Abstract
Histone deacetylase 6 (HDAC6), a unique cytoplasmic deacetylase, likely plays a role in neurodegeneration by coordinating cell responses to abnormal protein aggregation. Here we provide in vitro and in vivo evidence that HDAC6 interacts with tau, a microtubule-associated protein that forms neurofibrillary tangles in Alzheimer’s disease (AD). This interaction is mediated by microtubule binding domain on tau and SE14 domain on HDAC6. Treatment with tubacin, a selective inhibitor of tubulin deacetylation activity of HDAC6, did not disrupt HDAC6-tau interaction. Nonetheless tubacin treatment attenuated site-specific tau phosphorylation, as did shRNA-mediated knockdown of HDAC6. Proteasome inhibition potentiated HDAC6-tau interaction and facilitated the concentration and co-localization of HDAC6 and tau in a perinuclear aggresome-like compartment independent of HDAC6 tubulin deacetylase activity. Furthermore, we observed that in AD brains the protein level of HDAC6 was significantly increased. These findings establish HDAC6 as a tau-interacting protein and as a potential modulator of tau phosphorylation and accumulation.
Keywords: Alzheimer’s disease, phosphorylation, protein accumulation, tubacin, tubulin deacetylation
Introduction
Histone deacetylase 6 (HDAC6) is a unique member of class II HDACs (Verdin et al. 2003). Unlike other HDACs, HDAC6 has two complete catalytic deacetylase domains which largely determine its diverse substrates. In the cytoplasm where it is primarily localized, HDAC6 modulates both microtubule-dependent and actin-dependent cell motility by regulating the acetylation levels of α-tubulin and cortactin, an F-actin-binding protein (Hubbert et al. 2002, Zhang et al. 2003, Matsuyama et al. 2002, Zhang et al. 2007), respectively. Besides cytoskeleton proteins, HDAC6 also associates with and deacetylates heat shock protein 90 (Hsp90). HDAC6 deficiency results in hyperacetylation of Hsp90 and disruption of Hsp90-dependent maturation of client proteins (Kovacs et al. 2005, Murphy et al. 2005, Bali et al. 2005). HDAC6 also forms a complex with protein phosphatase 1 (PP1). Dissociation of the complex induced by the HDAC inhibitor trichostatin A (TSA) results in increased PP1-Akt association and decreased Akt phosphorylation (Brush et al. 2004, Chen et al. 2005).
In addition to its deacetylation activity, HDAC6 also plays a key role in communicating with the ubiquitin network through its unique C-terminal binder of ubiquitin zinc finger (BUZ), which is necessary and sufficient for binding polyubiquitin (Hook et al. 2002, Seigneurin-Berny et al. 2001, Boyault et al. 2006). By binding to both the microtubule-associated dynein motor and polyubiquitylated misfolded proteins, HDAC6 is able to facilitate transportation and concentration of protein aggregates to the aggresome, thus helping cells cope with abnormal accumulation of misfolded proteins (Kawaguchi et al. 2003). Furthermore, HDAC6 induces heat shock proteins to protect cells against potentially cytotoxic protein aggregation (Boyault et al. 2007b). Taken together, through different protein complexes, HDAC6 performs crucial functions in modulating a variety of cell signaling pathways and cell responses to stress.
Aberrant protein aggregation is a common feature shared by many neurodegenerative diseases, and recent studies have suggested that HDAC6 might be a key element in this process. HDAC6 was found concentrated in the Lewy bodies of Parkinson’s disease (PD) which are characterized by aggregated α-synuclein (Kawaguchi et al. 2003). In cell culture, the association of HDAC6 with polyubiquitylated mutant DJ-1, a protein involved in an early-onset form of PD, promoted the formation of DJ-1 containing aggresomes upon proteasome inhibition (Olzmann et al. 2007). Autophagic clearance of aggregated huntingtin in a neuronal cell model of Huntington’s disease (HD) required the presence of HDAC6, which recruited autophagic degradation machineries to inclusion bodies (Iwata et al. 2005). Interestingly, in a Drosophila model for a polyglutamine disease, HDAC6 rescued degeneration associated with proteasome impairment by mediating the autophagic degradation pathway (Pandey et al. 2007). Thus, HDAC6 appears to be an essential protein in coordinating the sequestration of protein aggregates and their clearance in neurodegeneration. However, the potential role of HDAC6 in Alzheimer’s disease (AD) has not yet been examined.
AD tau pathology includes neurofibrillary tangles (NFTs), dystrophic neurites and neuritic threads that are mainly composed of abnormally accumulated hyperphosphorylated microtubule-associated protein tau (Kosik et al. 1986, Grundke-Iqbal et al. 1986). Tau is predominantly expressed in neurons where its primary function is to promote tubulin polymerization and microtubule stability (Johnson & Bailey 2002). Hyperphosphorylation of tau has been suggested to impair its ability to bind and stabilize microtubules, and promote tau self-assembly and aggregation (Alonso et al. 1994, Alonso et al. 2001). Although many proteins such as protein kinases and phosphatases have been implicated in the development of tau pathology, the diverse functions of HDAC6 and the connection between tau and microtubules led us to examine whether HDAC6 also interacts with tau and thus could be involved in regulating tau pathology.
Here we report that HDAC6 interacts with tau in vitro, in situ and in human brain tissues, thus defining tau as an HDAC6 interacting protein. Inhibition of HDAC6 attenuated tau phosphorylation at T231, a critical site in regulating tau function (Cho & Johnson 2004). However, inhibition of HDAC6 did not disrupt HDAC6-tau interaction nor did it hinder the perinuclear accumulation of tau upon proteasome inhibition. Furthermore, we show that the protein level of HDAC6 in AD brain is significantly increased compared with normal brain.
Materials and Methods
Constructs, reagents and antibodies
pcDNA3.1(−)-tau containing human tau with four microtubule binding repeats but without exon 2 and 3 has been described previously (Cho & Johnson 2003). For C-terminal V5 tagged tau constructs, full-length tau and truncated fragments 1–372 (N+RD), 244–441 (RD+C), 1–243 (N), 244–372 (RD) were amplified and subcloned to pcDNA3.1/V5-HisA (Invitrogen, Carlsbad, CA, USA). For tau expression in bacteria, tau was subcloned to pGEX-6P-2 vector (Amersham Biosciences, Piscataway, NJ, USA) with glutathione S-transferase (GST) tagged to the N-terminus of tau (Ding et al. 2006). pMH-HDAC6 was generated by subcloning the full-length coding region of HDAC6 cDNA from pCMV-HDAC6 (Origene, Rockville, MD, USA) to pMH vector with a C-terminal HA tag (Roche, Indianapolis, IN, USA). Truncated HDAC6 1–1091 (ΔBUZ) and 1–840 (CD) were amplified and cloned into the pMH vector. Catalytically inactive HDAC6 (dm) was generated by introducing H216A/H611A double mutations using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA). Tubacin and niltubacin were kindly provided by Dr. Stuart L. Schreiber (Harvard University, Cambridge, MA, USA). TSA and MG132 were from Sigma (St. Louis, MO, USA) and Calbiochem (San Diego, CA, USA), respectively. The following tau antibodies were used in this study: the mouse monoclonal antibody Tau5 (from Dr. L. Binder) (Carmel et al. 1996) and a rabbit polyclonal tau antibody (Dako, Carpinteria, CA, USA) which are both phospho-independent tau antibodies; and the phospho-dependent monoclonal tau antibodies including PHF-1 (from Dr. P. Davies) for phosphorylated S396/S404 (Otvos et al. 1994); AT180 (Pierce, Rockford, IL, USA) for phosphorylated T231 (Goedert et al. 1994, Hoffmann et al. 1997); 12E8 (from Dr. P. Seubert) for phosphorylated S262 (Seubert et al. 1995). HA antibody HA-7 (Sigma) was used in immunoprecipitation and immunocytochemistry; HA antibody HA.11 (Covance, Emeryville, CA, USA) was used in immunoblotting. The rabbit polyclonal HDAC6 antibody H-300 was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Other antibodies used include anti-V5 (Invitrogen), anti-GST (Roche), anti-actin (Millipore, Billerica, MA, USA), anti-α-tubulin B-5-1-2 and anti-acetylated α-tubulin 6-11B-1 (Sigma).
