase antibody at a 1:5000 dilution for 45 minutes at room temperature, and washed again. For detection of α-tubulin, membranes were incubated with stripping buffer (62.5 mM Tris pH 6.7, 2% sodium dodecyl sulfate, 0.7% β-mercaptoethanol) at 50°C for 1 hour and washed. Membranes were probed with anti-α-tubulin at a 1:500 dilution overnight at 4°C, washed, and probed with secondary donkey anti-mouse IgG horseradish peroxidase antibody as described above. Protein was detected using Western Lightning Western Blot Chemiluminescence reagent and visualized on a Kodak Image Station 440.
2.6 Statistical Analysis
1-way ANOVA was used to determine statistical significance between control and treatment values (GraphPad InStat Version 3.06). Dunnett’s multiple comparisons post hoc testing was used to establish significance among the distinct selenocompound treatment groups compared to the control samples with p < 0.05 considered significant.
3. Results
3.1 Toxicity of selenocompounds in lung cells
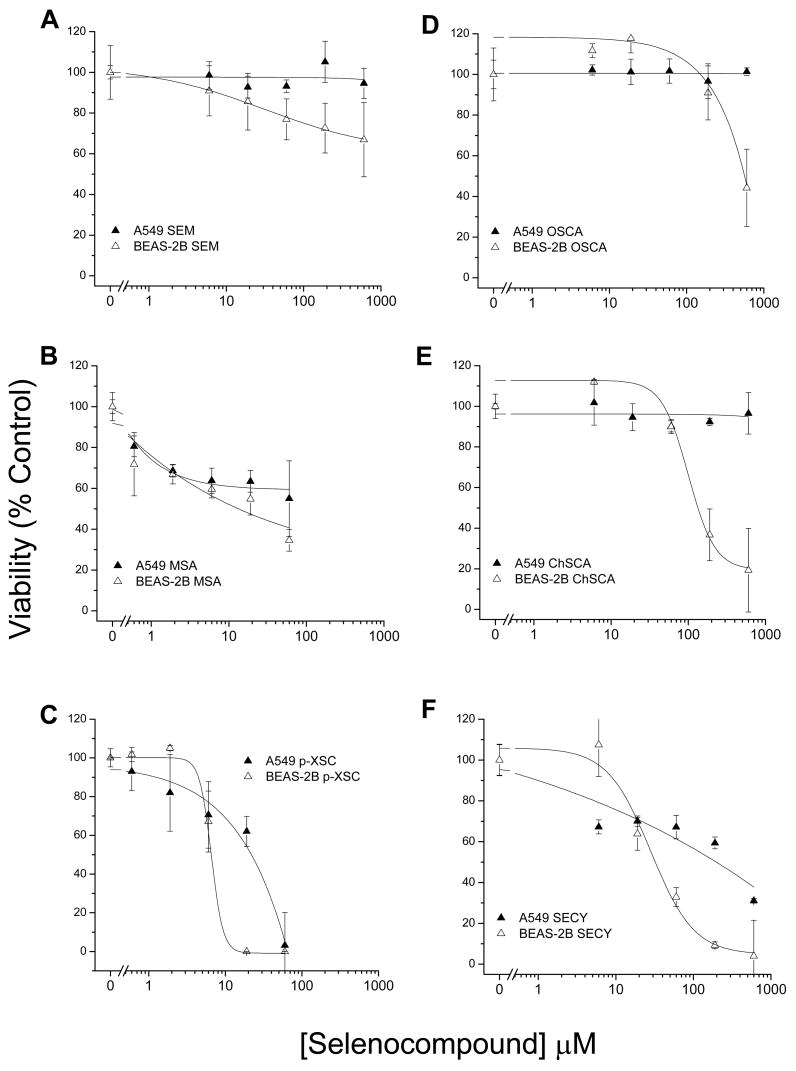
Differential toxicity of the selenocompounds was observed between the BEAS-2B and A549 cell lines (fig. 1). The majority of the selenocompounds demonstrated greater toxicity towards BEAS-2B cells than to A549 cell line. Several selenocompounds did not markedly affect A549 cell viability. The most notable exceptions were p-XSC, and to a lesser extent (only at higher concentrations), selenocystine. In the more sensitive BEAS-2B cells, p-XSC was highly toxic (100% lethal at > 10 μM). Selenocystine required concentrations of >100 μM to achieve 100% lethality. Within the 24 hr time period of evaluation, methylseleninic acid decreased cell viability at low doses (<2 μM) in both cell lines, but did not demonstrate the level of toxicity observed with p-XSC. The selenazolidines, ChSCA and OSCA, demonstrated only minor toxicity at doses >100 μM. In the BEAS-2B cells, the selenazolidines showed an enhancement of cell viability compared with control at doses <20 μM. Selenomethioinine showed very little (BEAS-2B) or no (A549) toxicity.
Figure 1. Human lung cell line viability following treatment with selenocompounds.
Dose response curves for A549 cells (filled triangles) and BEAS-2B cells (open triangles) treated with; (A) SEM, (B) MSA, (C) p-XSC, (D) OSCA, (E) ChSCA, and (F) SECY for 24 hrs. p-XSC and MSA were evaluated at concentrations between 0 to 60 μM while all other selenocompounds were evaluated at concentrations between 0 to 600 μM. Symbols represent the mean viability of triplicate measures as a percentage of control with standard deviations.
