Abstract
We previously assessed the motivational properties of pups relative to those of cocaine in parturient female rats (dams) across the postpartum period and demonstrated that the larger subset of dams in early postpartum (PPD8) preferred the pup-associated chamber, whereas the majority of dams tested in late postpartum (PPD16) preferred the cocaine-associated chamber [Mattson, B.J., Williams, S., Rosenblatt, J.S., Morrell, J.I. 2001. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav. Neurosci., 115, 683-694; Seip, K.M., Morrell, J.I. 2007. Increasing the incentive salience of cocaine challenges preference for pup- over cocaine-associated stimuli during early postpartum: place preference and locomotor analyses in the lactating female rat. Psychopharmacology 194, 309-319]. The present study uses a dual-choice conditioned place preference to ask how the progression of the postpartum period, including natural pup development, influences maternal motivation for pups. Preferences for cued chambers associated with pups that were age-matched to the postpartum stage of the dam in contrast to a stimulus with little incentive salience were higher during the early than the late postpartum, suggesting that the incentive salience of pups diminishes as the postpartum period progresses. Preferences of the early postpartum dams deprived of pups for 15 min, 2, 6, 12 or 22 hrs prior to conditioning and testing did not differ statistically but there was a trend of more pup preference after 22 hr deprivation; pup age was not an important factor in early postpartum. In marked contrast, late postpartum dams only exhibited robust pup-associated place preference when they were conditioned with young (4-7 day-old) pups or after a 22 hr period of deprivation from contemporaneous pups. Together these results suggest that both forces are at work in the mother-pup dyad, changes in the pups as they develop and changes in the physiological and endocrine state of the female as she progresses through the postpartum period.
Keywords: Maternal behavior, Maternal motivation, Conditioned place preference, Postpartum female, Pup incentive salience
Introduction
Our previous work used a dual-choice conditioned place preference (CPP) procedure to assess the motivational properties of pups relative to those of cocaine in maternal female rats across the postpartum period (Mattson et al., 2001, 2003; Seip and Morrell, 2007). When given a choice between chambers associated with pups or cocaine, the larger subset of dams tested during early postpartum (PPD8) preferred the pup-associated chamber, whereas the majority of dams tested in late postpartum (PPD16) preferred the cocaine-associated chamber (Mattson et al., 2001; Seip and Morrell, 2007). These prior experiments were not designed to determine whether an independent change in the incentive salience of the pups, cocaine or in both were at work. We have since found that the CPP preference of dams for cocaine- over saline-associated cues is remarkably stable across the postpartum period (Seip et al., in press).
It is our working hypothesis that changing maternal motivation for pups is a key drive in our original findings, that it wanes as the postpartum period progresses, and that this is mainly due to the changing dynamic of the mother-pup dyad produced by synchronized changes in the physiological state of female and in the developing needs of the pup. The present study examines the two distinct postpartum periods we have previously used in our explorations, early and late postpartum. We use place preference as a measure of initial phases of the motivational state (pup seeking), with the time the females spend in pup associated chamber at test as measure of maternal motivation. Of note is that CPP eliminates the consummatory components i.e. expression of maternal behavior during the test of maternal motivation, since preference for pup-associated chamber is measured in the absence of the pups.
In a series of experiments that use conditioned place preference, Fleming and colleagues (1994) demonstrated that both postpartum and virgin females during the first ten days of maternal responsiveness, exhibited marked pup-associated place preference, using a two-chambered choice of a chamber associated with pups versus an empty chamber associated with no specific unconditioned stimulus. Although as little as ten minutes of pup-deprivation before conditioning resulted in pup-associated place preference, more females had stronger preference after 23 hours of pup-deprivation prior to conditioning (Fleming et al., 1994). Magnusson and Fleming (1995) also demonstrated that physical interaction with pups during conditioning sessions was essential in establishing this pup-associated place preference, in particular access to chemosensory and somatosensory inputs from the pups.
We have conducted two sets of experiments using our prior procedures based on a three chambered place preference apparatus (Mattson et al., 2001; Seip and Morrell, 2007). Experiment I explores the motivational state of the maternal female rat in early (days 4-8) versus late (days 12-16) postpartum for the natural pup stimulus that is pups that are age-matched to the postpartum stage of the test subject. To explore the motivational state at these two time points we examined the CPP preferences for pup- versus object-associated chambers of independent groups of females which were subjected to systematically varied deprivation from pups prior to being conditioned with pups as stimuli. Experiment II examines the impact of pup development on maternal motivation with two different pup-stimulus arrangements. Experiment IIA examines two independent groups of early postpartum dams, one group conditioned with age-matched pups, and a second group conditioned with older pups (12-15 days old), as well as two independent groups of late postpartum dams, one group conditioned with age-matched pups, and a second conditioned with younger pups (4-7 days old). The alternative conditioning in all these groups was to a neutral empty chamber. Experiment IIB examines the preference of early and late postpartum dams conditioned to associate 4-7 day old pups with one chamber, and to associate 12-15 day-old pups with the other chamber.
Methods
General methods
Subjects
The subjects (n=90) were primiparous postpartum female Sprague-Dawley rats (original stock from Charles River Laboratories, Kingston, NY) bred in our colony at the Laboratory Animal Facility (LAF) of Rutgers University, Newark Campus, accredited by the American Association for Accreditation of Laboratory Animal Care. At the start of procedures (Mayer and Rosenblatt, 1998), nulliparous females (90 to 120 days old) in behavioral estrus were housed overnight with sexually experienced males. Before giving birth, pregnant females were housed individually in polyethylene shoebox cages (41.9 cm long×20.3 cm wide×20.3 cm high) lined with fresh woodchip bedding (Beta chip, Northeastern Products Corp., Warrensburg, NY), with continuous access to water and food (Lab Diet 2008, PMI Nutrition International, LLC, Brentwood, MO). The day after parturition, litters were culled to eight pups per dam. All females were kept on a 12-hour light/dark cycle (with lights on at 0700) at 22 °C±1 °C. All procedures were in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985).
Conditioned place preference procedure
Apparatus
The place preference apparatus consisted of three equal-sized chambers (27.9 cm long×21.6 cm wide×24.1 cm high) made of clear Plexiglas, with red Plexiglas walls dividing the apparatus into three chambers: left, center, and right; the ceiling was wire mesh. Each side chamber contained unique contextual cues of wallpaper (vertical or horizontal stripes constructed by alternating black and white tape 1.9 cm wide, on the three clear walls) and tactile flooring, either small paper squares (Alpha-Dri, W. F. Fisher & Sons) or corncob pieces (Bed-o-Cobs, The Andersons, Maumee, OH) on either rough or smooth disposable paper bedding (Tech-Board, W. F. Fisher & Sons, Bound Brook, NJ). Chambers were interconnected through circular openings (like a porthole) 7.5 cm in diameter, 4.5 cm from the apparatus floor, at free run and test these openings allowed the subjects access to all three chambers. During conditioning this opening was closed so that the subject was confined to one uniquely cued chamber. A mirror was positioned above the apparatus for unobtrusive behavioral observation by investigators.
Pre-conditioning baseline session
Dams were exposed to the place preference apparatus before conditioning to identify pre-existing chamber preferences, either on day 1 (early) or 9 (late) postpartum. Dams were placed into the center chamber of the apparatus and allowed to explore all three chambers of the apparatus for 30 min. The time the dam spent in each chamber was recorded manually by observers.