Cell lines and transfection
Human embryonic kidney cells (HEK) and HEK cells stably expressing tau (HEK-tau) were grown at 37°C in DMEM/F-12 medium (Cellgro, Manassas, VA, USA) supplemented with 5% FBS (HyClone, Logan, UT, USA), 2 mM L-glutamine, 10 U/ml penicillin and 100 U/ml streptomycin (Cellgro, Manassas, VA, USA). HEK-tau cells stably expressing shRNA for HDAC6 were established by transfecting pRS vector containing HDAC6 shRNA (Origene) into HEK-tau cells and selecting with 1 μg/ml puromycin (Sigma). The immortalized mouse cortical neuronal cell line CN1.4 expressing tau (CN-tau) (Krishnamurthy & Johnson 2004) was maintained at 33°C in DMEM supplemented with 8% FBS, 2 mM L-glutamine, 10 U/ml penicillin, 100 U/ml streptomycin and 50 μg/ml hygromycin B (Roche). CN-tau cells were treated with 1 μg/ml doxycycline (Roche) for 48 h to induce tau expression. For transfection, Effectene Reagent was used according to the manufacturer’s instructions (Qiagen, Valencia, CA, USA).
Cell lysis and immunoblotting
Cells were collected in lysis buffer (50 mM Tris·Cl pH 7.4, 150 mM NaCl, 1% NP-40, 1 mM EDTA, 1 mM NaF, 1 mM Na3VO4, 1 mM PMSF and 1 μg/ml of leupeptin, aprotinin and pepstatin), incubated on ice for 20 min and centrifuged at 10,000 g for 10 min at 4°C. Protein concentrations were determined using the bicinchoninic acid assay (Pierce). Equal amounts of proteins were diluted with 2 × protein loading buffer (0.25 M Tris·Cl pH 6.8, 2% SDS, 5 mM EDTA, 5 mM EGTA, 10% glycerol, 25 mM dithiothreitol and 0.01% bromophenol blue), incubated in a boiling water bath for 5 min, separated on SDS-polyacrylamide gels, transferred to nitrocellulose membrane and immunoblotted.
Brain tissue homogenization
Postmortem frozen cerebral cortical and hippocampal tissues from AD (3 females, 3 males aged 76 ± 2.9 years), age-matched normal control brains (two females, 5 males aged 69 ± 5.1 years) and one young normal brain (CTL-Y) were obtained from the Neuropathology Core Laboratory at the University of Alabama at Birmingham (UAB). The CTL-Y case was used as an internal control for comparing protein intensities on different blots. The postmortem intervals for the AD and control cases were 6.1±1.2 h and 13.3±4.6 h, respectively. The Brain tissues were homogenized in glass tissue grinders with homogenization buffer (50 mM Tris·Cl pH 7.4, 150 mM NaCl, 0.1% Tween-20, 10% glycerol, 1 mM EDTA, 1 mM NaF, 1 mM Na3VO4, 1 mM PMSF and 1 μg/ml of leupeptin, aprotinin and pepstatin), and incubated on ice for 30 min (Gandhi et al. 2006). The unhomogenized pellet was discarded after centrifuging at 3,000 g for 5 min at 4°C and the supernatant was saved as total homogenate. The protocol was approved by UAB Institutional Review Board for Human Use.
Co-immunoprecipitation
Dynabeads M-280 sheep anti-mouse IgG (Invitrogen) were washed twice in PBS pH 7.4 containing 0.1% BSA, followed by incubation with appropriate primary antibody overnight at 4°C with gentle shaking. After washing three times in PBS containing 0.1% BSA, the primary antibody conjugated Dynabeads were further incubated with 150 μg cell lysate or 500 μg human brain homogenate at 4°C for 3 h with gentle shaking, followed by washing five times in PBS containing 300 mM NaCl and 0.5% NP-40 for cell lysate immunoprecipitation or in homogenization buffer for brain homogenate immunoprecipitation. Proteins attached to Dynabeads were eluted with 2 × protein loading buffer, boiled for 5 min, subjected to SDS-PAGE and blotted with antibodies for tau and HDAC6.
GST pull-down assay
The recombinant GST and GST-tau fusion proteins were expressed in E. coli strain BL21(DE3) after induction overnight with 0.3 mM IPTG at 16°C, followed by bacteria lysis in PBS containing 1% Triton X-100 and brief sonication (Ding et al. 2006). Glutathione sepharose 4B were incubated with crude bacterial lysate containing GST and GST-tau for 2 h at 4°C with gentle shaking, and then washed three times in PBS, followed by incubation with HEK cell lysate expressing HDAC6-HA for 2 h at 4°C with gentle shaking. After washing five times in PBS containing 300 mM NaCl and 0.5% NP-40, proteins were eluted with 2 × protein loading buffer, boiled for 5 min, subjected to SDS-PAGE and blotted with antibodies for HA and GST.
Immunocytochemistry
Cells were washed with PBS, fixed in 4% paraformaldehyde for 30 min at room temperature and permeabilized in 0.5% Triton X-100 in PBS for 5 min. After washing cells with PBS, cells were incubated in blocking buffer (4% BSA in PBS) for 90 min followed by incubation with primary antibodies diluted in blocking buffer for 90 min. After washing with PBS, cells were incubated with fluorescent dye-labeled secondary antibodies diluted in blocking buffer for 90 min. Nuclei were stained with DAPI in PBS for 10 min. Slides were washed with PBS and mounted with mounting medium (Thermo Scientific, Waltham, MA, USA). Images were captured with an AxioCamMR3 camera (Zeiss Corp., Thornwood, NY, USA) connected to a Zeiss Axio Observer D1 microscope with an EC Plan-Neofluor 40X/1.3 oil M27 objective and exported in TIFF format using AxioVision software. Brightness and contrast were adjusted with Zeiss LSM image browser. For quantification, at least nine fields of each slide were randomly selected and the percentage of cells with perinuclear accumulated tau was counted. At least two hundred cells transfected with tau in each group were counted.
Immunohistochemistry
Formalin-fixed and paraffin-embedded hippocampal sections from two AD brains and two age-matched control brains were obtained from the Department of Neuropathology at the University of Rochester Medical Center (URMC). Sections were deparaffinized in Histoclear (Electron Microscopy Sciences, Hatfield, PA) twice for 10 min each, then rehydrated sequentially in isopropanol three times for 5 min each and in running water and deionized water for 5 min each. Antigen retrieval was achieved by heating sections immersed in 10 mM sodium citrate buffer pH 6.0 containing 0.05% Tween-20 for 30 min in a steamer, followed by cooling down for 20 min. After three 5-min rinses in PBS with 0.05% Tween-20, sections were incubated in blocking buffer (1% BSA, 2% goat serum, 0.1% Triton X-100 and 0.05% Tween-20 in PBS) at room temperature for 2 h, and then with primary antibodies diluted in PBS with 1% BSA at 4°C for 40 h. Sections were rinsed three times in PBS with 0.05% Tween-20 and incubated with fluorescent dye-labeled secondary antibodies diluted in PBS at room temperature for 2 h, followed by nuclear staining with DAPI for 10 min. After three 5-min rinses in PBS with 0.05% Tween-20, sections were mounted with mounting medium. Images were captured with an AxioCamHR camera connected to a Zeiss Axio Observer D1 microscope with an Achroplan 20X/0.45 Ph2 objective and exported in TIFF format using AxioVision software. Brightness and contrast were adjusted with Zeiss LSM image browser.
Statistic analysis
Tau phosphorylation and immunoprecipitation ratios (Figures 4, 5) were analyzed using one-way ANOVA, followed by post hoc t tests. Comparisons of perinuclear tau inclusion formation (Figure 6) and HDAC protein expression in patient samples (Figure 7) were performed using Student’s t test, and values were considered significantly different when p<0.05.
