Abstract
Problem
The endocervix is a major target of Chlamydia trachomatis (CT) infection, but little is known about the immune repertoire in this tissue, or its response to these common bacteria.
Method of Study
Using a cytobrush, we isolated cells from the endocervix of 20 women during CT infection, and post-antibiotic treatment. Endocervical swabs and blood were taken in parallel. Endocervical cells were enumerated, and endocervical and blood T cells immunophenotyped. CT was genotyped by sequence analysis of the OmpA gene, and quantified by culture.
Results
CT genotypes were D, E, F and Ia, and infectious burden varied considerably. Endocervical T cell and neutrophil numbers were highly elevated during infection, with both CD4 and CD8 T cell subsets accumulating. Regardless of the presence or absence of infection, the endocervical cell infiltrate was dominated by effector memory T cells, and the numbers of CCR5 and CD103 expressing T cells was significantly higher than in the blood. HLA-DR expression by endocervical T cells was significantly increased during infection.
Conclusions
The human endocervix exhibits a distinct cellular response to C.trachomatis infection that can be longitudinally evaluated by cytobrush sampling. Infecting organisms can be sampled and analyzed in parallel.
Keywords: Chlamydia trachomatis, endocervix, human, T lymphocyte
Introduction
Chlamydia trachomatis serovars D-K are tropic for columnar epithelial cells of the genital tract, and the endocervix is the most commonly infected site in women.1,2 Over 80% of women are asymptomatic, hence the majority of women who are infected remain undiagnosed and untreated. While many women are believed to resolve chlamydial infection, this is thought to take several months to several years. 3–5 In women who are chronically infected, the bacteria are more likely to ascend into the endometrium and fallopian tubes, and this can result in pelvic inflammatory disease, tubal infertility and ectopic pregnancy. 1,2
In the Chlamydia muridarum model of genital chlamydial infection, CD4 T cells are the predominant T lymphocyte subpopulation that accumulate in the reproductive tract during infection,6,7 and interferon-gamma secreting cells, predominantly CD4 T cells, are the major cell population involved in resolving the disease.8,9 We know much less about anti-chlamydial cellular immunity in the human genital tract where chlamydial infection is so much more prolonged, and this is compounded by our limited knowledge of the endocervical immune repertoire. While studies have indicated that the cervix is a component of the common mucosal system, at least with respect to B cell homing, it does have some unusual features, for example, there is a predominance of IgG rather than IgA. 10,11 Some studies have also indicated that the human cervix may not share the same homing receptors as classical immune sites. 12,13 The influence of female sex steroids on many aspects of immunity,14 the common existence of other genital coinfections, and exposure to most antigens in the context of seminal plasma, a fluid rich in immunomodulatory cytokines,15 clearly make this a unique and complex environment for the induction of immunity, effector function and in the generation of T cell memory. This is almost certainly a result of the need to be tolerant to sperm and to paternal alloantigen. 15
By utilizing methodology that enables longitudinal sampling of cells in the human endocervix, we were able to quantify and characterize the cellular response of the endocervix to Chlamydia trachomatis infection by comparing samples taken from women during infection, and one and 2 months after successful antibiotic treatment. These samples also enabled us to use multiparameter flow cytometry to phenotype and compare endocervical T cells sampled during and after infection, and to compare them with concurrently collected peripheral blood T cells. These studies, and the associated development of techniques, should provide a basis to help develop our knowledge of cellular immunity to C.trachomatis, and other sexually-transmitted pathogens, in the human endocervix.
Materials and Methods
Study population and Clinic Procedures
Institutional Review Board approval for this study was obtained from LSU Health Sciences Center. Women aged 18–28 years and attending the Delgado STD Clinic or the New Orleans Family Planning Clinic, or who were being screened for participation in a genital herpes vaccine study, were asked to participate if they had a high probability of chlamydial infection based on the following criteria: a recent positive screening test for Chlamydia, recent sexual contact with a male suspected of chlamydial infection, or a mucopurulent cervicitis (cervical friability and/or yellow discharge) (MPC) on routine examination. The exclusion criteria were as follows: pregnancy, underlying chronic disease, use of steroids or antibiotics within the last 2 weeks, sexual intercourse within the last 12 hours, current menstrual bleeding, documented infection with human immunodeficiency virus, or a history of genital herpes.
Women were asked to provide a baseline (infected, untreated), sample at the time of enrollment. If they were C.trachomatis positive by a urine NAAT test and an endocervical culture, they were also asked to return for a first follow-up sample approximately one month after the baseline visit. This was timed, wherever possible, to be at the same point in their menstrual cycle as the baseline sample. A second follow-up sample was also requested at 2 months post-infection if the women provided a first follow-up sample. Women were excluded from the analyses if they were C.trachomatis negative or Neisseria gonorrhoeae or Trichomonas vaginalis positive at their baseline visit, if they failed to provide a first follow-up sample, or if they were C.trachomatis, N. gonorrhoeae or T. vaginalis positive at follow-up. All Chlamydia-infected women were treated with azithromycin or doxycycline on the day of the baseline visit, or as soon as their NAAT test was run.
