Abstract
Pulmonary hypertension is a devastating disease, which leads to right heart failure. Serotonin (5-HT) plays important roles in the pathogenesis of pulmonary hypertension and pulmonary vascular remodeling. The role of 5-HT in right heart failure, however, is unknown. Since oxidative stress may mediate heart failure, the present study examined the effects of 5-HT on protein oxidation in the adult rat right heart ventricle. Treatment of perfused isolated hearts with 5-HT resulted in the promotion of protein carbonylation, specifically in the right ventricle, but not in the left. While no differences between right and left ventricular antioxidant enzymes and 5-HT receptors/transporter were detected, monoamine oxidase A (MAO-A) expression and activity were found to be lower in the right ventricle compared to the left. These results indicate that differences in neither the reactive oxygen species (ROS) scavenging ability, 5-HT membrane signaling capacity, nor MAO-dependent production of hydrogen peroxide are responsible for varied 5-HT-mediated protein carbonylation in right and left ventricles. Rather, lower MAO-A in the right heart might preserve cytosolic 5-HT which triggers other mechanisms for ROS production. Consistently, inhibition of MAO-A resulted in the promotion of protein carbonylation. We propose that low MAO-A, thus reduced degradation of 5-HT, increases the intracellular 5-HT activity in the right ventricle, leading to the promotion of protein carbonylation.
Keywords: 5-hydroxytryptamine, monoamine oxidase, protein carbonylation, pulmonary hypertension, reactive oxygen species, right heart, signal transduction
Introduction
Pulmonary hypertension is a disorder of elevated blood pressure in the lung circulation. The condition can occur without a known cause or in association with other heart and lung diseases such as chronic obstructive pulmonary disease, interstitial lung disease, left ventricular failure, congenital heart defects and thromboembolic obstruction [1,2]. Other triggers of pulmonary hypertension include collagen vascular disease, liver disease, HIV infection, inflammatory disorders, and drugs and toxins including appetite suppressants, cocaine and methamphetamine [3]. It is a devastating disease with mean survival after diagnosis remains to be only 2 – 3 years [4]. Pathologic features of pulmonary hypertension include excess vasoconstriction as well as pulmonary vascular remodeling, leading to progressively increased pulmonary vascular impedance [5]. The increased pulmonary vascular resistance results in right ventricular hypertrophy and ultimately right ventricular failure and death. While right ventricular failure is the major cause of death among pulmonary hypertension patients, biology of the right heart is not well understood [6].
Serotonin (5-hydroxytryptamine; 5-HT) has been recognized as a major contributor for the development of pulmonary hypertension through its ability to induce pulmonary vascular vasoconstriction and to promote growth of pulmonary vascular smooth muscle cells. The role of 5-HT in the development of pulmonary hypertension was recognized in fawn-hooded rats with a genetic deficit in 5-HT platelet storage, high plasma levels of 5-HT, and increased susceptibility to developing pulmonary hypertension [7]. Similarly, in patients with pulmonary arterial hypertension, high levels of plasma 5-HT were detected [8]. Further studies demonstrated that: an intravenous infusion of 5-HT during exposure of rats to hypoxia increased the development of pulmonary hypertension [9]; 5-HT transporter-deficient mice developed less hypoxic pulmonary hypertension [10]; and 5-HT transporter overexpressing mice had enhanced hypoxia-induced pulmonary vascular remodeling, right ventricular pressure, and right ventricular hypertrophy [11]. In pulmonary artery smooth muscle cells, 5-HT has been shown to promote the generation of reactive oxygen species (ROS) [12,13], which regulate signal transduction [14-17]. While the roles of 5-HT in alterations of pulmonary vasculature and thus the development of pulmonary hypertension have been documented, 5-HT actions on the right heart are unknown. Recently, 5-HT was found to promote the generation of ROS in isolated ventricular myocyte preparations obtained from both right and left ventricles [18,19].
ROS can oxidize various biological molecules including proteins, DNA and lipids as well as small molecules. During various oxidative stress conditions, protein oxidation often affects protein functions. Carbonylation is one of oxidation processes, which occur on proteins often in response to metal-catalyzed formation of hydroxyl radicals [20]. 2,4-Dinitrophenylhydrazine (DNPH) can react with carbonyl groups of ketone and aldehyde and form a hydrazone derivative. Protein carbonyl measurements with DNPH have been well used to assess protein oxidation in various oxidative stress and disease conditions.
Because of the importance of 5-HT in pulmonary hypertension that leads to right-sided ventricular hypertrophy and failure, the present study examined the effects of 5-HT on protein carbonylation, specifically in the right ventricle. We found that the administration of 5-HT to isolated hearts resulted in right ventricle-specific actions to promote protein carbonylation.
Materials and Methods
Animals
Male Sprague-Dawley rats (250 – 300 g) were used in this study in accordance with institutional guidelines. Hearts were rapidly removed from rats anesthetized with isoflurane inhalation. For the perfusion, the aorta was cannulated and coronary arteries were perfused with modified Krebs-Henseleit (KH) buffer, containing (in mM): 118.0 NaCl, 4.7 KCl, 1.7 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25.0 NaHCO3 and 10.0 glucose, continuously bubbled with 95% O2 and 5% CO2 (pH 7.4, 37°C) [21]. In these preparations, vena cavas were also tied to perfuse right ventricle and large pulmonary artery (with right ventricular pressure being < 10 mmHg). After equilibration, 5-HT (Sigma Chemical, St. Louis, MO) or clorgyline (Sigma) was perfused.
Western blot analysis
To prepare total tissue homogenates, right and left ventricular walls were dissected and solubilized with 50 mM Hepes solution (pH 7.4) containing 1% (v/v) Triton X-100, 4 mM EDTA, 1 mM sodium fluoride, 0.1 mM sodium orthovanadate, 1 mM tetrasodium pyrophosphate, 2 mM PMSF, 10 μg/mL leupeptin, and 10 μg/mL aprotinin. Equal protein amounts of homogenates were electrophoresed through a reducing SDS polyacrylamide gel and electroblotted onto a nitrocellulose membrane [22,23]. The membrane was blocked and incubated with IgG’s for 5-HT transporter (SERT), 5-HT receptor 1B (SR-1B), 5-HT receptor 4 (SR-4), monoamine oxidase-A (MAO-A), MAO-B, glycealdehyde 3-phosphate dehydrogenase (GADPH) (Santa Cruz Biotechnology, Santa Cruz, CA), catalase (Calbiochem, San Diego, CA) and catechol-O-methyltransferase (BD Transduction Laboratories, San Jose, CA), and the detection was made with HRP-linked secondary antibodies and ECL System (Amersham Life Science, Arlington Heights, IL). Levels of protein carbonyl groups were measured using OxyBlot Protein Oxidation Detection Kit (Millipore, Billerica, MA).