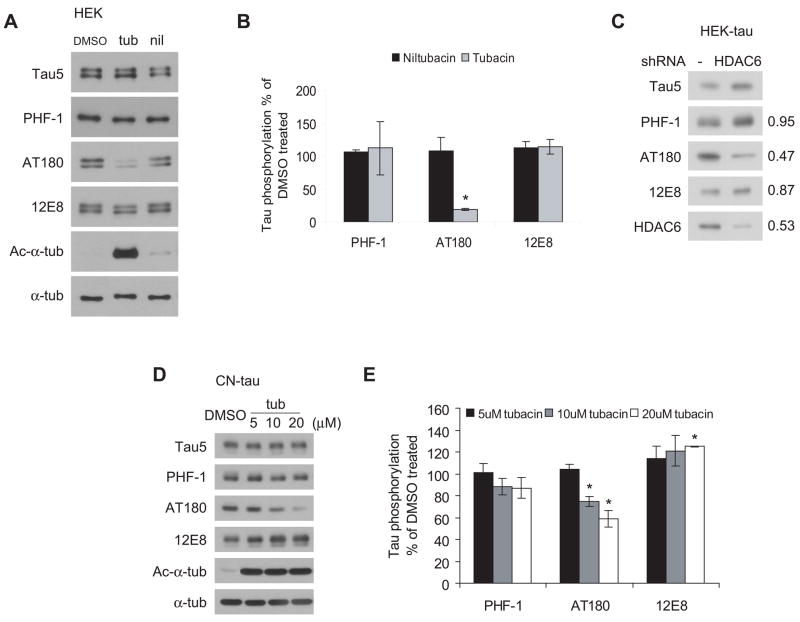
Figure 4. Down-regulation of HDAC6 attenuates tau phosphorylation in a site-specific manner.
A, Representative immunoblots showing that tubacin treatment attenuates tau phosphorylation at the AT180 site in HEK cells. HEK cells were transfected with tau and treated for 5 h with DMSO, 20 μM tubacin or 20 μM niltubacin. Cell lysates were immunoblotted with Tau5 for total tau and with PHF-1, AT180 and 12E8 for tau phosphorylated at S396/S404, T231 and S262, respectively. α-tubulin was blotted as a loading control. B, Quantification of relative levels of phosphorylated tau from three independent experiments described in panel A (mean ± SEM). Values were normalized to total tau levels (Tau5) and expressed as percentages of DMSO treated group. C, Knocking down HDAC6 in HEK-tau cells attenuated tau phosphorylation at the AT180 site. Cell lysates from HEK-tau cell lines expressing control vector or shRNA for HDAC6 were immunoblotted as indicated. Shown on the right are the ratios of phosphorylated tau in HDAC6-knockdown cells versus control cells after normalized to total tau. D, Tubacin treatment attenuated tau phosphorylation at AT180 site in CN-tau cells in a dose-dependent manner. CN-tau cells were treated with doxycycline for 48 h to induce tau expression followed by treatment with DMSO or 5, 10, 20 μM tubacin for 5 h. Cell lysates were immunoblotted as indicated. E, Quantification of relative levels of phosphorylated tau from three independent experiments described in panel D (mean ± SEM). Values were normalized to total tau level immunoblotted by Tau5 and expressed as percentages of DMSO treated group. *p<0.05 versus DMSO treated group.
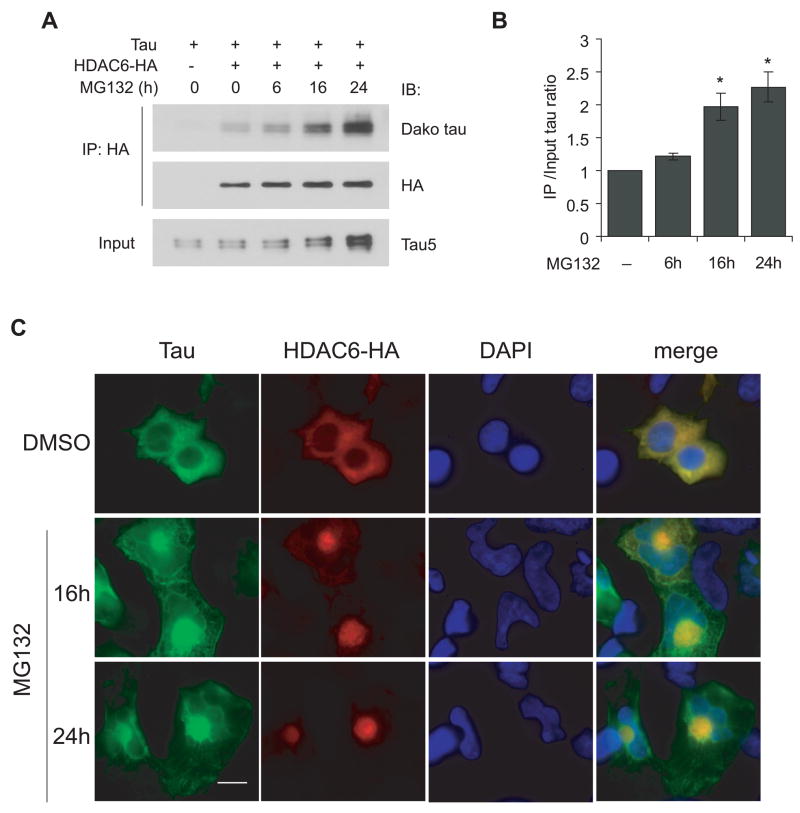
Figure 5. Proteasome inhibition potentiates HDAC6-tau interaction and perinuclear co-localization.
A, HEK cells were transfected with tau and HDAC6-HA and treated with 1 μM MG132 for 0, 6, 16 or 24 h. Cell lysates were immunoprecipitated with HA antibody and immunoblotted as indicated. B, Quantification of the ratios of co-immunoprecipitated tau versus total input tau from three independent experiments described in panel A (mean ± SEM). Values were normalized to untreated group. Co-immunoprecipitated tau increased significantly after 16 h treatment compared with both untreated and 6 h treatment groups. *p<0.05. C, Perinuclear co-localization of HDAC6 and tau in MG132 treated cells. HEK cells were transfected with tau and HDAC6-HA and treated with 1 μM MG132 for 16 or 24 h followed by immunostaining with antibodies for tau (green) and HA (red). Nuclei were stained with DAPI (blue). Scale bar, 10 μm.
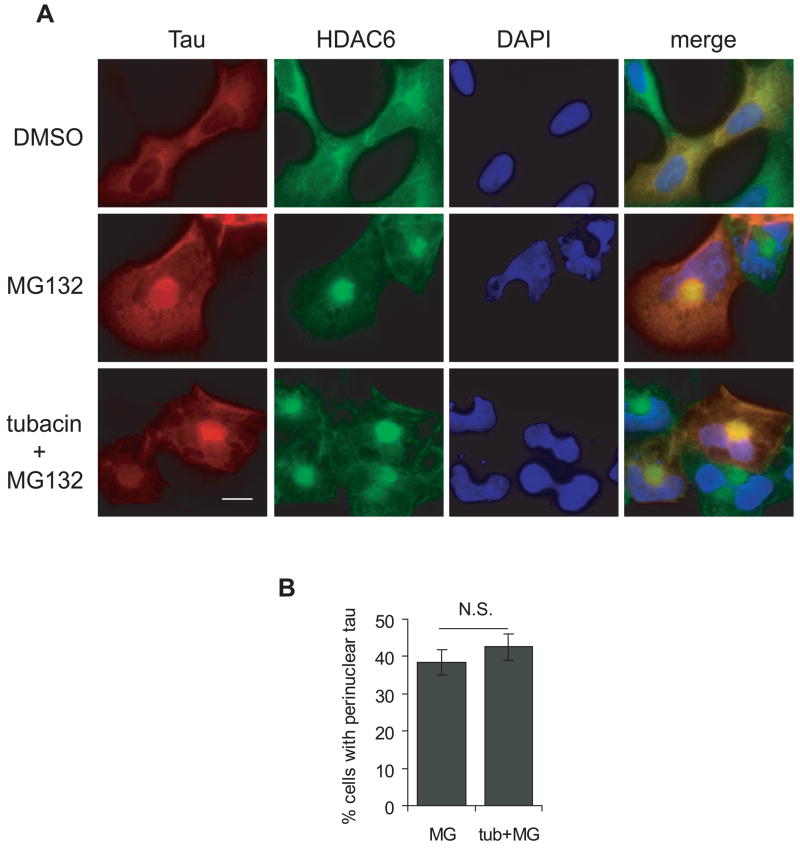
Figure 6. Tubulin deacetylase activity of HDAC6 is not required for perinuclear tau accumulation.
