Abstract
Heightened impulsivity and differential sensitivity to a drug's behavioral effects are traits that, individually, have been associated with chronic drug use and dependence. Here, we used an animal model to test whether individual differences in cocaine-induced activity are predictive of impulsive choice behavior. Adult, male Sprague-Dawley rats were given cocaine (10 mg/kg, i.p.) and classified into low or high cocaine responders (LCRs and HCRs, respectively) based on their locomotor response in an open-field arena. Rats were then trained in a delay-discounting task that offers a choice between immediately delivered, but smaller reinforcements, or larger reinforcements that are delivered after a delay. We also examined the effects of amphetamine (AMPH; 0.3-1.0 mg/kg) and the 5-HT1A agonist 8-OH-DPAT (0.3-1.0 mg/kg) on delay discounting. Lastly, all rats were retested in the open-field to determine if phenotypes were stable. We observed baseline differences in choice behavior between the groups, with HCRs behaving more impulsively (i.e., choosing the small reinforcement) compared to LCRs. AMPH decreased choice of the large reinforcement in LCRs, but did not alter choice in HCRs. Impulsive choice was increased in both phenotypes following 8-OH-DPAT, with LCRs exhibiting changes across a wider range of delays. When cocaine-induced open-field behavior was retested, responses in LCRs were similar whereas HCRs showed evidence of tolerance. Our results suggest that differential sensitivity to cocaine-induced locomotion is predictive of impulsivity and the potential neurobiological differences in LCRs and HCRs may provide insight into mechanisms contributing to vulnerability for chronic drug use and/or dependence.
Keywords: cocaine, individual differences, delay-discounting, locomotor activity, 8-OH-DPAT, amphetamine, rat
1. Introduction
Impulsivity is a complex, multifaceted trait that is broadly defined by a lack of behavioral inhibition, which includes premature and poorly controlled actions, and impulsive choice, where decisions are poorly conceived and sensitive to delayed rewards (see Evenden, 1999; Winstanley et al., 2006a for more detailed accounts). Individuals who abuse drugs tend to exhibit heightened levels of these traits, particularly with regards to impulsive decision-making (Bornovalova et al., 2005; Coffey et al., 2003; Heil et al., 2006; Kirby and Petry, 2004; Lejuez et al., 2007; Moeller et al., 2002; Verdejo-Garcia et al., 2007). It has been suggested that a high level of impulsivity develops as a result of repeated exposure to abused drugs, and this in turn facilitates the development and/or maintenance of addiction (Jentsch and Taylor, 1999). Evidence supporting this hypothesis comes in part from studies using animal models, where repeated exposure to cocaine in rats leads to an increase in impulsive choice behavior relative to that observed in saline-treated controls (Paine et al., 2003; Roesch et al., 2007; Simon et al., 2007), and from clinical studies, where individuals who have been chronically exposed to cocaine show increases in impulsive choice relative to non-drug users or drug abstainers (for review, see Bickel and Marsch, 2001 and Reynolds, 2006).
An alternative and not necessarily mutually exclusive hypothesis is that a high level of impulsivity is a pre-existing trait whose multidimensional components overlap with other traits or behaviors that also confer enhanced vulnerability to chronic drug use and/or dependence (Bechara, 2005; Dalley et al., 2007a, b; Kreek et al., 2005). An example of one such candidate trait is differential sensitivity to a drug's behavioral effects. In humans, for example, differences in initial sensitivity to cocaine are predictive of long-term use and dependence (Davidson et al., 1993; Haertzen et al., 1983; Schafer and Brown, 1991), and a recent study has indicated that individuals who reported a high degree of “liking” or “wanting” on their initial use of cocaine had a significantly increased risk of cocaine abuse (Lambert et al., 2006). In rodents, a reliable indicator of initial sensitivity to psychostimulants is drug-induced locomotor activity in an open-field arena. Cocaine, in particular (Briegleb et al., 2004), has variable stimulant effects on behavior, such that outbred rats can readily be classified as low or high cocaine responders (LCRs or HCRs, respectively) based on their response to a single treatment with cocaine (Sabeti et al., 2002; Gulley et al., 2003; Gulley, 2007). Interestingly, these individual differences are related to differences in the function of dopamine transporters (DATs) in the dorsal striatum and nucleus accumbens (Sabeti et al., 2002, 2003), and not to pharmacokinetic factors (Gulley et al., 2003). Subsequent studies have shown that the LCR/HCR phenotype can be used to predict behavior in a food-reinforced operant task (Gulley, 2007), as well as conditioned place preference for cocaine (Allen et al., 2007).
In the current study, we used rats to examine if differential sensitivity to the locomotor activating effects of cocaine were predictive of one component of impulsivity, impulsive choice behavior. Rats were first characterized as LCRs and HCRs based on their response to 10 mg/kg cocaine in an open-field arena. They were then trained in a delay-discounting task that offered a choice between immediately delivered, but smaller reinforcements, or larger reinforcements that were delivered after a delay of up to 60 s. After 34 daily training sessions, we performed a series of drug challenges in order to determine if impulsive choice could be altered in a differential manner between LCRs and HCRs. First, rats were given amphetamine (AMPH; 0.3-1.0 mg/kg) or saline prior to the start of daily delay-discounting sessions. AMPH has been shown previously to increase or decrease delay-discounting behavior (Cardinal et al., 2000; Evenden and Ryan, 1996, 1999; van Gaalen et al., 2006), and it has been suggested that an individual's baseline level of impulsive choice behavior influences this response (Barbelivien et al., 2008; Winstanley et al., 2003). Rats were then challenged with the 5-HT1A agonist 8-OH-DPAT (0.3-1.0 mg/kg) or saline prior to their daily session. 8-OH-DPAT has been shown to increase impulsive choice (Winstanley et al., 2005; but see Evenden and Ryan, 1999; Poulos et al., 1996) and it is not clear if this effect depends on the baseline level of impulsive choice. Lastly, all rats were re-tested in the open-field for their response to cocaine in order to assess if the phenotypic differences in cocaine response that were established initially remained stable following the ∼3 months time period that elapsed during the course of the study.