The following samples were taken at each visit: urine for pregnancy testing and Chlamydia trachomatis and N. gonorrhoeae screening, peripheral blood drawn into EDTA vacutainers for isolation of mononuclear cells, and pelvic samples taken in the following order: two sequential cervical cytobrushes, each one a gentle 360° sweep of the cervical os, which was immediately placed in a 3.5ml of RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 30μg/ml Nystatin (Sigma, St. Louis, MO) and 100μg/mlGentamycin (Invitrogen), for isolation of endocervical cells; one endocervical swab placed immediately in 2ml of 0.2M sucrose phosphate transport medium16 for C. trachomatis culture and genotyping; one vaginal swab for a wet mount preparation and Gram stain preparation, and one vaginal swab immediately placed in an InPouch (BioMed Diagnostics Inc., San Jose, CA) for T. vaginalis culture.
STD screening
Urine specimens were used to determine the presence of C.trachomatis and N.gonorrhoeae DNA using the BD ProbeTec assay as instructed by the manufacturer (Beckton Dickinson, Franklin Lakes, NJ). A vaginal wet preparation was made in the clinic, and bacterial vaginosis (BV) was diagnosed by Gram stain, with a Nugent’s score of ≥ 7 being positive.17 InPouch culture for T. vaginalis screening was read at baseline, 48 and 72 hours.17
Swab specimens in endocervical medium were immediately frozen until required for semi-quantitative culture and genotyping of C.trachomatis. Semi-quantitative culture was performed using a modification of Barnes et al on near-confluent monolayers of McCoy cells grown on cover slips in one dram vials. 16 In brief, immediately prior to infection, 30μl of 0.1% DEAE-Dextran was added to a vial, which was incubated at 35°C for approximately 30 minutes. Medium was aspirated and 300μl of endocervical swab transport media (one sixth of the total volume) was inoculated onto the monolayer, and the vials were spun at 1,200×g for 60 minutes at room temperature. The inoculum was then aspirated, replaced by 1.5 ml of MEM-based maintenance medium containing 0.5 μg/ml cyclohexamide (Sigma), and vials were incubated for 72 hours at 35°C. The coverslip was then stained using an anti-Chlamydia LPS-FITC conjugated monoclonal antibody according to the manufacturers instructions (Meriflour, Meridian Bioscience, Cincinnati, OH), and the number of inclusion forming units (IFU) in 20 high power fields (hpf) were counted. Total IFU per endocervical swab were calculated from the following formula: Mean number of inclusions/hpf × number of fields per coverslip × factor which was derived from the volume of the inoculum.
PCR amplification and sequencing of the C.trachomatis ompA gene
A nested PCR approach was used that enabled sequencing to be performed directly from clinical specimens. In brief, genomic DNA was extracted from 300–600μl endocervical medium by using the High Pure PCR Template Preparation Kit (Roche Applied Science, Indianapolis, IN) following the manufacturers’ instructions. All amplifications were performed with AmpliTaq Gold DNA polymerase (Applied Biosystems, Foster City, CA) and a touch-down protocol as described previously.18 Most specimens were amplified by a single-round of PCR using primers 90UF (5′-GGACATCTTGTCTGGCTTTAACT-3′) and 220DR (5′-GCGCTCAAGTAGACCGATATAGTA-3′). For several specimens that showed very faint bands on agarose gels, a nested PCR was performed with primers 60UF (5′-CCGCCAGAAAAAGATAG-3′) and 80DR (5′-CCAGAAACACGGATAGTGTTATTA-3′) and a thermal cycling profile as follows: 1 cycle of 95°C for 9 min, 30 cycles of 94°C for 45 sec, 52°C for 1 min, and 72°C for 1 min 30 s. All PCR products were purified by using the DNA Clean and Concentrator-5 Kit (Zymo Research, Orange, CA) and sequenced directly from two directions. Each sequence was then aligned with those representing the known ompA sequences.
Processing of cytobrush and blood specimens
Cytobrushes were processed within 3 hrs of collection, as previously described.19 In brief, cytobrushes were vortexed, and if substantial mucus was present they were treated with 2mM DTT (Sigma, St Louis, MO) in HBSS (Invitrogen) with 4% BSA (Sigma) for 10 min at room temperature, then washed twice and resuspended in 2ml of RPMI 1650 supplemented with 10% FBS. If necessary, cells were then filtered through a 40μm Nylon cell strainer. Red blood cells (RBC), leukocytes and neutrophils (PMNs) were enumerated by light microscopy, the latter using the Endtz test.20 Peripheral blood mononuclear cells (PBMNC) were isolated on a Ficoll gradient (Amersham Pharmacia Biotech, Uppsala, Sweden AB).
Flow cytometry
T cell subpopulations in blood and endocervix were analyzed for expression of phenotypic markers by using fluorescent antibodies specific for the following: CD3, CD4, CD8, HLA-DR, β7, CD45RA, CD45RO, CCR5, CD103 (αE) and CCR7 (all BD Biosciences Pharmingen, San Diego, CA) and CD49d (α4) (Immunotech, Beckman Coulter, Marseille, France). Cells were stained and analyzed for expression of surface markers by flow cytometry on a FACSCalibur flow cytometer (Becton Dickinson) as previously described.19 The antibody to CD3 was included in every tube, and T lymphocytes were gated based on forward and side scatter, and expression of this marker. PBMNC stained with only one specific antibody were used to compensate for overlapping signals between different fluorescence channels. Isotype controls were used to determine quadrant markers. A single CD3 staining with 10% of the endocervical sample was always included for calculation of CD3 numbers.