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA (1 μg) extracted from the left or right ventricle using TRIZOL was reverse-transcribed by oligo(dT) priming and MMLV reverse transcriptase (Applied Biosystems, Foster City, CA). The resultant cDNA was amplified using Taq DNA polymerase (Invitrogen, Carlsbad, CA) and visualized on a 1.5% agarose gel using ethidium bromide. PCR primers for rat MAO-A were 5’ primer, 5′-GTG GCT CTT CTC TGC TTT GT-3′; 3′ primer, 5′-AGT GCC AAG GGT AGT GTG TAT CA-3′ which produce a 500 bp product [24]. Denaturing was performed at 95°C for 1 min, annealing was for 1 min at 56°C and polymerase reaction was for 2 min at 72°C (30 cycles). 28S rRNA was measured as an internal control.
Measurement of MAO activity
Enzymatic activities of MAO-A and –B were measured using the method previously described [25]. The assay mixture contained 1.3 mg/ml protein of ventricular homogenates, 1.5 units horseradish peroxidase (Type X; Sigma), 0.1 M borate buffer (pH 8.7), 60 μM homovanilic acid, 1 mM EDTA, 0.2 mg/ml sodium azide, and 1 mM substrates including tyramine (Fisher Scientific, Pittsburgh, PA; for total MAO activity), octopamine (Sigma; for MAO-A activity) and benzylamine (Sigma; for MAO-B activity). Enzymatic reactions at 37°C were initiated by adding one of the substrates, and were stopped by adding 15 volumes of 0.1 M NaOH after 0, 20, 40 and 60 min. Fluorescence intensity due to hydrogen peroxide (H2O2) production was recorded using a Quantech Fluorimeter (Barnstead International, Dubuque, IA) with anexcitation wavelength at 315 nm and emission at 425 nm, and the amount of H2O2 was estimated using a standard curve.
Measurement of catalase activity
H2O2 scavenging activity in heart homogenates was measured spectrophotometrically by following the decrease in the H2O2 concentration over 60 sec (linear least-squares fittings) at 240 nm (ε240 = 43.6 M−1cm−1) [26].
Measurement of glutathione peroxidase activity
The heart homogenates were mixed with 0.1 M Tris-HCl, 0.5 mM EDTA buffer (pH 8.0), 2 mM GSH, 1 unit GSH reductase (Sigma) and 0.2 mM NADPH (Sigma), and incubated at 37°C. After 2 min incubation, t-butyl hydroperoxide was added to make final concentration to 70 μM, and oxidation of NADPH (ε340 = 6.22 mM−1cm−1) was continuously monitored spectrophotometrically at 340 nm for 2 min [27].
Statistical analysis
Means ± SEM were calculated. Statistically significant differences between two groups were determined by the Student’s t-test. Significant differences between all groups were computed by ANOVA with a Student-Newman-Keuls post hoc test. P < 0.05 was considered to be significant.
Results
5-HT promotes protein oxidation in the right ventricle
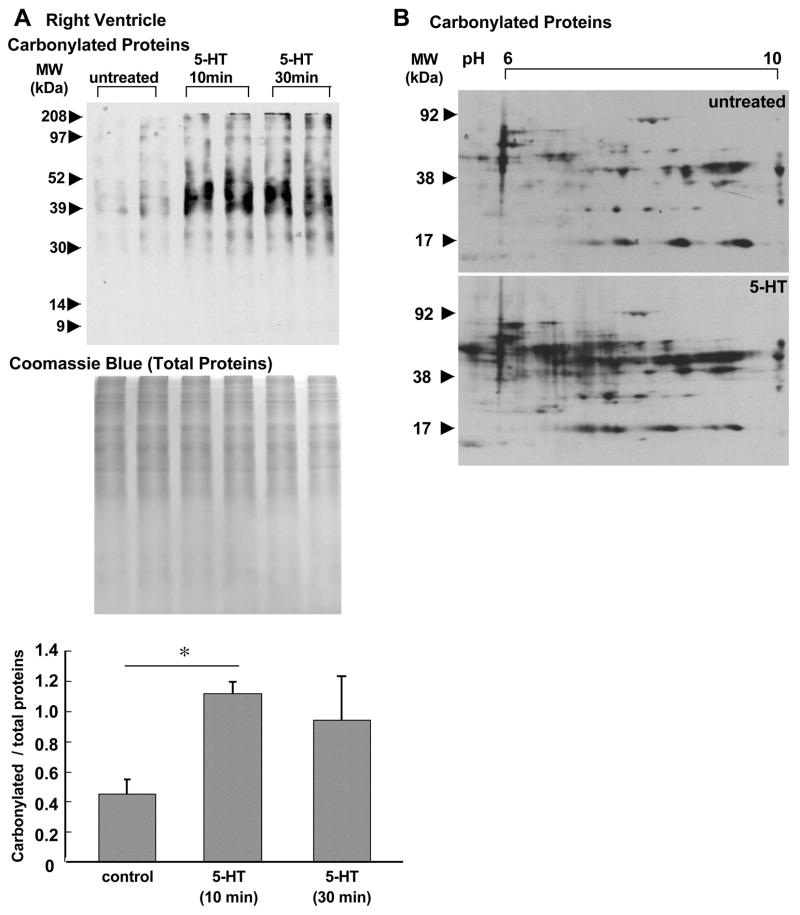
Since 5-HT is elevated in pulmonary hypertension patients [8] and produces ROS [12,13,18,19], this vasoactive amine may also mediate the development of right ventricular hypertrophy and/or failure by affecting the right heart. Thus, we tested the hypothesis that 5-HT may promote oxidative stress in the right ventricle. We have previously shown that protein carbonylation can be induced by 5-HT or endothelin-1 in pulmonary artery smooth muscle [28–30]. Thus, we monitored carbonylation as an indication of protein oxidation in response to 5-HT. Isolated rat hearts were subjected to retrograde perfusion through coronary artery, and 5-HT was administered. Ten or 30 min after the initiation of 5-HT perfusion, the right ventricular wall was dissected and homogenized, and protein samples were derivatized with DNPH to label carbonyl groups. The samples were subjected to SDS-PAGE and immunoblotting with the antibody which interacts with DNPH-derivatized proteins. Fig. 1A shows that, in the right ventricle, 5-HT caused marked increases in carbonylation of number of proteins. Two-fold increase in the level of total carbonylated proteins was observed after treatment with 5-HT. Coomassie Blue staining showed that there are no appreciable changes in the level of total protein expression. 5-HT-mediated carbonylation of various proteins was also visible in 2-dimensional gel electrophoresis, followed by immunoblotting (Fig. 1B). These results suggest that 5-HT promotes protein oxidation in the right ventricle.