A, HEK cells were transfected with tau and treated with or without 2.5 μM tubacin for 8 h prior to treatment with 1 μM MG132 for 16 h, followed by immunostaining with Tau5 antibody for tau (red) and an antibody for HDAC6 (green). Nuclei were stained with DAPI (blue). Scale bar, 10 μm. B, Quantification of the percentage of cells containing perinuclear accumulated tau in MG132 treated cells and in tubacin and MG132 co-treated cells from three independent experiments (mean ± SEM). N.S., not significant.
Figure 7. Increased HDAC6 protein levels in AD cerebral cortex and hippocampus.
A, B, Representative HDAC6 immunoblots of homogenates from postmortem frozen cerebral cortex (A) and hippocampus (B) of control (CTL) and AD brains. Actin was blotted as a loading control. C, Quantification of relative HDAC6 protein levels in cerebral cortex and hippocampus from seven age-matched normal control brains (5 males and 2 females, average age =69 yr) and six AD brains (3 males and 3 females, average age =76 yr). HDAC6 protein levels were normalized to actin and expressed as percentages of CTL-Y which was used as an internal control for comparing protein levels from different blots (mean ± SEM). *p<0.05 versus control.
Results
HDAC6 interacts with tau
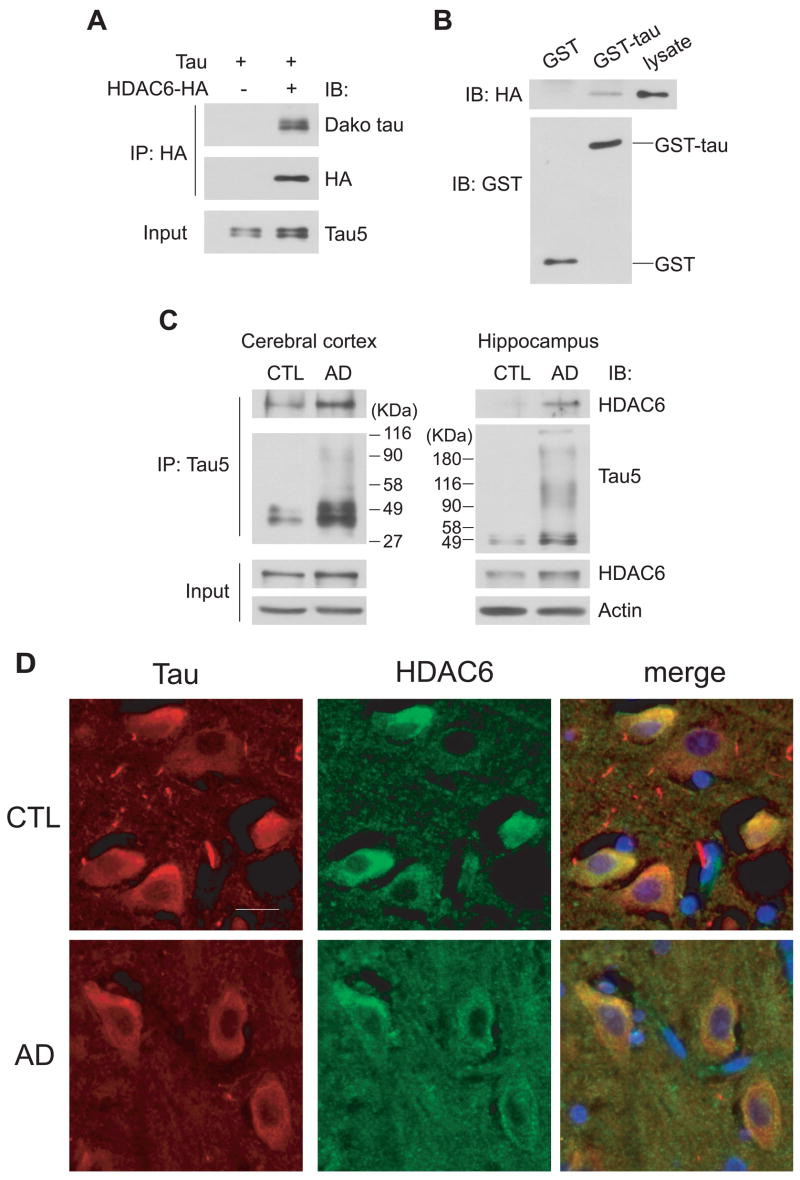
To determine whether HDAC6 interacts with tau, co-immunoprecipitation experiments were carried out using lysates from HEK cells that were transiently transfected with tau and HDAC6-HA. HDAC6-HA was immunoprecipitated by an anti-HA antibody and the co-precipitates were probed for tau. Immunoprecipitates from cells transfected with tau alone were used as negative controls. As shown in Figure 1A, tau co-immunoprecipitated with HDAC6-HA, suggesting that tau may interact with HDAC6. This interaction was confirmed by an in vitro GST pull-down assay. In this assay, lysates from HEK cells transiently transfected with HDAC6-HA were incubated with GST or GST-tau conjugated glutathione beads and proteins pulled down were immunoblotted with antibodies for HA and GST. As shown in Figure 1B, HDAC6-HA could only be pulled down by GST-tau but not GST alone. To examine whether the HDAC6-tau interaction occurs in vivo, homogenates from frozen cerebral cortical and hippocampal tissues from AD brains and age-matched normal brains were immunoprecipitated with the Tau5 antibody. The co-precipitates were probed for HDAC6. As expected, there was an increased amount of tau of high molecular weight immunoprecipitated from AD brains, consistent with the abnormal accumulation and hyperphosphorylation of tau in AD (Grundke-Iqbal et al. 1986, Kosik et al. 1986). Interestingly, HDAC6 co-immunoprecipitated with tau in control and AD cerebral cortex as well as AD hippocampus (Figure 1C). Furthermore, immunohistochemistry was performed on brain sections from AD patients and age-matched controls to examine the distribution of tau and HDAC6. As shown in Figure 1D, tau and HDAC6 partially co-localized in neurons in the CA3 areas of both control and AD hippocampus. Together, the data demonstrate that tau and HDAC6 interact with each other in vitro, in cells and in human brain tissue.
Figure 1. HDAC6 interacts with tau.
A, Tau co-immunoprecipitates with HDAC6 in situ. Cell lysates from HEK cells transiently transfected with tau and HDAC6-HA or tau alone were immunoprecipitated with an anti-HA antibody followed by immunoblotting with antibodies for tau and HA. Total tau input was shown by immunoblotting cell lysates with Tau5 antibody. B, HDAC6 is pulled down by GST-tau in vitro. Cell lysates from HEK cells transiently transfected with HDAC6-HA were incubated with GST or GST-tau conjugated glutathione beads and proteins pulled down were immunoblotted with antibodies for HA and GST. C, Tau co-immunoprecipitates with HDAC6 in vivo. Frozen cerebral cortical (left) and hippocampal (right) tissues from AD brains and age-matched normal control (CTL) brains were homogenized and immunoprecipitated with Tau5 antibody followed by immunoblotting with Tau5 and an anti-HDAC6 antibody. Total HDAC6 input was shown by immunoblotting homogenates with anti-HDAC6 and actin blots were also shown as loading controls. D, Tau co-localizes with HDAC6 in vivo. Sections of postmortem hippocampal tissues from two age-matched control brains (CTL) (2 males) and two AD brains (1 male and 1 female) were immunostained with Tau5 antibody for tau (red) and an antibody for HDAC6 (green). Nuclei were stained with DAPI (blue). Representative images of CA3 areas of one case from each group are shown. Scale bar, 20 μm.
