Abstract
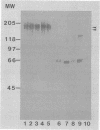
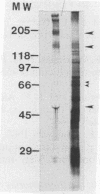
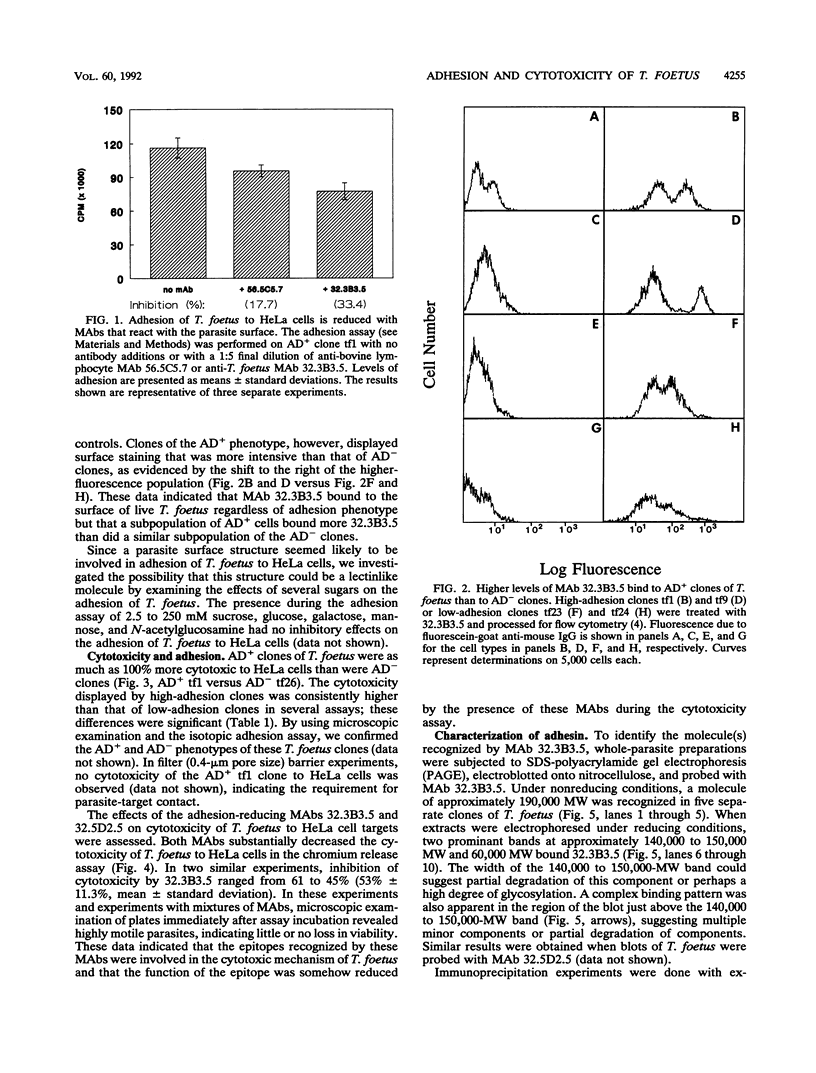
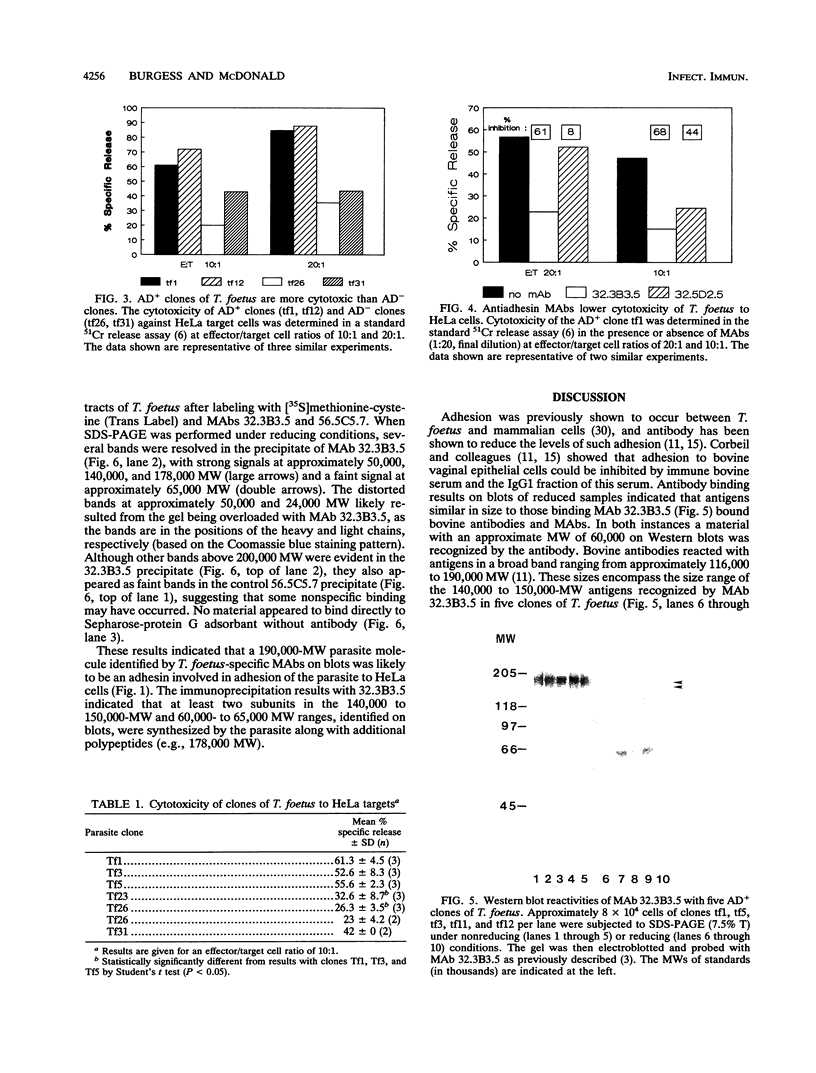
The relationship of Tritrichomonas foetus adhesion to mammalian cells and cytotoxicity to these targets was investigated. High-adherence and low-adherence lines of T. foetus, derived by repeated adhesion to HeLa cells, showed high and low cytotoxicity, respectively, to HeLa cells. When parasites were separated from targets by membranes (0.4-microns pore size), no cytotoxicity was detectable. Monoclonal antibodies elicited against T. foetus that lowered adhesion also lowered parasite-mediated cytotoxicity. Flow cytometry experiments revealed that the levels of an adhesion- and cytotoxicity-blocking antibody bound to the surface of high-adherence clones of T. foetus were higher than those in low-adherence clones. Western blots of parasite extracts separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis were probed with an anti-T. foetus antibody. A molecule with a molecular weight of approximately 190,000 composed of subunits with molecular weights of approximately 140,000 to 150,000 and approximately 65,000 was identified. Immunoprecipitation experiments with metabolically labeled T. foetus and the same antibody confirmed that similar subunits were synthesized by the parasite. These results indicate that adhesion of T. foetus to mammalian cells is an important step in cytotoxic damage of these targets and that a surface adhesin on the parasite is involved in the adhesion mechanism.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Garza G. E. Specific nature of Trichomonas vaginalis parasitism of host cell surfaces. Infect Immun. 1985 Dec;50(3):701–708. doi: 10.1128/iai.50.3.701-708.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Pearlman E. Pathogenic Trichomonas vaginalis cytotoxicity to cell culture monolayers. Br J Vener Dis. 1984 Apr;60(2):99–105. doi: 10.1136/sti.60.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo R., Engbring J., Alderete J. F. Molecular basis of host epithelial cell recognition by Trichomonas vaginalis. Mol Microbiol. 1992 Apr;6(7):853–862. doi: 10.1111/j.1365-2958.1992.tb01536.x. [DOI] [PubMed] [Google Scholar]
- Baker P. E., Knoblock K. F. Bovine costimulator. I. Production kinetics, partial purification, and quantification in serum-free Iscove's medium. Vet Immunol Immunopathol. 1982 Jul;3(4):365–379. doi: 10.1016/0165-2427(82)90020-4. [DOI] [PubMed] [Google Scholar]
- Burgess D. E. Clonal and geographic distribution of a surface antigen of Tritrichomonas foetus. J Protozool. 1988 Feb;35(1):119–122. doi: 10.1111/j.1550-7408.1988.tb04090.x. [DOI] [PubMed] [Google Scholar]
