Abstract
Purpose
Radiation-induced heart disease (RIHD) is a severe side effect of thoracic radiotherapy. This study examined the effects of PTX and α-tocopherol on cardiac injury in a rat model of RIHD.
Methods and Materials
Male Sprague-Dawley rats received fractionated local heart irradiation with a daily dose of 9 Gy for 5 days and were observed for six months after irradiation. Rats were treated with a combination of pentoxifylline (PTX, 100 mg/kg/day) and α-tocopherol (20 IU/kg/day) and received these compounds either from one week before until six months after irradiation or starting three months after irradiation, a time point at which histopathological changes become apparent in our model of RIHD.
Results
Radiation-induced increases in left ventricular diastolic pressure (in mmHg: 35 ± 6 after sham-irradiation, 82 ± 11 after irradiation) were significantly reduced by PTX and α-tocopherol (early treatment: 48 ± 7, late treatment: 53 ± 6). PTX and α-tocopherol significantly reduced deposition of collagen types I (radiation only: 3.5 ± 0.2 μm2 per 100 μm2, early treatment: 2.7 ± 0.8, late treatment: 2.2 ± 0.2) and III (radiation only: 13.9 ± 0.8, early treatment: 11.0 ± 1.2, late treatment: 10.6 ± 0.8). On the other hand, radiation-induced alterations in heart/body weight ratios, myocardial degeneration, left ventricular mast cell densities, and most echocardiographic parameters were not significantly altered by PTX and α-tocopherol.
Conclusions
treatment with PTX and α-tocopherol may have beneficial effects on radiation-induced myocardial fibrosis and left ventricular ex vivo function, both when started before irradiation and when started later during the process of RIHD.
Keywords: heart, radiation, pentoxifylline, α-tocopherol, fibrosis
Introduction
Radiation-induced heart disease (RIHD) is a potentially life-threatening side effect of radiotherapy of thoracic and chest wall tumors when all or part of the heart is included in the radiation field. RIHD presents clinically several years after irradiation, and the disease process is progressive. Randomized studies show a significant increase in cardiac events in patients at least 10 years after treatment with radiotherapy for thoracic and chest wall tumors. Specifically, in patients treated before the 1980’s, a 2-fold increase in cardiovascular mortality is seen after radiotherapy for Hodgkin’s disease (1) and breast cancer (2). In patients treated in the 1980’s and 1990’s, a significant increase is found in cardiovascular mortality in patients treated with radiotherapy for left-sided breast cancer when compared to patients treated for right-sided breast cancer (3). Moreover, reduced mortality from breast cancer is often offset by an increase in mortality from heart disease in irradiated patients (4,5). The heart continues to be a major organ at risk in thoracic radiotherapy despite recent advances in radiation delivery and treatment planning techniques. Nonetheless, there is currently no method or approach for preventing or reversing RIHD.
A prominent clinical manifestation of RIHD is myocardial fibrosis (3,6). Pentoxifylline (PTX), a phosphodiesterase inhibitor first developed as a rheological agent, has been shown to reduce radiation fibrosis in clinical studies when administered alone (7) or in combination with α-tocopherol (vitamin E) (8,9). In many of these studies treatment with PTX and α-tocopherol is started after radiation-induced fibrosis has become clinically manifest. On the other hand, not all studies show a beneficial effect of PTX and vitamin E on radiation fibrosis (10). Moreover, it is suggested that PTX may enhance radiation sensitivity when administered during radiation (11,12).
In this study, we examined the effects of PTX in combination with α-tocopherol on cardiac structure and function after localized fractionated heart irradiation in rats. The efficacy of PTX and α-tocopherol was tested both when treatment was started one week before irradiation, and when treatments was started three months after irradiation, a time point at which histopathological changes including myocardial fibrosis are apparent in our animal model (13).
Methods and Materials
Rat heart irradiation
All procedures in this study were approved by the Institutional Animal Care and Use Committee of the University of Arkansas for Medical Sciences. A total of 72 male Sprague-Dawley rats (180–200 g) were obtained from Harlan (Indianapolis, IN). All animals were maintained in our Division of Laboratory Medicine on a 12:12 light-to-dark cycle with free access to food and water.
After two weeks of acclimatization, rats were anesthetized with 2.5% isoflurane and irradiated with a Seifert Isovolt 320 X-ray machine (Seifert X-Ray Corporation, Fairview Village, PA) operated at 250 kV and 15 mA with 3 mm aluminum and 1.85 mm copper added filtration, at a dose rate of 1.17 Gy/min. Dosimetry was performed using an electrometer (Model 206, CNMC, Nashville, TN, calibration factor 1,000 nC/reading) and a cylindrical ionization chamber with volume 0.13 mL (Model N31005, PTW, Hicksville, NY, calibration factor 28.98 R/nC). Calibration of the instruments was performed by the University of Wisconsin Accredited Dosimetry Calibration Laboratory (Madison, WI). The measured Half-Value Layer of the beam used for these experiments was 2.8 mm Cu.
A daily dose of 9 Gy or 0 Gy was administered for 5 consecutive days. This radiation dose corresponds to approximately 21 Gy single dose and was chosen to produce moderate to severe RIHD (14). Radiation was delivered locally on the heart using parallel opposed fields (anterior-posterior 1:1) with a 19 mm diameter, while the rest of the animal was shielded with lead plates.