3.2 Alterations in cellular redox parameters
Since selenium is redox active, the cellular redox state was determined following exposure to selenocompounds using cytometric assays. To avoid confusion due to high cell death, selenocompound concentrations that resulted in less than 25% loss of viability were selected for use in these experiments. Since p-XSC was much more toxic than the other compounds assessed, it was not included in these or any further analyses.
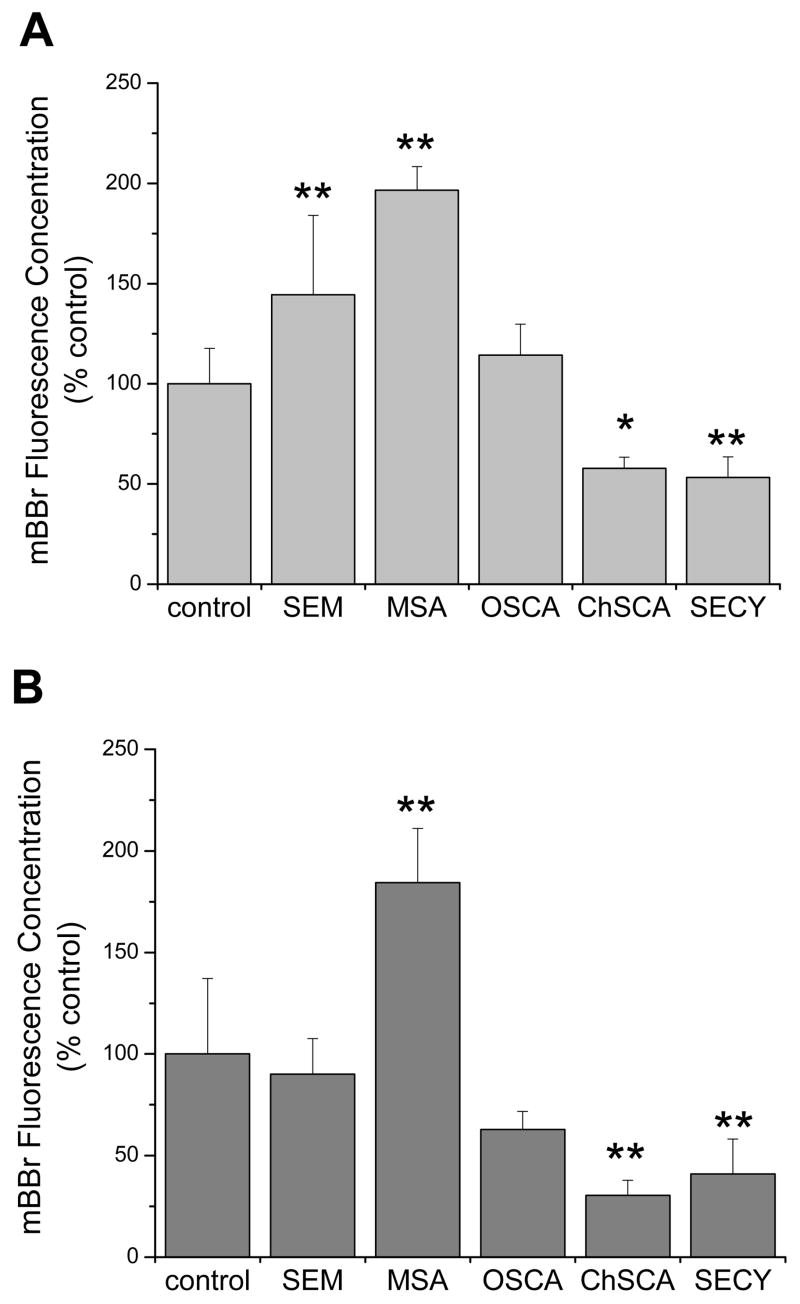
Cellular free thiol levels were determined as a measure of reducing capacity in a time dependent assay using the fluorophore monobromobimane (mBBr, fig. 2). As glutathione levels can change with cell size (Keij et al., 1999), the fluorescence values were normalized to the electronic volume of the cells so that differences in thiol content rather than cell volume would be measured. After a 24 hr incubation with 2.5 μM methylseleninic acid, both cell lines showed significant increases in mBBr fluorescence. Selenomethionine treatment resulted in an increase in mBBr fluorescence in the A549 cells but not in the BEAS-2B cells. In contrast to methylseleninic acid, ChSCA, which spontaneously hydrolyzes to selenocysteine and potentially forms selenocystine, produced a significant decrease in mBBr fluorescence. The other selenazolidine investigated, OSCA, had no statistically significant effect in either cell line.
Figure 2. Thiol status following treatment with selenocompounds in human lung cells.
Analysis of (A) A549 cells and (B) BEAS-2B cells using the fluorophore mBBr as a measure of cellular thiol status. Cells were incubated with 40 μM mBBr for 5 min prior to cytometric analysis. Selenocompounds were used at concentrations that resulted in less than 25% decreases in viability. Concentrations used were: SEM, 100 μM; OSCA, 100 μM; MSA, 5 μM (A549) or 2.5 μM (BEAS-2B); ChSCA, 100 μM (A549) or 50 μM (BEAS-2B); SECY, 100 μM (A549) or 25 μM (BEAS-2B). Bars represent the mean mBBr fluorescence concentrations normalized to the control with standard deviations. The control mean mBBr fluorescence concentrations were 0.267 and 0.203 with coefficient of variation of 13% and 12% for A549 and BEAS-2B, respectively. Samples with significant differences from the control are marked with asterisks: *, P<0.05; **, P<0.01.