2. Methods
2.1 Animals
Male Sprague-Dawley rats (n = 16), bred in our animal facility from stock rats obtained from Harlan (Indianapolis, IN), were housed individually starting at ∼2 months of age and were 3-3.5 months old (300-490 g) at the start of experiments. They were maintained on a 12:12 h light: dark cycle (lights on at 0800) with experimental sessions conducted between 0900 and 1800 h. Rats were handled five times for 15 min intervals prior to being used in experiments. With the exception of periods when rats were undergoing operant training and testing, food was available ad libitum. Water was always available ad libitum. All experimental procedures were approved by the IACUC at the University of Illinois, Urbana-Champaign and were consistent with the Principles of Laboratory Animal Care (NIH Publication no. 85-23).
2.2 Apparatus
Locomotor activity was measured in an open-field chamber that consisted of a transparent, Plexiglas box (40.6 × 40.6 × 40.6 cm) surrounded by photobeams (Coulbourn Instruments; Allentown, PA). Each apparatus was connected to a computer operating software (TruScan, v. 2.01; Coulbourn Instruments) that recorded all horizontal and vertical beam breaks (100 ms sampling rate). The horizontal beam breaks, or coordinate changes, were converted into distance traveled (cm). The chambers were individually contained inside sound-attenuating cubicles (76 × 80 × 63 cm). Each cubicle contained a speaker (76 mm dia.) fixed to one wall, two ceiling-mounted white lights (4 W) for dim illumination, and a ceiling-mounted camera between the two lights. White nose (70 dB) was played continuously through the speakers when rats were in the testing room.
Operant behavior was monitored in standard operant chambers (Coulbourn Instruments). One wall of the chamber contained two retractable levers that were positioned on either side of a centrally located food trough. Infrared detectors were used to monitor head entries into the food trough. White cue lights were located above each lever. A white houselight was located near the top of the chamber on the opposite wall.
2.2.1. Initial behavioral characterization: Locomotor activity in the open-field
After a 30-min acclimation period in the testing room, rats were placed in the open-field chambers for 90 min. They were then removed from the chamber, injected (i.p.) with (−) cocaine HCl (10 mg/kg) and placed back into the chamber for an additional 60 min. This dose was chosen based on previous studies (Sabeti et al, 2002; Gulley et al, 2003) showing that it is optimal for inducing the widest range of behavioral responses in male Sprague-Dawley rats. After the testing session, rats were returned to the colony room and their access to food was restricted so that they were reduced to 85% of their free-feeding weight over the course of several days. Rats were maintained at 85-90% of free-feeding weight for the duration of operant training and testing.
2.2.2. Delay-discounting behavior
Seven days after the open-field test, rats were trained in overnight sessions (2100 to 0900 hours) to respond on either of two available levers for a food pellet (45-mg; Bio-Serv F0021 or F0042) on a fixed ratio schedule (FR1) of reinforcement. After they displayed approximately equal responding on both levers, rats were moved to the next training phase (1-h sessions, between 0900 and 1700 h). Trials began with levers retracted, and the food trough illuminated by a cue light. A nosepoke into the trough resulted in the extension of one randomly selected lever, with a subsequent lever press response reinforced by delivery of a food pellet. After 3-4 days of training at this stage, rats began the final stage of training.
Training in the delay-discounting task was done in daily, 100 min sessions that consisted of five blocks of 12 trials. Each trial lasted 100 s and began with the illumination of the house light and cue light located in the food trough. Rats were required to nosepoke into the trough within 10 s, whereupon a single lever would be presented randomly and a response was reinforced with food pellet delivery. The amount of food delivered was pre-assigned to a lever, such that responses on one (e.g., left side lever) resulted in a larger, delayed reward of four pellets and responses on the other (e.g., right side lever) resulted in a small, immediate reward of one pellet. Assignment of reward magnitude to levers was counterbalanced across groups, but remained consistent for each rat. After a lever response, the house light turned off, the levers retracted, and the cue light above the lever was illuminated until food was delivered. If the rat either failed to nosepoke within 10 s or respond on the presented lever, the trial was recorded as an omission. On omission trials, the levers remained retracted and the chamber was returned to an intertrial interval (ITI) state until the beginning of the next trial. After completing or omitting the first two forced-choice trials (which served as exemplars for each block), 10 free-choice trials were presented where both levers were extended. Delays for the delivery of large rewards increased with each block of trials (0, 10, 20, 40, and 60 seconds, respectively). Each animal participated in one session per day, for a minimum of 34 training sessions.
Following the last training session, the effects of AMPH or 8-OH-DPAT on task behavior were assessed in two testing blocks. For the first testing block, rats were given injections of vehicle (saline; 1 ml/kg, i.p.) or AMPH (0.3, 0.6, 1.0 mg/kg, i.p.) 5 min before they were placed in the operant chamber for a test session. Saline or drug injections were given in an alternating fashion over six consecutive daily sessions (i.e., SDSDSD, where S = saline and D = drug). The order of drug doses was chosen based on a Latin square design, with a particular order assigned to each rat randomly. Rats were given a five day break after the last AMPH test session. Subsequently, they were allowed a session without any injections, followed on the next day by the start of the second testing block. For these tests, saline or 8-OH-DPAT (0.3, 0.6. and 1.0 mg/kg, i.p.) was given using the same procedure as was used in the first testing block.