Conditioning phase
Early and late postpartum dams were exposed to each unconditioned stimuli once a day for four consecutive days of conditioning during postpartum days 4-7 or 12-15, respectively; each stimulus for 2 hrs at a time. Stimulus-cue pairings were counter-balanced, with specific individuals using one sequence or the other throughout conditioning, as was the order in which dams were conditioned with each stimulus.
Post-conditioning test session
Dams were tested for their chamber preference on the day after the final conditioning session, either on postpartum day 8 or 16. Dams were placed into the center chamber of the apparatus, and allowed exploration of all three chambers for 60 min, with no unconditioned stimuli present. During the testing period, the time the female spent in each chamber was recorded manually by the experimenter. Also, the pattern of activity and exploratory behaviors, such as sniffing, rearing, locomotion, and grooming were noted.
Methods Specific for Experiment I – effect of pup deprivation on CPP
Independent groups of test dams were subjected to a systematic variation in duration of pup deprivation, prior to being conditioned to associate one chamber with unique contextual cues with pup stimuli age-matched to postpartum day of subject. These test dams were also conditioned to associate a different uniquely cued chamber with pup-sized objects. Dams are exposed to the center chamber only during preconditioning baseline session, and during the post conditioning test session; it is not used for conditioning. Thus this center chamber provides a relatively novel, neutral third chamber choice, ensuring that choice of a stimulus associated chamber is not a forced choice to avoid the other stimulus associated chamber.
Early postpartum dams were conditioned on postpartum days 4-7 and tested on postpartum day 8, after being deprived of pups for 15 min (essentially during repositioning of pups for conditioning, n=8), or 2 hrs (n=9), or 6 hrs (n=7) or 12 hrs (n=8) or 22 hrs (n=8). Late postpartum dams were conditioned on postpartum days 12-15 and tested on postpartum day 16, after being deprived of pups for 2 hrs (n=7), or 6 hrs (n=8) or 22 hrs (n=8). Litters were co-mingled prior to the start of experimental procedures for each group and these dams were given 8 age-matched pups to their postpartum day for home cage litters.
Pup Stimul were 4-7 or 12-15 days old, age-matched to the postpartum stage of the early or late postpartum dams, respectively. If dams were in a group deprived of pups for 2 hrs or less prior to conditioning, their pups were also deprived of maternal care for the same time. If dams were in a group deprived of pups for 6, 12, or 22 hrs before conditioning, the pups used in those conditioning sessions were deprived of care giving by dams only for 2-4 hours before use in conditioning. During the remaining time that they were away from their test dam, these pups were placed with foster dams. After conditioning, all experimental dams were reunited with pups in their home cage, except dams in the 22 hr deprivation condition which were returned to their home-cage alone. Thus, once conditioning began, the only time dams in the 22 hr group physically interacted with pups was during the pup-conditioning sessions.
Object Stimuli were pup-sized pieces of bubble® tubing (Kendall Oxford, Durometer (Shore A) PVC tubing, product # 8889-224153). Subjects were initially provided with these objects in their home cages on the day of parturition, ensuring equal familiarity as with pups prior to conditioning. Subjects were not neophobic to the objects and frequently hoarded objects, used them as nesting material, buried, and chewed them. Dams were deprived of objects prior to conditioning with objects (2 hours) and testing on the pup deprivation schedule as above. In order to eliminate odors of home-cage or pups, only clean, new objects were used for conditioning. Pup-sized objects are frequently used in maternal behavior testing (Corodimas et al., 1992; Rosenblatt, 1975).
Methods specific for experiments IIA and B - effect of pup age on CPP
In Experiment IIA four groups of females were conditioned: (1) Early postpartum females (n=9) were conditioned to associate a uniquely cued chamber with 4-7 day-old pups and to associate a second uniquely cued chamber with no stimulus (empty chamber); (2) Early postpartum females (n=7) were conditioned to associate a uniquely cued chamber with 12-15 day-old pups and a second uniquely cued chamber with no stimulus; (3) Late postpartum females (n=7) were conditioned to associate one chamber with 12-15 day-old pups and a second chamber with no stimulus; (4) Late postpartum females (n=6) were conditioned to associate one chamber with 4-7 day-old pups and a second chamber with no stimulus. Thus at test the choice was always a chamber associated with pups or with no stimulus. Prior to this experiment we demonstrated that there was no impact of offering an empty chamber or a chamber associated with objects as the alternative choice to pups in our CPP procedure (data not shown; P>.05). All other methods are as described in General Methods.
Experiment IIB uses two groups of females early (n=8) and late (n=8) postpartum. Each postpartum group was conditioned to associate one uniquely cued chamber with 4-7 day-old pups and a second differently cued chamber with 12-15 day-old pups. Thus in early dams the test examined their preference for a chamber associated with 4-7 day old pups (their contemporaneously aged pups) or a chamber associated with 12-16 day old pups (older pups). Late dams were examined for their preference for a chamber associated with 4-7 day old pups (younger pups) or a chamber associated with 12-16 day old pups (their contemporaneously aged pups).
In both Experiment IIA and IIB, to avoid the confound of preference for novelty that could occur in the conditioning procedure if the females were offered the non-matched pups only during conditioning, females were housed with a mixed litter of 8 pups, two non-matched pups together with 6 pups age-matched to their postpartum day. Females readily accepted non-age matched pups into these mixed litters and cared for them well without exception. All pups were healthy throughout the entire experimental procedure, with pups' weight gain within normal parameters for each age group.
Analyses and statistics
The CPP apparatus includes a third, central chamber of equal size and pre-conditioning chamber preference to the two side conditioning chambers; analysis included full consideration of time in this chamber. By providing a chamber choice with no conditioning, the third chamber ensures that conditioned chamber preferences are not the result of a forced choice between the two stimulus-associated side chambers (Mattson et al., 2001; Seip and Morrell, 2007).
The time spent in each chamber during pre- and postconditioning sessions was used to identify each dam's chamber preference. Subjects that spend at least 50% of the test time in one chamber and 25% more than the time spent in either of the two remaining chambers were categorized as preferring that chamber (Mattson et al., 2001, 2003). Four outcomes of preference using a three-chambered place preference apparatus are possible: a preference for the left, center, or right chamber or no preference for any chamber. Preference responses of individual subjects are presented as proportions of females per group. The time subjects spent in each chamber was averaged across each experimental group's response (mean±standard error of the mean (S.E.M)).
Categorical data from pre- and postconditioning chamber preferences of the individual dams were compared using the Chi-square goodness-of-fit test, followed by one-tailed test for significance of a proportion. Between-group chamber preference comparisons were examined using the Chi-square test when the expected frequency of each cell was >5, or the Fisher's Exact Test when the expected frequency of each cell was <5. The z-test for two proportions assessed between group differences in preference for a single chamber. To examine the time subjects spend in each chamber, analysis of variance (ANOVA) followed by Tukey's honest significant differences (HSD) post hoc test were performed to examine the main effects and interactions of groups. This was preceded by verification that variances of data were homogeneous. A statistical significance level of p<0.05 was used.
Chamber Preference Prior to Conditioning was assessed to verify that dams did not have a preexisting preference for one chamber of our CPP apparatus prior to conditioning. Between-group comparisons revealed that early and late postpartum dams did not differ in their preconditioning chamber preferences and times, and so the data from early and late groups were combined for a baseline response analysis (Figs. 1A and B). The majority (67%) of dams did not have a preexisting preference for any chamber (Fig. 1A). Side chamber times were similar, with center time slightly more than the time spent in the chamber that would become the non-pup-associated chamber (F (2,124)=3.24, p=0.0425; Tukey's HSD: p<0.05) (Fig. 1B). Thus, before conditioning, the apparatus was relatively neutral.
