Abstract
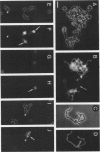
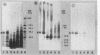
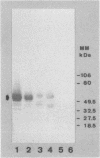
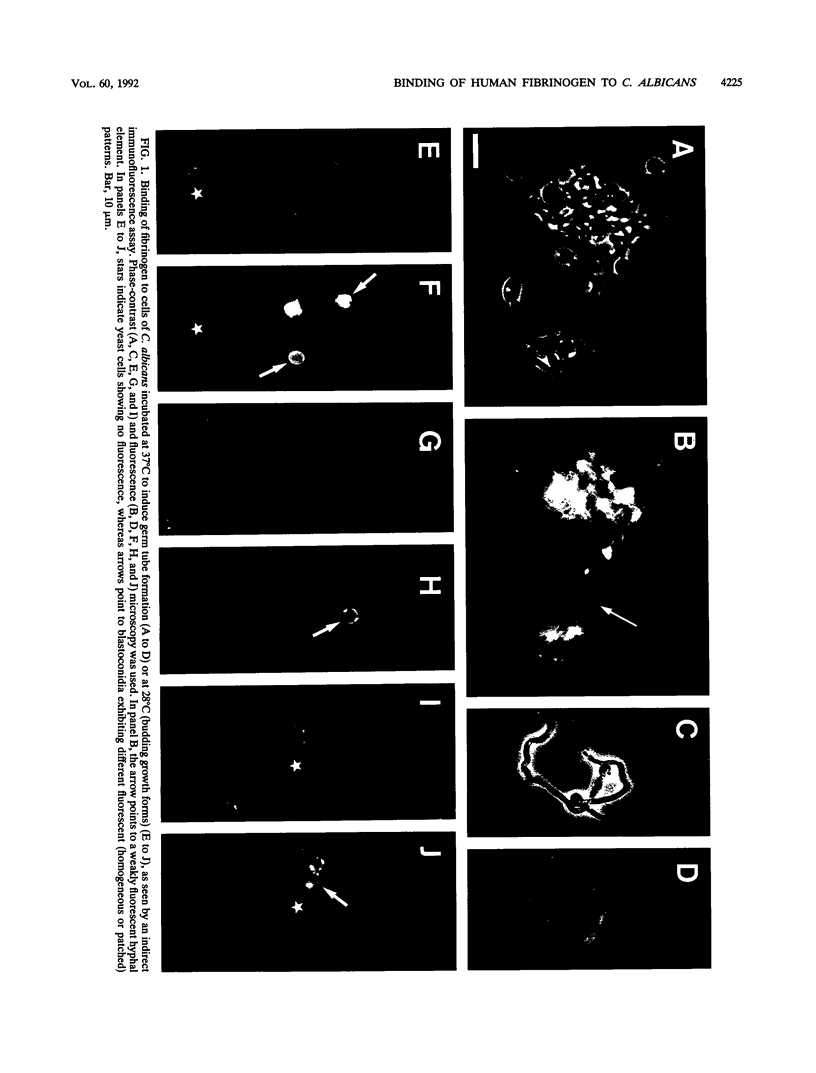
Treatment of both yeast (blastoconidia) and hyphal (blastoconidia with germ tubes) cells of Candida albicans with beta-mercaptoethanol (beta ME) releases a complex array of cell wall-bound proteins and glycoproteins. Analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western immunoblotting with fibrinogen-anti-fibrinogen antibody allowed the identification of a 58-kDa mannoprotein (mp58) in both extracts which specifically interacts with human fibrinogen. Treatment of intact cells with low concentrations of beta-glucanase (Zymolyase 20T) for short periods or with beta ME abolished or significantly reduced binding of fibrinogen. A rabbit polyclonal antiserum was raised against the purified mp58 species released by beta ME from germinated blastoconidia (PAb anti-mp58). By Western blotting, the antiserum cross-reacted with the homologous 58-kDa fibrinogen-binding mannoprotein present in beta ME extracts from blastoconidia, and by indirect immunofluorescence, the antiserum labelled both yeast cells and hyphae, yet reactivity was found primarily on the cell surface of filamentous forms. Immunostaining of human infected tissue sections with PAb anti-mp58 showed that the mp58 species is also expressed in vivo; in this case, the species is in the forms of both yeast and hyphal elements similarly labelled by the antiserum. Purified immunoglobulin G fraction from the antiserum did not alter the binding of fibrinogen as determined by a modified enzyme-linked immunosorbent assay and Western blotting. The N- and O-glycosidically linked carbohydrates represent 18 to 20% and 3 to 4%, respectively, of the molecular mass of the mp58. O-linked sugar residues may be involved in the interaction of the molecule with fibrinogen.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouali A., Robert R., Tronchin G., Senet J. M. Characterization of binding of human fibrinogen to the surface of germ-tubes and mycelium of candida albicans. J Gen Microbiol. 1987 Mar;133(3):545–551. doi: 10.1099/00221287-133-3-545. [DOI] [PubMed] [Google Scholar]
- Bouchara J. P., Tronchin G., Annaix V., Robert R., Senet J. M. Laminin receptors on Candida albicans germ tubes. Infect Immun. 1990 Jan;58(1):48–54. doi: 10.1128/iai.58.1.48-54.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone R. A., Braun P. C. Adherence and receptor relationships of Candida albicans. Microbiol Rev. 1991 Mar;55(1):1–20. doi: 10.1128/mr.55.1.1-20.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone R. A., Linehan L., Wadsworth E., Sandberg A. L. Identification of C3d receptors on Candida albicans. Infect Immun. 1988 Jan;56(1):252–258. doi: 10.1128/iai.56.1.252-258.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova M., Chaffin W. L. Cell wall glycoproteins of Candida albicans as released by different methods. J Gen Microbiol. 1991 May;137(5):1045–1051. doi: 10.1099/00221287-137-5-1045. [DOI] [PubMed] [Google Scholar]