2.2.3. Open-field behavior retest
After their final session in the delay-discounting task, rats were given access to food ad libitum. One week later, they were re-tested in the open-field with 10 mg/kg cocaine (i.p.). The same procedures used during the initial open-field behavioral characterization test were followed, including the time of day experiments were performed.
2.3. Drugs
The (−) cocaine HCl used in this study was obtained from NIDA (RTI International; Research Triangle Park, NC). d-Amphetamine sulfate and 8-OH-DPAT were obtained from Sigma-Aldrich (St. Louis, MO). Drugs were dissolved in sterile saline (0.9% NaCl), with dosages calculated based on the weight of the salt. All injections were given at a volume of 1 ml/kg.
2.4. Data Analyses
All analyses were conducted using SAS 9.1 (SAS Institute, Inc.; Cary, NC), SigmaStat 3.5 (Systat Software, Inc.; San Jose, CA), and SQL Server 2005 Developer (Microsoft; Seattle, WA). Cocaine-induced locomotor activity in the open-field was assessed by determining the cumulative activity during the first 30-min following injection. As in previous studies (Gulley et al., 2003; Gulley, 2007), rats with activity scores in the lower half of the population distribution were designated LCRs and those in the upper half were designated HCRs. The statistical significance of group differences in cocaine-induced activity was determined using two-way, mixed factor ANOVA, with group as the between-subjects factor and test session as the repeated measure. The locomotor response to a novel environment was also assessed by determining the cumulative activity during the first 30-min following placement into the open-field arena; group differences were analyzed with a one-way ANOVA.
Several measures were used to analyze delay-discounting behavior. Choice behavior was represented as mean choice of the large reward for free-choice trials only. The progression of task performance across sessions was assessed by obtaining these data during early acquisition (sessions 1-3), training midpoint (15-17), and stable baseline (sessions 35, 37, and 39). The stable baseline period encompassed the three sessions during which rats were given saline injections before they began the task. Data from each of these training endpoints were analyzed with two-way repeated-measures ANOVA (group x delay, as well as session x delay for sessions 32-34). In order for behavior to be considered stable, the session factor had to be non-significant, while the delay factor had to be significant. Data from tests of the acute effects of AMPH or 8-OH-DPAT on task performance were analyzed using multifactorial ANOVA with group as a between-subjects factor and dose and delay as within-subjects factors. Further ANOVAs were performed (with session and delay as within-subject factors) to test for stability in behavior between vehicle injections. When appropriate, Holm-Sidak post-hoc tests were performed. In all tests, p values less than 0.05 were considered significant.
3. Results
3.1. Open-field behavior
The first exposure to 10 mg/kg cocaine resulted in disparate locomotor activity that could be described by separating rats into LCRs and HCRs. As shown in Fig. 1, LCRs exhibited, on average, 61% less cocaine-induced activity compared to that observed in HCRs. This group difference in behavior was statistically significant (main effect of group: F1,14 = 27.0, p < 0.001). Novelty-induced activity was also lower in LCRs (10,206 ± 895 cm) compared to HCRs (12,822 ± 854 cm), but this difference was not statistically significant. Following the completion of training and testing in the delay-discounting task (see below), rats were retested in the open-field arena for their response to 10 mg/kg cocaine. During the ∼3 month period that elapsed between the first and second tests, these rats were never exposed to the open-field chambers or the testing room in which the chambers were housed. As shown in Fig. 1, we found that cocaine-induced activity in LCRs was similar in both tests. HCRs, in contrast, exhibited less activation on retest compared to their initial characterization. These effects were reflected in a significant interaction between group and test session (F1,14 = 6.47, p < 0.05). Cocaine-induced activity in LCRs and HCRs was not significantly different on retest.
Figure 1.
Locomotor activity (distance, in cm) after the first (Test 1) and second (Retest) injection with 10 mg/kg cocaine. The retest occurred following the conclusion of the operant behavior phase of the study, with ∼ 3 months separating the two tests. Shown is a scatter plot of the cumulative locomotor activity (mean shown as a horizontal line) for the 30-min following i.p. injection. The response during Test 1 was used to classify rats as LCRs or HCRs (n = 8/group). ### p < 0.001, compared to HCRs at Test1; ** p < 0.01, Test 1 compared to Retest within HCRs
3.2. Delay-discounting behavior
As shown in Fig. 2, there were differences in delay-discounting behavior between LCRs and HCRs. We observed significant main effects of group (F1,210 = 29.4, p < 0.001), training period (F2,210 = 11.3, p < 0.001), and delay (F4,210 = 29.5, p < 0.001). More specifically, group differences in delay-discounting were significant during the midpoint of training (sessions 15-17; group x delay interaction: F4,56 = 37.6, p < 0.05) and at stable baseline (sessions 35, 37, and 39; group x delay interaction: F4,56 = 30.7, p < 0.01), but not during the early phase of task acquisition (Fig. 2A-C). As expected, rats in both groups exhibited sensitivity to delay by reducing their responding on the large reward lever as their number of days in training increased. As shown in Fig. 2A, the sensitivity to delay was emerging in the earliest stages of task acquisition, primarily at the 40- and 60-s delays. As training progressed, rats in both groups chose the large reward almost exclusively when there was no delay, and progressively less often as delay increased. Furthermore, discounting was less steep in LCRs compared to HCRs. This was most apparent at the midpoint of training, where the groups differed at the 10, 20, and 40 s delays (Fig. 2B). At stable baseline, LCRs continued to choose the large reward more frequently than HCRs across these same delays, although the difference at 20 s was the only one that was statistically significant (Fig. 2C). The number of trial omissions was relatively low for both groups, with LCRs and HCRs omitting a total of 0.36 ± 0.27 and 0.33 ± 0.27 trials, respectively, across all delays during the midpoint sessions. The small group difference was not statistically significant, and neither were those at acquisition (LCRs: 1.81 ± 0.74 trials; HCRs: 0.65 ± 0.74 trials) or baseline (LCRs: 0.08 ± 0.06 trials; HCRs: 0.09 ± 0.06 trials).