Fig. 1.
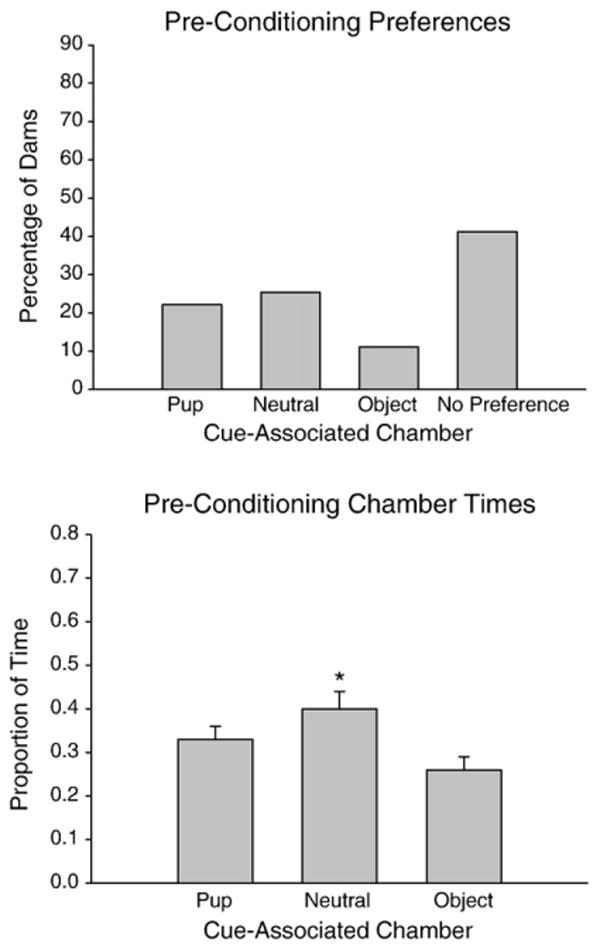
(A) Percentage of dams in each preference category and (B) proportion of time spent in the three-chambered conditioned place preference (CPP) apparatus during the pre-conditioning baseline session, prior to assigning dams to different stimulus-chamber assignment. Data pooled from all experimental groups.
Results
Experiment I - Effect of pup deprivation on CPP
Early postpartum dams
All groups of early postpartum females conditioned with pup stimuli age-matched to their postpartum day demonstrated a consistent and statistically significant change in chamber preference when compared to their baseline preferences prior to conditioning (15 min deprivation group χ2 (3, N=8)=17.08; 2 hr group χ2 (3, N=9)=12.58; 6 hr group χ2 (3, N=7)=7.61; 22 hr group χ2 (3, N=8)=30.83, all p<0.05; exception the 12 hr group χ2 (3, N=8)=3.09, p=ns). Approximately 50% of the dams in groups subjected to 15 min, 2, 6, or 12 hr of pup-deprivation before their daily conditioning with pups met our criterion as having a preference for the pup-associated chamber, while after 22 hr of deprivation this preference extended to 75% of the population (Fig. 2A). These five groups were not statistically different from each other in their chamber preferences or the proportion of time spent in each chamber, and so data from all five groups was pooled for analysis of early postpartum responses, as described below (Figs. 2A and B).
Fig. 2.
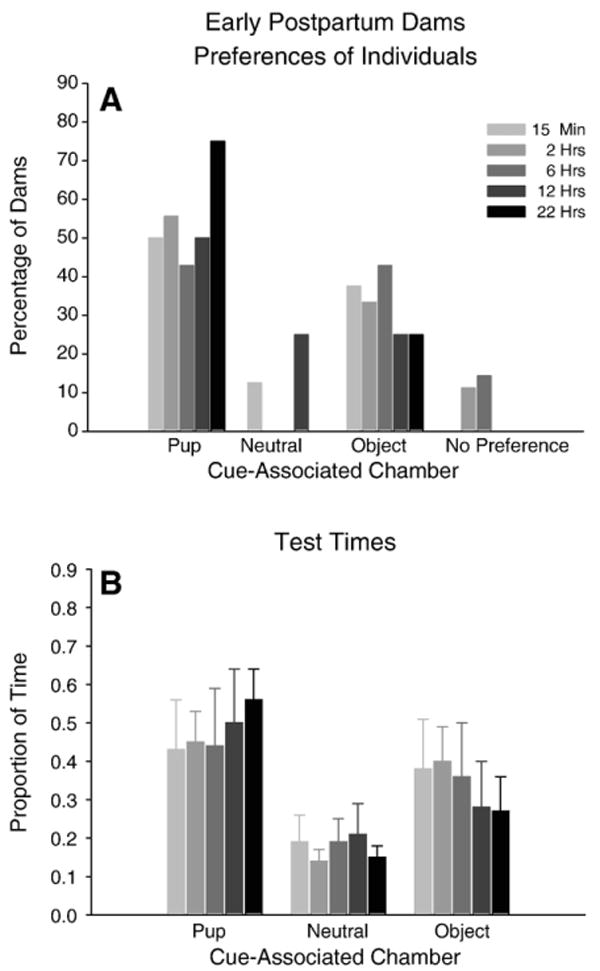
Early Postpartum Dams (A) Percentage of dams in each preference category after 15 min, 2, 6, 12, and 22 hrs of pup-deprivation prior to conditioning and testing. (B) Proportion of time spent by dams in each cue-associated chamber after 15 min, 2, 6, 12, and 22 hrs of pup-deprivation prior to conditioning and testing.
Conditioning had a substantial and statistically significant effect on chamber preference in early postpartum dams as reflected in these pooled data (χ2 (3, N=40)=48.61, p<0.0001). After conditioning, the majority of dams (55%) preferred the pup-associated chamber. This was a substantial and statistically significant increase over the percentage of dams (22%) with pre-conditioning preferences for that chamber (z=4.90, p<0.0001). In addition, a surprising number of dams (32%) were categorized as preferring the object-associated chamber after conditioning, a change from the original 13% observed prior to conditioning. This change was statistically significant (z=3.85, p<0.0001). Post-conditioning, few dams had no chamber preference or preferred the center chamber.
Conditioning consistently and statistically significantly altered the proportion of time early postpartum dams spent in particular chambers as reflected in the data pooled from the five groups (Two-way repeated measures ANOVA, conditioning×chamber time interaction: F (2, 78)=8.84, p=0.0004). After conditioning, dams spent the majority of their time in the pup-associated chamber, a statistically significant increase over the time spent in this chamber prior to conditioning (Tukey's HSD: p=0.026). The dams also spent a greater proportion of time in the object-associated chamber after conditioning, but this increase only approached statistical significance (Tukey's HSD: p=0.074). Time spent in the neutral center chamber significantly decreased after conditioning (Tukey's HSD: p<0.0001).
Analysis of the proportion of time the dams spent in the three chambers after conditioning revealed a statistically significant difference (One-way repeated measures ANOVA: F (2, 78)=8.18, p=0.006). Post-hoc analysis revealed that the dams spent a significantly greater proportion of time in the pup-associated chamber compared to the neutral chamber (Tukey's HSD: p<0.05). No other comparisons were statistically significant.