- Casanova M., Gil M. L., Cardeñoso L., Martinez J. P., Sentandreu R. Identification of wall-specific antigens synthesized during germ tube formation by Candida albicans. Infect Immun. 1989 Jan;57(1):262–271. doi: 10.1128/iai.57.1.262-271.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova M., Martìnez J. P., Chaffin W. L. Identification of germ tube cell wall antigens of Candida albicans. J Med Vet Mycol. 1991;29(4):269–272. doi: 10.1080/02681219180000391. [DOI] [PubMed] [Google Scholar]
- Casanova M., Martínez J. P., Chaffin W. L. Fab fragments from a monoclonal antibody against a germ tube mannoprotein block the yeast-to-mycelium transition in Candida albicans. Infect Immun. 1990 Nov;58(11):3810–3812. doi: 10.1128/iai.58.11.3810-3812.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassone A. Cell wall of Candida albicans: its functions and its impact on the host. Curr Top Med Mycol. 1989;3:248–314. doi: 10.1007/978-1-4612-3624-5_10. [DOI] [PubMed] [Google Scholar]
- Cheung A. L., Fischetti V. A. The role of fibrinogen in staphylococcal adherence to catheters in vitro. J Infect Dis. 1990 Jun;161(6):1177–1186. doi: 10.1093/infdis/161.6.1177. [DOI] [PubMed] [Google Scholar]
- Douglas L. J. Adhesion of Candida species to epithelial surfaces. Crit Rev Microbiol. 1987;15(1):27–43. doi: 10.3109/10408418709104446. [DOI] [PubMed] [Google Scholar]
- Elorza M. V., Marcilla A., Sentandreu R. Wall mannoproteins of the yeast and mycelial cells of Candida albicans: nature of the glycosidic bonds and polydispersity of their mannan moieties. J Gen Microbiol. 1988 Aug;134(8):2393–2403. doi: 10.1099/00221287-134-8-2393. [DOI] [PubMed] [Google Scholar]
- Elorza V., Mormeneo S., Garcia de la Cruz F., Gimeno C., Sentandreu R. Evidence for the formation of covalent bonds between macromolecules in the domain of the wall of Candida albicans mycelial cells. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1118–1125. doi: 10.1016/0006-291x(89)90789-4. [DOI] [PubMed] [Google Scholar]
- Fukayama M., Calderone R. A. Adherence of cell surface mutants of Candida albicans to buccal epithelial cells and analyses of the cell surface proteins of the mutants. Infect Immun. 1991 Apr;59(4):1341–1345. doi: 10.1128/iai.59.4.1341-1345.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen K. C., Lay J. G., Hazen B. W., Fu R. C., Murthy S. Partial biochemical characterization of cell surface hydrophobicity and hydrophilicity of Candida albicans. Infect Immun. 1990 Nov;58(11):3469–3476. doi: 10.1128/iai.58.11.3469-3476.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M., Vaudaux P. E., Pittet D., Auckenthaler R., Lew P. D., Schumacher-Perdreau F., Peters G., Waldvogel F. A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988 Oct;158(4):693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Kanbe T., Li R. K., Wadsworth E., Calderone R. A., Cutler J. E. Evidence for expression of the C3d receptor of Candida albicans in vitro and in vivo obtained by immunofluorescence and immunoelectron microscopy. Infect Immun. 1991 May;59(5):1832–1838. doi: 10.1128/iai.59.5.1832-1838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. J., Sandin R. L. Influence of growth conditions on Candida albicans adhesion, hydrophobicity and cell wall ultrastructure. J Med Vet Mycol. 1988 Apr;26(2):79–92. [PubMed] [Google Scholar]