Figure 2.
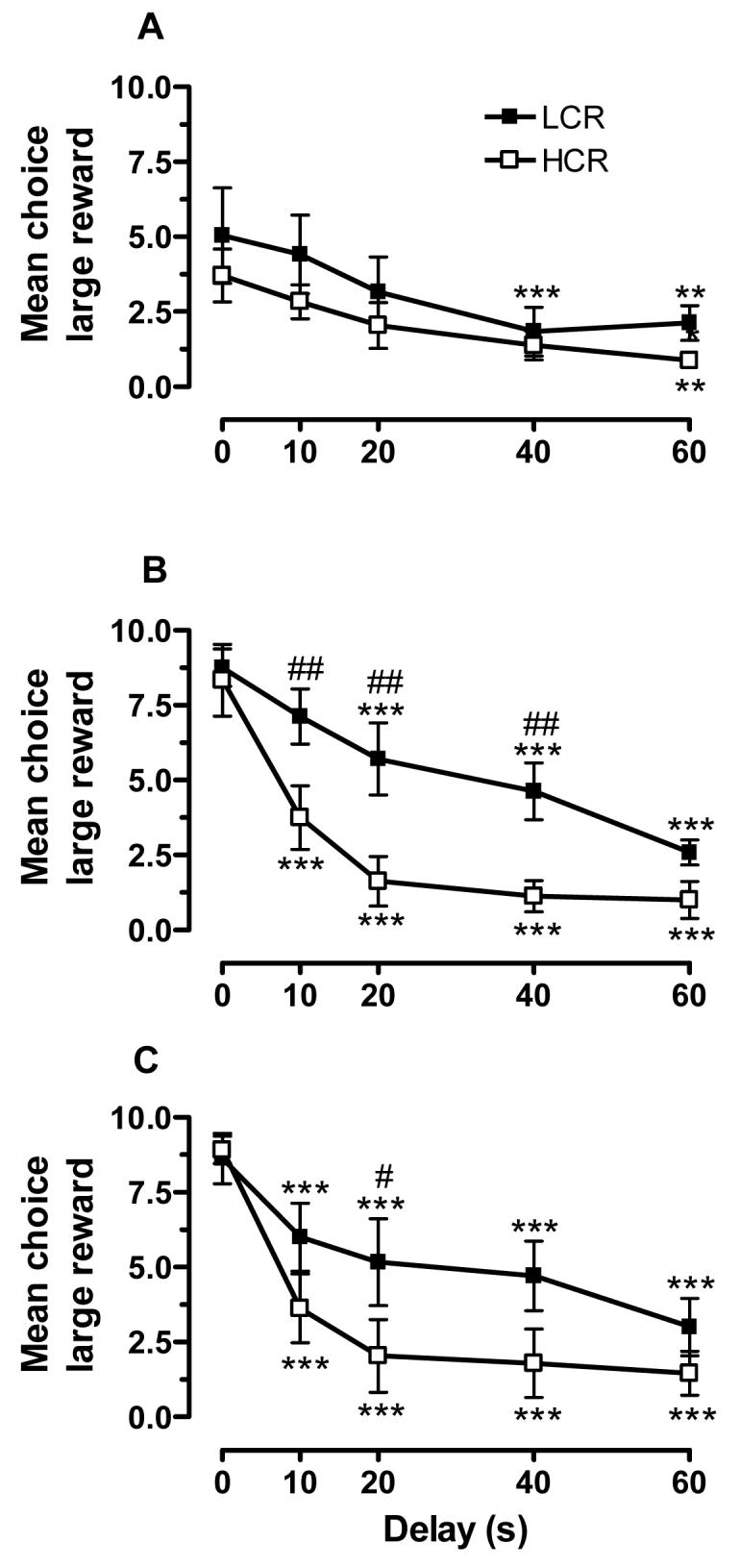
Choice behavior during early acquisition (sessions 1-3; A), training midpoint (sessions 15-17; B), and at stable baseline (vehicle sessions 35, 37, and 39; C) for rats classified as LCRs or HCRs (n = 8/group). Shown is the mean choice of the large reward (± SEM) at each delay during the three sessions that were within these training time periods. ** p < 0.01 and *** p < 0.001, compared to 0 s; # p < 0.05 and ## p < 0.01, compared to HCRs at the indicated time points
3.2.1. Effects of AMPH on delay-discounting
When AMPH was administered 5 min before the start of the task, it significantly altered choice behavior (Fig. 3). Overall ANOVA revealed significant main effects of group (F1,280 = 18.3, p < 0.001) and delay (F4,280 = 50.8, p < 0.001), as well as a group x delay interaction (F4,280 = 3.20, p < 0.01). Subsequent analysis of within-group AMPH effects using a two-way ANOVA and post-hoc tests revealed dose-dependent changes in LCRs (dose: F3,84 = 3.28, p < 0.05), with a trend for a dose x delay interaction (F12,84 = 1.81, p = 0.06). As shown in Fig. 3A for LCRs, 1.0 mg/kg AMPH decreased choice of the large reward compared to that observed following vehicle injection. The most robust effect was at the 20-s delay. There were no significant effects of any of the tested AMPH doses on choice behavior in HCRs. For number of trial omissions, overall ANOVA revealed a significant main effect of dose (F3,280 = 10.6, p < 0.001) and a delay x dose interaction (F12,280 = 3.05, p < 0.001). Subsequent analysis revealed that the 1.0 mg/kg dose of AMPH was significantly different from vehicle, but only in HCRs and only at the 20-s delay (Fig. 3C).
Figure 3.