Late postpartum dams
Conditioning markedly altered chamber preferences of all three groups of late postpartum dams (2 hr deprivation group χ2 (3, N=7)=19.14; 6 hr group: χ2 (3, N=8)=41.85; 22 hr group: χ2 (3, N=8)=29.27, all p<0.01). Moreover, the chamber times of each group changed significantly as a function of conditioning (Two-way repeated measures ANOVA, session×chamber time interaction: 2 hr: F (2, 12)=3.91, p<0.05; 6 hr: F (2, 14)=3.85, p<0.05; 22 hr: F (2, 14)=5.02, p<0.05).
There were interesting and statistically significant differences in chamber preferences after conditioning that depended upon the time the dams were deprived of pups prior to conditioning and testing (Fig. 3A). Seventy-five percent of the dams in the group deprived of pups for 22 hr met the criterion for pup-associated chamber preference, while only approximately 14% of the dams deprived of pups up to 6 hr preferred this chamber (Fisher's Exact Test; p=0.0190; z=2.98, p=0.0014; Fig. 3A). Furthermore, approximately 80% of the dams deprived of pups up to 6 hr prior to conditioning met the criterion for object-associated chamber preference compared to just 13% of the dams that were deprived of pups for 22 hr (Fisher's Exact Test; p=0.0089; z=3.11, p<0.001; Fig. 3A). Few late postpartum dams had no chamber preference after conditioning, and few preferred the center chamber.
Fig. 3.
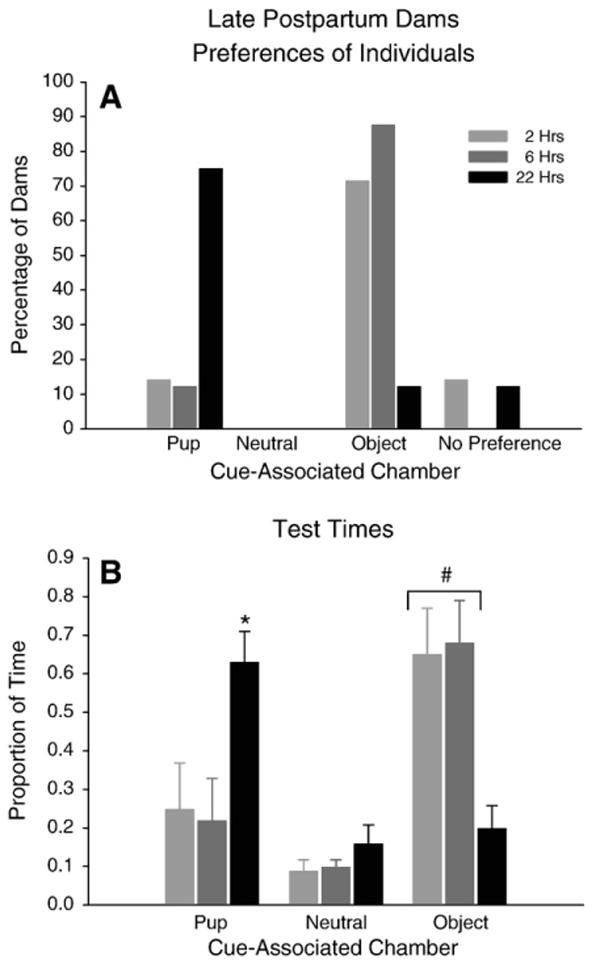
Late Postpartum Dams (A) Percentage of dams in each preference category after 2, 6, and 22 hrs of pup-deprivation prior to conditioning and testing. (B) Proportion of time spent by dams in each cue-associated chamber after 2, 6, or 22 hrs of pup-deprivation prior to conditioning and testing.
The proportion of time spent in each stimulus-associated chamber also differed remarkably as a function of the length of pup-deprivation between these three groups (Two-way repeated measures ANOVA, deprivation time×chamber time interaction: F (4, 40)=6.25, p=0.0047; Fig. 3B). When the proportion of time spent in each chamber was compared with individual one-way ANOVAs, dams in the 22 hr group spent a significantly greater proportion of time in the pup-associated chamber compared to dams in the 2 and 6 hr groups (F (2, 20)=5.30, p=0.014; Tukey's test: HSD: p<0.05). In contrast, dams deprived of pups up to 6 hr spent a significantly greater proportion of time in the object-associated chamber compared to dams in the 22 hr group (F (2, 20)=7.98, p=0.003; Tukey's HSD: p<0.05).
Comparison of early and late postpartum preferences after up to 6 hrs of Pup Deprivation
Since early postpartum dams deprived of pups for 15 min, 2, or 6 hr prior to conditioning did not differ statistically (Figs. 2A & B above), data from these groups were pooled for this analysis (Figs. 4A & B). Similarly, the data for the late postpartum dams deprived of pups for 2 or 6 hrs (Figs. 3A & B) were pooled for this analysis (Figs. 4A & B).
Fig. 4.
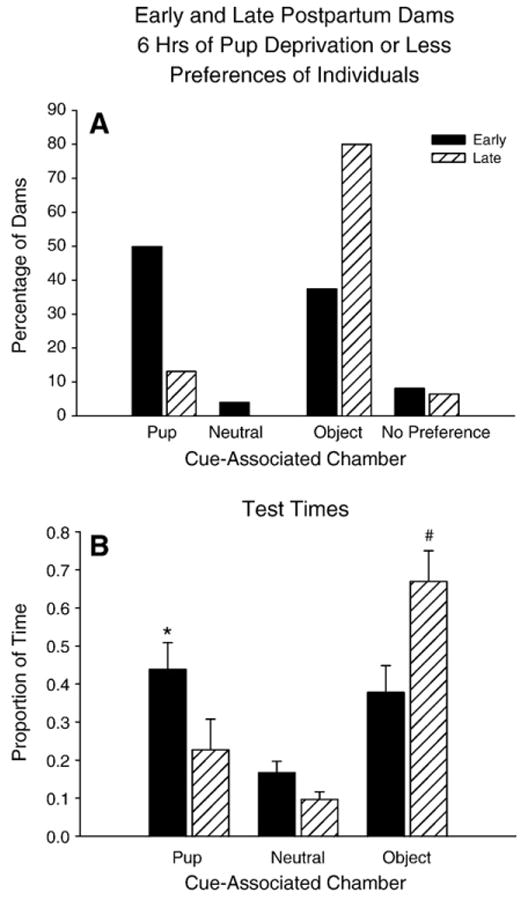
Early and Late Postpartum dams after 6 hrs of pup-deprivation or less. (A) Percentage of early (cross-hatched bars) and late (solid bars) postpartum dams in each preference category after conditioning. (B) Proportion of time spent by early and late postpartum dams in each cue-associated chamber after conditioning.
These pooled data (Fig. 4A) provides a simplified analysis demonstrating that the chamber preferences of early postpartum dams were markedly and statistically significantly different from the chamber preferences of late postpartum dams. Fifty percent of early postpartum dams preferred the pup-associated chamber compared to just 13% of the late postpartum dams (χ2 (1, N=39)=5.39, p=0.0378; z=2.34, p=0.0096). Conversely, 80% of the late postpartum dams preferred the object-associated chamber compared to 38% of the early postpartum dams (χ2 (1, N=39)=6.71, p=0.0096; z=2.59, p=0.0048).
Similarly, the proportion of time spent in each chamber also differed as a function of the postpartum period (Two-way repeated measures ANOVA, postpartum period×chamber time interaction: F (2, 74)=5.54, p=0.0165) (Fig. 4B). Early postpartum dams spent almost twice the time in the pup-associated chamber compared to late postpartum dams (Tukey's HSD: p=0.05). In contrast, late postpartum dams spent a significantly greater proportion of time in the object-associated chamber compared to early postpartum dams (Tukey's HSD: p=0.01).