Data analysis
Descriptive statistics were used to summarize the different cell populations and T cell subpopulations in endocervical and blood samples of women during infection and after resolution (treatment) of infection. Comparison of the frequency and phenotype of T cells between endocervix and blood compartments and between infected and treated endocervix were made by the non-parametric Wilcoxon Signed Rank test. Correlations were tested with the Spearman correlation coefficient. All statistical analyses were performed using the SAS software (version 9.1, SAS Institute).
Results
Study population and characteristics of endocervical C.trachomatis infection
A total of 57 women were recruited into the study, and C.trachomatis infection was diagnosed by urine NAAT in 37 (64.5%) individuals. Twenty C. trachomatis positive women were included in the final analyses (54% of all infected women). The other C.trachomatis-infected women were excluded for the following reasons: 1 (2.7%) was chlamydia NAAT positive, but culture negative, at the baseline visit; 3 (8.1%), were chlamydia positive at the first follow-up; 2 (5.4%) were co-infected with N. gonorrhoeae and 5 (13.5%) with T. vaginalis at the baseline or first follow-up visits and 6 (16.2%) women did not return for a follow-up visit.
The median age of the twenty women was 22.4 years (range 19–28 years), 18/20 (90%) were African American and the remaining 10% were Caucasian. Six (30%) of these women self-reported a previous history of chlamydial infection within the last 1–5 years, and 11 of the 20 participants (55%) were recruited into the study as they had tested positive for C.trachomatis by NAAT in the previous 6 weeks (median time from NAAT testing to enrollment/treatment 14 days, range 8–38 days). Eight (40%) presented with MPC at their baseline visit while none were MPC positive at first follow-up. Eight (40%) presented with BV at their baseline visit, and 6 (30%) at first follow-up. Asymptomatic carriage of yeast was noted in 2 (10%) women at baseline and 3 (15%) at first follow-up. Four of the women (20%) were using Depoprovera for contraception and four (20%) were on OCPs when they were enrolled in the study. These contraceptive practices remained consistent at the follow-up visits. The median time from the baseline, infected visit to the first follow-up visit was 35 days (range 14–113 days).
Semi-quantitative culture of C.trachomatis indicated infectious burden in the endocervix varied considerably, with the number of chlamydial inclusion forming units (IFU) cultured from the baseline visit swab ranging from 14 to 121,560 (median IFU 7,300) (Figure 1). The C.trachomatis ompA genotypes infecting this cohort of women were those typically reported in the US for genital infection,21 and were as follows: D (39%), E (11%), F (33%) and Ia (17%). The majority (80%) of the D genotype patients belonged to the subtype D/B-120 (GenBank accession number X62918). Two patients could not be genotyped due to sample losses because of Hurricane Katrina.
Figure 1. C. trachomatis genotype and infectious burden in the endocervix.
Semi-quantitative culture of endocervical Chlamydia trachomatis was performed by 48-hour passage of one sixth of each endocervical swab specimen on near-confluent layers of HeLa cells treated with cycloheximide. The number of inclusion forming units (IFU) per swab were calculated after immunostaining fixed cultures with a FITC-conjugated antibody specific chlamydial LPS. Genomic DNA was extracted from a portion of the remaining specimen, and the C.trachomatis ompA gene amplified by PCR and sequenced.
C. trachomatis infection induces a significant cellular infiltrate in the endocervix
When we isolated and enumerated cells from endocervical cytobrushes, we observed a dramatic difference between the baseline samples taken during active chlamydial infection and the post-antibiotic treated samples taken at the first follow-up visit (Table I). Of particular note was the marked elevation in the number of T cells (CD3) cells at the baseline visit (p=0.003). Neutrophil (p=0.05) and red blood cell numbers (p=0.05) were also significantly higher at this visit.
Table I.
Cellularity of the human endocervix sampled by cytobrush during C.trachomatis infection, and at follow-up after antibiotic treatment.
| Parameter | Baseline visit a | First follow-up visit a | Pb |
|---|---|---|---|
| CD3 × 104 | 2.7
(8.1 ± 11.5) |
0.8
(1.2 ± 1.7) |
0.003 |
| PMN × 106 | 1.8
(2.8 ± 2.8) |
0.4
(1.0 ± 1.5) |
0.05 |
| RBC × 106 | 1.3
(15.0 ± 35.1) |
0.1
(0.9 ± 1.8 |
0.05 |
| Inclusion forming Units (IFU) × 103 | 7.0
(25.0 ± 39.0) |
0
(0 ± 0) |