Fig. 1. Effects of 5-HT on protein carbonylation in the right ventricle.
Isolated rat hearts were subjected to retrograde perfusion on a Langendorff apparatus. After 30 min equilibration with KH buffer, the solution containing 1 μM 5-HT in KH buffer was perfused for 10 or 30 min. (A) The right ventricular wall was dissected and homogenized. Samples were derivatized with DNPH to label carbonyl moieties and subjected to SDS-PAGE and immunoblotting with the antibody which interacts with DNPH-derivatized proteins. Total protein levels were visualized by Coomassie Blue staining. Bar graphs represent means ± SEM (n = 3). (*) denotes values significantly different from each other at P < 0.05. (B) DNPH-derivatized right ventricular homogenates from perfused hearts untreated or treated with 5-HT for 10 min were subjected to isoelectric focusing followed by SDS-PAGE to obtain 2-dimensional gel electrophoresis data. After electrophoresis, slab gels were electroblotted to nitrocellulose membranes. The membrane underwent immunoblotting with the antibody, which interacts with DNPH-derivatized proteins.
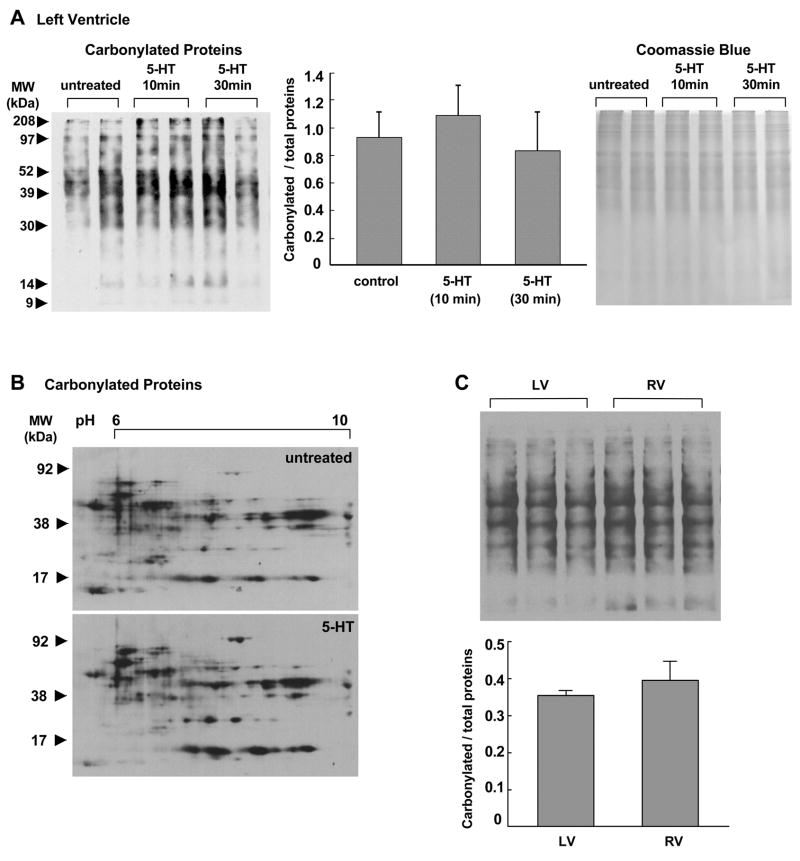
In contrast to the right ventricle, in the left ventricle, effects of 5-HT were minimal. As shown in Fig. 2A, no significant increase in total protein carbonyls was caused in response to 5-HT treatment. In contrast to dramatic changes in carbonylated protein spots as seen in the right ventricle (Fig. 1B), the effects of 5-HT on carbonylated proteins in the left ventricle were minimal as shown in two dimensional gel electrophoresis data (Fig. 2B). Examinations of right and left ventricular samples in the same gel revealed that there are no significant differences in basal levels of protein carbonylation (Fig. 1C). These results suggest that the right and left ventricles are different in their responses to 5-HT-mediated protein carbonylation.
Fig. 2. Effects of 5-HT on protein carbonylation in the left ventricle.
Isolated rat hearts were subjected to retrograde perfusion on a Langendorff apparatus. After 30 min equilibration with KH buffer, the solution containing 1 μM 5-HT in KH buffer was perfused for 10 or 30 min. (A) The left ventricular walls were dissected and homogenized. Samples were derivatized with DNPH to label carbonyl moieties and subjected to SDS-PAGE and immunoblotting with the antibody which interacts with DNPH-derivatized proteins. Total protein levels were visualized by Coomassie Blue staining. Bar graphs represent means ± SEM (n = 3). (B) DNPH-derivatized left ventricular homogenates from perfused hearts untreated or treated with 5-HT for 10 min were subjected to isoelectric focusing followed by SDS-PAGE to obtain 2-dimensional gel electrophoresis data. After electrophoresis, slab gels were electroblotted to nitrocellulose membranes. The membrane underwent immunoblotting with the antibody, which interacts with DNPH-derivatized proteins. (C) Right ventricle (RV) and left ventricle (LV) from untreated rat hearts were derivatized with DNPH, followed by SDS-PAGE and immunoblotting with the antibody that interacts with DNPH-derivatized proteins. Bar graphs represent means ± SEM (n = 3).