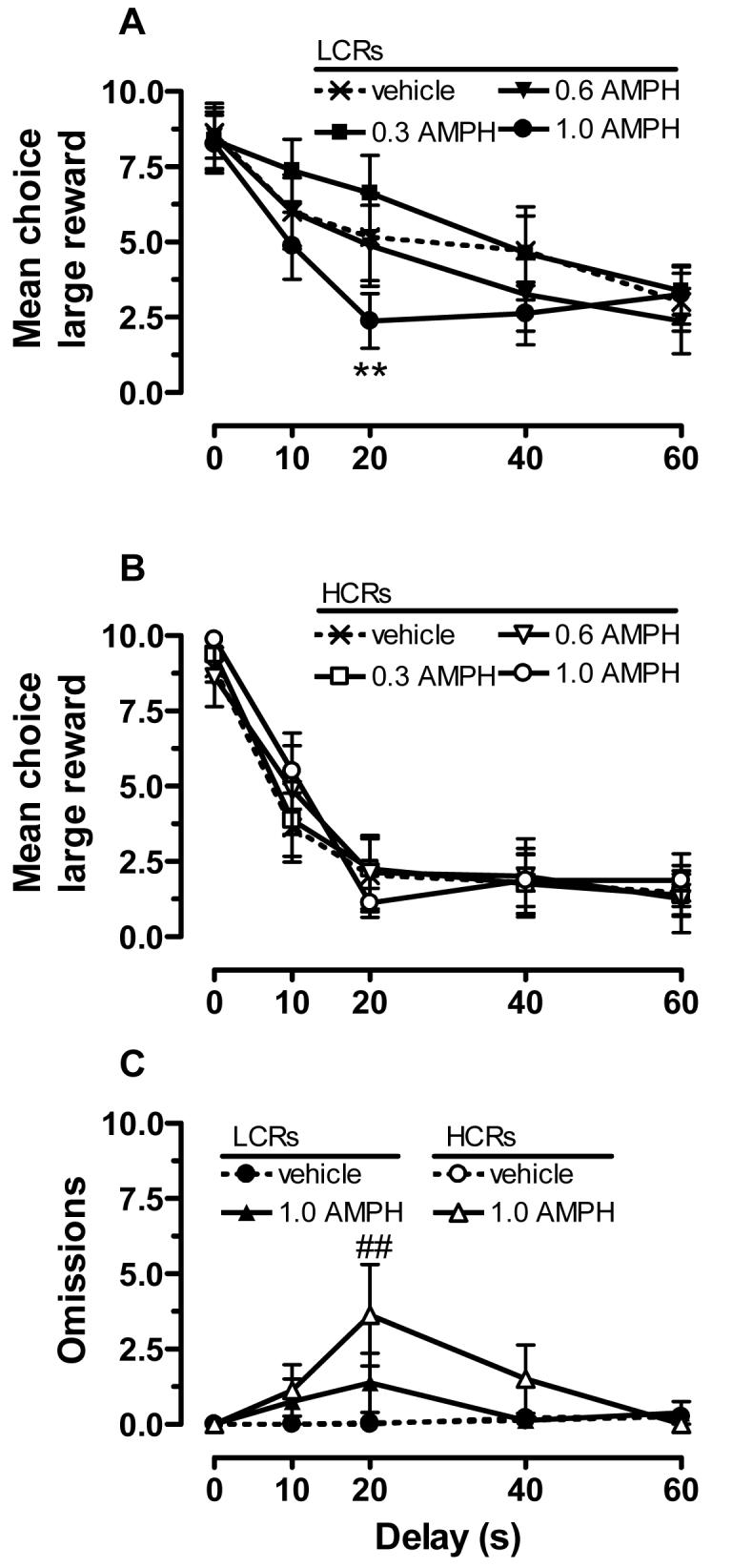
The effects of AMPH (0.3-1.0 mg/kg) on delay-discounting behavior in LCRs and HCRs (n = 8/group). Shown in A and B is the mean choice of the large reward (± SEM) across delays for LCRs (A) and HCRs (B). Vehicle injection data are the same as that shown in Fig. 2C. The mean number of trial omissions (± SEM) as a function of delay is shown in C. Only the 1.0 mg/kg dose of AMPH is shown because there were no significant increases in omissions at 0.3 or 0.6 mg/kg. ** p < 0.01, compared to vehicle at the 20-s delay; ## p < 0.01, compared to vehicle at the 20-s delay for HCRs
The latency to nosepoke for the presentation of levers, as well as latency to respond on a lever, was altered to a modest extent by AMPH. For these analyses, data were collapsed across delay because latencies remained relatively stable across the session. We observed significant main effects of dose for both measures (nosepoke latency: F3,42 = 6.13, p < 0.001; lever press latency: F3,42 = 2.94, p < 0.05). As shown in Table 1, the 0.3 mg/kg dose significantly decreased response latencies in LCRs. The number of nosepoke responses that rats made during periods when food pellets were not available—during ITIs and during delays following responses on the large reward lever—was not significantly different between LCRs and HCRs following vehicle injection or after AMPH treatment (data not shown).
Table 1.