Responses after 22 hrs of Pup Deprivation
In marked contrast to the dams deprived of pups for less time (above), the preference responses of the early and late postpartum dams were remarkably similar after 22 hr of pup-deprivation (Fig. 5A), with seventy-five percent of the dams in each group preferring the pup-associated chamber. The two groups spent their time after conditioning similarly, with both spending the major proportion of the test time in the pup-associated chamber (Two-way repeated measures ANOVA, main effect for chamber: F (2, 28)=16.40, p=0.0002) (Fig. 5B). Furthermore, these dams spent a significantly greater proportion of time in the pup-associated chamber compared to both the center chamber and the object-associated chamber (One-way repeated measures ANOVA: F (2, 30)=17.04, p<0.0001; Tukey's HSD: all p<0.05).
Fig. 5.
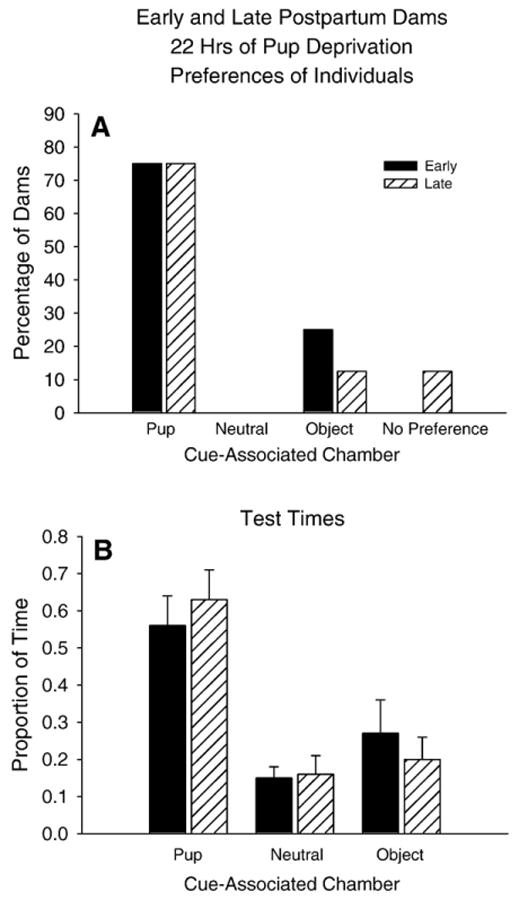
Early and Late Postpartum dams after 22 hrs of pup-deprivation. (A) Percentage of early and late postpartum dams in each preference category after conditioning. (B) Proportion of time spent by early and late postpartum dams in each cue-associated chamber after conditioning.
Experiment II Effects of pup age on CPP for pups
Experiment IIA
All early and late postpartum groups showed a statistically significant change when chamber preferences before conditioning were compared to those after conditioning [early postpartum dams with 4-7 day-old pups: χ2 (3, N=9)=8.66 or 12-15 day-old pups: χ2 (3, N=7)=16.29; late postpartum dams with 4-7 day-old pups: χ2 (3, N=6)=20.21 or 12-15 day-old pups: χ2(3, N=7)=24.83 - all p<0.05]. More dams in both early postpartum groups and the late postpartum females conditioned with younger pups (4-7 day old) preferred the pup-associated chamber after conditioning than prior to conditioning (early PP dams with 4-7 day-old pups: z=2.46, and 12-15 day-old pups: z=2.56; late PP dams with 4-7 day-old pups: z=2.09 - all p<0.05). Late postpartum group conditioned with age-matched pups did not exhibit this pup-associated chamber preference after conditioning.
After conditioning, most early postpartum dams exhibited a pup-associated chamber preference regardless of the age of pup stimuli used for conditioning (Fig. 6A) such that preference responses were similar in early postpartum dams conditioned with age-matched or older pups. As expected, few late postpartum dams exhibited a preference for age-matched pups (Fig. 6A). In notable contrast, the majority of late postpartum dams conditioned with younger pups preferred the pup-associated cues, thus having a preference pattern comparable to that of early postpartum dams. Pup-associated chamber times followed the subjects preferences (main chamber effect: F (2,48)=4.06, p<0.05 - early PP dams with 4-7 day-old pups>early PP dams with 12-15 day-old pups>late PP dams with 4-7 day-old pups>late PP dams with 12-15 day-old pups, Tukey's HSD: all p<0.05), while the opposite was true for the empty chamber (Tukey's HSD: all p<0.05) (Fig. 6B). There were no substantial differences within postpartum groups in the mean time spent in the pup- and empty-associated chambers (Fig. 6B).
Fig. 6.
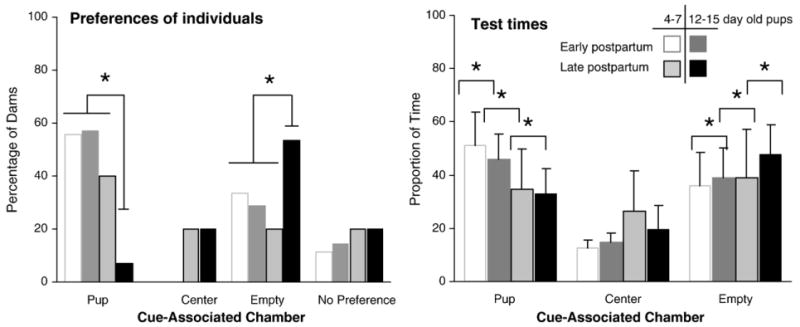
Effect of age of the pups on place preference in early and late postpartum dams: (A) Percentage of early and late postpartum dams in each preference category after conditioning with 4-7 day-old and 12-15 day-old pups. (B) Proportion of time spent by early and late postpartum dams in each cue-associated chamber after conditioning with 4-7 day-old and 12-15 day-old pups.
Experiment IIB
When early and late postpartum dams were given a direct choice between one chamber associated with 4-7 days-old pups and the alternative chamber associated with 2-15 days-old pups, neither of the postpartum groups show a preference of one chamber over the other (data not illustrated). That is, both early and late postpartum dams had about equal preference for the two pup-associated chambers, and no preference for the center chamber. Moreover, there were no substantial differences within groups in the mean time spent in any of the chambers.
Discussion
Preferences for cued chambers associated with pups age-matched to the postpartum stage of the dam were higher during the early than the late postpartum period, suggesting that maternal motivation to seek pups diminishes as the postpartum period progresses. Early postpartum females had equally robust pup-associated place preference regardless of the age of the pups used for conditioning. Further, the length of pup deprivation did not significantly impact pup-associated preference response in early dams. In notable contrast, robust pup-associated place preference could only be revealed in late postpartum if these dams were conditioned with young pups or when conditioned with age-matched pups only if these dams were pup deprived for a prolonged period (22 hrs). These results suggest that the motivation of the female is partly dependent upon the developing needs of the pups, and partly a function of the postpartum stage and that there is considerable plasticity in maternal motivation that is likely adaptive for the survival of the pups.