The SE14 domain of HDAC6 binds the RD domain of tau
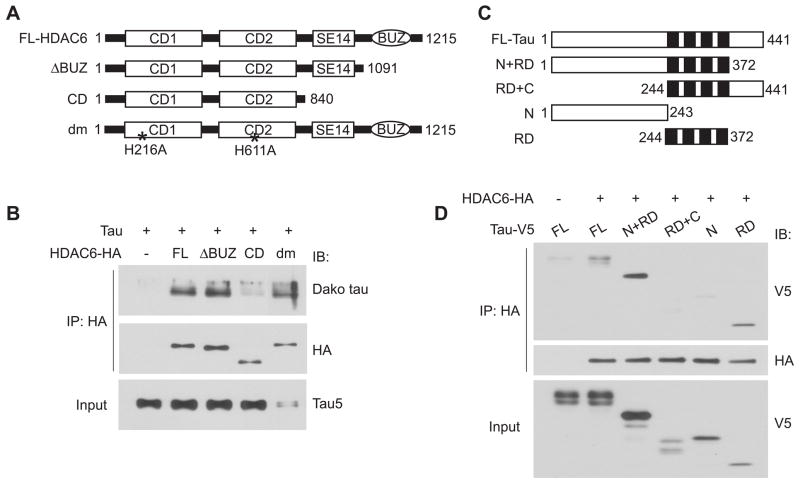
The diverse functions of HDAC6 are determined by its unique structure. The two catalytic domains at the N-terminus confer its deacetylase activity towards not only histones in the nucleus but also α-tubulin, cortactin and Hsp90 in the cytoplasm (Hubbert et al. 2002, Zhang et al. 2007, Kovacs et al. 2005). The BUZ domain at the C-terminus is able to bind to polyubiquitin (Hook et al. 2002), and the Ser/Glu tetradecapeptide repeat domain (SE14) between the second catalytic domain and the BUZ domain was found to be involved in retaining HDAC6 in the cytoplasm (Bertos et al. 2004). To identify the domain necessary for HDAC6-tau interaction, we made HA tagged HDAC6 deletion constructs that either lack the BUZ domain (ΔBUZ) or only contain the catalytic domains (CD). The catalytically inactive double mutant H216A/H611A (dm) was also included to test whether the deacetylase activity is required for the interaction (Figure 2A). These constructs together with tau were co-transfected into HEK cells followed by co-immunoprecipitation assays. We found that tau co-immunoprecipitated with full-length HDAC6, ΔBUZ but not CD alone (Figure 2B). Thus, the SE14 domain but not the BUZ domain is required for HDAC6 to interact with tau, whereas the catalytic domains alone are not sufficient for the interaction. Tau also co-immunoprecipitated well with the catalytically inactive mutant (dm) (Figure 2B), suggesting that the deacetylase activity of HDAC6 is not required for HDAC6-tau interaction.
Figure 2. Mapping the interacting domains on HDAC6 and tau.
A, Schematic diagram of C-terminal HA tagged full-length, truncated and catalytically inactive HDAC6 constructs. FL, full-length; BUZ, binder of ubiquitin zinc finger; SE14, Ser/Glu tetradecapeptide repeat domain; CD, catalytic domain; dm, double mutant. B, SE14 domain on HDAC6 is required for efficient interaction with tau. Cell lysates from HEK cells transfected with tau and different HDAC6 constructs were immunoprecipitated with HA antibody and immunoblotted as indicated. Total tau input was shown by immunoblotting cell lysates with Tau5 antibody. C, Schematic diagram of C-terminal V5 tagged full-length and truncated tau constructs. N, N-terminal region; C, C-terminal region; RD, microtubule binding repeat domain. D, Microtubule binding repeat domain on tau is necessary and sufficient for interaction with HDAC6. Cell lysates from HEK cells transfected with HDAC6-HA and different tau constructs were immunoprecipitated with HA antibody and immunoblotted as indicated. Total tau input was shown by immunoblotting cell lysates with anti-V5.
Considering that the BUZ domain mainly binds to the polyubiquitin portion of polyubiquitylated proteins (Hook et al. 2002), the dispensability of this domain in HDAC6-tau interaction indicates that HDAC6 interacts with tau via a particular domain on tau independent of the polyubiquitin chain. Therefore, to define the interacting domain on tau, we made V5 tagged tau constructs that lack either the C-terminus (N+RD) or N-terminus (RD+C), or only contain the N-terminus (N) or microtubule-binding repeat domain (RD) (Figure 2C). In co-immunoprecipitation assays, full-length tau, N+RD or RD alone co-immunoprecipitated with HDAC6, whereas the N-terminus (amino acids 1–243) alone did not efficiently co-precipitate with HDAC6 (Figure 2D), suggesting that the RD (amino acids 244–372) is necessary and sufficient for mediating HDAC6-tau interaction. However, the C-terminus (amino acids 373–441) of tau appeared to attenuate the interaction of tau with HDAC6 as there was a slight increase in co-immunoprecipitated N+RD compared with full-length tau, and the RD+C did not co-immunoprecipitate with HDAC6 compared with RD alone (Figure 2D).
Inhibition of HDAC6 does not disrupt HDAC6-tau interaction but attenuates tau phosphorylation at T231
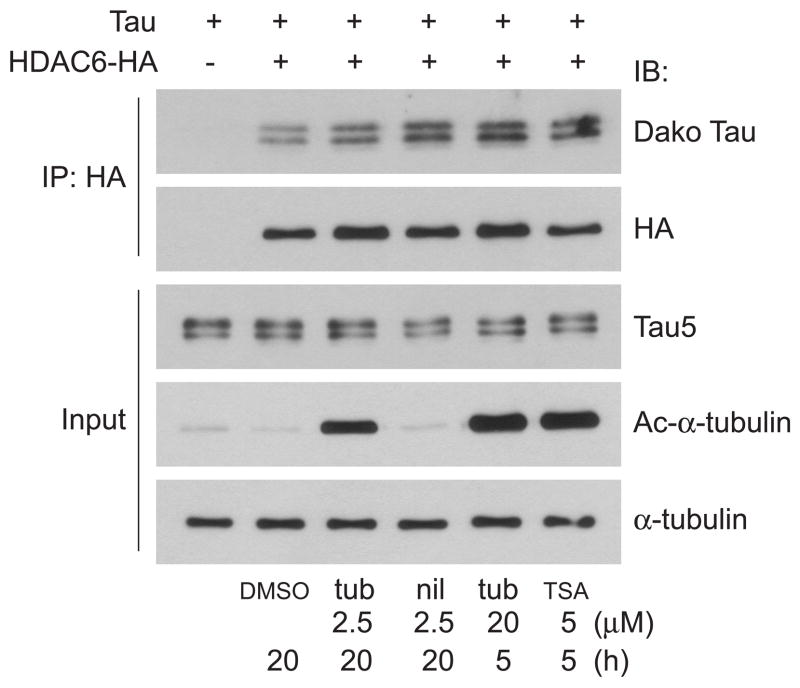
TSA treatment or catalytic inactivation of HDAC6 blocks its interaction with PP1 (Brush et al. 2004). To test whether TSA has a similar effect on HDAC6-tau interaction, HEK cells were transfected with tau and HDAC6-HA and treated with TSA followed by co-immunoprecipitation. The increase of acetylated a-tubulin confirmed efficient inhibition of HDAC6 activity by TSA. However, unlike the HDAC6-PP1 interaction which is disrupted by TSA treatment, the HDAC6-tau interaction was not impaired by TSA as tau co-immunoprecipitated well with HDAC6 even in the presence of TSA (Figure 3). Since TSA inhibits not only HDAC6 but also other HDACs, tubacin, a specific inhibitor of the tubulin deacetylase activity of HDAC6, and its inactive analogue niltubacin (Haggarty et al. 2003) were used. Similar to TSA treatment, tubacin inhibited HDAC6 activity as demonstrated by increased acetylated a-tubulin. However, it did not disrupt HDAC6-tau interaction (Figure 3). These data support the finding above that tau interacts with the catalytically inactive HDAC6 mutant, further demonstrating that the deacetylase activity of HDAC6 is not required for HDAC6-tau interaction.
Figure 3. Inhibition of HDAC6 by tubacin and TSA does not disrupt HDAC6-tau interaction.
HEK cells were transfected with tau and HDAC6-HA or tau alone and then treated with DMSO (20 h), tubacin (2.5 μM for 20 h or 20 μM for 5 h), niltubacin (2.5 μM for 20 h) or TSA (5 μM for 5 h). Cell lysates were immunoprecipitated with HA antibody and immunoblotted as indicated. Total tau and acetylated α-tubulin input were shown by immunoblotting cell lysates with Tau5 and anti-acetylated α-tubulin, respectively. α-tubulin was also blotted as a loading control. tub: tubacin; nil: niltubacin.