Pentoxifylline and α-tocopherol administration
Rats were treated with a combination of PTX (100 mg/kg body weight/day) (Sigma-Aldrich, St Louis MO) and α-tocopherol (in the form of dl-α-tocopheryl acetate, 20 IU/kg body weight/day). These compounds were added to the standard rodent chow TD8640 by Harlan-Teklad. Rats were randomly divided into six treatment groups: 1) sham-irradiation and regular chow; 2) sham-irradiation and customized chow administered from one week before until six months after sham-irradiation; 3) sham-irradiation and customized chow from three months after until six months after sham-irradiation; 4) 5×9 Gy and regular chow; 5) 5×9 Gy and customized chow from one week before until six months after irradiation (“early treatment”); 6) 5×9 Gy and customized chow from three months after until six months after irradiation (“late treatment”).
Echocardiography
At six months after irradiation, cardiac function was assessed with echocardiography using the Vevo 770 high-resolution in vivo micro imaging system (VisualSonics, Toronto, Canada). The VisualSonics RMV 716 Scanhead with center frequency 17.5 MHz, frequency band 11.5–23.5 MHz and focal length 17.5 mm was used. Rats were anesthetized with 3% isoflurane and maintained at 1.8% isoflurane delivered through a nose cone during the procedure. All hair was removed from the chest with clippers followed by Nair lotion hair remover (Church and Dwight, Princeton, NJ). Rats were positioned on a heated platform that records both ECG and breathing pattern. The chest was covered with ultrasound transmission gel (Aquasonic, Parker Laboratories, Fairfield, NJ) and the heart was visualized at the parasternal short axis at the mid left ventricular level, verified by the presence of prominent papillary muscles. M-mode recordings were stored for determination of echocardiographic parameters with the Vevo cardiac software package. Care was taken to avoid fragments of M-mode recordings taken during inhalation.
Langendorff perfused rat heart preparation
Langendorff studies were performed as described before (14). Hearts were isolated from rats in each of the six treatment groups and immediately perfused via the aorta with an oxygenated Krebs-Henseleit solution (37°C) at a flow rate of 8 ml/g heart/min. Both atria were removed and the ventricles were paced with platinum contact electrodes positioned on the interventricular septum to obtain a heart rate of 250 beats/min. A fluid-filled balloon catheter was placed in the left ventricle and left ventricular diastolic pressure, peak systolic pressure, +dP/dtmax (rate of contraction), and −dP/dtmax (rate of relaxation) were measured at various preload balloon volumes between 80 μl and 300 μl (a range that elicited maximum contractility in all preparations). Coronary pressure was monitored continuously with a Statham pressure transducer. All data were digitized and analyzed with the use of acquisition and analysis software (CODAS; DataQ Instruments, Akron, Ohio, USA). After Langendorff studies the hearts were weighed and processed for histology and immunohistochemistry.
According to a method of Radke et al (15), pressure data obtained from Langendorff preparations were converted into wall stress to correct for possible changes in left ventricular geometry. Left ventricular wall stress was calculated as: Left ventricular pressure/[(left ventricular wall volume/balloon volume +1)2/3 −1]. Left ventricular wall volume was calculated as: heart weight/1.05, assuming that heart weight reflects mainly left ventricular weight.
Histology and immunohistochemistry
Hearts were fixed in methanol Carnoy’s solution (60% methanol, 30% chloroform, 10% acetic acid) and embedded in paraffin to obtain 5 μm cross sections.
For determination of mast cell numbers, sections were stained with 0.5% Toluidine Blue (14). The total mast cell number in 10 ocular fields with a 10× objective was determined. This area covered almost the entire left ventricular area. Mast cell density was calculated as number of mast cells per area (mm2).
Immunohistochemistry was performed with methods established and optimized in our laboratory (16,17). Sections were deparaffinized and rehydrated. Endogenous peroxidase was blocked with 1% H2O2 in methanol. Non-specific antibody binding was reduced by 10% normal rabbit or normal goat serum (Vector Laboratories, Burlingame, CA) in TBS containing 3% dry powdered milk and 0.2% BSA. Sections were incubated with one of the following primary antibodies: goat anti-collagen type I, goat anti-collagen type III (both Southern Biotechnology Associates, Birmingham, AL), each at 1:100, or pan-specific rabbit anti-transforming growth factor beta (TGF-β, R&D, Minneapolis, MN) at 1:300. After incubation with biotinylated goat anti-rabbit IgG or rabbit anti-goat IgG (Vector Laboratories, Burlingame, CA), both at 1:400, sections were incubated with avidin-biotin-peroxidase complex (Vector Laboratories) and visualized with 0.5 mg/ml 3,3-diaminobenzidine tetrahydrochloride (Sigma-Aldrich) and 0.003% H2O2 in TBS.
Sections were analyzed with an Axioskop transmitted light microscope (Carl Zeiss, Thornwood, NY) equipped with a color chilled 3CCD camera (Hamamatsu, Hamamatsu City, Japan). Quantitative assessment of areas immunoreactive for collagen type I, collagen type III or extracellular matrix-associated TGF-β was performed with computerized image analysis (Image-Pro Plus, Media Cybernetics, Silver Spring, MD) as validated before (18). For each heart the average area of 20 fields with a 40× objective was calculated.
Hematoxylin-eosin stained sections were used for assessment of total left ventricular area of myocardial degeneration (myocardial necrosis accompanied by inflammation) with computerized image analysis. Each heart was then given a score for area of myocardial degeneration, as follows: score 0 (no degeneration), score 1 (total area of degeneration 0 –20,000 μm2), score 2 (20,000–40,000 μm2), or score 3 (40,000–60,000 μm2).