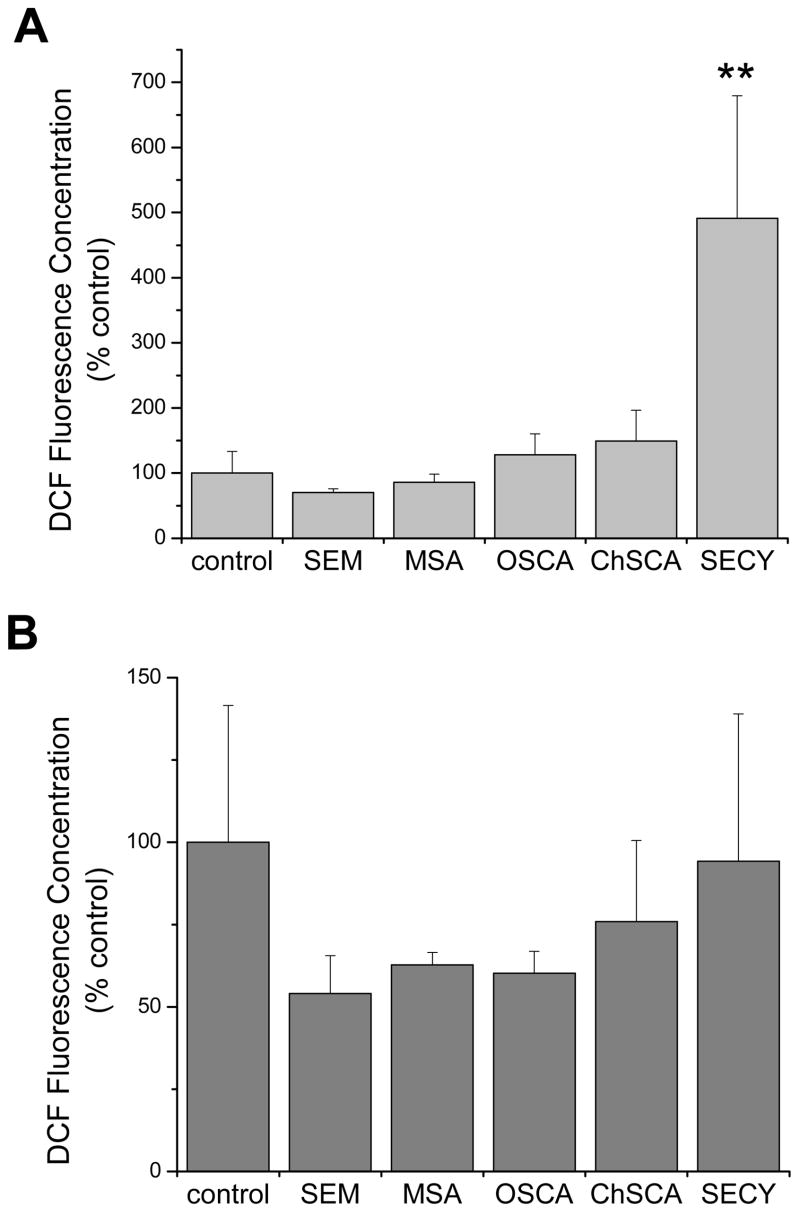
Cellular reactive oxygen species (ROS) levels were measured by the oxidation of DCFH to the fluorophore DCF (fig. 3). Again, the fluorescence concentration of cells treated with the selenocompounds was normalized to electronic cell volume. Only high concentrations (100 μM) of selenocystine resulted in a significant alteration in cellular ROS within this 24 hr period and only in A549 cells.
Figure 3. Generation of ROS in human lung cell lines following treatment with selenocompounds.
Analysis of (A) A549 cells and (B) BEAS-2B cells using the oxidation sensitive fluorophore DCFH as a measure of cellular ROS. Cells were incubated with DCFH-DA for 30 min. and then incubated with PI prior to cytometric analysis so that only PI-negative cells were assessed for DCF fluorescence. Cells were exposed to the selenocompounds for 24 hrs at the same concentrations as indicated in figure 2. Bars represent the mean DCF fluorescence concentrations normalized to the control with standard deviations. The control mean DCF fluorescence concentrations were 0.009 and 1.398 for A549 and BEAS-2B, respectively. Only SECY in the A549 cells demonstrated a significant difference (**, P<0.01) compared to the control.
The observed alterations in cellular redox status do not appear to result from a direct effect of the compounds since cellular redox status changes were not seen when cells were evaluated after 4 hrs of exposure (data not shown). The changes only develop over time and appear to reach steady state levels by 16 hrs since similar results were found at both 16 and 24 hrs (the 24 hr data is shown in fig. 2 and 3).