Antioxidant activities in right and left ventricles
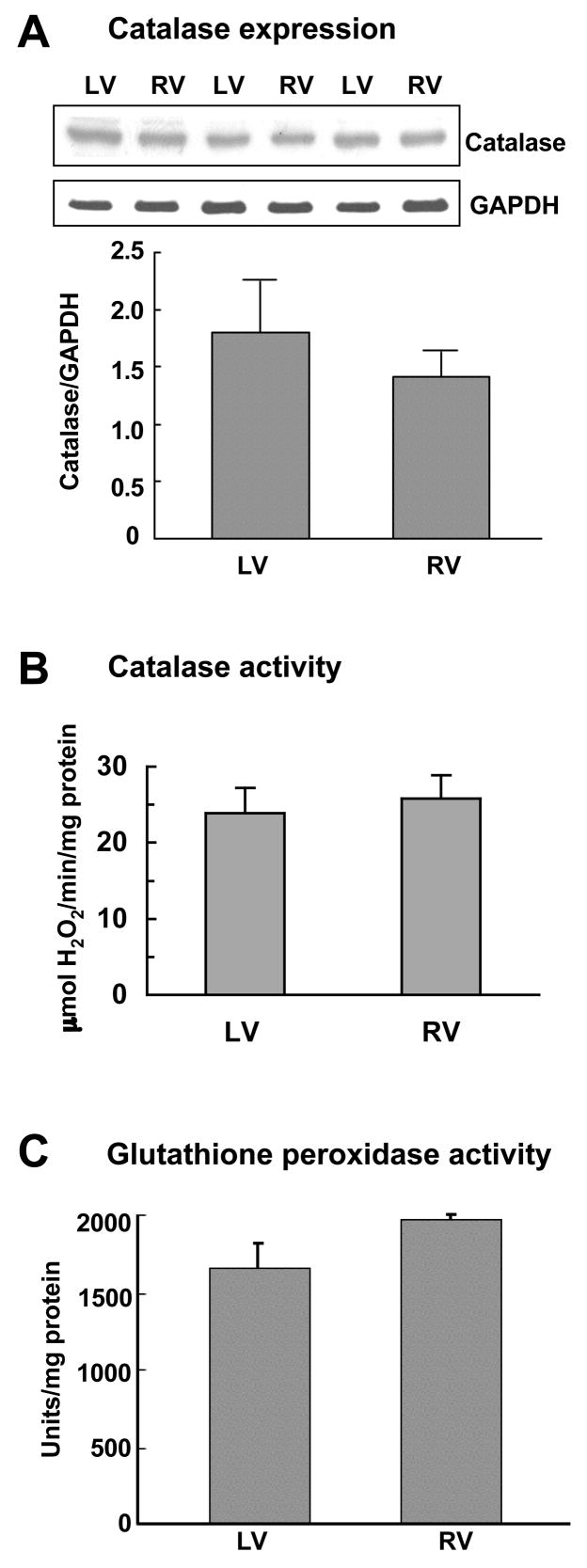
The enhanced activity of 5-HT-mediated protein carbonylation could possibly be due to lower cellular antioxidant activity in the right ventricle. Protein carbonylation is often formed via H2O2-mediated Fenton reaction and the generation of hydroxy radicals, thus the H2O2scavenging activities were specifically compared between right and left ventricles. We found no differences in catalase protein expression between the two ventricles (Fig. 3A). Similarly, catalase activity levels were found to be comparable between right and left ventricles (Fig. 3B). The total cellular glutathione-dependent peroxidase activity was also similar between the two ventricles (Fig. 3C). These results indicate that differences in H2O2 scavenging activity levels do not account for differential efficacies of 5-HT actions to promote protein carbonylaton in right and left ventricles.
Fig. 3. H2O2 scavenging activity in right and left ventricles.
Right (RV) and left (LV) ventricular walls were dissected and homogenized. (A) Protein expression levels of catalase (65 kDa) and loading control GAPDH (37 kDa) were monitored by immunoblotting. (B) Catalase activity was measured spectrophotometrically. (C) Glutathione-dependent peroxidase activity was monitored spectrophotometrically. Bar graphs represent means ± SEM (n = 3 – 6).
Differences in 5-HT regulatory molecules between right and left ventricles
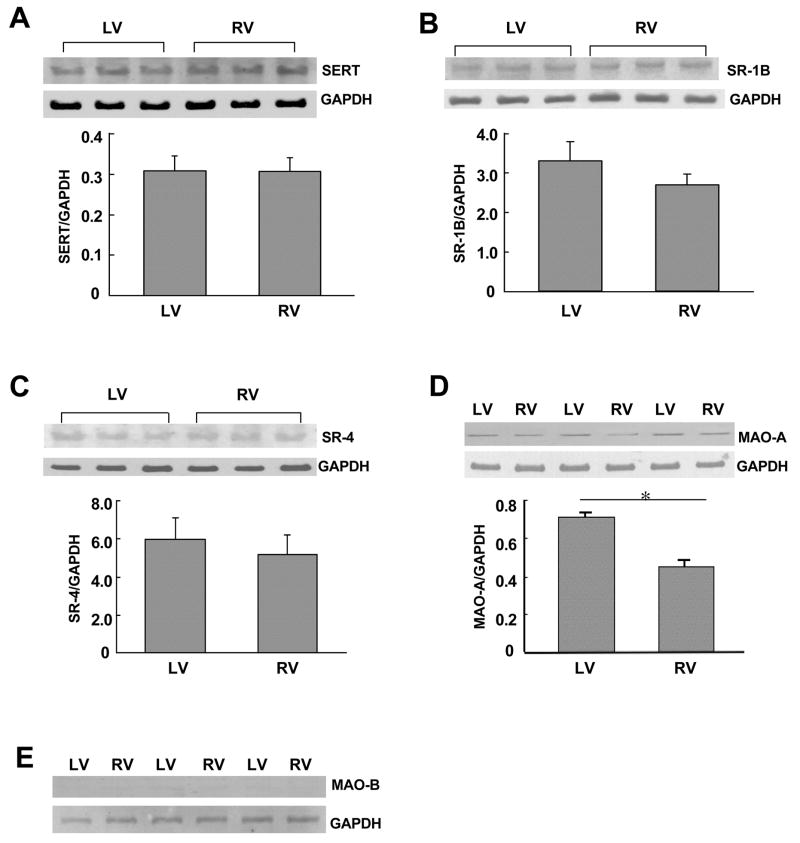
To understand the mechanism of the differences between right and left ventricular 5-HT-mediated protein carbonylation, levels of 5-HT signaling components were monitored. 5-HT signals either by interacting with membrane receptors or by being transported intracellularly through the 5-HT transporter. Thus, we first measured protein expression levels of 5-HT transporter and 5-HT receptors (types 1B and 4) and found no differences between right and left ventricles (Figs. 4A–4C). We then measured MAO-A protein expression and found that MAO-A is expressed significantly lower in the right ventricle compared to the left (Fig. 4D). This was rather surprising as we expected that the right ventricle, which exhibits greater 5-HT-mediated protein oxidation, might have higher levels of MAO-A that can produce ROS. MAO-B protein was not detectable in either right or left ventricle (Fig. 4E).