Cardiac fibroblast isolation and staining
Hearts were isolated from rats, immediately rinsed and minced in sterile saline, and incubated in HBSS containing 75 U/ml collagenase type II (Worthington Biochemical, Lakewood, NJ) in a trypsinizing flask (Wheaton Science Products, Millville, NJ) under continuous stirring with a star-headed stir-bar (Nalgene Labware, Rochester, NY) at 37°C. The first supernatant obtained after 10 min of incubation was discarded. Subsequent supernatants were collected every 10 min for a total of 40 min and immediately centrifuged at 1,300 g for 4 min. The pellets were resuspended in DMEM containing 10% FBS and antibiotics (Invitrogen, Carlsbad, CA), combined and maintained in a culture flask at 37°C in a humidified atmosphere of 5% CO2 and 95% air. After ten days of incubation colonies of cells were detected with the cellular morphology of fibroblasts. Cells were trypsinized and plated on cover slips. After three days of incubation, cover slips were rinsed with PBS and fixed in 4% methanol free formaldehyde (Pierce, Rockford, IL). After cell permeabilization with 0.1% Triton X-100 for 10 min, cover slips were incubated with FITC-phalloidin (Invitrogen) at 5 U/ml for 20 min to visualize actin filaments. Cells were counterstained with a DAPI nucleus stain and analyzed with an Axioskop 40 fluorescence microscope equipped with an Axiocam MRm camera (Carl Zeiss).
RNA isolation and real-time PCR
Hearts were isolated from three rats in each of the six treatment groups, snap-frozen in liquid nitrogen and subsequently stored at −80°C. Frozen tissue samples from the left ventricle were homogenized in Ultraspec™ RNA reagent (Biotecx Laboratories, Houston, TX), according to the manufacturer’s instructions. After treatment with RQ-DNAse I (Promega, Madison, WI) at 37°C for 30 min, cDNA was synthesized using the High Capacity cDNA Archive Kit™ (Applied Biosystems, Foster City, CA). Steady-state mRNA levels were measured with real-time quantitative PCR (TaqMan™) using the ABI Prism 7700 Sequence Detection System, TaqMan mastermix, TaqMan polymerase, and the pre-designed TaqMan Gene Expression Assay™ for rat TGF-β1 (Rn00572010_m1) or rat connective tissue growth factor (CTGF, Rn00573960_q1) (all Applied Biosystems, Foster City, CA).
Statistical analysis
Data were evaluated with the software package NCSS 2007 (NCSS, Kaysville, UT). Langendorff data were tested with repeated measures ANOVA. Frequencies of scores for myocardial degeneration were tested with a Chi-Square test. All other data were tested with two-way ANOVA, followed by Newman-Keuls individual comparisons. The criterion for significance was a p<0.05. Data are reported as average ± standard error of the mean (SEM).
Results
In vivo and ex vivo cardiac function
Radiation induced significant reductions both in heart weight (F=39.76, p<0.001) and in heart/body weight ratio (F=36.14, p<0.001) as measured at six months (Table 1). Treatment with PTX and α-tocopherol did not alter body weight or heart/body weight ratio in any of the treatment groups.
Table 1.
Body weights, heart weights, and heart/body weight ratios of rats in each treatment group at six months after (sham-) irradiation (average ± SEM, n=8).
| Treatment group | Body weight (g) | Heart weight (g) | Heart/body weight(mg/g) |
|---|---|---|---|
| 1: Sham, Regular chow | 468.9 ± 14.8 | 1.6 ± 0.1 | 3.4 ± 0.04 |
| 2: Sham, PTX early | 475.6 ± 13.6 | 1.5 ± 0.04 | 3.2 ± 0.1 |
| 3: Sham, PTX late | 482.5 ± 6.7 | 1.5 ± 0.02 | 3.2 ± 0.05 |
| 4: 5×9 Gy, Regular chow | 457.3 ± 12.8 | 1.4 ± 0.1* | 3.0 ± 0.1* |
| 5: 5×9 Gy, PTX early | 455.6 ± 8.4 | 1.3 ± 0.04 | 2.8 ± 0.06 |
| 6: 5×9 Gy, PTX late | 459.3 ± 8.7 | 1.3 ± 0.04 | 2.9 ± 0.1 |
Radiation induced a significant reduction in heart weight (F=39.76, p<0.001) and heart/body weight ratio (F=36.14, p<0.001).
Table 2 lists echocardiographic parameters obtained at six months after (sham-) irradiation. Radiation induced a significant increase in left ventricular anterior wall thickness, both in systole (F=6.64, p=0.02) and in diastole (F=5.46, p=0.03). Increased left ventricular wall thickness was accompanied by reduced left ventricular inner diameter in irradiated hearts, as observed both in systole (F=12.94, p=0.001) and diastole (F=4.87, p=0.04). Both ejection fraction and fractional shortening were significantly increased in irradiated hearts (F=12.82, p=0.001 and F=13.34, p=0.001, respectively), but stroke volume was not altered. In sham-irradiated hearts, PTX and α-tocopherol significantly increased left ventricular inner diameter, but only when measured in diastole (F=7.41, p=0.007), In addition, stroke volume was significantly increased in sham-irradiated animals (F=5.64, p=0.02). In irradiated hearts, left ventricular anterior wall thickness was reduced by PTX and α-tocopherol when measured in systole (F=5.25, p=0.02), but not when measured in diastole. None of the other echocardiographic parameters were significantly altered by PTX and α-tocopherol.
Table 2.