3.3 Effects of selenocompounds on subcellular organelles
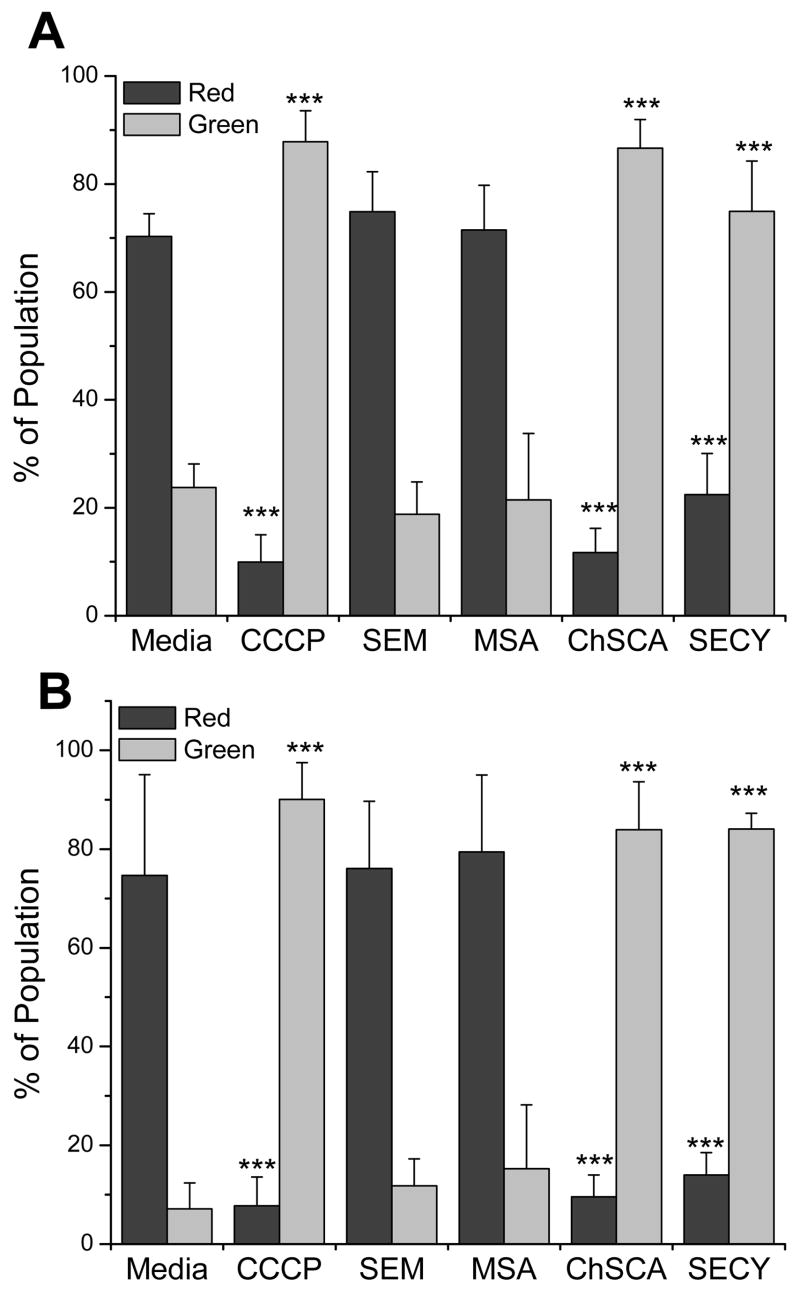
To better understand the mechanisms of toxicity seen in figure 1, selenocompound effects on the mitochondria and endoplasmic reticulum (ER) were evaluated. Of the selenazolidines, only ChSCA was used in these experiments since OSCA treated cells displayed minimal toxicity (fig. 1) and did not show significant differences in thiol status (fig. 2) or ROS (fig. 3). The mitochondrial potential was measured cytometrically using JC-1 (fig. 4). Selenomethionine and methylseleninic acid treatments did not alter the mitochondrial potential in either cell line. In a close parallel to effects on thiol status, selenocystine and ChSCA treatments both depolarized the mitochondrial membrane to an extent similar to the mitochondrial potential disrupter CCCP.
Figure 4. Depolarization of mitochondrial membrane potential by selenocompounds.
Analysis of (A) A549 cells and (B) BEAS-2B cells using the mitochondrial fluorophore JC-1 to cytometrically measure the mitochondrial membrane potential. CCCP (25 μM), a recognized mitochondrial membrane potential disrupter, was utilized as a positive control. Cells were incubated with selenocompounds for 24 hrs and the concentrations of the compounds used were: SEM, 100 μM; MSA, 5 μM; ChSCA, 200 μM (A549) or 100 μM (BEAS-2B); SECY, 100 μM (A549) or 50 μM (BEAS-2B). Bars represent the percentage of the cellular population with polarized mitochondria as indicated by red fluorescence (525 nm) or depolarized mitochondria as indicated by green fluorescence (575 nm) with standard deviations. Treatment with CCCP, ChSCA, and SECY resulted in a significant difference from control, (***, P<0.001).
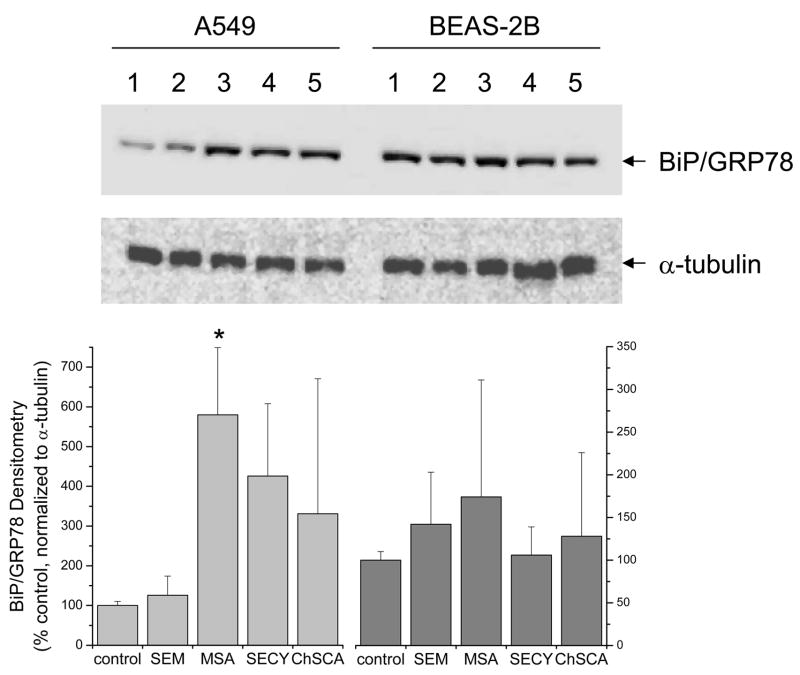
ER stress has been identified as a consequence of exposure to certain selenocompounds (Wu et al., 2005; Zu et al., 2006). Utilizing BiP/GRP78 protein expression as a marker of ER stress, only methylseleninic acid in A549 cells consistently induced BiP/GRP78 protein expression (fig. 5), while selenocystine and ChSCA displayed variable results that did not reach significance. In BEAS-2B cells, cells that appear to have a higher basal expression of BiP/GRP78 than A549 cells, these selenocompounds produced only minor changes in BiP/GRP78 expression that were not consistent among multiple experiments. Selenomethionine did not appear to induce ER stress in either cell line.