Fig. 4. Expression of 5-HT regulatory proteins in right and left ventricles.
Right (RV) and left (LV) ventricular walls were dissected and homogenized. The samples were subjected to immunoblotting with (A) 5-HT transporter (SERT; 70 kDa), (B) 5-HT1B receptor (SR-1B; 42 kDa), (C) 5-HT4 receptor (SR-4; 43 kDa), (D) MAO-A (61 kDa), and (E) MAO-B (60 kDa). Levels of GAPDH (37 kDa) were also monitored. The bar graph represents means ± SEM (n = 3 – 5). (*) denotes values significantly different from each other at P < 0.05.
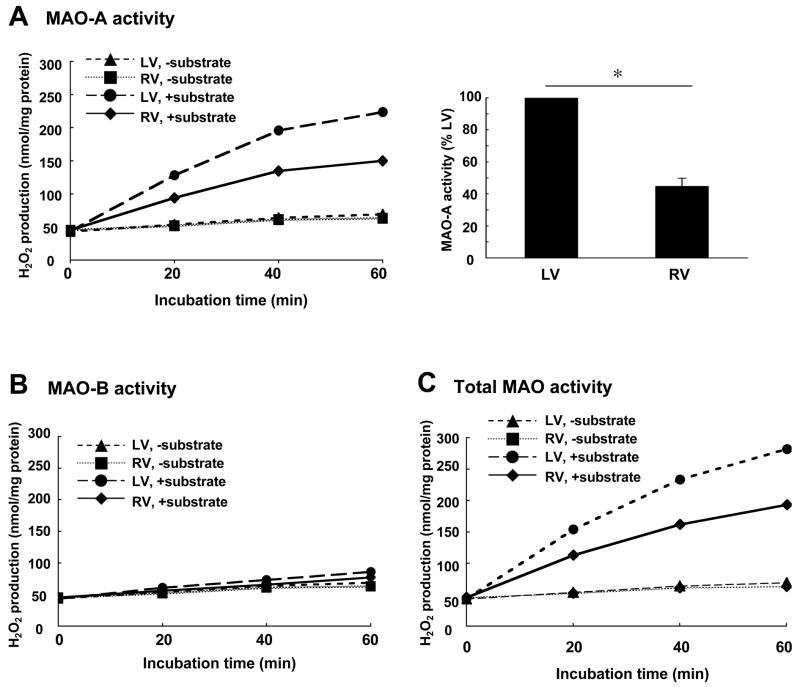
We further examined enzymatic activities of MAO in right and left ventricles and found consistent results with protein expression levels. Fig. 5A shows MAO-A activities in right and left ventricular homogenates using octopamine as a substrate. Right ventricular MAO-A activity was found to be significantly lower compared to the left ventricle. The use of benzylamine showed no detectable activity of MAO-B in either ventricle (Fig. 5B). The total MAO activity using tyramine provided results corresponding to those of MAO-A and MAO-B combined (Fig. 5C). These results demonstrate that protein expression and activity of MAO-A are lower in the right ventricle compared to the left. Consistent with these observations, basal intracellular 5-HT levels were found to be 2.5 fold higher in the right ventricle (15.0 ± 5.3 pmol/mg protein) compared to the left (5.8 ± 1.5 pmol/mg protein). Since the expression levels of another 5-HT catabolizing enzyme, catechol-O-methyltransferase (COMT) were not significantly different between right and left ventricles (data not shown), MAO-A is likely responsible for differential levels of 5-HT.
Fig. 5. MAO activity in right and left ventricles.
Right (RV) and left (LV) ventricular walls were dissected and homogenized. The activity of MAO was measured fluorometrically. (A) MAO-A activity with octopamine as a substrate. (B) MAO-B activity with benzylamine as a substrate. (C) Total MAO activity (MAO-A + MAO-B) with tyramine as a substrate. The bar graphs represent means ± SEM (n = 6). (*) denotes values significantly different from each other at P < 0.05.
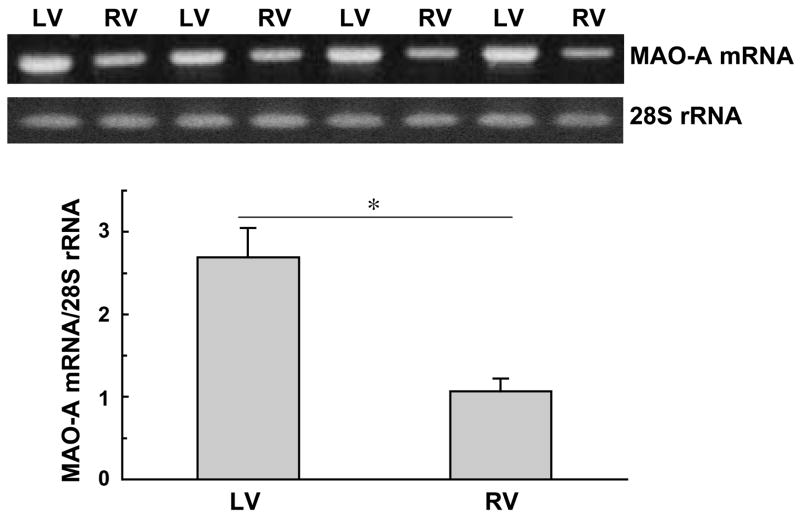
We next examined if lower protein expression of MAO-A in the right ventricle might be due to reduced mRNA levels. RNA was isolated from right and left ventricles, and RT-PCR was performed to monitor mRNA expression of MAO-A. We found that the MAO-A mRNA level was also lower in the right ventricle compared to the left (Fig. 6). These results suggest that MAO-A mRNA synthesis is reduced and/or MAO-A mRNA degradation rate is enhanced in the right ventricle compared to the left.