Selected echocardiographic parameters in each treatment group at six months after (sham-) irradiation (average ± SEM, n=5–6).
| Treatment group | LVAW* (mm) in systole in diastole | LVID* (mm) in systole in diastole | Ejection fraction(%) | Fractional shortening(%) | Stroke volume (μl) |
|---|---|---|---|---|---|
| 1: Sham, Regular chow | 3.4 ± 0.1
2.0 ± 0.1 |
3.8 ± 0.2
7.5 ± 0.1 |
77.4 ± 2.3 | 47.9 ± 2.2 | 235.4 ± 11.2 |
| 2: Sham, PTX early | 3.1 ± 0.2
1.8 ± 0.1 |
4.4 ± 0.2
8.2 ± 0.1† |
73.0 ± 2.9 | 44.1 ± 2.5 | 272.0 ± 10.6† |
| 3: Sham, PTX late | 3.3 ± 0.1
1.9 ± 0.02 |
4.4 ± 0.1
8.2 ± 0.1† |
73.4 ± 1.3 | 44.1 ± 1.1 | 281.6 ± 7.2† |
| 4: 5×9 Gy, Regular chow | 3.8 ± 0.1‡
2.2 ± 0.05‡ |
3.4 ± 0.1‡
7.4 ± 0.1‡ |
82.3 ± 0.7‡ | 53.3 ± 1.0‡ | 241.3 ± 8.7 |
| 5: 5×9 Gy, PTX early | 3.3 ± 0.1§
2.0 ± 0.1 |
3.5 ± 0.3
7.5 ± 0.4 |
78.8 ± 1.0 | 49.1 ± 1.1 | 244.9 ± 21.4 |
| 6: 5×9 Gy, PTX late | 3.4 ± 0.1§
2.1 ± 0.1 |
3.8 ± 0.2
7.9 ± 0.2 |
78.5 ± 1.7 | 49.0 ± 1.7 | 274.0 ± 11.4 |
LVAW: Left ventricular anterior wall thickness, LVID: Left ventricular inner diameter
PTX and α-tocopherol significantly increased left ventricular diastolic inner diameter (F=7.41, p=0.007) and stroke volume (F=5.64, p=0.02) in sham-irradiated hearts.
Radiation induced a significant increase in left ventricular anterior wall thickness in systole (F=6.64, p=0.02) and diastole (F=5.46, p=0.03), a reduction in left ventricular inner diameter in systole (F=12.94, p=0.001) and diastole (F=4.87, p=0.04), and increased ejection fraction (F=12.82, p=0.001) and fractional shortening (F=13.34, p=0.001).
PTX and α-tocopherol significantly reduced left ventricular systolic anterior wall thickness in irradiated hearts (F=5.25, p=0.02).
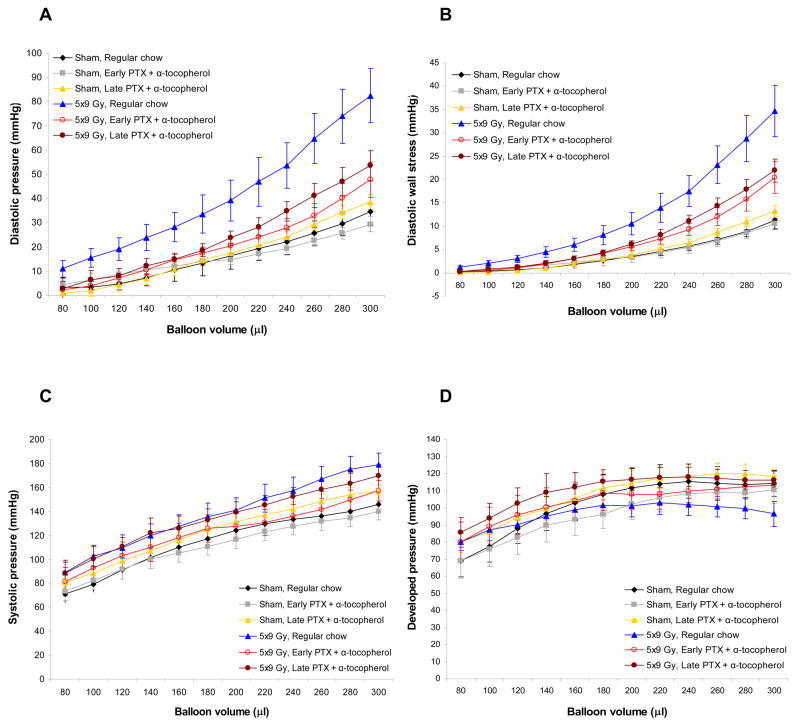
Figure 1 shows parameters measured with Langendorff isolated perfused hearts at six months after (sham-) irradiation. Radiation induced a significant increase in left ventricular diastolic pressure (F=10.85, p=0.002). PTX and α-tocopherol significantly reduced diastolic pressures within irradiated animals (F=4.34, p=0.03). Similar results were obtained when left ventricular diastolic pressures were converted into left ventricular wall stress (Figure 1B). All other Langendorff parameters, including coronary pressures, +dP/dtmax, and −dP/dtmax were not altered by radiation or by PTX and α-tocopherol (data not shown). No differences were found between the effects of early and late treatment with PTX and α-tocopherol on any of the in vivo or ex vivo cardiac measurements.
Figure 1.
Parameters obtained with Langendorff studies in each of the treatment groups at six months after (sham-) irradiation (average ± SEM, 5×9 Gy vehicle group: n=6, other groups: n=7–8). (A) Radiation-induced increases in diastolic pressure was significantly reduced by PTX and α-tocopherol (F=4.34, p=0.03). (B) Left ventricular diastolic wall stress calculated from left ventricular diastolic pressures. (C) No differences were found in left ventricular systolic pressures between any of the treatment groups. (D) No differences were found in left ventricular developed pressures between any of the treatment groups.