Mean latencies (s) to nosepoke for the initiation of a trial and to choose a lever, following injection of vehicle, AMPH or 8-OH-DPAT. SEM is given in parentheses.
| AMPH (mg/kg) |
8-OH-DPAT (mg/kg) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Vehicle | 0.3 | 0.6 | 1.0 | Vehicle | 0.3 | 0.6 | 1.0 | |
| LCR | ||||||||
| Nosepoke | 1.82 (0.25) | 1.34 (0.14)*** | 1.72 (0.19) | 1.77 (0.18) | 1.55 (0.17) | 1.87 (0.18) | 2.42 (0.53)** | 2.77 (0.48)*** |
| Lever press | 2.82 (0.26) | 2.11 (0.23)** | 2.71 (0.27) | 2.68 (0.28) | 2.50 (0.25) | 2.92 (0.49) | 3.90 (0.73) | 4.64 (0.89) |
| HCR | ||||||||
| Nosepoke | 1.57 (0.14) | 1.30 (0.10) | 1.38 (0.10) | 1.41 (0.11) | 1.55 (0.10) | 1.81 (0.12) | 2.20 (0.19) | 2.26 (0.24) |
| Lever press | 2.33 (0.33) | 2.07 (0.26) | 2.29 (0.31) | 2.28 (0.35) | 2.47 (0.25) | 3.34 (0.33) | 5.25 (0.87)** | 4.28 (0.82) |
p<0.01
p<0.001, compared to vehicle
3.2.2. Effects of 8-OH-DPAT on delay-discounting
When 8-OH-DPAT was administered 5 min before the start of the task, it significantly reduced choice of the large reward, with a relatively greater effect in LCRs (Fig. 4A). An overall ANOVA revealed significant main effects of group (F1,280 = 34.2, p < 0.001), dose (F3,280 = 12.3, p < 0.001], and delay (F4,280 = 37.0, p < 0.001). Subsequent analysis of 8-OH-DPAT within groups revealed that the two highest doses significantly decreased choice of the large reward in LCRs, and in particular, at the 0-, 10-, and 20-s delays. The effect of 1.0 mg/kg 8-OH-DPAT was also significant at the 40-s delay. In HCRs, all three doses reduced responding on the large reward lever, but this effect was only significant at the 0-s delay (Fig. 4B). The changes in choice behavior produced by 8-OH-DPAT were associated with increases in trial omissions, which were evident in both groups at the 0.6 and 1.0 mg/kg doses (Fig. 4C). Statistical analysis revealed a trend for a main effect of dose in LCRs (F3,84 = 2.57, p = 0.08) and a significant main effect of dose (F3,84 = 4.99, p < 0.01) and a delay x dose interaction (F12,84 = 3.93, p < 0.001) in HCRs.
Figure 4.

The effects of 8-OH-DPAT (0.3-1.0 mg/kg) on delay-discounting behavior in LCRs or HCRs (n = 8/group). Shown in A and B is the mean choice of the large reward (± SEM) across delays for LCRs (A) and HCRs (B). The mean number of trial omissions (± SEM) as a function of delay is shown in C. The 0.3 mg/kg dose is not shown because it did not significantly increase trial omissions at any of the delays. For A: * p < 0.05, comparison between 0.6 mg/kg and vehicle at the indicated delay; # p < 0.05, comparison between 1.0 mg/kg and vehicle at the indicated delay; For B: **p<.01 and *** p<.001, compared to vehicle at the 0-s delay. For C: * p < 0.05, comparison between 0.6 mg/kg and vehicle at the indicated delay for HCRs; # p < 0.05, comparison between 1.0 mg/kg and vehicle at the indicated delay for HCRs
The latency to nosepoke for the presentation of levers, as well as to respond on a lever, was also altered by 8-OH-DPAT. Similar to the analysis of AMPH effects, these data were collapsed across delay because latencies remained stable across the session. We found significant main effects of dose for both measures (nosepoke latency: F3,42 = 8.14, p < 0.001; lever press latency: F3,37 = 6.31, p < 0.05], with the 0.6 and 1.0 mg/kg doses having significant effects compared to vehicle injections (Table 1). There was no significant effect of 8-OH-DPAT on the total number of nosepoke responses that rats made during periods when food pellets were not available.
4. Discussion
A primary goal of this study was to investigate a potential overlapping relationship between individual differences in the initial sensitivity to cocaine-induced behavior and impulsive choice. Using a delay-discounting operant procedure, we observed baseline differences in impulsive choice behavior in rats with disparate locomotor responses to 10 mg/kg cocaine in an open-field arena. Specifically, HCRs showed a greater sensitivity to delay, and thus behaved more impulsively, compared to LCRs. When AMPH was administered prior to delay-discounting sessions, we found that the highest dose tested (1.0 mg/kg) increased impulsive choice in LCRs without influencing the number of trial omissions. None of the tested doses had a statistically significant effect on impulsive choice in HCRs, although the highest dose significantly increased trial omissions. When rats were tested for the effects of 8-OH-DPAT, which occurred one week after the last AMPH test session, we found significant increases in impulsive choice in both phenotypes, although HCRs were only affected at the 0-s delay. Taken together, our results suggest that initial sensitivity to the locomotor effects of cocaine can be used to predict subsequent behavior in a delay-discounting task that examines trait behavior associated with drug use and dependence (Dalley et al., 2007a). Furthermore, they show that drugs previously shown to alter impulsive choice in rats (e.g., Cardinal et al., 2000; Evenden and Ryan, 1996, 1999; van Gaalen et al., 2006; Winstanley et al., 2005) have differential effects in LCRs compared to HCRs.
While ours is the first published study to assess the relationship between individual differences in cocaine-induced locomotion and impulsivity, several studies have investigated individual differences in response to novelty as a predictor of impulsive behavior (Bardo et al., 2006; Dellu-Hagedorn, 2006; Stoffel and Cunningham, 2008). For example, Stoffel and Cunningham (2008) examined the relationship between locomotor activation induced by exposure to an inescapable novel environment and impulsive behavior in a differential reinforcement of low rates of responding (DRL) task. They found that novelty phenotype was predictive of the behavioral inhibition displayed, such that the high novelty responders (HRs) were more impulsive than low novelty responders (LRs). In addition, HRs and LRs were equally sensitive to cocaine-induced increases in impulsivity. Although it was not the focus of the present study, we analyzed the relationship between novelty responses and impulsivity by re-characterizing our rats as LRs or HRs based on their locomotor behavior during the first 30 min of the habituation period on their first open-field test. We found no significant differences in delay-discounting behavior in LRs and HRs (data not shown). Notably, the lack of overlap between the novelty and cocaine response phenotypes has been described previously in male Sprague-Dawley rats (Gulley et al., 2003; Gulley, 2007), and suggests the LCR/HCR classification is somewhat distinct from one based on novelty and may be mediated by different neural mechanisms. It is also noteworthy that different methods of assessing impulsive behavior in rats (e.g., delay-discounting compared to DRL tasks) are likely to be focusing on particular aspects of impulsivity, and the neural mechanisms of impulsive behavior are not likely to be identical (Evenden, 1999; Winstanley et al., 2006a).