0.0001 |
Data presented are median values (mean + standard deviation)
Signed Rank test comparing baseline to follow-up, p> 0.05, not significant
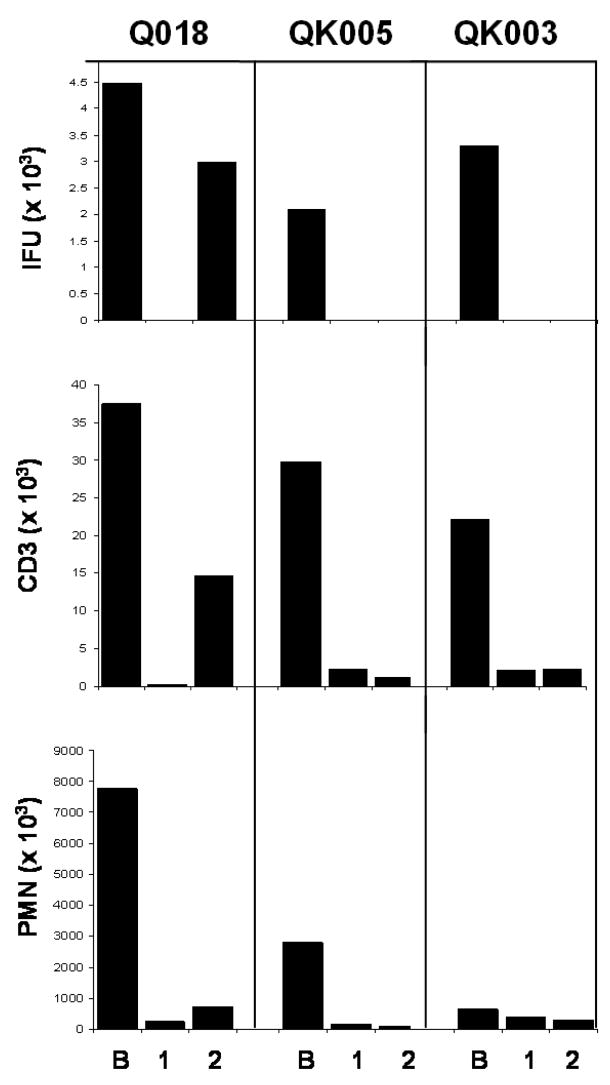
To eliminate the possibility that higher cell numbers seen during infection actually reflected an abnormally low cellularity in the first follow-up visit sample due to an effect of antibiotic treatment, we were able to follow-up on 9 individuals who returned for a second post-treatment visit approximately 1 month after the first (median time from the first to the second follow-up visit was 30 days, range 20 to 87 days). Sample inclusion criteria were the same as for the first follow-up. No significant differences were seen in any cell population between first and second follow-up visits, indicating that the cell counts observed in the first follow-up visit samples reflected a normal uninfected endocervix. In contrast to these patients, one woman was re-infected with C.trachomatis at the second follow-up, and this was accompanied by a concomitant dramatic increase in endocervical T cells. (Figure 2). OmpA sequencing revealed the infecting genotype at the second follow-up to be the same as the baseline visit (Ia).
Figure 2. Longitudinal analysis of cellularity and infectious burden in the endocervix of patients with resolved infection and reinfection in the endocervix.
Endocervical swabs were used for quantitating and genotyping C.trachomatis and cytobrushes were used to assess CD3 and neutrophil (PMN) numbers during infection (B), and at approximately 1 months (1) and 2 months (2) post-antibiotic treatment of the infection.
We next determined if there were any associations between the cell types retrieved from the cytobrush, clinical findings and bacterial counts (IFU). T cell numbers correlated very well with neutrophil numbers at the baseline visit (r=0.82, p<0.0001), but no other significant associations between cell types were noted at baseline or the first follow-up visit. Surprisingly, a clinical diagnosis of MPC at baseline did not correlate with neutrophil count, or any other cell count. Neither was there an association between any cell type, or the clinical diagnosis of MPC, with IFU. Because BV is a condition that is highly prevalent in our population, we also determined whether BV impacted upon the cellularity of the endocervix at the time of infection, or at the subsequent first follow-up visit. No significant differences in cellular parameters were seen between BV-positive or BV-negative women in the presence or absence of active chlamydial infection. Neither did BV appear to have an impact on the IFU number.
Both CD4 and CD8 T cells are recruited into the endocervix during C. trachomatis infection, and are predominantly effector memory cells
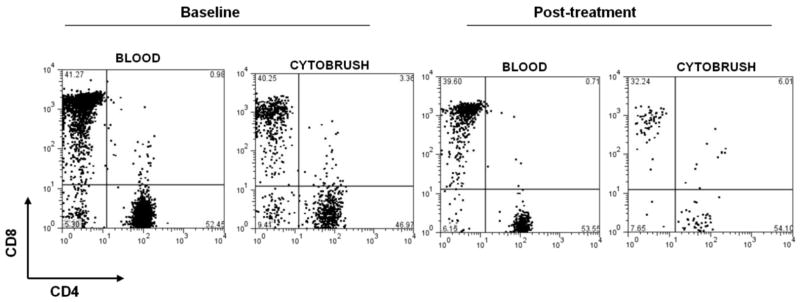
Using flow cytometry, we next examined the relative ratio of CD4+ to CD8+ T cells in endocervix and blood, and found this to be similar at the two sites both during infection, and at the treated first follow-up visit (Figure 3 and Table I). No significant difference were observed in the CD4/CD8 ratio between the infected and treated peripheral blood T cell populations, and a similar observation was made when infected and treated endocervical T cell populations were compared. Because T cell numbers are highly elevated in the endocervix during infection, this indicates that both subpopulations are recruited to and/or expand in the endocervix during chlamydial infection.
Figure 3. CD4 and CD8 expression by endocervical and peripheral blood T cells.
Expression of CD4 and CD8 was assessed on cells isolated from endocervical cytobrush specimens and peripheral blood by flow cytometry from women during C.trachomatis infection, and again after successful antibiotic treatment. Numbers indicate the proportion of CD3+ T cells expressing CD4 or CD8.
In contrast to the CD4+/CD8+ ratio, there was a striking difference in the relative ratio of CD45RO+/CD45RA+ T cells between blood and endocervix at both infected and treated timepoints, with the endocervix being entirely dominated by CD45RO-expressing cells, which is indicative of a memory phenotype (Table II). No significant difference was seen, however, in the ratio between infected and first follow-up endocervical T cell populations or between infected and first follow-up blood T cell populations. The lymph node homing receptor CCR7 was almost completely absent from the endocervical T cell population both during infection, and after treatment, providing further evidence that the majority of the cell population was comprised of effector memory cells (Table II).