Myocardial structure and mRNA levels
Seven hearts in each treatment group were scored for area of myocardial degeneration on a scale from 0 to 3. The distribution of scores among sham-irradiated hearts was as follows: 5 hearts received score 0, one heart received score 1, and one heart received score 2. Among irradiated hearts, 3 hearts received score 0, one heart received score 2, and 3 hearts received score 3. Although treatment with PTX and α-tocopherol showed a trend towards smaller areas of myocardial degeneration, with none of the hearts receiving a score above 1, the differences did not quite reach statistical significance (Chi-Square: p=0.06).
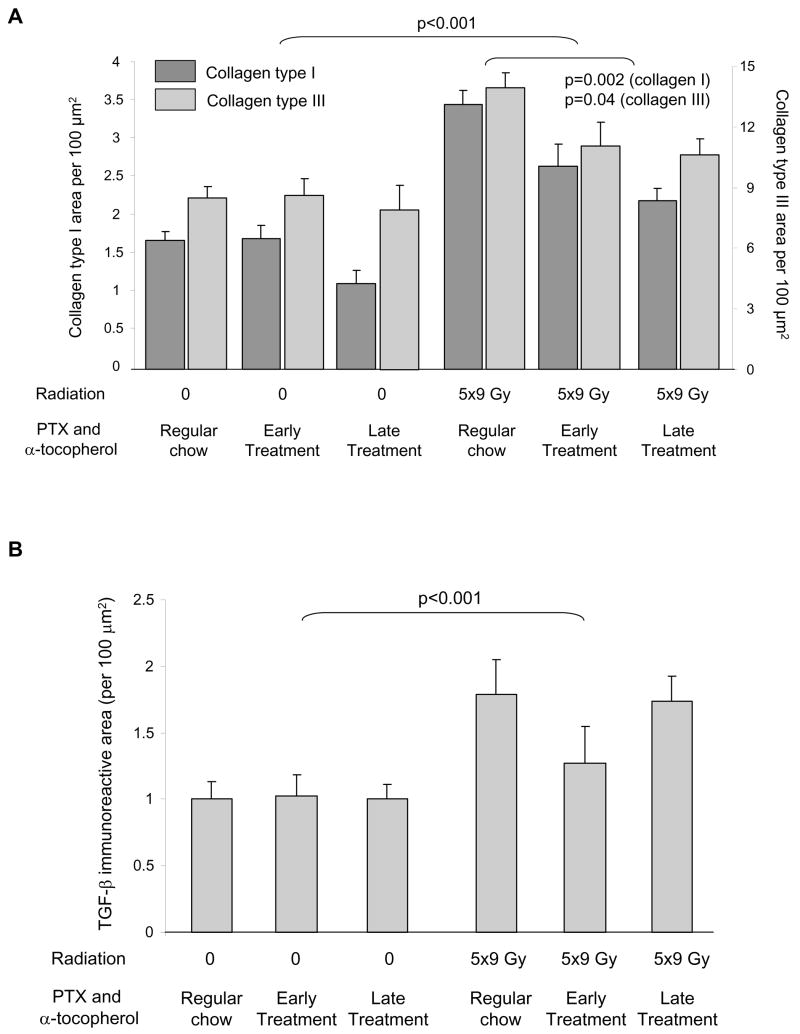
Radiation induced significant increases in deposition of both collagen type I and collagen type III (Figure 2A, F=69.97, p<0.001 and F=23.49, p<0.001, respectively). PTX and α-tocopherol significantly reduced both types of collagen in irradiated animals (collagen type I: F=8.68, p=0.002 and collagen type III: F=3.85, p=0.04), although areas were not completely reduced to levels in sham-irradiated hearts. No differences were found between the effects of early and late treatment with PTX and α-tocopherol.
Figure 2.
Left ventricular immunoreactive areas of collagens and TGF-β, as measured by quantitative immunohistochemistry in each of the treatment groups at six months after (sham-) irradiation (average ± SEM, n=7). (A) Radiation-induced increases in interstitial collagen types I and III areas were significantly reduced by PTX and α-tocopherol. (B) Radiation-induced increases in extracellular matrix-associated TGF-β were not significantly altered by PTX and α-tocopherol.
In addition to collagens, radiation induced a significant increase in extracellular matrix-associated TGF-β (Figure 2B, F=13.04, p<0.001). Treatment with PTX and α-tocopherol did not significantly alter TGF-β immunoreactive area in sham-irradiated hearts or in irradiated hearts. In addition, no differences were found in relative left ventricular mRNA levels of TGF-β1 or CTGF between any of the experimental groups (data not shown).
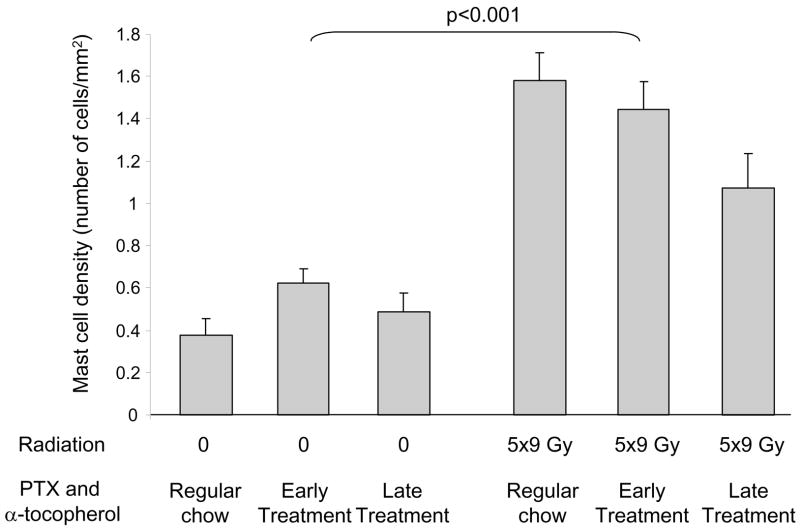
Radiation induced an increase in left ventricular mast cell density (F=71.63, p<0.001). Treatment with PTX and α-tocopherol did not significantly alter mast cell density in sham-irradiated hearts or in irradiated hearts (Figure 3).