Fig. 6. MAO-A mRNA expression in right and left ventricles.
Right (RV) and left (LV) ventricular walls were dissected and homogenized in Trizol. Total RNA was isolated and reverse transcribed. PCR was subsequently performed to detect MAO-A mRNA levels as well as 28S rRNA. The bar graph represents means ± SEM of the ratio of MAO-A mRNA to 28S rRNA (n = 6). (*) denotes values significantly different from each other at P < 0.05.
Effects of MAO-A inhibitor
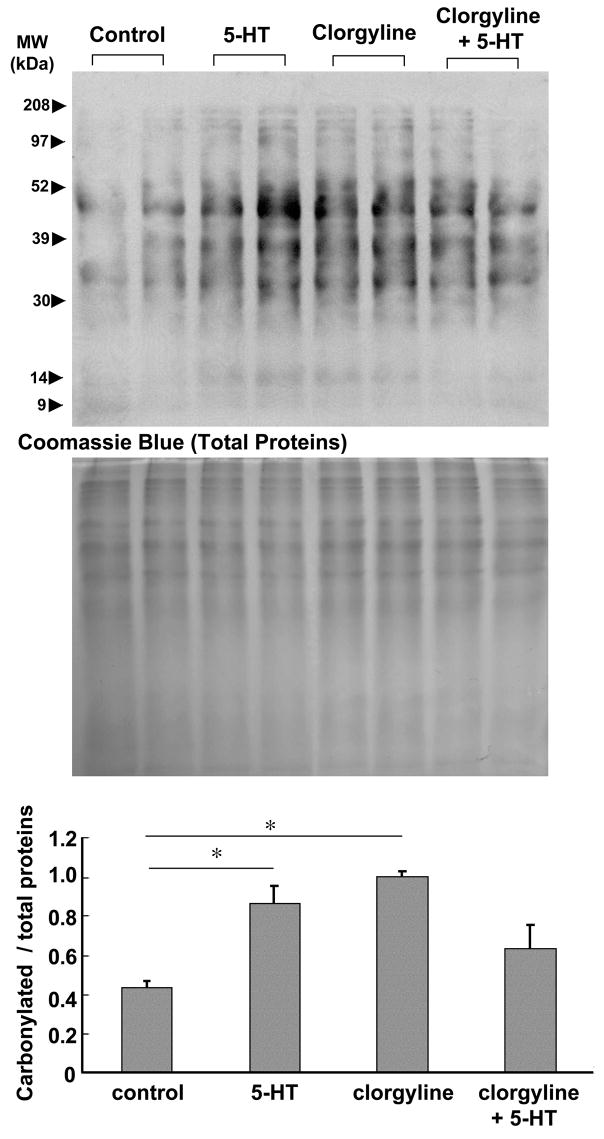
The functions of MAO-A include: (i) degradation of intracellular 5-HT and (ii) production of H2O2. Since the MAO-A level is lower in the right ventricle where 5-HT can elicit higher levels of protein oxidation, we hypothesized that an important function of MAO-A might be to regulate levels of intracellular 5-HT which might signal to activate other ROS generators. To determine whether 5-HT-mediated protein oxidation is MAO-A-dependent or the function of MAO-A is to regulate availability of intracellular 5-HT, we perfused clorgyline, an MAO-A inhibitor, through the isolated hearts and examined protein carbonylation in the right ventricle. Pre-treatment with clorgyline (10 μM) for 30 min did not attenuate the promotion of right ventricular protein carbonlylation by 5-HT (Fig. 7), suggesting that MAO-A may not be the mediator of 5-HT-induced protein oxidation. Moreover, the perfusion of the heart with clorgyline alone for 30 min promoted protein carbonylation. These results are consistent with the hypothesis that MAO-A inhibition preserves intracellular 5-HT which in turn promotes protein carbonylation by activating other ROS producing systems.
Fig. 7. Effects of MAO-A inhibition on protein carbonylation in the right ventricle.
Isolated rat hearts were subjected to retrograde perfusion on a Langendorff apparatus. After equilibration, hearts were perfused with 5-HT (1 μM) for 10 min, clorgyline (10 μM) for 30 min, or clorgyline pre-treatment for 30 min plus 5-HT for 10 min. Right ventricular wall was dissected and homogenized. Protein samples were derivatized with DNPH and subjected to SDS-PAGE and immunoblotting with the antibody, which interacts with DNPH-derivatized proteins. The bar graph represents means ± SEM (n = 3). (*) denotes values significantly different from each other at P < 0.05.
Discussion
Pulmonary hypertension is a devastating disease without cure. While right ventricular failure is the major cause of death among pulmonary hypertension patients, information on the pathophysiologic properties of the right side of the heart is lacking [6], limiting the development of therapeutic strategies to treat right heart failure. We hypothesize that some of the factors involved in the development of pulmonary vascular remodeling might also serve to mediate right ventricular hypertrophy and failure. As a support for this hypothesis, the present study demonstrated that 5-HT, an important mediator of pulmonary vasoconstriction and pulmonary vascular remodeling, promoted protein carbonylation specifically in the right ventricle, but not in the left.
In bovine pulmonary artery smooth muscle cells, Fanburg and co-workers have shown that 5-HT produces superoxide and promotes cell proliferation [12,31]. They have also shown that an MAO inhibitor iproniazid promotes cell proliferation, rather than inhibiting 5-HT actions [31], suggesting that MAO may not be involved as a mediator of this signal transduction pathway. In contrast, Lawrie et al. [14] have reported that MAO-A is a mediator of 5-HT-induced H2O2 production in human pulmonary artery smooth muscle cells. Similarly, in isolated cardiac myocytes, 5-HT has been shown to elicit H2O2 signaling through MAO-A [18,19].