Table II.
Comparison of T cell phenotypes between endocervical (En) and peripheral blood (PB) compartments and between C.trachomatis-infected and antibiotic-treated follow-up, endocervix and blood samples.
| CD3 Parameter a | Infected En | Infected PB | Pb | First follow-up En c | First follow-up PB c | Pb |
|---|---|---|---|---|---|---|
| CD4+/CD8+ | 1.0 | 1.0 | ns | 1.2 | 1.0 | ns |
| CD45RA+/CD45RO+ | 0.13 | 1.3 | .0001 | 0.16 | 1.4 | .0001 |
| %CCR7+ | 3.6 | 45.3 | .001 | 0 | 16.9 | .0625 |
| %CD4+ CD103+(αE) | 9.2 | 0.7 | .0001 | 7.6 | 0.6 | .0028 |
| %CD8+ CD103+(αE) | 49.1 | 2.6 | .0001 | 45.2 | 2.5 | .001 |
| %CD49d+ β7+ (α4β7) | 12.2 | 43.3 | .0001 | 8.6 | 38.4 | .0001 |
| %CCR5+ | 58.0 | 15.6 | .002 | 56.3 | 21.9 d | .0313 |
| %HLA-DR+ | 28.0 | 2.7 | .0001 | 14.7 e | 2.7 | .0001 |
Data presented are median values
Wilcoxon Signed Rank test comparing endocervix-derived T cell population to peripheral blood T cell population, p> 0.05, not significant
Wilcoxon Signed Rank test comparing T cell population in endocervical or blood during C.trachomatis infection with follow-up, antibiotic treated sample, p> 0.05, not significant. All comparisons were not significant except for
Infected peripheral blood significantly different from treated, follow-up blood (p=0.0273) and
Infected endocervix significantly different from treated, follow-up endocervix (p=0.0267)
Endocervical T cells express some the classical markers associated with mucosal homing and retention
We next examined expression of CD103 (αE), the alpha chain of the αEβ7 integrin that mediates T cell adhesion to epithelial cells in the intestine via interactions with E-cadherin.22 This was separately determined in CD4 and CD8 T cells due to its differential expression in these subpopulations in the intestine.23 We found that expression was 10-fold higher in both cervical T cell subpopulations compared to blood, but there was no difference between infected and treated samples. (Table II). We also looked at the number of T cells co-expressing α4 (CD49d) and β7, which together form the α4β7 integrin, and which is associated with the homing of T cells to mucosal sites such as the gastrointestinal tract.24 We found co-expression of α4 and β7was significantly lower in the cervical T cells than in peripheral T cells, and this was observed in both infected and treated cervices (Table II). In contrast to this, CCR5, an inflammatory homing receptor, was significantly elevated in both infected and treated cervical T cell populations compared to blood (Table II).
Endocervical T cells become highly activated during C.trachomatis infections
T cells situated in the Chlamydia-negative, treated, endocervix had a much higher expression of HLA-DR compared to those in peripheral blood (Table II and Figure 4). Chlamydial infection also further elevated the expression of HLA-DR in the endocervical T cell population, and this was the one marker surveyed in this study to show a difference between infected and first follow-up endocervix. The difference in T cell activation status between infected and treated patient’s time-points was not seen in the peripheral blood. (Table II and Figure 4).
Figure 4. HLA-DR expression by endocervical and peripheral blood T cells.
Expression of the activation marker HLA-DR was assessed on cells isolated from endocervical cytobrush specimens and peripheral blood by flow cytometry from women during C.trachomatis infection, and again after successful antibiotic treatment. Numbers indicate the proportion of CD3+ T cells expressing HLA-DR.
Discussion
By using an endocervical cytobrush, we were able to collect samples of endocervical cells to assess the changes that occur to the human endocervix during C. trachomatis infection, and after resolution of infection by antibiotic treatment. These samples, taken concurrently with an endocervical swab that can be used for semi-quantitative assessment and genotyping of the infecting organism, create a very useful tool to longitudinally track the local host response to infection by this complex organism.
The cohort investigated in this study represent is typical of most women infected with C.trachomatis. A number of these women had a previous self-reported history of chlamydial infection, and the majority had been enrolled in the study when recalled to the clinic after a positive NAAT test. The observations we recorded were therefore unlikely to be early, acute immune responses to infection, but rather, ongoing acquired responses to infections of at least 2 weeks duration, and in some cases, a secondary immune response to a re-infection. While neutrophil, RBC and T cell numbers were all significantly elevated in the infected endocervix when compared to the treated endocervix, the greatest change was seen in the T cell number. We also observed a correlation between T cell and neutrophil numbers, but only during infection. Our findings suggest that at this stage of infection, the presence of high numbers of T cells in the endocervical samples may be a very accurate cellular correlate of chlamydial infection. While neutrophil numbers were also increased during infection, they were less significantly so than T cells, possibly because the study subjects were not seen in the first few days of a primary infection. It is also possible that neutrophils, as cellular components of the innate immune response, may be a more variable and less predictable indicator than T cells, as they can be transiently triggered by common events such as minor physical trauma or challenge by vaginal bacteria, and which do not result in the activation of an acquired immune response.