Figure 3.
Left ventricular mast cell densities in each of the treatment groups at six months after (sham-) irradiation (average ± SEM, n=7). Radiation-induced increases in left ventricular mast cell densities were not significantly altered by PTX and α-tocopherol.
Cytoskeletal changes in isolated cardiac fibroblasts
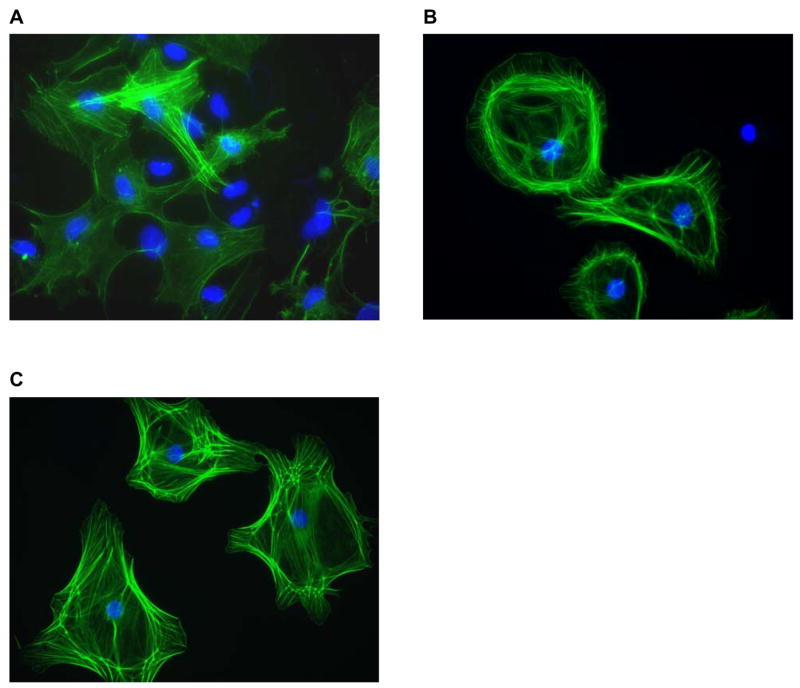
Cardiac fibroblasts were isolated from rats in group 1 (sham-irradiated rats on regular chow), group 4 (irradiated rats on regular chow), and group 6 (irradiated rats treated with PTX and α-tocopherol from three to six months after irradiation). Figure 4 shows representative images of fibroblasts stained with FITC-phalloidin to visualize actin filaments. Fibroblasts isolated from irradiated hearts showed more distinguished actin filaments than fibroblasts isolated from sham-irradiated hearts, consistent with the formation of stress fibers. Fibroblasts isolated from irradiated rats treated with PTX and α-tocopherol showed a cytoskeletal structure similar to fibroblasts from irradiated rats on regular chow. Fibroblasts from rats in group 5 (irradiated rats treated with PTX and α-tocopherol starting one week before irradiation) were not investigated.
Figure 4.
Representative images of fibroblasts isolated from rat hearts from different treatment groups at six months after (sham-) irradiation (40× objective). Cells were stained with FITC-phalloidin to visualize actin and DAPI to visualize cell nuclei. (A) Fibroblasts from sham-irradiated rat on regular chow. (B) Fibroblasts from irradiated rat on regular chow. (C) Fibroblasts from irradiated rat in the late PTX and α-tocopherol treatment group. Radiation-induced stress fiber formation in fibroblasts from irradiated hearts was not altered by PTX and α-tocopherol.
Discussion
This study used a rat model of fractionated local heart irradiation to assess the effects of PTX and α-tocopherol on cardiac radiation injury. At six months after irradiation hearts showed increased ex vivo left ventricular diastolic pressures, indicative of left ventricular dysfunction. Radiation-induced diastolic dysfunction, which has been shown to occur in this animal model of RIHD, is possibly caused by reduced left ventricular size and increased deposition of myocardial collagen. A reduction in left ventricular size may also lead to increased fractional shortening (15) as was observed with echocardiography. Treatment with PTX and α-tocopherol was started one week before irradiation, or three months after irradiation, a time point at which histopathological changes including myocardial fibrosis are apparent in our animal model (13). Both treatment protocols induced a significant improvement in radiation-induced myocardial fibrosis and left ventricular diastolic dysfunction. Other parameters of radiation injury, including reduced heart/body weight ratio and myocardial degeneration, were not significantly altered by PTX and α-tocopherol. These results suggest that, although PTX and α-tocopherol reduce radiation-induced myocardial fibrosis, they may not have a beneficial effect on all manifestations of RIHD. On the other hand, group sizes may have been too small to detect a significant difference in area of myocardial degeneration. Although PTX and α-tocopherol did not significantly reduce myocardial mast cell densities, mast cells are suggested to play a beneficial role in cardiac radiation injury in the rat (14) and, hence, a reduction in mast cell density may not be desirable.