AMPH had differential effects on impulsive choice in our tests, with increases observed in LCRs and no effect observed in HCRs. These results were somewhat surprising given previous reports that suggested AMPH, at the doses tested here, decreases impulsivity (Barbelivien et al., 2008; Cardinal et al., 2000; deWit et al., 2002; Winstanley et al., 2003; van Gaalen et al., 2006). However, AMPH's effects on delay-discounting behavior are not unequivocal; it has been shown to increase (Evenden and Ryan, 1996; Cardinal et al., 2000) or have little to no effect on impulsive choice (Barbelivien et al., 2008; Uslaner and Robinson, 2006). One factor that has been suggested as a potential contributor to these inconsistent results is differences in the baseline level of delay-discounting that rats exhibit. In a previous study (Winstanley et al., 2003), AMPH was most effective at altering task behavior in rats that expressed a high baseline level of impulsivity. In our study, however, HCR rats showed the greatest sensitivity to delay at baseline and AMPH had no significant effect on their choice of the large reward across delays. These results are consistent with those from a recent study by Barbelivien et al. (2008). AMPH did increase the number of trial omissions at the highest dose, which is consistent with an enhanced sensitivity to AMPH-induced disruptions in motor behavior that might be expected in rats with high locomotor responses to cocaine. Interestingly, in that same study (Winstanley et al., 2003), the authors reported that AMPH's effects on impulsive choice were attenuated in a group of rats that were depleted of their forebrain 5-HT levels. Thus, impulsive choice might not only depend on baseline levels of impulsivity, but also on intact neurotransmitter systems. The results from the present study do offer some insight into the consequences of the neurobiological differences between LCRs and HCRs. Because the DAT is a primary pharmacological target of AMPH, and alterations in DA signaling are important for behavior in the delay-discounting task (Floresco et al., 2007; van Gaalen et al., 2006), the differential effects we observed for AMPH in LCRs compared to HCRs are a likely reflection of known differences in DAT function between these phenotypes (Sabeti et al., 2003; Briegleb et al., 2004). Importantly, impulsive choice behavior may be influenced by an interaction between DAergic and 5-HTergic function (Winstanley et al., 2005, 2006b), and this could be relevant for differences in choice behavior between LCRs and HCRs.
Direct stimulation of the 5-HT1A receptor through administration of the selective agonist 8-OH-DPAT reduced responding for the large reinforcement in both groups, with LCRs choosing it less frequently across a wide range of delays (10-40 s) at both the 0.6 and 1.0 mg/kg doses. The effect in HCRs was seen at all three tested doses, but only when there was no delay in reinforcement delivery. Increases in impulsive choice following 1.0 mg/kg 8-OH-DPAT have been reported previously (Winstanley et al., 2005), and this effect is partly related to the drug's activation of both postsynaptic 5-HT receptors and somatodendritic autoreceptors. The lack of effect at the 10-60 s delays in HCRs may be due to a floor effect as these rats were already demonstrating steep delay discounting curves (i.e., highly impulsive) at baseline. Additional studies will be necessary to determine if differences in 5-HT systems between LCRs and HCRs contribute to some of the differences in behavior in these phenotypes. Noteworthy in this regard are a number of recent studies highlighting the importance of 5-HT systems in cocaine-induced locomotor activity (Bubar and Cunningham, 2006; Carey et al., 2001, 2005; Liu and Cunningham, 2005), as well as timing processes (Ho et al., 2002) and sensitivity to delayed reinforcement (Mobini et al., 2000; Wogar et al., 1993). It will also be important to determine if the effects of 8-OH-DPAT on delay-discounting are influenced by changes in other behavioral processes, such as altered motivation for feeding or disrupted motor processes.
After the conclusion of the final delay-discounting sessions – which was approximately three months after the first cocaine injection was given – rats were retested in the open-field with 10 mg/kg cocaine. The behavior of LCRs was not significantly different on retest compared to the first test, whereas HCRs exhibited a relative decrease in cocaine-induced locomotor activity. The stability of the LCR phenotype, and the apparent tolerance evident in HCRs, is consistent with a previous study where LCRs and HCRs were retested following a seven day period where they were given extensive habituation to the testing environment (Gulley et al., 2003). In the present study, it is possible that either the AMPH and/or 8-OH-DPAT treatment in the context of the delay-discounting task had an effect on subsequent locomotor behavior in the open-field retest. This is unlikely for several reasons. First, the retest was given a week after the last drug treatment to prevent any carry-over effects. Second, administration of these drugs at low to moderate doses tends to potentiate locomotor activity when given alone or prior to cocaine (Carey et al., 2002, 2004; Chen and Reith, 1995; De La Garza and Cunningham, 2000; Herges and Taylor, 1998; Müller et al., 2003; Schenk et al., 1991). Lastly, the highest dose of 8-OH-DPAT has been shown to inhibit locomotor activity (Przegalinski and Filip, 1997), but this was true only when it was co-administered with cocaine. Thus, at this point, we speculate that the differences in locomotor activity on retest reflect distinct underlying neural adaptations that may be pre-existing and relate to findings from rats with a history of repeated drug exposure. This hypothesis is based on findings that behavioral sensitization is evident in LCRs, while HCRs show a lack of sensitization (Sabeti et al., 2003; unpublished observations). Interestingly, Ben-Shahar et al. (2005) reported differential behavioral responses to the locomotor stimulating effects of cocaine in animals who were previously given short or extended access to self-administer cocaine. Specifically, they reported sensitization in rats with short access compared to tolerance in those with extended access. This escalation model of repeated drug self-administration is associated with addictive behaviors shown in humans (Ahmed and Koob, 1998; Ben-Shahar et al., 2004, 2005).