Chlamydiae were cultured from all the women included in the study, but an extremely wide range of infectious burden was estimated by IFU, and this did not correlate with numbers of neutrophils or T cells isolated from the endocervix. Because this culture technique was semi-quantitative, and many clinical strains may vary in their in vitro growth potential, it would be of value to incorporate a quantitative PCR-based assay into these kind of studies in the future. Infecting genotypes were D, E, F and Ia, which are the 4 most common reported for genital infections in the US. 21 Interestingly, however, the most common infecting genotypes in our cohort of women was D, which was only the third most prevalent genotype reported in a large 3-city study, which included New Orleans, and was undertaken between 1995–7.21 This may suggest a shift of genotypes in the New Orleans population.
Histological analyses in the mouse model have indicated that primary and secondary C.muridarum infection is accompanied by an influx of T cells into the genital tract, and that these cells are predominantly CD4 positive.6,7 Elegant knockout studies have also shown that Th1 CD4 cells are pivotal in clearing infection. 8,9 In contrast, both CD4 and CD8 T cells accumulate in the cervix and upper genital tract of the guinea-pig during primary and secondary C. muridarum infection,25 an observation also made in macaque fallopian tubes after C.trachomatis infection.26,27 Historical studies of the human genital tract during sexually transmitted infection have demonstrated influxes of leukocytes into the genital tract28,29 and, more recently, several cross-sectional studies have demonstrated the presence of enhanced T cell numbers in C.trachomatis infection.30,31 This is the first time, to our knowledge, of a longitudinal study showing that culture positive C.trachomatis infection in the endocervix of young women is accompanied by a influx of both CD4 and CD8 T cells, and that antibiotic treatment of infection results in the disappearance of these cells, presumably due to the reduction in bacteria by the antibiotic. This data strengthens a conclusion drawn from animal models of chlamydial infection, namely that the association of T cells with infection indicate that is an important parameter in the response to infection. 6,9,32,33 Furthermore, the absence of T cells when infection is no longer present may also support the concept drawn from animal models that susceptibility to reinfection may relate loss of T cells in the genital tract.6,9,32,33 It may also be of relevance in a current debate as to whether antibiotic treatment might curtail the induction of natural immunity, and hence might render women even more susceptible to re-infection.5
With respect to other phenotypic characteristics of the endocervical repertoire, we noted that, similar to other mucosal sites and in contrast to systemic T cells, this tissue is almost exclusively populated by an experienced memory cell population.34,35 Though there is a high level of T cell activation in the uninfected endocervix, there is a marked increase in the number of such cells during culture positive C. trachomatis infection, suggesting further activation by the infection. Also, similar to the GI tract, we noted that a significant proportion of T cells expressed CD103. In contrast, we found that few endocervical T cells co-expressed α4 and β7, which are the integrins that combine to make the classical homing marker which is used by GI homing cells, and which is retained by T cells in the tissue.34 This observed lack of α4 and β7 co-expression may well be due to α4 down-regulation36,37 by these cells once in the endocervix or, alternatively, may indicate the use of an unrecognized alternate dominant homing receptor. In contrast, the number of T cells expressing CCR5 in the endocervix was much higher than in the blood, thus supporting other studies that suggest that this is a generic homing receptor for inflamed tissue sites.38,39 The elevated number of T cells found in the endocervix during chlamydial infection, concomitant with their high CCR5 expression, is compatible with what is known of the susceptibility of the endocervix to HIV infection. Our homing receptor observations should be explored by focused homing studies as they have very important implications for the design of STD vaccines.
Studies of human immunity to an organism which is usually asymptomatic, and therefore the time of the initiating event largely unknown, and that is cleared over a relatively long time-period but may only generate short term immunity, are challenging. However, while animal models lead us to key concepts in immunity, there are major differences in the course of the infection in humans, and we must also rise to the challenge of human research. In this study, we chose to focus on the immune events that occur in the endocervix, the most commonly infected site in women, and where infection remains localized. While our endocervical cytobrush technique is limited by the number of cells it can retrieve, with the advent of multi-parameter flow cytometry, it can be used to ask very focused questions. The power is also amplified by the fact that it is relatively non-invasive and samples can be taken longitudinally at every 3 weeks or so. In addition, concurrent swabs can be taken for analysis and quantification of C.trachomatis, and for other sexually-transmitted organisms.
Acknowledgments
This study was supported by NIH grant 5U19AI061972 and a grant from the Louisiana Board of Regents (HEF [2001–04]-04). Flow cytometry was performed in the Immunology Core in the Section of Pulmonary Medicine/CCM, Department of Medicine, LSU HSC, which is in part supported by NIH grant HL76100. We thank Judy Burnett BS, Mary Welch BS and Cathy Cammarata BS for excellent technical assistance, Karen Lenzyck RN, Julia Miller RN and Rebecca Lillis MD for help with patient recruitment and Priscilla Wyrick PhD and Paul Fidel PhD for critical reading of the manuscript.