PTX has been shown to reduce fibrosis caused by stimuli other than radiation (19,20). The mechanisms by which PTX inhibits development of fibrosis, or maybe even reverses fibrosis, are not exactly known. Interestingly, PTX inhibits intracellular signaling in response to TGF-β (21,22) and CTGF (23), two growth factors that are considered to play an important role in radiation-induced fibrosis (24,25). Some studies have shown a reduced deposition of TGF-β in response to PTX and α-tocopherol (26). In our study, on the other hand, no significant changes were found in left ventricular extracellular matrix-associated TGF-β or left ventricular mRNA levels of TGF-β1. The lack of changes in TGF-β1 transcript levels may have been caused by the late time point of investigation (27). PTX is also known to reduce endothelial dysfunction and prevent downregulation of endothelial cell surface thrombomodulin (TM) associated with endothelial dysfunction (28,29). Although endothelial dysfunction and downregulation of TM have long been implicated in both early and late normal tissue radiation injury (30), their roles in RIHD have not been investigated extensively. Nevertheless, it may be speculated that PTX has beneficial effects on radiation injury via alterations in endothelial function and TM.
Fibroblasts isolated from irradiated hearts exhibited more distinct actin filaments than fibroblasts isolated from sham-irradiated hearts, indicative of stress fiber formation. The formation of cytoplasmic actin stress fibers is considered to be associated with the formation of myofibroblasts (31), cells that show increased collagen synthesis. Interestingly, even though treatment with PTX and α-tocopherol reduced collagen deposition in irradiated hearts, it did not appreciably alter the cytoskeletal structure in fibroblasts from these hearts. However, because cells were not cultured in the presence of PTX or α-tocopherol, it cannot be excluded that in vivo effects of these agents on cardiac fibroblasts were reversed during the time of cell culture.
In conclusion, treatment with a combination of PTX and α-tocopherol significantly improved radiation-induced myocardial fibrosis and left ventricular diastolic dysfunction in a rat model of RIHD, both when started before irradiation, and when started at a time point at which histopathological changes have already become apparent. More studies are needed to determine mechanisms of action.
Acknowledgments
This work was supported by grants from the Lance Armstrong Foundation (LAF06SY4 to MB) and the National Institutes of Health (CA83719 to MH-J). The authors acknowledge Ashwini Kulkarni for excellent technical support and Dr. Sue A. Theus and Kimberly Henning of the Central Arkansas Veterans Healthcare System for excellent support in animal care and small animal echocardiography.
Footnotes
Conflicts of Interest Notification Actual or potential conflicts of interest do not exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aleman BM, van den Belt-Dusebout AW, Klokman WJ, et al. Long-term cause-specific mortality of patients treated for Hodgkin’s disease. J Clin Oncol. 2003;21:3431–3439. doi: 10.1200/JCO.2003.07.131. [DOI] [PubMed] [Google Scholar]
- 2.Hooning MJ, Aleman BM, van Rosmalen AJ, et al. Cause-specific mortality in long-term survivors of breast cancer: A 25-year follow-up study. Int J Radiat Oncol Biol Phys. 2006;64:1081–1091. doi: 10.1016/j.ijrobp.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 3.Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–565. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 4.Cuzick J, Stewart H, Rutqvist L, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol. 1994;12:447–453. doi: 10.1200/JCO.1994.12.3.447. [DOI] [PubMed] [Google Scholar]
- 5.Early Breast Cancer Trialists Collaborative Group. Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 2000;355:1757–1770. [PubMed] [Google Scholar]
- 6.Heidenreich PA, Hancock SL, Vagelos RH, Lee BK, Schnittger I. Diastolic dysfunction after mediastinal irradiation. Am Heart J. 2005;150:977–982. doi: 10.1016/j.ahj.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 7.Okunieff P, Augustine E, Hicks JE, et al. Pentoxifylline in the treatment of radiation-induced fibrosis. J Clin Oncol. 2004;22:2207–2213. doi: 10.1200/JCO.2004.09.101. [DOI] [PubMed] [Google Scholar]
- 8.Hille A, Christiansen H, Pradier O, et al. Effect of pentoxifylline and tocopherol on radiation proctitis/enteritis. Strahlenther Onkol. 2005;181:606–614. doi: 10.1007/s00066-005-1390-y. [DOI] [PubMed] [Google Scholar]
- 9.Delanian S, Porcher R, Rudant J, Lefaix JL. Kinetics of response to long-term treatment combining pentoxifylline and tocopherol in patients with superficial radiation-induced fibrosis. J Clin Oncol. 2005;23:8570–8579. doi: 10.1200/JCO.2005.02.4729. [DOI] [PubMed] [Google Scholar]
- 10.Gothard L, Cornes P, Earl J, et al. Double-blind placebo-controlled randomised trial of vitamin E and pentoxifylline in patients with chronic arm lymphoedema and fibrosis after surgery and radiotherapy for breast cancer. Radiother Oncol. 2004;73:133–139. doi: 10.1016/j.radonc.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Zywietz F, Bohm L, Sagowski C, Kehrl W. Pentoxifylline enhances tumor oxygenation and radiosensitivity in rat rhabdomyosarcomas during continuous hyperfractionated irradiation. Strahlenther Onkol. 2004;180:306–314. doi: 10.1007/s00066-004-1198-1. [DOI] [PubMed] [Google Scholar]