Figure 5. Induction of BiP/GRP78 expression by selenocompounds in human lung cells.
Cell lysates were homogenized and 10 μg of protein was loaded onto a NuPAGE 10% Bis-Tris gel and transferred to a PVDF membrane for immunochemical analysis as described in the Methods. Cells were incubated with the selenocompounds for 24 hrs at the same concentrations indicated in figure 4. Immunochemical analysis of α-tubulin was utilized as a loading control. Immunochemical analysis of BiP/GRP78 was evaluated as an indicator of the unfolded protein response. A representative blot of triplicate experiments is shown in the upper panel and densitometry for all experiments is shown in the lower panel with MSA demonstrating a significant difference compared to the control (*, P<0.05).
4. Discussion
Distinct selenocompounds have also demonstrated unexplained variability in cancer prevention model systems. One potential reason for differences observed among selenocompounds is their distinct metabolism determining the selenocompounds to which target tissues are exposed (Suzuki et al., 2006a; 2006b; 2006c; 2006d). While metabolism in the liver and kidney plays a major role in determining which selenocompounds are presented to other tissues, it is clear that selenoamino acids are distributed intact to other organs in vivo (Suzuki et al., 2006a; 2006b). In human lung cell lines various selenocompounds have demonstrated antiproliferative activities; for example, p-XSC and methylseleninic acid induced cell cycle arrest and apoptosis (El-Bayoumy et al., 2006; Swede et al., 2003), while selenomethionine enhanced the radiation-mediated cell killing (Shin et al., 2007). These studies are consistent with potential antitumor activities. Nevertheless, in the widely used murine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) lung tumor model, inorganic sodium selenite was ineffective (el-Bayoumy et al., 1993; Li et al., 2005) while the organic selenocompound, p-XSC, reduced the tumor burden in NNK-induced lung cancer in mice and rats (el-Bayoumy et al., 1993; el-Bayoumy et al., 1996; Prokopczyk et al., 1996). Certain selenium containing amino acids, including selenocystine and many selenazolidines (selenocysteine prodrugs) were effective against NNK-induced lung tumors (Franklin et al., 2007; Li et al., 2005). However, Se-methylselenocysteine, a methylated form of selenocysteine that is an effective producer of methylselenol (Suzuki et al., 2006b; Suzuki et al., 2008), lacked activity in this model (el-Bayoumy et al., 1993; Li et al., 2005).
Of the selenium-containing amino acids, the diselenide, selenocystine, was clearly the most toxic and demonstrated an oxidative activity in cells by decreasing free thiols in both A549 and BEAS-2B cells (fig. 2), as well as overtly increasing ROS levels in A549 cells (fig. 3). The selenocysteine prodrugs, OSCA and ChSCA, were considerably less toxic than selenocystine (fig. 1), and their redox modulation of the lung cell lines may reflect the differences in the expected metabolism of these compounds. ChSCA is expected to spontaneously hydrolyze to selenocysteine while OSCA is expected to require enzymatic degradation by 5-oxo-L-prolinase to release selenocysteine (Short et al., 2003). OSCA displayed less redox modulation than ChSCA, especially in A549 cells. Indeed, there are reports of oxoprolinase levels differing in the tissue of normal lung and lung cancer, with oxoprolinase expression decreasing in lung cancer compared to normal lung tissue (Chen et al., 1998). The cellular redox alterations observed with ChSCA may suggest that a more rapid release of selenocysteine from ChSCA may result in the generation of some selenocystine.
Selenomethionine, the currently preferred agent in human selenium cancer prevention studies, does not show efficacy in chemoprevention studies in animal models of lung cancer (Li et al., 2005; Prokopczyk et al., 1997), and has not proven effective as a cancer prevention agent in the follow-up studies of human lung cancer (Duffield-Lillico et al., 2002; Reid et al., 2002). In contrast to methylseleninic acid and the selenazolidines, selenomethionine did not modulate redox parameters measured in the experiments herein, except for a modest increase in cellular thiol levels in A549 cells (figs. 2 & 3), and did not affect the mitochondrion or ER subcellular compartments. A possible explanation for the lack of efficacy of selenomethionine may be due to incorporation in protein synthesis (Duffield-Lillico et al., 2002), i.e. replacing methionine, rather than metabolic conversion of the selenium component via the transselenation pathway to selenide, and then into newly synthesized selenoproteins. Other selenocompounds that have demonstrated preclinical efficacy in lung cancer models await clinical scrutiny.