References
- 1.Brunham RC. Human Immunity to Chlamydiae. In: Stephens RS, editor. Chlamydia: Intracellular Biology, Pathogenesis and Immunity. Washington DC: American Society for Microbiology; 1999. pp. 211–238. [Google Scholar]
- 2.Brunham B, Rey-Ladino J. Immunology of Chlamydia infection: Implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5:149–161. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 3.Golden MR, Schillinger JA, Markowitz L, St Louis ME. Duration of untreated genital infections with Chlamydia trachomatis: a review of the literature. Sex Transm Dis. 2000;27:329–337. doi: 10.1097/00007435-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Molano M, Meijer CJLM, Weiderpass E, Arsian A, Posso H, Fransceschi F, et al. The natural course of Chlamydia trachomatis infection in asymptomatic Colombian women: a five year follow-up study. J Infect Dis. 2005;191:907–916. doi: 10.1086/428287. [DOI] [PubMed] [Google Scholar]
- 5.Rekart ML, Brunham RC. Debate. Epidemiology of chlamydial infection: Are we losing ground? Sex Transm Inf. 2008 Jan 23; doi: 10.1136/sti.2007.027938. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Kelly KA, Rank RG. Identification of homing receptors that mediate the recruitment of CD4 T cells to the genital tract following intravaginal infection with Chlamydia trachomatis. Infect Immunol. 1997;65:5198–5208. doi: 10.1128/iai.65.12.5198-5208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison SG, Morrison RP. In situ analysis of the evolution of the primary response in murine Chlamydia trachomatis infection. Infect Immunol. 2002;70:2741–2751. doi: 10.1128/iai.68.5.2870-2879.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotter TW, Ramsey KH, Miranpuri GS, Poulsen CE, Byrne GI. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon knockout mice. Infect Immunol. 1997;68:2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rank RG. The role of the CD4 T cell in the host response to Chlamydia. In: Bavoil PM, Wyrick PB, editors. Chlamydia genomics and pathogenesis. Norfolk, UK: Horizon Bioscience; 2006. pp. 365–377. [Google Scholar]
- 10.Kutteh WH, et al. Secretory immune system of the female reproductive tract: I Immunoglobulin and secretory component containing cells. Obstet Gynecol. 1988;71:56–60. [PubMed] [Google Scholar]
- 11.Kutteh WH, et al. Variations in immunoglobulins and IgA subclasses of human uterine cervical secretions around the time of ovulation. Clin Exp Immunol. 1996;104:538–542. doi: 10.1046/j.1365-2249.1996.36742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson EL, Rudin A, Wassen L, Holmgren J. Distribution of lymphocytes and adhesion molecules in human cervix and vagina. Immunology. 1999;96:272–277. doi: 10.1046/j.1365-2567.1999.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansen FE, Baekkevold ES, Carlsen HS, Farstad IN, Soler D, Brandtzaeg P. Regional induction of adhesion molecules and chemokine receptors explains disparate homing of human B cells to systemic and mucosal effector sites: dispersion from tonsils. Blood. 2005;106:593–600. doi: 10.1182/blood-2004-12-4630. [DOI] [PubMed] [Google Scholar]
- 14.Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract; cellular responses and interactions. J Acquir Immune Defic Syndr. 2005;206:365–335. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 15.Robertson SA, Ingman WV, O’Leary S, Sharkey DJ, Tremellen KP. Transforming growth factor beta- a mediator of immune deviation in seminal plasma. J Reprod Immunol. 2002;57:109–128. doi: 10.1016/s0165-0378(02)00015-3. [DOI] [PubMed] [Google Scholar]
- 16.Barnes RC, Katz BP, Rolfs RT, Batteiger B, Caine V, Jones RB. Quantitative culture of endocervical Chlamydia trachomatis. J Clin Microbiol. 1990;28:774–780. doi: 10.1128/jcm.28.4.774-780.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kissinger PJ, Dumestre J, Clark RA, Wenthold L, Mohammed H, Hagensee ME, Martin DH. Vaginal swabs versus lavage for detection of Trichomonas vaginalis and bacterial vaginosis among HIV-positive women. Sex transm Dis. 2005;32:227–230. doi: 10.1097/01.olq.0000151416.56717.7d. [DOI] [PubMed] [Google Scholar]
- 18.Ma L, Kutty G, Jia Q, Kovacs JA. Characterization of variants of the gene encoding the p55 antigen in Pneumocystis from rats and mice. J Med Microbiol. 2003;52:955–960. doi: 10.1099/jmm.0.05131-0. [DOI] [PubMed] [Google Scholar]
- 19.Quayle AJ, Shah M, CuUvin S, Politch J, Chou C, Anderson DJ, Tuomala R, Crowley-Nowick P, Duerr A. Implications of blood contamination for assessment of local cellular immunity in the endocervix. AIDS Res Human Retroviruses. 2004;20:543–546. doi: 10.1089/088922204323087796. [DOI] [PubMed] [Google Scholar]
- 20.Endtz AW. A rapid staining method for differentiating granulocytes from ‘germinal cells’ in Papanicolaou-stained semen. Acta Cytol. 1974;18:2–7. [PubMed] [Google Scholar]