- Klotz S. A., Drutz D. J., Zajic J. E. Factors governing adherence of Candida species to plastic surfaces. Infect Immun. 1985 Oct;50(1):97–101. doi: 10.1128/iai.50.1.97-101.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee K. L., Buckley H. R., Campbell C. C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida Albicans. Sabouraudia. 1975 Jul;13(2):148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- Linehan L., Wadsworth E., Calderone R. Candida albicans C3d receptor, isolated by using a monoclonal antibody. Infect Immun. 1988 Aug;56(8):1981–1986. doi: 10.1128/iai.56.8.1981-1986.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Ribot J. L., Casanova M., Martinez J. P., Sentandreu R. Characterization of cell wall proteins of yeast and hydrophobic mycelial cells of Candida albicans. Infect Immun. 1991 Jul;59(7):2324–2332. doi: 10.1128/iai.59.7.2324-2332.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J. P., Gil M. L., Casanova M., Lopez-Ribot J. L., Garcia De Lomas J., Sentandreu R. Wall mannoproteins in cells from colonial phenotypic variants of Candida albicans. J Gen Microbiol. 1990 Dec;136(12):2421–2432. doi: 10.1099/00221287-136-12-2421. [DOI] [PubMed] [Google Scholar]
- Martinez J. P., Lopez-Ribot J. L., Gil M. L., Sentandreu R., Ruiz-Herrera J. Inhibition of the dimorphic transition of Candida albicans by the ornithine decarboxylase inhibitor 1,4-diaminobutanone: alterations in the glycoprotein composition of the cell wall. J Gen Microbiol. 1990 Oct;136(10):1937–1943. doi: 10.1099/00221287-136-10-1937. [DOI] [PubMed] [Google Scholar]
- Page S., Odds F. C. Binding of plasma proteins to Candida species in vitro. J Gen Microbiol. 1988 Oct;134(10):2693–2702. doi: 10.1099/00221287-134-10-2693. [DOI] [PubMed] [Google Scholar]
- Philippidis P., Naiman J. L., Sibinga M. S., Valdes-Dapnea M. A. Disseminated intravascular coagulation in Candida albicans septicemia. J Pediatr. 1971 Apr;78(4):683–686. doi: 10.1016/s0022-3476(71)80476-6. [DOI] [PubMed] [Google Scholar]
- Poulain D., Hopwood V., Vernes A. Antigenic variability of Candida albicans. Crit Rev Microbiol. 1985;12(3):223–270. doi: 10.3109/10408418509104430. [DOI] [PubMed] [Google Scholar]
- Rotrosen D., Calderone R. A., Edwards J. E., Jr Adherence of Candida species to host tissues and plastic surfaces. Rev Infect Dis. 1986 Jan-Feb;8(1):73–85. doi: 10.1093/clinids/8.1.73. [DOI] [PubMed] [Google Scholar]
- Saxena A., Calderone R. Purification and characterization of the extracellular C3d-binding protein of Candida albicans. Infect Immun. 1990 Feb;58(2):309–314. doi: 10.1128/iai.58.2.309-314.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerl K. G., Calderone R. A., Segal E., Sreevalsan T., Scheld W. M. In vitro binding of Candida albicans yeast cells to human fibronectin. Can J Microbiol. 1984 Feb;30(2):221–227. doi: 10.1139/m84-033. [DOI] [PubMed] [Google Scholar]
- Tronchin G., Bouchara J. P., Robert R. Dynamic changes of the cell wall surface of Candida albicans associated with germination and adherence. Eur J Cell Biol. 1989 Dec;50(2):285–290. [PubMed] [Google Scholar]
- Tronchin G., Robert R., Bouali A., Senet J. M. Immunocytochemical localization of in vitro binding of human fibrinogen to Candida albicans germ tube and mycelium. Ann Inst Pasteur Microbiol. 1987 Mar-Apr;138(2):177–187. doi: 10.1016/0769-2609(87)90194-3. [DOI] [PubMed] [Google Scholar]