Perhaps the most characteristic feature of methylseleninic acid treatment was the pronounced increase in cellular thiols which was observed by increased monobromobimane fluorescence in both A549 and BEAS-2B cells (fig. 2). methylseleninic acid is generated naturally by oxidative cleavage from selenosugars (Ogra et al., 2003; Suzuki et al., 2006e), and there has been considerable interest in this metabolite as a donor for methylselenol in cancer prevention. The mechanisms of methylseleninic acid-mediated cancer prevention have been rigorously pursued, and indicate that the methylselenol metabolite is crucial for activity (Ip et al., 2000). Mechanistic studies point to induction of the UPR in the ER as a likely cellular locus of action (Wu et al., 2005; Zu et al., 2006). Our data with methylseleninic acid are consistent with these previous studies and this stress on the ER was validated by the increase in BiP/GRP78 protein expression, particularly in A549 cells (fig. 5).
Selenium compounds can display differential effects in cancer and non-cancer cells; however the mechanisms that delineate these differences are not clear. Previous studies have demonstrated selenium-mediated effects in premalignant and neoplastic rat and canine mammary cells but minimal effects in normal cells (Fico et al., 1986; Ip et al., 2000). In addition, another study has demonstrated differences between normal and transformed human cell lines treated with selenite (Abdullaev et al., 1992). In the experiments described herein, A549 cells, a commonly used lung adenocarcinoma-derived cell line, and BEAS-2B cells, normal lung epithelial cells virally transformed for immortal growth in culture that generally display a non-tumorigenic phenotype (Iizasa et al., 1993; Reddel et al., 1993) were utilized to examine selenocompound selective sensitivity. We observed differences in the viability and redox modulation by distinct selenocompounds between these cells. The differential toxicity between the A549 cells and the BEAS-2B cells suggests that the non-tumorigenic line is more susceptible to the selenocompounds. This may suggest that these compounds may not be effective as anti-cancer agents alone, but may have a more pronounced effect earlier in the transformation process. Also, these differences mirror the basal redox states that reflect the mutational status of KEAP-1, a negative regulator of the antioxidant gene activator NRF2, in A549 cells (Singh et al., 2006).
Based on the differences in redox modulation by distinct selenocompounds, we suggest that these differences may provide a basis for distinct mechanisms of action in tumor models. Of the redox modulating selenocompounds, we speculate that selenocystine and the selenazolidines may activate an anti-oxidant response. The selenazolidines show particular promise due to their minimal toxicity and cancer prevention activity.
Acknowledgments
We gratefully acknowledge Dr. Jeanette Roberts and colleagues for the synthesis of the selenazolidines. This project was supported by a USPHS Grants CA115616 (PJM) and GM058913 (MRF).
Footnotes
Abbreviations: CCCP, carbonyl cyanide 3-chlorophenylhydrazone; CCK-8, Cell Counting Kit-8; ChSCA, 2-cyclohexylselenazolidine-4-(R)-carboxylic acid; DMSO, dimethylsulfoxide; LHC medium, Lechner and LaVeck medium; MSA, methylseleninic acid; mBBr, monobromobimane; NPC, Nutritional Prevention of Cancer; OSCA, 2-oxoselenazolidine-4(R)-carboxylic acid; p-XSC, 1,4-phenylenebis (methylene)selenocyanate; PBS, phosphate buffered saline; ROS, reactive oxygen species; SELECT, Selenium and Vitamin E Cancer Prevention Trial; SeCys, selenocysteine; SECY, selenocystine; SEM, selenomethionine; 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide, JC-1; UPR, unfolded protein response.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdullaev FI, MacVicar C, Frenkel GD. Inhibition by selenium of DNA and RNA synthesis in normal and malignant human cells in vitro. Cancer Lett. 1992;65:43–49. doi: 10.1016/0304-3835(92)90211-d. [DOI] [PubMed] [Google Scholar]
- Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annu Rev. 2005;11:127–152. doi: 10.1016/S1387-2656(05)11004-7. [DOI] [PubMed] [Google Scholar]
- Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ, Yang CS, Zheng SF, Gail M, Li GY, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85:1483–1492. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- Chen X, Schecter RL, Griffith OW, Hayward MA, Alpert LC, Batist G. Characterization of 5-oxo-L-prolinase in normal and tumor tissues of humans and rats: a potential new target for biochemical modulation of glutathione. Clin Cancer Res. 1998;4:131–138. [PubMed] [Google Scholar]
- Clark LC, Cantor KP, Allaway WH. Selenium in forage crops and cancer mortality in U.S. counties. Arch Environ Health. 1991;46:37–42. doi: 10.1080/00039896.1991.9937427. [DOI] [PubMed] [Google Scholar]