Our data accord well with the CPP findings of Fleming (et al., 1994) in dams up to day 10 postpartum and with our prior data using dual reinforcers in a choice CPP paradigm in both early and late postpartum (Mattson et al., 2001; Seip and Morrell, 2007). Our data also accord well with data from other approaches which show that dams in the first half of the postpartum period learn complex mazes (Simons, 1924; Szymanski and Munn, 1950), or cross electrified grids to gain access to pups (Nissen, 1930; Warden, 1931), and are more willing than maternal virgin females to retrieve pups from mazes (Bridges et al., 1972; Fahrbach and Pfaff, 1982; Gandelman et al., 1970; Stern and Mackinnon, 1976). Operant procedure approaches show that early postpartum females bar press to gain access to pups at the amazing rate of 1-4 pups a minute, a response that continues for hours and results in retrieval of hundreds of pups, when pups less than 8 days old are the stimuli (Hauser and Gandelman, 1985; Lee et al., 2000; Wilsoncroft, 1969). In a rare examination of the later postpartum period, Hauser and Gandelman (1985) found that operant responding for pups decreased as postpartum progressed, a finding that also accords well with our current and prior (Mattson et al., 2001; Seip and Morrell, 2007) data suggesting diminished maternal motivation in the late postpartum.
Tests of motivation other than CPP examine both the initial appetitive state, and the appetitive state subsequent to achieving the desired stimulus, as actions to obtain pups or nest material occur interspersed with the consummatory aspects of the behavior (Lee et al., 2000; Van Hemel, 1973; Oley and Slotnick, 1970). These consummatory responses necessarily impact on the subsequent appetitive state of the female. Indeed pup-reinforced bar-pressing decreased when the females were prevented from retrieving the pups to the nest afterwards. Regardless of whether the approach used to examine maternal motivation examines only the initial appetitive state or the more complex state subsequent to acquiring the stimulus the motivational responsiveness of the dam changes as the postpartum period progresses, with a clear decrease in motivational responses in the context of normally developing pups (Lonstein and Morrell, 2007).
Our current data and that of others show that younger pups are more salient stimuli for postpartum rodents (Mayer and Rosenblatt, 1980; Noirot, 1964a,b, 1965; Stern and Mackinnon, 1978; Reisbick et al., 1975). Older pups elicit fewer maternal responses suggesting that they are less attractive to dams (Rosenblatt, 1965). We posit that both the changing nature of the pup stimulus and the changing status of the postpartum female are at work; as pups develop, they presumably impact differently on the physiological and endocrine state of the female as she progresses through the postpartum period.
It is interesting that only the place preference choice associated with the greatest stimulus difference revealed the motivational preference for younger pups. This was the choice between either a cued-chamber associated with pups versus another associated with no stimulus. Since the relative incentive value of a stimulus is importantly influenced by the contrast among stimuli (Flaherty, 1996), we posit that this level of stimulus contrast was key to a working assay. Specifically we posit that the procedure in which the dam had to choose between a cued chamber associated with younger versus another associated with older pups (Experiment II B), did not offer sufficient stimulus contrast and was therefore not sufficiently sensitive.
We noted that the predominant behavior observed during each pup-conditioning session was nursing. The demands that developing pups place on their female's milk production do increase across postpartum (Babicky et al., 1970; Hanwell and Linzell 1972; Numan and Insel, 2003; Lehrman, 1961) such that in the later period the pups demand approximately five times greater milk volumes than in early postpartum (Hall and Rosenblatt, 1977). However we chose our early versus late postpartum groups and litter size to be well within the time in which pups are completely dependent upon their dams for all their nutritional needs, as well documented by prior work (Rosenblatt et al., 1985). Since our litters are normally weaned at day 21 postpartum, five days after the last test in these experiments we do not believe that decreased motivation seen in the late group was simply related to the initial phases of the weaning process.
In the case of the substantial preference response evoked in postpartum dams by the 22 hour pup deprivation, we have also considered that the prolonged pup deprivation might evoke higher pup-associated place preference for a physiological reason connected with nursing. Possibly the mammary glands of the postpartum females were engorged with milk after being separated from pups from 22 hrs, but not after shorter intervals. While it is a formal possibility that the relief from intra-mammary pressure was the factor that made the 22 hr condition most rewarding, particularly in late postpartum, the fact that maternal virgins deprived of their pups for 23 hours show marked, if not identically high, levels of pup preference compared to postpartum nursing females (Fleming et al., 1994) argues against this possibility.
During the postpartum period there are dramatic endocrine changes which may influence the brain's motivational systems. During early postpartum levels of estradiol are consistently low while levels of progesterone increase to moderately high while levels of prolactin are consistently high (Smith and Neill, 1977; Taya and Greenwald, 1982). In contrast, the late postpartum period is characterized by increasing levels of estradiol (which reach levels of the evening of diestrus-2 in the cycling female by day 20 postpartum), high but decreasing levels of progesterone, and low levels of prolactin. While the role of these hormones in the underpinnings of maternal motivation remains to be determined there is a particularly suggestive parallel between high maternal motivation and high prolactin levels in early postpartum. Circulating prolactin is high on postpartum days 4-10, declines sharply by postpartum day 12, and is low on postpartum days 12-21 (Taya and Greenwald, 1982). Thus, in our procedures early postpartum dams are conditioned and tested when levels of prolactin are high, whereas late postpartum dams are conditioned and tested when levels of prolactin are low. Prolactin has been shown to facilitate the rapid onset of maternal behavior in the rat (Bridges et al., 1985, 1990; Bridges, 1996), and this may extend to a role in maternal motivation. We posit that these effects on the brain would be independent of the peripheral role of prolactin in the regulation of milk production and nursing responses.
Whether ultimately driven by hormones or independent of hormonal influences, a differential response of the brain's neurochemistry likely mediates the differential place preference responses associated with young pups or when conditioning with contemporaneous pups is carried out after a prolonged period of pup-deprivation. While our understanding of the role of dopamine in mediation of reward is still developing (Wise, 2004; Salamone et al., 2007), the mesolimbic dopamine (DA) system is critically involved in the regulation of maternal responsiveness to pups. Interaction with pups after a separation period increases extracellular DA release within the nucleus accumbens (NA) of the dams (Champagne et al., 2004; Hansen et al., 1993) and restores the behavioral deficits in pup-care activities in dams with DA depletions in the ventral striatum (Hansen 1994; Hansen et al., 1993) or treated with DA antagonists (Keer and Stern, 1999; Stern and Taylor, 1991). Also, natural variations in the expression of maternal behavior (i.e. high vs low licking/grooming) are correlated with changes in the magnitude of DA signal in the nucleus accumbens (Champagne et al., 2004). Further, interaction with younger pups or demanding pups that have a stronger incentive salience for the dams induces more robust expression of maternal behavior (Pereira and Ferreira, 2006; Reisbick et al., 1975; Stern and Levine, 1972). Such pups can also stimulate good maternal behavior in dams with impaired maternal expression due to DA antagonist (Pereira and Ferreira, 2006). Furthermore, D1 DA receptor agonists increase active maternal behaviors and preference for pup-associated cues in late postpartum (Pereira et al., 2007, in press).
Taking all of these data into consideration, we hypothesize that pup-induced activation of the DA system is not sufficient to promote highly motivated behaviors in late postpartum dams which are interacting with contemporaneously aged pups. Possibly there was greater activation in this system in late postpartum dams interacting with younger pups, and with age-matched pups after prolonged periods of deprivation. We speculate that this increase in DA response makes the pup-associated cued chamber more reinforcing. By contrast, shorter intervals of pup-deprivation (6 hrs or less) may have induced sub-threshold dopamine release in the ventral striatum of the later but not the earlier postpartum females during conditioning, therefore rendering the pup-associated cues less reinforcing to the later postpartum dams.