- Burgess D. E., Knoblock K. F., Daugherty T., Robertson N. P. Cytotoxic and hemolytic effects of Tritrichomonas foetus on mammalian cells. Infect Immun. 1990 Nov;58(11):3627–3632. doi: 10.1128/iai.58.11.3627-3632.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess D. E., Knoblock K. F. Identification of Tritrichomonas foetus in sections of bovine placental tissue with monoclonal antibodies. J Parasitol. 1989 Dec;75(6):977–980. [PubMed] [Google Scholar]
- Burgess D. E. Tritrichomonas foetus: preparation of monoclonal antibodies with effector function. Exp Parasitol. 1986 Oct;62(2):266–274. doi: 10.1016/0014-4894(86)90031-7. [DOI] [PubMed] [Google Scholar]
- Calderone R. A., Braun P. C. Adherence and receptor relationships of Candida albicans. Microbiol Rev. 1991 Mar;55(1):1–20. doi: 10.1128/mr.55.1.1-20.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casta e Silva Filho F., de Souza W., Lopes J. D. Presence of laminin-binding proteins in trichomonads and their role in adhesion. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8042–8046. doi: 10.1073/pnas.85.21.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbeil L. B., Hodgson J. L., Jones D. W., Corbeil R. R., Widders P. R., Stephens L. R. Adherence of Tritrichomonas foetus to bovine vaginal epithelial cells. Infect Immun. 1989 Jul;57(7):2158–2165. doi: 10.1128/iai.57.7.2158-2165.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND L. S. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol. 1957 Aug;43(4):488–490. [PubMed] [Google Scholar]
- Gitler C., Mirelman D. Factors contributing to the pathogenic behavior of Entamoeba histolytica. Annu Rev Microbiol. 1986;40:237–261. doi: 10.1146/annurev.mi.40.100186.001321. [DOI] [PubMed] [Google Scholar]
- Hale T. L. Genetic basis of virulence in Shigella species. Microbiol Rev. 1991 Jun;55(2):206–224. doi: 10.1128/mr.55.2.206-224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson J. L., Jones D. W., Widders P. R., Corbeil L. B. Characterization of Tritrichomonas foetus antigens by use of monoclonal antibodies. Infect Immun. 1990 Sep;58(9):3078–3083. doi: 10.1128/iai.58.9.3078-3083.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger J. N., Ravdin J. I., Rein M. F. Contact-dependent cytopathogenic mechanisms of Trichomonas vaginalis. Infect Immun. 1985 Dec;50(3):778–786. doi: 10.1128/iai.50.3.778-786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood B. C., North M. J., Scott K. I., Bremner A. F., Coombs G. H. The use of a highly sensitive electrophoretic method to compare the proteinases of trichomonads. Mol Biochem Parasitol. 1987 May;24(1):89–95. doi: 10.1016/0166-6851(87)90119-8. [DOI] [PubMed] [Google Scholar]