While we found no difference in the expression of H2O2 scavenging enzymes, 5-HT transporter, or 5-HT receptors; the MAO-A level was found to be lower in the right ventricle compared to the left. Our results using an inhibitor of MAO-A support our hypothesis that a low MAO-A activity in the right ventricle maintains the higher cytosolic 5-HT level, which in turn activates alternative ROS generators.
We have previously found, in pulmonary artery smooth muscle cells, that protein carbonylation can be promoted by mediators of pulmonary hypertension, 5-HT and endothelin-1 [28–30]. Thus, we measured carbonyl formation as a means to assess protein oxidation. Two-electron reduction of O2 to directly form H2O2 via MAO does not involve the formation of superoxide. Superoxide generation, not only produces H2O2 via dismutation reaction, but also reduces iron ion to the ferrous form, which catalyzes Fenton reaction, forming hydroxyl radicals. Since protein carbonylation is often produced through the actions of hydroxyl radicals, the generation of superoxide that forms H2O2 as well as reduces iron may be necessary. This explains the ineffectiveness of MAO-produced H2O2 in forming carbonylated proteins. Therefore, we propose that, in the right ventricle, the ability of MAO-A to degrade intracellular 5-HT, rather than the production of H2O2, is critical for the regulation of 5-HT-mediated protein carbonylation. While further investigations are needed to determine the source of superoxide generated in response to 5-HT in the right ventricle, cytosolic 5-HT may promote auto-oxidation [32] and/or protein serotonylation [33,34] that might activate other oxidases. While our in vitroexperiments in test tubes did not support the occurrence of auto-oxidation of 5-HT to form H2O2(data not shown), these experiments may not account for cellular factors/mechanisms, which might be needed for 5-HT auto-oxidation.
While differences between right and left sides of the heart are not well recognized, there are some differences. The structures as well as the mechanics of the two ventricles are different; the left ventricle forms an oval shaped chamber with thick ventricular wall, and the right ventricle is crescent shaped with thin ventricular wall [6]. Moreover, the two ventricles are formed from different progenitor cells during development; first heart field cells form the left ventricle, and second heart field cells become the right ventricle with differential expression of signaling and transcriptional regulators during cardiac development [35]. Thus, mechanisms for cardiac hypertrophy and failure in right and left ventricles may be different. The present study demonstrated that right and left ventricles have differential regulatory mechanisms for 5-HT; specifically, increased intracellular 5-HT in the right ventricle due to reduced MAO-A might promote protein carbonylation, and this may be important for the setting of right ventricular changes in response to pulmonary hypertension.
Acknowledgments
This work was supported in part by NIH grants HL67340 and HL72844 (to YJS).
Abbreviations
- 5-HT
5-hydroxytryptamine; serotonin
- DNPH
dinitrophenylhydrazine
- GAPDH
glycealdehyde 3-phosphate dehydrogenase
- H2O2
hydrogen peroxide
- KH
Krebs-Henseleit
- MAO
monoamine oxidase
- ROS
reactive oxygen species
- RT-PCR
reverse transcription-polymerase chain reaction
- SERT
serotonin transporter
- SR
serotonin receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655–1665. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 2.Runo JR, Loyd JE. Primary pulmonary hypertension. Lancet. 2003;361:1533–1544. doi: 10.1016/S0140-6736(03)13167-4. [DOI] [PubMed] [Google Scholar]
- 3.Gaine SP, Rubin LJ. Primary pulmonary hypertension. Lancet. 1998;352:719–725. doi: 10.1016/S0140-6736(98)02111-4. [DOI] [PubMed] [Google Scholar]
- 4.Simonneau G, Galiè N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M, Rich S, Fishman A. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:S5–S12. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 5.Chemla D, Castelain V, Herve P, Lecarpentier Y, Brimioulle S. Haemodynamic evaluation of pulmonary hypertension. Eur Respir J. 2002;20:1314–1331. doi: 10.1183/09031936.02.00068002. [DOI] [PubMed] [Google Scholar]
- 6.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB. National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114:1883–1891. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 7.Sato K, Webb S, Tucker A, Rabinovitch M, Obrien RF, McMurtry IF, Stelzne TJ. Factors influencing the idiopathic development of pulmonary hypertension in the Fawn-Hooded rat. Am Rev Respir Dis. 1992;145:793–797. doi: 10.1164/ajrccm/145.4_Pt_1.793. [DOI] [PubMed] [Google Scholar]
- 8.Herve P, Launay JM, Scrobohaci ML, Brenot F, Simonneau G, Petitpretz P, Poubeau P, Cerrina J, Duroux P, Drouet L. Increased plasma serotonin in primary pulmonary hypertension. Am J Med. 1995;99:249–254. doi: 10.1016/s0002-9343(99)80156-9. [DOI] [PubMed] [Google Scholar]
- 9.Eddahibi S, Raffestin B, Pham I, Launay JM, Aegerter P, Sitbon M, Adnot S. Treatment with 5-HT potentiates development of pulmonary hypertension in chronically hypoxic rats. Am J Physiol. 1997;272:H1173–H1181. doi: 10.1152/ajpheart.1997.272.3.H1173. [DOI] [PubMed] [Google Scholar]