Little is known about how other neurotransmitters or neuromodulators might be impacting maternal motivation. Since there is a suckling-induced release of β-endorphin (Bridges, 1996; Selmanoff and Gregerson, 1986), possibly this release might be greater after longer deprivation periods which might increase the reinforcing properties of the pup stimulus during conditioning. Different deprivation times may alter the amount of ACTH released upon reuniting with pups as others have shown that both postpartum state and pup separation are significant forces in ACTH regulation (Walker et al., 1992). Varied ACTH responsiveness may contribute to varied pup-associated place preference responses either by impact on learning or possibly by reducing the positive incentive salience of the stimulus (Broadbear et al., 2002).
Our place preference results accord with the progression of expression of maternal behavior in postpartum. There is a decline in care giving such that the robust activity of early postpartum is reduced starting around day 12 postpartum (Stern and Levine, 1972; Reisbick et al., 1975; Rosenblatt et al., 1985; Rosenblatt, 1965). Early postpartum dams voluntarily spend approximately 70% of their time with the pups, with only short intervals (minutes) away from pups. In contrast late postpartum, dams spend only 38% of their time with the pups in a pattern of much short times with and much longer time (hours) away from pups (Grota and Adler, 1969, 1974; Pereira et al., 2007, in press). While during this late postpartum period infant rats are increasingly exploratory (Smith and Morrell, 2007), they are also vigilantly barricaded in the maternal nest by the dam both when she is in the nest and when she leaves (Barnett, 1963; Galef, 1971, 1977; Galef and Clark, 1971; Pereira et al., 2007, in press; Small 1899), suggesting a plasticity in the responses of the dams. Female rats are very successful mothers across a wide variety of environmental demands, and we speculate that plasticity in their motivation shapes the expression of maternal behavior adaptively for the survival of the pups.
References
- Babicky A, Ostadalova I, Kolar PJ, Bibr B. Use of radioisotope techniques for determining the weaning period in experimental animals. Physiol Bohemoslov. 1970;19:457–467. [PubMed] [Google Scholar]
- Barnett SA. The rat: A study in behaviour. Aldine; Chicago: 1963. [Google Scholar]
- Bridges RS. Biochemical basis of parental behavior in the rat. In: Rosenblatt JS, Snowdon CT, editors. Advances in the Study of Behavior. Vol. 25. Academic Press; New York: 1996. pp. 215–242. [Google Scholar]
- Bridges RS, DiBiase R, Loundes DD, Doherty PC. Prolactin stimulation of maternal behavior in female rats. Science. 1985;227:782–784. doi: 10.1126/science.3969568. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Numan M, Ronsheim PM, Mann PE, Lupini CE. Central prolactin infusions stimulate maternal behavior in steroid-treated, nulliparous female rats. Proc Natl Acad Sci U S A. 1990;87:8003–8007. doi: 10.1073/pnas.87.20.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges R, Zarrow MX, Gandelman R, Denenberg VH. Differences in maternal responsiveness between lactating and sensitized rats. Dev Psychobiol. 1972;5:123–127. doi: 10.1002/dev.420050205. [DOI] [PubMed] [Google Scholar]
- Broadbear JH, Winger G, Rice KC, Woods JH. Antalarmin, a putative CRH-RI antagonist, has transient reinforcing effects in rhesus monkeys. Psychopharmacology. 2002;164:268–276. doi: 10.1007/s00213-002-1187-y. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24:4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corodimas KP, Rosenblatt JS, Morrell JI. The habenular complex mediates hormonal stimulation of maternal behavior in rats. Behav Neurosci. 1992;106:853–865. doi: 10.1037//0735-7044.106.5.853. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Pfaff DW. Hormonal and neural mechanisms underlying maternal behavior in the rat. In: Pfaff DW, editor. The Physiological Mechanisms of Motivation. Springer-Verlag; New York: 1982. pp. 253–285. [Google Scholar]
- Flaherty C. Incentive relativity. Cambridge University Press; New York: 1996. [Google Scholar]
- Fleming AS, Korsmit M, Deller M. Rat pups are potent reinforcers to the maternal animal: effects of experience, parity, hormones, and dopamine function. Psychobiology. 1994;22:44–53. [Google Scholar]
- Galef BG. Social effects in the weaning of domestic pups. J Comp Physiol Psychol. 1971;75:358–362. [Google Scholar]
- Galef BG. Mechanisms for the social transmission of food preferences from adult to weanling rats. In: Barker LM, Best M, Domjan M, editors. Learning Mechanisms in Food Selection. Baylor; Waco: 1977. pp. 123–150. [Google Scholar]
- Galef BG, Jr, Clark MM. Social factors in the poison-avoidance and feeding behavior of wild and domesticated rat pups. J Comp Physiol Psychol. 1971;75:341–357. doi: 10.1037/h0030937. [DOI] [PubMed] [Google Scholar]
- Gandelman R, Zarrow MX, Denenberg VH. Maternal behavior: differences between mother and virgin mice as a function of the testing procedure. Dev Psychobiol. 1970;3:207–214. doi: 10.1002/dev.420030308. [DOI] [PubMed] [Google Scholar]
- Grota LJ, Adler R. Continous recording of maternal behavior in rattus norvegicus. Anim Behav. 1969;17:722–729. [Google Scholar]
- Grota LJ, Ader R. Behavior of lactating rats in a dual-chambered maternity cage. Horm Behav. 1974;5:275–282. doi: 10.1016/0018-506x(74)90014-2. [DOI] [PubMed] [Google Scholar]
- Hall WG, Rosenblatt JS. Suckling behavior and intake control in the developing rat pup. J Comp Physiol Psychology. 1977;91:1232–1247. [Google Scholar]
- Hansen S. Maternal behavior of female rats with 6-OHDA lesions in the ventral striatum: characterization of the pup retrieval deficit. Physiol Behav. 1994;55:615–620. doi: 10.1016/0031-9384(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Hansen S, Bergvall AH, Nyiredi S. Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol Biochem Behav. 1993;45:673–676. doi: 10.1016/0091-3057(93)90523-v. [DOI] [PubMed] [Google Scholar]
- Hauser H, Gandelman R. Lever pressing for pups: evidence for hormonal influence upon maternal behavior of mice. Horm Behav. 1985;19:454–468. doi: 10.1016/0018-506x(85)90041-8. [DOI] [PubMed] [Google Scholar]
- Hanwell A, Linzell JL. A simple technique for measuring the rate of milk secretion in the rat. Comp Biochem Physiol A. 1972;43:259–270. doi: 10.1016/0300-9629(72)90184-3. [DOI] [PubMed] [Google Scholar]
- Keer SE, Stern JM. Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiol Behav. 1999;67:659–669. doi: 10.1016/s0031-9384(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res. 2000;108:215–231. doi: 10.1016/s0166-4328(99)00170-9. [DOI] [PubMed] [Google Scholar]
- Lehrman DS. Hormonal regulation of parental behavior in birds and infrahuman mammals. In: Young WC, editor. Sex and internal secretions. Williams and Wilkins; Baltimore: 1961. pp. 1268–1382. [Google Scholar]