Although tubacin treatment had no effect on HDAC6-tau interaction, it might affect tau phosphorylation indirectly by modulating microtubule dynamics and other HDAC6-protein complexes. To test this hypothesis, HEK cells transfected with tau were treated with 20 μM tubacin or niltubacin for 5 h and the cell lysates were immunoblotted with both phospho-independent and -dependent tau antibodies. Interestingly, as shown in Figure 4A and 4B, tubacin treatment attenuated tau phosphorylation at T231 detected by AT180 antibody, whereas tau phosphorylation at S396/S404 detected by PHF-1 and at S262 detected by 12E8 were not changed when compared with DMSO and niltubacin. Similar changes in tau phosphorylation were also observed in HDAC6-knockdown HEK cells that stably express tau (Figure 4C). To further confirm the effects of tubacin on tau phosphorylation, we used immortalized mouse cortical neuronal cell line CN1.4 expressing tau under the control of a doxycycline-inducible promoter (Krishnamurthy & Johnson 2004). Tubacin treatment significantly attenuated tau phosphorylation at T231 in a dose-dependent manner whereas the phosphorylation at S396/S404 was not changed (Figure 4C, 4D). In contrast to the results in HEK cells, tau phosphorylation at S262 was increased slightly in response to tubacin treatment (Figure 4C, 4D). Together, these data demonstrate that in cell culture models, treatment with the HDAC6 inhibitor tubacin can attenuate site-specific tau phosphorylation without disrupting HDAC6-tau interaction.
Proteasome inhibition potentiates HDAC6-tau interaction and co-localization independent of tubulin deacetylase activity of HDAC6
Previous studies have shown a critical role for HDAC6 in aggresome formation and autophagic degradation in response to aberrant protein accumulation (Kawaguchi et al. 2003, Iwata et al. 2005, Pandey et al. 2007). Given that proteasome inhibition and aberrant accumulation of tau in AD brain have been well documented (Lam et al. 2000, Keck et al. 2003), we tested whether HDAC6 might be involved in abnormal tau accumulation under pathological conditions. HEK cells were transfected with tau and HDAC6-HA followed by treatment with 1 μM of the proteasome inhibitor MG132 for 0, 6, 16 or 24 h. Tau proteins were probed after immunoprecipitating the cell lysates with anti-HA antibody. Both co-immunoprecipitated tau and total tau level increased in a time-dependent manner (Figure 5A). We therefore quantified the fold increase in co-immunoprecipitated tau and total tau in MG132 treated groups in comparison to the untreated group. The ratio of co-immunoprecipitated tau to total tau was significantly increased after 16 h treatment and reached a maximum after 24 h (Figure 5B), suggesting that proteasome inhibition potentiated HDAC6-tau interaction. Immunocytochemistry showed that in contrast to the diffuse distribution of HDAC6 and tau throughout the cytoplasm in DMSO treated cells, these two proteins were concentrated and co-localized in a perinuclear aggresome-like compartment in MG132 treated cells (Figure 5C).
To test whether tubulin deacetylase activity is required for the perinuclear co-localization of HDAC6 and tau, HEK cells transfected with tau alone were pre-treated with 2.5 μM tubacin for 8 h to inhibit endogenous HDAC6 activity prior to MG132 treatment for 16 h. Immunocytochemistry showed that even in the presence of tubacin, HDAC6 and tau were still concentrated and co-localized in the perinuclear loci (Figure 6A). To quantify the effects of tubacin, the number of cells containing perinuclear accumulated tau were counted. There was no significant difference between the MG132-only treated group and the tubacin and MG132 co-treated group (Figure 6B), suggesting that the perinuclear accumulation of tau upon proteasome inhibition is not dependent on the tubulin deacetylase activity of HDAC6.
Increase of HDAC6 in AD brains
In AD brains, tubulin acetylation is reduced in neurons containing NFTs (Hempen & Brion 1996), whereas tau is abnormally accumulated and hyperphosphorylated. HDAC6 is a key deacetylase for a-tubulin, and the results we have presented so far imply that HDAC6 might be involved in regulating tau phosphorylation and accumulation. Therefore, we tested whether the level of HDAC6 might be deregulated in AD brains. Homogenates from AD and age-matched control cerebral cortex (Figure 7A) and hippocampus (Figure 7B) were immunoblotted for HDAC6 and actin. To quantify, a young normal brain (CTL-Y) was used as an internal control for comparing protein intensities on different blots. HDAC6 protein levels were normalized to actin and expressed as percentages of CTL-Y. We observed that compared with controls, the protein levels of HDAC6 were increased by 52% in AD cortex and by 91% in AD hippocampus (Figure 7C).
Discussion
HDAC6 performs pivotal functions in modulating the dynamic of the cytoskeleton network (Gao et al. 2007, Tran et al. 2007, Zhang et al. 2007) and controlling various cell responses to stress (Kawaguchi et al. 2003, Boyault et al. 2007b, Kwon et al. 2007). Recent studies show that HDAC6 also plays critical roles in neurodegenerative diseases, especially polyglutamine diseases, by participating in aggresome formation and coordinating the ubiquitin proteasome system and autophagic degradation (Iwata et al. 2005, Olzmann et al. 2007, Pandey et al. 2007). However, the connection at the molecular level between HDAC6 and key proteins involved in neurodegeneration has not been examined. In this report, we identified tau as a novel interacting partner of HDAC6 and provided evidence linking HDAC6 with tau phosphorylation and accumulation.
Previous studies have shown α-tubulin, cortactin, Hsp90, dynein, p97/VCP and Ras-GTPase-activating protein SH3 domain-binding protein 1 (G3BP) as binding partners of HDAC6 (Hubbert et al. 2002, Zhang et al. 2007, Kovacs et al. 2005, Kawaguchi et al. 2003, Seigneurin-Berny et al. 2001, Kwon et al. 2007). Here we show tau is also a bona fide interacting protein of HDAC6 as they associate in vitro, in cell culture models as well as in human brain tissue (Figure 1). The interaction is mediated by the Ser/Glu tetradecapeptide domain SE14 on HDAC6 and the microtubule binding domain on tau whereas the C-terminus of tau appears to inhibit HDAC6-tau interaction (Figure 2). One possible explanation for this inhibition is that removal of the N-terminus of tau allows tighter binding of the C-terminus to the microtubule binding domain (Jeganathan et al. 2006), which could potentially disallow interaction with other proteins. This is supported by the data showing that the construct missing the C-terminus still binds to HDAC6. Further, given that the C-terminus of tau has also been shown to inhibit tau assembly and polymerization (Abraha et al. 2000), it would be interesting to determine whether HDAC6 can hinder the assembly of tau filaments by competing for the C-terminal truncated tau (e.g., caspase-cleaved tau) (Gamblin et al. 2003). Tau co-immunoprecipitated efficiently with the catalytically inactive HDAC6 mutant and inactivation of HDAC6 by either tubacin or TSA did not disrupt HDAC6-tau interaction (Figure 3). Therefore, their interaction is not dependent on the deacetylase activity of HDAC6. This is similar to the HDAC6-G3BP interaction (Kwon et al. 2007) but different than the HDAC6-PP1 interaction that requires active HDAC6 (Brush et al. 2004). These findings further support the hypothesis that by forming different protein complexes, HDAC6 functions as far more than just as a deacetylase.
Although we have clearly demonstrated that there is an interaction between HDAC6 and tau, the physiological relevance of this interaction requires further clarification. Nonetheless, our studies indicate that HDAC6 plays a role in modulating tau phosphorylation, albeit indirectly. This is supported by the finding that inhibition of HDAC6 activity by tubacin, as well as shRNA-mediated knockdown, attenuated tau phosphorylation (Figure 4). Although the underlying molecular basis is still unknown, there are three possible mechanisms to explain this observation. (i) Inactivation or knockdown of HDAC6 dissociates HDAC6-protein phosphatase complexes so that more phosphatases are available for tau dephosphorylation. A similar mechanism was suggested previously for Akt dephosphorylation induced by disrupted HDAC6-PP1 association (Chen et al. 2005, Brush et al. 2004). (ii) Hyperacetylation of α-tubulin in these cells might alter the dynamic of microtubules and thus indirectly modulate the activities and recruitment of relevant tau kinases and phosphatases. (iii) Increased acetylation of unknown substrates and association-dissociation of other HDAC6-protein complexes induced by HDAC6 inactivation might also be involved in regulating tau phosphorylation.