- 10.Eddahibi S, Hanoun N, Lanfumey L, Lesch KP, Raffestin B, Hamon M, Adnot S. Attenuated hypoxic pulmonary hypertension in mice lacking the 5-hydroxytryptamine transporter gene. J Clin Invest. 2000;105:1555–1562. doi: 10.1172/JCI8678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacLean MR, Deuchar GA, Hicks MN, Morecroft I, Shen S, Sheward J, Colston J, Loughlin L, Nilsen M, Dempsie Y, Harmar A. Overexpression of the 5-hydroxytryptamine transporter gene: effect on pulmonary hemodynamics and hypoxia-induced pulmonary hypertension. Circulation. 2004;109:2150–2155. doi: 10.1161/01.CIR.0000127375.56172.92. [DOI] [PubMed] [Google Scholar]
- 12.Lee SL, Wang WW, Fanburg BL. Superoxide as an intermediate signal for serotonin-induced mitogenesis. Free Radic Biol Med. 1998;24:855–858. doi: 10.1016/s0891-5849(97)00359-6. [DOI] [PubMed] [Google Scholar]
- 13.Lee SL, Wang WW, Finlay GA, Fanburg BL. Serotonin stimulates mitogen-activated protein kinase activity through the formation of superoxide anion. Am J Physiol. 1999;277:L282–L291. doi: 10.1152/ajplung.1999.277.2.L282. [DOI] [PubMed] [Google Scholar]
- 14.Lawrie A, Spiekerkoetter E, Martinez EC, Ambartsumian N, Sheward WJ, MacLean MR, Harmar AJ, Schmidt AM, Lukanidin E, Rabinovitch M. Interdependent serotonin transporter and receptor pathways regulate S100A4/Mts1, a gene associated with pulmonary vascular disease. Circ Res. 2005;97:227–235. doi: 10.1161/01.RES.0000176025.57706.1e. [DOI] [PubMed] [Google Scholar]
- 15.Lee SL, Simon AR, Wang WW, Fanburg BL. H2O2 signals 5-HT-induced ERK MAP kinase activation and mitogenesis of smooth muscle cells. Am J Physiol. 2001;281:L646–L652. doi: 10.1152/ajplung.2001.281.3.L646. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki YJ, Day RM, Tan CC, Sandven TH, Liang Q, Molkentin JD, Fanburg BL. Activation of GATA-4 by serotonin in pulmonary artery smooth muscle cells. J Biol Chem. 2003;278:17525–17531. doi: 10.1074/jbc.M210465200. [DOI] [PubMed] [Google Scholar]
- 18.Bianchi P, Kunduzova O, Masini E, Cambon C, Bani D, Raimondi L, Seguelas MH, Nistri S, Colucci W, Leducq N, Parini A. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation. 2005;112:3297–3305. doi: 10.1161/CIRCULATIONAHA.104.528133. [DOI] [PubMed] [Google Scholar]
- 19.Bianchi P, Pimentel DR, Murphy MP, Colucci WS, Parini A. A new hypertrophic mechanism of serotonin in cardiac myocytes: receptor-independent ROS generation. FASEB J. 2005;19:641–643. doi: 10.1096/fj.04-2518fje. [DOI] [PubMed] [Google Scholar]
- 20.Stadtman ER, Levine RL. Protein oxidation. Ann N Y Acad Sci. 2000;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki YJ, Nagase H, Day RM, Das DK. GATA-4 regulation of myocardial survival in the preconditioned heart. J Mol Cell Cardiol. 2004;37:1195–1203. doi: 10.1016/j.yjmcc.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Park AM, Suzuki YJ. Effects of intermittent hypoxia on oxidative stress-induced myocardial damage in mice. J Appl Physiol. 2007;102:1806–1814. doi: 10.1152/japplphysiol.01291.2006. [DOI] [PubMed] [Google Scholar]
- 23.Park AM, Nagase H, Vinod Kumar S, Suzuki YJ. Acute intermittent hypoxia activates myocardial cell survival signaling. Am J Physiol. 2007;292:H751–H757. doi: 10.1152/ajpheart.01016.2006. [DOI] [PubMed] [Google Scholar]
- 24.Maurel A, Hernandez C, Kunduzova O, Bompart G, Cambon C, Parini A, Francés B. Age-dependent increase in hydrogen peroxide production by cardiac monoamine oxidase A in rats. Am J Physiol. 2003;284:H1460–H1467. doi: 10.1152/ajpheart.00700.2002. [DOI] [PubMed] [Google Scholar]
- 25.Seiler N, Moulinoux JP, Havouis R, Toujas L. Characterization of amine oxidase activities in macrophages from human peripheral blood. Biochem Cell Biol. 1995;73:275–281. doi: 10.1139/o95-034. [DOI] [PubMed] [Google Scholar]
- 26.Beers RF, Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- 27.Yamamoto Y, Takahashi K. Glutathione peroxidase isolated from plasma reduces phospholipid hydroperoxides. Arch Biochem Biophys. 1993;305:541–545. doi: 10.1006/abbi.1993.1458. [DOI] [PubMed] [Google Scholar]
- 28.Wong CM, Suzuki YJ. Protein carbonyl formation by endothelin-1 in vascular smooth muscle cells. Proc Am Thorac Soc. 2006;3:A855. (abstract) [Google Scholar]
- 29.Wong CM, Suzuki YJ. Serotonin promotes redox signaling via protein carbonylation in pulmonary artery smooth muscle. Am J Resp Crit Care Med. 2007;175:A41. (abstract) [Google Scholar]
- 30.Wong CM, Cheema AK, Zhang L, Suzuki YJ. Protein carbonylation as a novelmechanism in redox signaling. Circ Res. 2008;102:310–318. doi: 10.1161/CIRCRESAHA.107.159814. [DOI] [PubMed] [Google Scholar]
- 31.Lee SL, Wang WW, Moore BJ, Fanburg BL. Dual effect of serotonin on growth of bovine pulmonary artery smooth muscle cells in culture. Circ Res. 1991;68:1362–1368. doi: 10.1161/01.res.68.5.1362. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Reyes E, Mason RP. Characterization of the structure and reactions of fee radicals from serotonin and related indoles. J Biol Chem. 1981;256:2427–2432. [PubMed] [Google Scholar]
- 33.Walther DJ, Peter JU, Winter S, Holtje M, Paulmann N, Grohmann M, Vowinckel J, Alamo-Bethencourt V, Wilhelm CS, Ahnert-Hilger G, Bader M. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet α-granule release. Cell. 2003;115:851–862. doi: 10.1016/s0092-8674(03)01014-6. [DOI] [PubMed] [Google Scholar]
- 34.Guilluy C, Rolli-Derkinderen M, Tharaux PL, Melino G, Pacaud P, Loirand G. Transglutaminase-dependent RhoA activation and depletion by serotonin in vascular smooth muscle cells. J Biol Chem. 2007;282:2918–2928. doi: 10.1074/jbc.M604195200. [DOI] [PubMed] [Google Scholar]
- 35.Srivastava D. Making or breaking the heart: from lineage determination to morphogenesis. Cell. 2006;126:1037–1048. doi: 10.1016/j.cell.2006.09.003. [DOI] [PubMed] [Google Scholar]