- Luaces A. L., Barrett A. J. Affinity purification and biochemical characterization of histolysin, the major cysteine proteinase of Entamoeba histolytica. Biochem J. 1988 Mar 15;250(3):903–909. doi: 10.1042/bj2500903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonson I. M., Clark B. L., Dufty J. H. Early pathogenesis and pathology of Tritrichomonas foetus infection in virgin heifers. J Comp Pathol. 1976 Jan;86(1):59–66. doi: 10.1016/0021-9975(76)90028-1. [DOI] [PubMed] [Google Scholar]
- Petri W. A., Jr, Ravdin J. I. Protection of gerbils from amebic liver abscess by immunization with the galactose-specific adherence lectin of Entamoeba histolytica. Infect Immun. 1991 Jan;59(1):97–101. doi: 10.1128/iai.59.1.97-101.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri W. A., Jr, Smith R. D., Schlesinger P. H., Murphy C. F., Ravdin J. I. Isolation of the galactose-binding lectin that mediates the in vitro adherence of Entamoeba histolytica. J Clin Invest. 1987 Nov;80(5):1238–1244. doi: 10.1172/JCI113198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri W. A., Jr, Snodgrass T. L., Jackson T. F., Gathiram V., Simjee A. E., Chadee K., Chapman M. D. Monoclonal antibodies directed against the galactose-binding lectin of Entamoeba histolytica enhance adherence. J Immunol. 1990 Jun 15;144(12):4803–4809. [PubMed] [Google Scholar]
- Picha K. S., Baker P. E. Bovine T lymphocytes. I. Generation and maintenance of an interleukin-2-dependent, cytotoxic T-lymphocyte cell line. Immunology. 1986 Jan;57(1):131–136. [PMC free article] [PubMed] [Google Scholar]
- Ravdin J. I., Croft B. Y., Guerrant R. L. Cytopathogenic mechanisms of Entamoeba histolytica. J Exp Med. 1980 Aug 1;152(2):377–390. doi: 10.1084/jem.152.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin J. I., Guerrant R. L. Role of adherence in cytopathogenic mechanisms of Entamoeba histolytica. Study with mammalian tissue culture cells and human erythrocytes. J Clin Invest. 1981 Nov;68(5):1305–1313. doi: 10.1172/JCI110377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhyan J. C., Stackhouse L. L., Quinn W. J. Fetal and placental lesions in bovine abortion due to Tritrichomonas foetus. Vet Pathol. 1988 Sep;25(5):350–355. doi: 10.1177/030098588802500503. [DOI] [PubMed] [Google Scholar]
- Schurig G. G., Duncan J. R., Winter A. J. Elimination of genital vibriosis in female cattle by systemic immunization with killed cells or cell-free extracts of Campylobacter fetus. J Infect Dis. 1978 Oct;138(4):463–472. doi: 10.1093/infdis/138.4.463. [DOI] [PubMed] [Google Scholar]
- Silva Filho F. C., de Souza W. The interaction of Trichomonas vaginalis and Tritrichomonas foetus with epithelial cells in vitro. Cell Struct Funct. 1988 Aug;13(4):301–310. doi: 10.1247/csf.13.301. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Talbot J. A., Nielsen K., Corbeil L. B. Cleavage of proteins of reproductive secretions by extracellular proteinases of Tritrichomonas foetus. Can J Microbiol. 1991 May;37(5):384–390. doi: 10.1139/m91-062. [DOI] [PubMed] [Google Scholar]