Interestingly, the dephosphorylation of tau occurred at T231 but not S396/404 or S262 (Figure 4). It is noteworthy that in a mouse model of AD, treatment with nicotinamide, an inhibitor of Sir2, a class III HDAC member, also caused tau dephosphorylation at T231, which was partially responsible for cognitive improvements following the treatment (Green et al. 2007). T231 is an important site that has been implicated in the development of tau pathology. For instance, T231 is hyperphosphorylated in paired helical filament in AD brain but not in normal brain (Goedert et al. 1994). Phosphorylation at T231 plays a critical role in regulating the ability of tau to bind and stabilize microtubules (Cho & Johnson 2004) and might also promote tau hyperphosphorylation at other sites (Lin et al. 2007). The underlying mechanism of the site-specific dephosphorylation is not clear. Using site-specific pseudophosphorylated tau constructs (Ding et al. 2006) in co-immunoprecipitation assays, we did not observe differential interaction between HDAC6 and the different pseudophosphorylated tau proteins (data not shown). Further studies on the relationship of HDAC6, acetylated tubulin and tau kinases and phosphatases are needed to provide mechanistic insight into the regulation of tau phosphorylation by HDAC6.
In addition to hyperphosphorylation, tau becomes abnormally accumulated in dystrophic neurites around senile plaques and in NFTs in the AD brain. Existence of chaperones, ubiquitin ligase, and proteasome components in tau pathology clearly indicates that various cellular machineries might be actively utilized to cope with abnormal tau accumulation (Dou et al. 2003, Petrucelli et al. 2004, Fergusson et al. 1996). Our study suggests that HDAC6 might play a role as well in this process. In a cell culture model, proteasome inhibition potentiated HDAC6-tau interaction (Figure 5A, 5B) and induced accumulation and co-localization of HDAC6 and tau in a perinuclear aggresome-like compartment (Figure 5C). Taking this data together with the demonstrated role of HDAC6 in facilitating aggresome formation from other aggregation-prone proteins (Kawaguchi et al. 2003, Olzmann et al. 2007, Kwon et al. 2007), we speculate that HDAC6 might sequester abnormally accumulated tau in a certain compartment as a neuronal response to specific pathological stressors. It is also interesting to note that HDAC6 appears to be required for the autophagy-mediated clearance of mutant huntingtin aggregates in a cell model (Iwata et al. 2005).
We also observed up-regulation of HDAC6 in AD brain that correlates with tau hyperphosphorylation and aggregation (Figure 7). However, the cause and functional consequence of this up-regulation are not known. The cellular concentration of HDAC6 and p97/VCP, an AAA-ATPase chaperone, is thought to determine the fate of polyubiquitylated proteins and an excess of HDAC6 would favor the accumulation of ubiquitylated protein aggregates and inclusion body formation (Boyault et al. 2006, Boyault et al. 2007a). Considering the proteasome impairment in AD brain (Lam et al. 2000, Keck et al. 2003) and the link between HDAC6 and autophagy (Iwata et al. 2005, Pandey et al. 2007), it can be hypothesized that HDAC6 up-regulation might on one hand, facilitate sequestration of ubiquitylated protein aggregates and recruitment of autophagic machinery to clear aggregates; but on the other hand, decrease tubulin acetylation and increase tau phosphorylation by decreasing the availability of protein phosphatases for tau dephosphorylation. Therefore, although the initial effect of an increase in HDAC6 levels may be protective, prolonged increases may negatively impact neuronal cell survival in AD and other tauopathies.
Acknowledgments
We thank Dr. Stuart L. Schreiber for providing tubacin and niltubacin. We gratefully acknowledge the provision of frozen human brain tissue by the Neuropathology Core Laboratory of the UAB Alzheimer Disease Research Center which is supported by National Institute of Aging Grant P50 AG16582, and human brain tissue sections by the Department of Neuropathology at URMC and the patients who participated in URMC tissue donation program. We thank Fran Vito for excellent assistance in immunohistochemistry, and Dr. Linda M. Callahan and Eileen Johnson, MS, RN for assistance in case analysis. This work was supported by NIH grant NS051279 and a grant from the Alzheimer’s Association.
Abbreviations used
- AD
Alzheimer’s disease
- BUZ
binder of ubiquitin zinc finger
- CD
catalytic domains
- dm
double mutant
- GST
glutathione S-transferase
- HDAC6
histone deacetylase 6
- NFTs
neurofibrillary tangles
- PP1
protein phosphatase 1
- RD
microtubule-binding repeat domain
- SE14
Ser/Glu tetradecapeptide domain
- TSA
trichostatin A
References
- Abraha A, Ghoshal N, Gamblin TC, Cryns V, Berry RW, Kuret J, Binder LI. C-terminal inhibition of tau assembly in vitro and in Alzheimer’s disease. J Cell Sci. 2000;113(Pt 21):3737–3745. doi: 10.1242/jcs.113.21.3737. [DOI] [PubMed] [Google Scholar]
- Alonso A, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci U S A. 2001;98:6923–6928. doi: 10.1073/pnas.121119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso AC, Zaidi T, Grundke-Iqbal I, Iqbal K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc Natl Acad Sci U S A. 1994;91:5562–5566. doi: 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bali P, Pranpat M, Bradner J, et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem. 2005;280:26729–26734. doi: 10.1074/jbc.C500186200. [DOI] [PubMed] [Google Scholar]
- Bertos NR, Gilquin B, Chan GK, Yen TJ, Khochbin S, Yang XJ. Role of the tetradecapeptide repeat domain of human histone deacetylase 6 in cytoplasmic retention. J Biol Chem. 2004;279:48246–48254. doi: 10.1074/jbc.M408583200. [DOI] [PubMed] [Google Scholar]
- Boyault C, Gilquin B, Zhang Y, Rybin V, Garman E, Meyer-Klaucke W, Matthias P, Muller CW, Khochbin S. HDAC6-p97/VCP controlled polyubiquitin chain turnover. Embo J. 2006;25:3357–3366. doi: 10.1038/sj.emboj.7601210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyault C, Sadoul K, Pabion M, Khochbin S. HDAC6, at the crossroads between cytoskeleton and cell signaling by acetylation and ubiquitination. Oncogene. 2007a;26:5468–5476. doi: 10.1038/sj.onc.1210614. [DOI] [PubMed] [Google Scholar]
- Boyault C, Zhang Y, Fritah S, et al. HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates. Genes Dev. 2007b;21:2172–2181. doi: 10.1101/gad.436407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brush MH, Guardiola A, Connor JH, Yao TP, Shenolikar S. Deactylase inhibitors disrupt cellular complexes containing protein phosphatases and deacetylases. J Biol Chem. 2004;279:7685–7691. doi: 10.1074/jbc.M310997200. [DOI] [PubMed] [Google Scholar]
- Carmel G, Mager EM, Binder LI, Kuret J. The structural basis of monoclonal antibody Alz50’s selectivity for Alzheimer’s disease pathology. J Biol Chem. 1996;271:32789–32795. doi: 10.1074/jbc.271.51.32789. [DOI] [PubMed] [Google Scholar]
- Chen CS, Weng SC, Tseng PH, Lin HP. Histone acetylation-independent effect of histone deacetylase inhibitors on Akt through the reshuffling of protein phosphatase 1 complexes. J Biol Chem. 2005;280:38879–38887. doi: 10.1074/jbc.M505733200. [DOI] [PubMed] [Google Scholar]
- Cho JH, Johnson GV. Glycogen synthase kinase 3beta phosphorylates tau at both primed and unprimed sites. Differential impact on microtubule binding. J Biol Chem. 2003;278:187–193. doi: 10.1074/jbc.M206236200. [DOI] [PubMed] [Google Scholar]
- Cho JH, Johnson GV. Primed phosphorylation of tau at Thr231 by glycogen synthase kinase 3beta (GSK3beta) plays a critical role in regulating tau’s ability to bind and stabilize microtubules. J Neurochem. 2004;88:349–358. doi: 10.1111/j.1471-4159.2004.02155.x. [DOI] [PubMed] [Google Scholar]