- Clark LC, Combs GF, Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, Krongrad A, Lesher JL, Jr, Park HK, Sanders BB, Jr, Smith CL, Taylor JR. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. Jama. 1996;276:1957–1963. [PubMed] [Google Scholar]
- Duffield-Lillico AJ, Reid ME, Turnbull BW, Combs GF, Jr, Slate EH, Fischbach LA, Marshall JR, Clark LC. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002;11:630–639. [PubMed] [Google Scholar]
- El-Bayoumy K, Das A, Narayanan B, Narayanan N, Fiala ES, Desai D, Rao CV, Amin S, Sinha R. Molecular targets of the chemopreventive agent 1,4-phenylenebis(methylene)selenocyanate in human non small cell lung cancer. Carcinogenesis. 2006 doi: 10.1093/carcin/bgi328. [DOI] [PubMed] [Google Scholar]
- el-Bayoumy K, Upadhyaya P, Desai DH, Amin S, Hecht SS. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone tumorigenicity in mouse lung by the synthetic organoselenium compound, 1,4-phenylenebis(methylene)selenocyanate. Carcinogenesis. 1993;14:1111–1113. doi: 10.1093/carcin/14.6.1111. [DOI] [PubMed] [Google Scholar]
- el-Bayoumy K, Upadhyaya P, Desai DH, Amin S, Hoffmann D, Wynder EL. Effects of 1,4-phenylenebis(methylene)selenocyanate, phenethyl isothiocyanate, indole-3-carbinol, and d-limonene individually and in combination on the tumorigenicity of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in A/J mouse lung. Anticancer Res. 1996;16:2709–2712. [PubMed] [Google Scholar]
- Fico ME, Poirier KA, Watrach AM, Watrach MA, Milner JA. Differential effects of selenium on normal and neoplastic canine mammary cells. Cancer Res. 1986;46:3384–3388. [PubMed] [Google Scholar]
- Franklin MR, Moos PJ, El-Sayed WM, Aboul-Fadl T, Roberts JC. Pre- and post-initiation chemoprevention activity of 2-alkyl/aryl selenazolidine-4(R)-carboxylic acids against tobacco-derived nitrosamine (NNK)-induced lung tumors in the A/J mouse. Chem Biol Interact. 2007;168:211–220. doi: 10.1016/j.cbi.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge K, Yang G. The epidemiology of selenium deficiency in the etiological study of endemic diseases in China. Am J Clin Nutr. 1993;57:259S–263S. doi: 10.1093/ajcn/57.2.259S. [DOI] [PubMed] [Google Scholar]
- Hedley DW, Chow S. Evaluation of methods for measuring cellular glutathione content using flow cytometry. Cytometry. 1994;15:349–358. doi: 10.1002/cyto.990150411. [DOI] [PubMed] [Google Scholar]
- Iizasa T, Momiki S, Bauer B, Caamano J, Metcalf R, Lechner J, Harris CC, Klein-Szanto AJ. Invasive tumors derived from xenotransplanted, immortalized human cells after in vivo exposure to chemical carcinogens. Carcinogenesis. 1993;14:1789–1794. doi: 10.1093/carcin/14.9.1789. [DOI] [PubMed] [Google Scholar]
- Ip C, Thompson HJ, Ganther HE. Selenium modulation of cell proliferation and cell cycle biomarkers in normal and premalignant cells of the rat mammary gland. Cancer Epidemiol Biomarkers Prev. 2000;9:49–54. [PubMed] [Google Scholar]
- Ip C, Thompson HJ, Zhu Z, Ganther HE. In vitro and in vivo studies of methylseleninic acid: evidence that a monomethylated selenium metabolite is critical for cancer chemoprevention. Cancer Res. 2000;60:2882–2886. [PubMed] [Google Scholar]
- Keij JF, Bell-Prince C, Steinkamp JA. Simultaneous analysis of relative DNA and glutathione content in viable cells by phase-resolved flow cytometry. Cytometry. 1999;35:48–54. doi: 10.1002/(sici)1097-0320(19990101)35:1<48::aid-cyto7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2′, 7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- Li L, Xie Y, El-Sayed WM, Szakacs JG, Franklin MR, Roberts JC. Chemopreventive activity of selenocysteine prodrugs against tobacco-derived nitrosamine (NNK) induced lung tumors in the A/J mouse. J Biochem Mol Toxicol. 2005;19:396–405. doi: 10.1002/jbt.20105. [DOI] [PubMed] [Google Scholar]
- Li L, Xie Y, El-Sayed WM, Szakacs JG, Roberts JC. Characteristics of selenazolidine prodrugs of selenocysteine: toxicity, selenium levels, and glutathione peroxidase induction in A/J mice. Life Sci. 2004;75:447–459. doi: 10.1016/j.lfs.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Mark SD, Qiao YL, Dawsey SM, Wu YP, Katki H, Gunter EW, Fraumeni JF, Jr, Blot WJ, Dong ZW, Taylor PR. Prospective study of serum selenium levels and incident esophageal and gastric cancers. J Natl Cancer Inst. 2000;92:1753–1763. doi: 10.1093/jnci/92.21.1753. [DOI] [PubMed] [Google Scholar]
- Novoselov SV, Calvisi DF, Labunskyy VM, Factor VM, Carlson BA, Fomenko DE, Moustafa ME, Hatfield DL, Gladyshev VN. Selenoprotein deficiency and high levels of selenium compounds can effectively inhibit hepatocarcinogenesis in transgenic mice. Oncogene. 2005;24:8003–8011. doi: 10.1038/sj.onc.1208940. [DOI] [PubMed] [Google Scholar]
- Ogra Y, Hatano T, Ohmichi M, Suzuki KT. Oxidative production of monomethylated selenium from the major urinary selenometabolite, selenosugar. J Anal At Spectrom. 2003;18:1252–1255. [Google Scholar]