- 12.Vernimmen F, Verheye-Dua F, du Toit H, Bohm L. Effect of pentoxifylline on radiation damage and tumor growth. Strahlenther Onkol. 1994;170:595–601. [PubMed] [Google Scholar]
- 13.Boerma M, Zurcher C, Esveldt I, Schutte-Bart CI, Wondergem J. Histopathology of ventricles, coronary arteries and mast cell accumulation in transverse and longitudinal sections of the rat heart after irradiation. Oncology Reports. 2004;12:213–219. doi: 10.3892/or.12.2.213. [DOI] [PubMed] [Google Scholar]
- 14.Boerma M, Wang J, Wondergem J, et al. Influence of mast cells on structural and functional manifestations of radiation-induced heart disease. Cancer Res. 2005;65:3100–3107. doi: 10.1158/0008-5472.CAN-04-4333. [DOI] [PubMed] [Google Scholar]
- 15.Radke MH, Peng J, Wu Y, et al. Targeted deletion of titin N2B region leads to diastolic dysfunction and cardiac atrophy. Proc Natl Acad Sci U S A. 2007;104:3444–3449. doi: 10.1073/pnas.0608543104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boerma M, Fiser WP, Hoyt G, et al. Influence of mast cells on outcome after heterotopic cardiac transplantation in rats. Transpl Int. 2007;20:256–265. doi: 10.1111/j.1432-2277.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 17.James JD, Hauer-Jensen M. Effects of fixative and fixation time for quantitative computerized image analysis of immunohistochemical staining. Journal of histotechnology. 2001;22:109–111. [Google Scholar]
- 18.Richter KK, Langberg CW, Sung CC, Hauer-Jensen M. Association of transforming growth factor beta (TGF-beta) immunoreactivity with specific histopathologic lesions in subacute and chronic experimental radiation enteropathy. Radiother Oncol. 1996;39:243–251. doi: 10.1016/0167-8140(95)01735-6. [DOI] [PubMed] [Google Scholar]
- 19.Rajendran R, Rani V, Shaikh S. Pentoxifylline therapy: a new adjunct in the treatment of oral submucous fibrosis. Indian J Dent Res. 2006;17:190–198. doi: 10.4103/0970-9290.29865. [DOI] [PubMed] [Google Scholar]
- 20.Desmouliere A, Xu G, Costa AM, et al. Effect of pentoxifylline on early proliferation and phenotypic modulation of fibrogenic cells in two rat models of liver fibrosis and on cultured hepatic stellate cells. J Hepatol. 1999;30:621–631. doi: 10.1016/s0168-8278(99)80192-5. [DOI] [PubMed] [Google Scholar]
- 21.Hung KY, Huang JW, Chen CT, Lee PH, Tsai TJ. Pentoxifylline modulates intracellular signalling of TGF-beta in cultured human peritoneal mesothelial cells: implications for prevention of encapsulating peritoneal sclerosis. Nephrol Dial Transplant. 2003;18:670–676. doi: 10.1093/ndt/gfg141. [DOI] [PubMed] [Google Scholar]
- 22.Fang CC, Yen CJ, Chen YM, et al. Pentoxifylline inhibits human peritoneal mesothelial cell growth and collagen synthesis: effects on TGF-beta. Kidney Int. 2000;57:2626–2633. doi: 10.1046/j.1523-1755.2000.00123.x. [DOI] [PubMed] [Google Scholar]
- 23.Lin SL, Chen RH, Chen YM, et al. Pentoxifylline attenuates tubulointerstitial fibrosis by blocking Smad3/4-activated transcription and profibrogenic effects of connective tissue growth factor. J Am Soc Nephrol. 2005;16:2702–2713. doi: 10.1681/ASN.2005040435. [DOI] [PubMed] [Google Scholar]
- 24.Martin M, Lefaix J, Delanian S. TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? International Journal of Radiation Oncology, Biology, Physics. 2000;47:277–290. doi: 10.1016/s0360-3016(00)00435-1. [DOI] [PubMed] [Google Scholar]
- 25.Vozenin-Brotons MC, Milliat F, Sabourin JC, et al. Fibrogenic signals in patients with radiation enteritis are associated with increased connective tissue growth factor expression. Int J Radiat Oncol Biol Phys. 2003;56:561–572. doi: 10.1016/s0360-3016(02)04601-1. [DOI] [PubMed] [Google Scholar]
- 26.Lefaix JL, Delanian S, Vozenin MC, et al. Striking regression of subcutaneous fibrosis induced by high doses of gamma rays using a combination of pentoxifylline and alpha-tocopherol: an experimental study. Int J Radiat Oncol Biol Phys. 1999;43:839–847. doi: 10.1016/s0360-3016(98)00419-2. [DOI] [PubMed] [Google Scholar]
- 27.Kruse JJ, Bart CI, Visser A, Wondergem J. Changes in transforming growth factor-beta (TGF-beta1), procollagen types I and III mRNA in the rat heart after irradiation. Int J Radiat Biol. 1999;75:1429–1436. doi: 10.1080/095530099139296. [DOI] [PubMed] [Google Scholar]
- 28.Seigneur M, Dufourcq P, Belloc F, et al. Influence of pentoxifylline on membrane thrombomodulin levels in endothelial cells submitted to hypoxic conditions. J Cardiovasc Pharmacol. 1995;25 Suppl 2:S85–S87. doi: 10.1097/00005344-199500252-00018. [DOI] [PubMed] [Google Scholar]
- 29.Normandin L, Herve P, Brink C, et al. L-arginine and pentoxifylline attenuate endothelial dysfunction after lung reperfusion injury in the rabbit. The Paris-Sud University Lung Transplant Group. Ann Thorac Surg. 1995;60:646–650. doi: 10.1016/0003-4975(95)00484-3. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Boerma M, Fu Q, Hauer-Jensen M. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J Gastroenterol. 2007;13:3047–3055. doi: 10.3748/wjg.v13.i22.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinz B, Gabbiani G. Mechanisms of force generation and transmission by myofibroblasts. Curr Opin Biotechnol. 2003;14:538–546. doi: 10.1016/j.copbio.2003.08.006. [DOI] [PubMed] [Google Scholar]