- Ding H, Matthews TA, Johnson GV. Site-specific phosphorylation and caspase cleavage differentially impact tau-microtubule interactions and tau aggregation. J Biol Chem. 2006;281:19107–19114. doi: 10.1074/jbc.M511697200. [DOI] [PubMed] [Google Scholar]
- Dou F, Netzer WJ, Tanemura K, Li F, Hartl FU, Takashima A, Gouras GK, Greengard P, Xu H. Chaperones increase association of tau protein with microtubules. Proc Natl Acad Sci U S A. 2003;100:721–726. doi: 10.1073/pnas.242720499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson J, Landon M, Lowe J, Dawson SP, Layfield R, Hanger DP, Mayer RJ. Pathological lesions of Alzheimer’s disease and dementia with Lewy bodies brains exhibit immunoreactivity to an ATPase that is a regulatory subunit of the 26S proteasome. Neurosci Lett. 1996;219:167–170. doi: 10.1016/s0304-3940(96)13192-x. [DOI] [PubMed] [Google Scholar]
- Gamblin TC, Chen F, Zambrano A, et al. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2003;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S, Muqit MM, Stanyer L, et al. PINK1 protein in normal human brain and Parkinson’s disease. Brain. 2006;129:1720–1731. doi: 10.1093/brain/awl114. [DOI] [PubMed] [Google Scholar]
- Gao YS, Hubbert CC, Lu J, Lee YS, Lee JY, Yao TP. Histone deacetylase 6 regulates growth factor-induced actin remodeling and endocytosis. Mol Cell Biol. 2007;27:8637–8647. doi: 10.1128/MCB.00393-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Crowther RA, Cohen P, Vanmechelen E, Vandermeeren M, Cras P. Epitope mapping of monoclonal antibodies to the paired helical filaments of Alzheimer’s disease: identification of phosphorylation sites in tau protein. Biochem J. 1994;301(Pt 3):871–877. doi: 10.1042/bj3010871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K, Martinez-Coria H, Steffan JS, Schreiber SS, Thompson LM, LaFerla FM. Oral nicotinamide treatment induces ubiquitin-dependent degradation of phospho-tau and restores cognitive function in a mouse model of Alzheimer disease. Abstract of 37th Annual Meeting of Society for Neuroscience; 2007. Program No. 157.15. [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci U S A. 2003;100:4389–4394. doi: 10.1073/pnas.0430973100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempen B, Brion JP. Reduction of acetylated alpha-tubulin immunoreactivity in neurofibrillary tangle-bearing neurons in Alzheimer’s disease. J Neuropathol Exp Neurol. 1996;55:964–972. doi: 10.1097/00005072-199609000-00003. [DOI] [PubMed] [Google Scholar]
- Hoffmann R, Lee VM, Leight S, Varga I, Otvos L., Jr Unique Alzheimer’s disease paired helical filament specific epitopes involve double phosphorylation at specific sites. Biochemistry. 1997;36:8114–8124. doi: 10.1021/bi970380+. [DOI] [PubMed] [Google Scholar]
- Hook SS, Orian A, Cowley SM, Eisenman RN. Histone deacetylase 6 binds polyubiquitin through its zinc finger (PAZ domain) and copurifies with deubiquitinating enzymes. Proc Natl Acad Sci U S A. 2002;99:13425–13430. doi: 10.1073/pnas.172511699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- Iwata A, Riley BE, Johnston JA, Kopito RR. HDAC6 and microtubules are required for autophagic degradation of aggregated huntingtin. J Biol Chem. 2005;280:40282–40292. doi: 10.1074/jbc.M508786200. [DOI] [PubMed] [Google Scholar]
- Jeganathan S, von Bergen M, Brutlach H, Steinhoff HJ, Mandelkow E. Global hairpin folding of tau in solution. Biochemistry. 2006;45:2283–2293. doi: 10.1021/bi0521543. [DOI] [PubMed] [Google Scholar]
- Johnson GV, Bailey CD. Tau, where are we now? J Alzheimers Dis. 2002;4:375–398. doi: 10.3233/jad-2002-4505. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell. 2003;115:727–738. doi: 10.1016/s0092-8674(03)00939-5. [DOI] [PubMed] [Google Scholar]
- Keck S, Nitsch R, Grune T, Ullrich O. Proteasome inhibition by paired helical filament-tau in brains of patients with Alzheimer’s disease. J Neurochem. 2003;85:115–122. doi: 10.1046/j.1471-4159.2003.01642.x. [DOI] [PubMed] [Google Scholar]
- Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1986;83:4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs JJ, Murphy PJ, Gaillard S, et al. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell. 2005;18:601–607. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy PK, Johnson GV. Mutant (R406W) human tau is hyperphosphorylated and does not efficiently bind microtubules in a neuronal cortical cell model. J Biol Chem. 2004;279:7893–7900. doi: 10.1074/jbc.M311203200. [DOI] [PubMed] [Google Scholar]
- Kwon S, Zhang Y, Matthias P. The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response. Genes Dev. 2007;21:3381–3394. doi: 10.1101/gad.461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam YA, Pickart CM, Alban A, Landon M, Jamieson C, Ramage R, Mayer RJ, Layfield R. Inhibition of the ubiquitin-proteasome system in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2000;97:9902–9906. doi: 10.1073/pnas.170173897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YT, Cheng JT, Liang LC, Ko CY, Lo YK, Lu PJ. The binding and phosphorylation of Thr231 is critical for Tau’s hyperphosphorylation and functional regulation by glycogen synthase kinase 3beta. J Neurochem. 2007;103:802–813. doi: 10.1111/j.1471-4159.2007.04792.x. [DOI] [PubMed] [Google Scholar]
- Matsuyama A, Shimazu T, Sumida Y, et al. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. Embo J. 2002;21:6820–6831. doi: 10.1093/emboj/cdf682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PJ, Morishima Y, Kovacs JJ, Yao TP, Pratt WB. Regulation of the dynamics of hsp90 action on the glucocorticoid receptor by acetylation/deacetylation of the chaperone. J Biol Chem. 2005;280:33792–33799. doi: 10.1074/jbc.M506997200. [DOI] [PubMed] [Google Scholar]
- Olzmann JA, Li L, Chudaev MV, Chen J, Perez FA, Palmiter RD, Chin LS. Parkin-mediated K63-linked polyubiquitination targets misfolded DJ-1 to aggresomes via binding to HDAC6. J Cell Biol. 2007;178:1025–1038. doi: 10.1083/jcb.200611128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otvos L, Jr, Feiner L, Lang E, Szendrei GI, Goedert M, Lee VM. Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J Neurosci Res. 1994;39:669–673. doi: 10.1002/jnr.490390607. [DOI] [PubMed] [Google Scholar]
- Pandey UB, Nie Z, Batlevi Y, et al. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2007;447:859–863. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- Petrucelli L, Dickson D, Kehoe K, et al. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum Mol Genet. 2004;13:703–714. doi: 10.1093/hmg/ddh083. [DOI] [PubMed] [Google Scholar]
- Seigneurin-Berny D, Verdel A, Curtet S, Lemercier C, Garin J, Rousseaux S, Khochbin S. Identification of components of the murine histone deacetylase 6 complex: link between acetylation and ubiquitination signaling pathways. Mol Cell Biol. 2001;21:8035–8044. doi: 10.1128/MCB.21.23.8035-8044.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert P, Mawal-Dewan M, Barbour R, et al. Detection of phosphorylated Ser262 in fetal tau, adult tau, and paired helical filament tau. J Biol Chem. 1995;270:18917–18922. doi: 10.1074/jbc.270.32.18917. [DOI] [PubMed] [Google Scholar]
- Tran AD, Marmo TP, Salam AA, et al. HDAC6 deacetylation of tubulin modulates dynamics of cellular adhesions. J Cell Sci. 2007;120:1469–1479. doi: 10.1242/jcs.03431. [DOI] [PubMed] [Google Scholar]
- Verdin E, Dequiedt F, Kasler HG. Class II histone deacetylases: versatile regulators. Trends Genet. 2003;19:286–293. doi: 10.1016/S0168-9525(03)00073-8. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yuan Z, Zhang Y, et al. HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol Cell. 2007;27:197–213. doi: 10.1016/j.molcel.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li N, Caron C, Matthias G, Hess D, Khochbin S, Matthias P. HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. Embo J. 2003;22:1168–1179. doi: 10.1093/emboj/cdg115. [DOI] [PMC free article] [PubMed] [Google Scholar]