- Prokopczyk B, Amin S, Desai DH, Kurtzke C, Upadhyaya P, El-Bayoumy K. Effects of 1,4-phenylenebis(methylene)selenocyanate and selenomethionine on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced tumorigenesis in A/J mouse lung. Carcinogenesis. 1997;18:1855–1857. doi: 10.1093/carcin/18.9.1855. [DOI] [PubMed] [Google Scholar]
- Prokopczyk B, Cox JE, Upadhyaya P, Amin S, Desai D, Hoffmann D, el-Bayoumy K. Effects of dietary 1,4-phenylenebis(methylene)selenocyanate on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced DNA adduct formation in lung and liver of A/J mice and F344 rats. Carcinogenesis. 1996;17:749–753. doi: 10.1093/carcin/17.4.749. [DOI] [PubMed] [Google Scholar]
- Reddel RR, Salghetti SE, Willey JC, Ohnuki Y, Ke Y, Gerwin BI, Lechner JF, Harris CC. Development of tumorigenicity in simian virus 40-immortalized human bronchial epithelial cell lines. Cancer Res. 1993;53:985–991. [PubMed] [Google Scholar]
- Reid ME, Duffield-Lillico AJ, Garland L, Turnbull BW, Clark LC, Marshall JR. Selenium supplementation and lung cancer incidence: an update of the nutritional prevention of cancer trial. Cancer Epidemiol Biomarkers Prev. 2002;11:1285–1291. [PubMed] [Google Scholar]
- Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997;411:77–82. doi: 10.1016/s0014-5793(97)00669-8. [DOI] [PubMed] [Google Scholar]
- Shamberger RJ, Willis CE. Selenium distribution and human cancer mortality. CRC Crit Rev Clin Lab Sci. 1971;2:211–221. doi: 10.3109/10408367109151308. [DOI] [PubMed] [Google Scholar]
- Shin SH, Yoon MJ, Kim M, Kim JI, Lee SJ, Lee YS, Bae S. Enhanced lung cancer cell killing by the combination of selenium and ionizing radiation. Oncol Rep. 2007;17:209–216. [PubMed] [Google Scholar]
- Short MD, Xie Y, Li L, Cassidy PB, Roberts JC. Characteristics of selenazolidine prodrugs of selenocysteine: toxicity and glutathione peroxidase induction in V79 cells. J Med Chem. 2003;46:3308–3313. doi: 10.1021/jm020496q. [DOI] [PubMed] [Google Scholar]
- Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E, Brock MV, Biswal S. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki KT, Somekawa L, Kurasaki K, Suzuki N. Simultaneous tracing of 76Se-selenite and 77Se-selenomethionine by absolute labeling and speciation. Toxicol Appl Pharmacol. 2006a;217:43–50. doi: 10.1016/j.taap.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Suzuki KT, Doi C, Suzuki N. Metabolism of 76Se-methylselenocysteine compared with that of 77Se-selenomethionine and 82Se-selenite. Toxicol Appl Pharmacol. 2006b;217:185–195. doi: 10.1016/j.taap.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Suzuki KT, Kurasaki K, Ogawa S, Suzuki N. Metabolic transformation of methylseleninic acid through key selenium intermediate selenide. Toxicol Appl Pharmacol. 2006c;215:189–197. doi: 10.1016/j.taap.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Suzuki KT, Ohta Y, Suzuki N. Availability and metabolism of 77Se-methylseleninic acid compared simultaneously with those of three related selenocompounds. Toxicol Appl Pharmacol. 2006d;217:51–62. doi: 10.1016/j.taap.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Suzuki KT, Somekawa L, Suzuki N. Distribution and reuse of 76Se-selenosugar in selenium-deficient rats. Toxicol Appl Pharmacol. 2006e;216:303–308. doi: 10.1016/j.taap.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Suzuki KT, Tsuji Y, Ohta Y, Suzuki N. Preferential organ distribution of methylselenol source Se-methylselenocysteine relative to methylseleninic acid. Toxicol Appl Pharmacol. 2008;227:76–83. doi: 10.1016/j.taap.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Swede H, Dong Y, Reid M, Marshall J, Ip C. Cell cycle arrest biomarkers in human lung cancer cells after treatment with selenium in culture. Cancer Epidemiol Biomarkers Prev. 2003;12:1248–1252. [PubMed] [Google Scholar]
- Taylor PR, Li B, Dawsey SM, Li JY, Yang CS, Guo W, Blot WJ. Prevention of esophageal cancer: the nutrition intervention trials in Linxian, China. Linxian Nutrition Intervention Trials Study Group. Cancer Res. 1994;54:2029s–2031s. [PubMed] [Google Scholar]
- Vinceti M, Wei ET, Malagoli C, Bergomi M, Vivoli G. Adverse health effects of selenium in humans. Rev Environ Health. 2001;16:233–251. doi: 10.1515/reveh.2001.16.4.233. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhang H, Dong Y, Park YM, Ip C. Endoplasmic reticulum stress signal mediators are targets of selenium action. Cancer Res. 2005;65:9073–9079. doi: 10.1158/0008-5472.CAN-05-2016. [DOI] [PubMed] [Google Scholar]
- Xie Y, Short MD, Cassidy PB, Roberts JC. Selenazolidines as novel organoselenium delivery agents. Bioorg Med Chem Lett. 2001;11:2911–2915. doi: 10.1016/s0960-894x(01)00590-x. [DOI] [PubMed] [Google Scholar]
- Zu K, Bihani T, Lin A, Park YM, Mori K, Ip C. Enhanced selenium effect on growth arrest by BiP/GRP78 knockdown in p53-null human prostate cancer cells. Oncogene. 2006;25:546–554. doi: 10.1038/sj.onc.1209071. [DOI] [PMC free article] [PubMed] [Google Scholar]