- 21.Millman K, Black CM, Stamm WE, Jones RB, Hook EW, 3, Martin DH, Bolan G, Tavare S, Dean D. Population-based genetic epidemiologic analysis of Chlamydia trachomatis serotypes and lack of association between ompA polymorphisms and clinical phenotypes. Microbes Infect. 2006;8:604–611. doi: 10.1016/j.micinf.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 23.Schön MP, Arya A, Murphy EA, Adams CM, Strauch UG, Agace WW, Marsal J, Donohue JP, Her H, Beier DR, Olson S, Lefrancois L, Brenner MB, Grusby MJ, Parker CM. Mucosal T lymphocyte numbers are selectively reduced in integrin αE (CD103)-deficient mice. J Immunol. 1999;162:6641–6649. [PubMed] [Google Scholar]
- 24.Erle DJ, Briskin MJ, Butcher EC, Garcia-Pardo A, Lazorovits AI, Tidswell M. Expression and function of the MAdCAM-1 receptor, integrin α4β7, on human leukocytes. J Immunol. 1994;153:517–528. [PubMed] [Google Scholar]
- 25.Rank RG, Bowlin AK, Kelly KA. Characterization of the lymphocyte response in the female genital tract during ascending chlamydial genital infection in the guinea pig model. Infect Immun. 2000;68:5293–5298. doi: 10.1128/iai.68.9.5293-5298.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Voorhis WC, Barrett LK, Sweeney YT, Kuo CC, Patton DL. Repeated Chlamydia trachomatis infection of Macaca nemestrina fallopian tubes produces a Th1-like cytokine response associated with fibrosis and scarring. Infect Immun. 1997;65:2175–2182. doi: 10.1128/iai.65.6.2175-2182.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Voorhis WC, Barrett LK, Sweeney YT, Kuo CC, Patton DL. Analysis of lymphocyte phenotype and cytokine activity in the inflammatory infiltrates of the upper genital tract of female macaques infected with Chlamydia trachomatis. J Infect Dis. 1996;174:1142. doi: 10.1093/infdis/174.3.647. [DOI] [PubMed] [Google Scholar]
- 28.Kiviat NB, Wolner-Hanssen P, Eschenbach DA, Wasserheit JN, Paavonen JA, Bell TA, Critchlow CW, Stamm WE, Moore DE, Holmes KK. Endometrial histopathology in patients with culture-proved upper genital tract infection and laparoscopically diagnosed acute salpingitis. Am J Surg Pathol. 1990;14:167–175. doi: 10.1097/00000478-199002000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Kiviat NB, Paavonen JA, Wolner-Hanssen P, Critchlow CW, Stamm WE, Douglas J, Eschenbach DA, Corey LA, Holmes KK. Histopathology of endocervical infection caused by Chlamydia trachomatis, herpes simplex virus, Trichomonas vaginalis, and Neisseria gonorrhoeae. Hum Pathol. 1990;21:831–837. doi: 10.1016/0046-8177(90)90052-7. [DOI] [PubMed] [Google Scholar]
- 30.Levine WC, Pope V, Bhoomkar A, Tambe P, Lewis JS, Zaidi AA, Farshy CE, Mitchell S, Talkington DF. Increase in endocervical CD4 lymphocytes among women with non-ulcerative sexually transmitted diseases. J Infect Dis. 1998;177:167–174. doi: 10.1086/513820. [DOI] [PubMed] [Google Scholar]
- 31.Mittal A, Rastogi S, Reddy BS, Verma S, Salhan S, Gupta E. Enhanced immunocompetent cells in chlamydial cervicitis. J Reprod Med. 2004;49:671–677. [PubMed] [Google Scholar]
- 32.Igietseme JU, Rank RG. Susceptibility to reinfection after a primary chlamydial genital infection is associated with a decrease of antigen-specific T cells in the genital tract. Infect Immun. 1991;59:1346–1351. doi: 10.1128/iai.59.4.1346-1351.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hawkins RA, Rank RG, Kelly KA. Expression of mucosal homing receptor alpha4beta7 is associated with enhanced migration to the Chlamydia-infected murine genital mucosa in vivo. Infect Immun. 68:5587–5594. doi: 10.1128/iai.68.10.5587-5594.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shacklett BL, Cox CA, Sandberg JK, Stollman NH, Jacobsen MA, Nixon DF. Trafficking of human immunodeficiency virus type-1 specific CD8+ T cells to gut-associated lymphoid tissue during chronic infection. J Virol. 2003;77:5631–5631. doi: 10.1128/JVI.77.10.5621-5631.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kourtis AP, Ibegbu CC, Theiler R, Xu Y-X, Bansil P, Jamieson DJ, Lindsay M, Butera S, Duerr A. Breast milk CD4+ T cells express high levels of C chemokine receptor 5 and CXC chemokine receptor 4 and are preserved in HIV-infected mothers receiving highly active antiretroviral therapy. J Infect Dis. 195:965–972. doi: 10.1086/512082. [DOI] [PubMed] [Google Scholar]
- 36.Hernández-Caselles T, Martinez-Esparza M, Lazarovits AI, Aparicio P. Specific regulation of VLA-4 and α4β7 integrin expression on human activated lymphocytes. J Immunol. 1996;156:3668–3677. [PubMed] [Google Scholar]
- 37.Meenan J, Spaans J, Grool TA, Pals ST, Tytgat GNJ, van Deventer SJH. Altered expression of α4β7, a gut homing integrin, by circulating and mucosal T cells in colonic mucosal inflammation. Gut. 1997;40:241–246. doi: 10.1136/gut.40.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kunkel EJ, Boisvert J, Murphy K, Vierra MA, Genovese MC, Wardlaw AJ, Greenberg HB, Hodge MR, Wu L, Butcher EC, Campbell JJ. Expression of the chemokine receptors CCR4, CCR5, and CXCR3 by human tissue infiltrating lymphocytes. Am J Pathol. 2002:347–355. doi: 10.1016/S0002-9440(10)64378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patterson BK, Landay A, Siegel JN, Flener Z, Pessis D, Chaviano A, Bailey RC. Susceptibility to human immunodeficiency virus-1 infection of human foreskin and cervical tissue grown in explant culture. Am J Pathol. 2002;161:867–873. doi: 10.1016/S0002-9440(10)64247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]