- Lonstein JS, Morrell JI. Neuroendocrinology and neurochemistry of maternal motivation and behavior. In: Blaustein J, editor. Behavioral Neurochemistry, Neuroendocrinology, and Molecular Neurobiology. Chapter 5 2007. [Google Scholar]; Lajtha A, editor. Handbook of Neurochemistry and Molecular Neurobiology. 3rd. pp. 2–51. Series Ed. [Google Scholar]
- Magnusson JE, Fleming AS. Rat pups are reinforcing to the maternal rat: role of sensory cues. Psychobiology. 1995;23:69–75. [Google Scholar]
- Mattson BJ, Williams S, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci. 2001;115:683–694. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Williams SE, Rosenblatt JS, Morrell JI. Preferences for cocaine- or pup-associated chambers differentiates otherwise behaviorally identical postpartum maternal rats. Psychopharm y (Berl) 2003;167:1–8. doi: 10.1007/s00213-002-1351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AD, Rosenblatt JS. Hormonal interaction with stimulus and situational factors in the initiation of maternal behavior in nonpregnant rats. J Comp Physiol Psychol. 1980;94:1040–1059. doi: 10.1037/h0077744. [DOI] [PubMed] [Google Scholar]
- Mayer AD, Rosenblatt JS. A method for regulating the duration of pregnancy and the time of parturition in Sprague-Dawley rats (Charles River CD strain) Dev Psychobiol. 1998;32:131–136. [PubMed] [Google Scholar]
- Nissen HW. A study of maternal behavior in the white rat by means of the obstruction method. J Genetic Psych. 1930;37:377–393. [Google Scholar]
- Noirot E. Changes in responsiveness to young in the adult mouse. I. The problematic effect of hormones. Animal Behav. 1964a;12:52–58. [Google Scholar]
- Noirot E. Changes in responsiveness in the adult mouse. IV. The effect of an initial contact with a strong stimulus. Animal Behav. 1964b;12:442–445. [Google Scholar]
- Noirot E. Changes in responsiveness to young in the adult mouse. III The effect of immediately preceding performances. Behaviour. 1965;24:318–325. doi: 10.1163/156853965x00084. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. The Neurobiology of Parental Behavior. Springer-Verlag; New York: 2003. [Google Scholar]
- Oley NN, Slotnick BM. Nesting material as a reinforcer for operant behavior in the rat. Psychonomic Sci. 1970;21:41–43. [Google Scholar]
- Pereira M, Ferreira A. Demanding pups improve maternal behavioral impairments in sensitized and haloperidol-treated lactating female rats. Behav Brain Res. 2006;175:139–148. doi: 10.1016/j.bbr.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Pereira M, Dziopa EI, Morrell JI. Expression of maternal behavior during the late postpartum period: Effect of D1 dopamine receptor stimulation. Parental Brain Conference; Boston, MA. 2007. Abstract. [Google Scholar]
- Pereira M, Seip K, Morrell JI. Maternal motivation across the postpartum period. In: Bridges R, editor. Neurobiology of the Parental Brain. Elsevier; in press. [Google Scholar]
- Reisbick S, Rosenblatt JS, Mayer AD. Decline of maternal behavior in the virgin and lactating rat. J Comp Physiol Psychol. 1975;89:722–732. doi: 10.1037/h0077059. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS. The basis of synchrony in the behavioral interaction between the mother and her offspring in the laboratory rat. In: Foss BM, editor. Determinants of Infant Behaviour III. Methuen; London: 1965. pp. 3–45. [Google Scholar]
- Rosenblatt JS. Selective retrieving by maternal and nonmaternal female rats. J Comp Physiol Psychol. 1975;88:678–686. doi: 10.1037/h0076410. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS, Mayer AD, Siegel HI. Maternal behavior among non-primate mammals. In: Adler N, Pfaff D, Goy RW, editors. Handbook of Behavioral Neurobiology Reproduction. Vol. 7. Plenum Press; New York: 1985. [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Seip KM, Morrell JI. Increasing the incentive salience of cocaine challenges preference for pup- over cocaine-associated stimuli during early postpartum: place preference and locomotor analyses in the lactating female rat. Psychopharmacology. 2007;194:309–319. doi: 10.1007/s00213-007-0841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seip KM, Pereira M, Wansaw MP, Reiss JI, Dziopa EI, Morrell JI. Incentive salience of cocaine remains remarkably stable across the postpartum period, and differs from virgin and male responses: place preference and locomotor analyses. Psychopharm In press. [Google Scholar]
- Selmanoff M, Gregerson KA. Suckling-induced prolactin release is suppressed by naloxone and simulated by beta-endorphin. Neuroendocrinology. 1986;42:255–259. doi: 10.1159/000124448. [DOI] [PubMed] [Google Scholar]
- Simons R. The relative effectiveness of certain incentives in animal learning. Comp Psychol Monogr. 1924;2 [Google Scholar]
- Small WS. Notes on the psychic development of the young rat. Am J Psychol. 1899;11:80–100. [Google Scholar]
- Smith KS, Morrell JI. Comparison of infant and adult rats in exploratory activity, diurnal patterns, and novel and anxiety-provoking environments. Behav Neurosci. 2007;12:449–461. doi: 10.1037/0735-7044.121.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MS, Neill JD. Inhibition of gonadotropin secretion during lactation in the rat: relative contribution of suckling and ovarian steroids. Biol Reprod. 1977;17:255–261. doi: 10.1095/biolreprod17.2.255. [DOI] [PubMed] [Google Scholar]
- Stern JM, Levine S. Pituitary-adrenal activity in the postpartum rat in the absence of suckling stimulation. Horm and Behav. 1972;3:237–246. doi: 10.1016/0018-506x(72)90037-2. [DOI] [PubMed] [Google Scholar]
- Stern JM, Mackinnon DA. Postpartum, hormonal, and nonhormonal induction of maternal behavior in rats: effects on T-maze retrieval of pups. Horm Behav. 1976;7:305–316. doi: 10.1016/0018-506x(76)90036-2. [DOI] [PubMed] [Google Scholar]
- Stern JM, Mackinnon DA. Sensory regulation of maternal behavior in rats: effects of pup age. Dev Psychobiol. 1978;11:579–586. doi: 10.1002/dev.420110607. [DOI] [PubMed] [Google Scholar]
- Stern JM, Taylor LA. Haloperidol inhibits maternal retrieval and licking, but enhances nursing behavior and litter weight gains in lactating rats. J Neuroendo. 1991;3:591–596. doi: 10.1111/j.1365-2826.1991.tb00323.x. [DOI] [PubMed] [Google Scholar]
- Szymanski JS, Munn NL. Handbook of Psychological Research on the Rat. Houghton-Mifflin; New York: 1950. p. 311. [Google Scholar]
- Taya K, Greenwald GS. Peripheral blood and ovarian levels of sex steroids in the lactating rat. Endocrinol Jpn. 1982;29:453–459. doi: 10.1507/endocrj1954.29.453. [DOI] [PubMed] [Google Scholar]
- Van Hemel SB. Pup retrieving as a reinforcer in nulliparous mice. J Exp Anal Behav. 1973;19:233–238. doi: 10.1901/jeab.1973.19-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CD, Lightman SL, Steele MK, Dallman MF. Suckling is a persistent stimulus to the adrenalcortical system in the rat. Endocrinology. 1992;130:115–125. doi: 10.1210/endo.130.1.1309321. [DOI] [PubMed] [Google Scholar]
- Warden CJ. Animal Motivation Studies, The Albino Rat. Columbia Press; New York: 1931. [Google Scholar]
- Wilsoncroft WE. Babies by bar-press: maternal behavior in the rat. Behav Res Meth Instrum. 1969;1:229–230. [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]