In summary, we found that two traits previously associated with those who abuse cocaine and other drugs, namely initial sensitivity to drug-induced behavior and impulsivity, are closely related to one another in a rat model of these behaviors. These findings highlight the importance of studying individual differences, especially in the context of pre-disposing traits that overlap with other behaviors that can potentially be related to the vulnerability for drug dependence. It is possible that the disparate drug-induced locomotor responses and ensuing differences in delay-discounting behavior are the result of distinct underlying neural mechanisms. Future studies should focus on how pre-existing traits interact with drug-induced neuroadaptations, as they have the potential to provide a better understanding of the underlying neurobiological mechanisms contributing to individual differences in drug abuse potential. It will also be important to determine how differential sensitivity to cocaine predicts behavior in other experimental paradigms that assess other aspects of the multifaceted trait of impulsivity, such as the five-choice serial reaction time task, differential reinforcement of low rate of responding, or fixed consecutive number schedule.
Acknowledgements
The authors thank Eric Vega for his technical assistance and Lauren Sehy and Ashley Light for help with the collection of pilot data. Financial support for this work was provided by the University of Illinois, Urbana-Champaign and a predoctoral fellowship to JJS (T32 NIH/HD007333).
Footnotes
Author Disclosures
Role of Funding Source
Funding for this study and the preparation of the manuscript was provided by the University of Illinois and a predoctoral fellowship to JJS (T32 NIH/HD007333). The University or NIH had no further role in study design, collection, or analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Conflict of Interest
All authors declare that they have no conflicts of interest.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Allen RM, Everett CV, Nelson AM, Gulley JM, Zahniser NR. Low and high locomotor responsiveness to cocaine predicts intravenous cocaine conditioned place preference in male Sprague-Dawley rats. Pharmacol Biochem Behav. 2007;86:37–44. doi: 10.1016/j.pbb.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbelivien A, Billy E, Lazarus C, Kelche C, Majchrzak M. Rats with different profiles of impulsive choice behavior exhibit differences in responses to caffeine and d-amphetamine and in medial prefrontal cortex 5-HT utilization. Behav Brain Res. 2008;187:273–283. doi: 10.1016/j.bbr.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Cain ME, Bylica KE. Effect of amphetamine on response inhibition in rats showing high or low response to novelty. Pharmacol Biochem Behav. 2006;85:98–104. doi: 10.1016/j.pbb.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A. The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res. 2004;995:46–54. doi: 10.1016/j.brainres.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Moscarello JM, Jacob B, Roarty MP, Ettenberg A. Prolonged daily exposure to i.v. cocaine results in tolerance to its stimulant effects. Pharmacol Biochem Behav. 2005;82:411–416. doi: 10.1016/j.pbb.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Bornovalova MA, Daughters SB, Hernandez GD, Richards JB, Lejuez CW. Differences in impulsivity and risk-taking propensity between primary users of crack cocaine and primary users of heroin in a residential substance-use program. Exp Clin Psychopharmacol. 2005;13:311–318. doi: 10.1037/1064-1297.13.4.311. [DOI] [PubMed] [Google Scholar]
- Briegleb SK, Gulley JM, Hoover BR, Zahniser NR. Individual differences in cocaine- and amphetamine-induced activation of male Sprague-Dawley rats: contribution of the dopamine transporter. Neuropsychopharmacology. 2004;29:2168–2179. doi: 10.1038/sj.npp.1300536. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Curr Top Med Chem. 2006;6:1971–1985. doi: 10.2174/156802606778522131. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology (Berl) 2000;152:362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Carey RJ, De Palma G, Damianopoulos E. 5-HT1A agonist/antagonist modification of cocaine stimulant effects: implications for cocaine mechanisms. Behav Brain Res. 2002;132:37–46. doi: 10.1016/s0166-4328(01)00383-7. [DOI] [PubMed] [Google Scholar]
- Carey RJ, DePalma G, Damianopoulos E. Cocaine and serotonin: a role for the 5-HT(1A) receptor site in the mediation of cocaine stimulant effects. Behav Brain Res. 2001;126:127–133. doi: 10.1016/s0166-4328(01)00253-4. [DOI] [PubMed] [Google Scholar]
- Carey RJ, Depalma G, Damianopoulos E, Muller CP, Huston JP. The 5-HT1A receptor and behavioral stimulation in the rat: effects of 8-OHDPAT on spontaneous and cocaine-induced behavior. Psychopharmacology (Berl) 2004;177:46–54. doi: 10.1007/s00213-004-1917-4. [DOI] [PubMed] [Google Scholar]
- Carey RJ, DePalma G, Damianopoulos E, Shanahan A, Muller CP, Huston JP. Evidence that the 5-HT1A autoreceptor is an important pharmacological target for the modulation of cocaine behavioral stimulant effects. Brain Res. 2005;1034:162–171. doi: 10.1016/j.brainres.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Chen NH, Reith ME. Monoamine interactions measured by microdialysis in the ventral tegmental area of rats treated systemically with (+/−)-8-hydroxy-2-(di-npropylamino)tetralin. J Neurochem. 1995;64:1585–1597. doi: 10.1046/j.1471-4159.1995.64041585.x. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007a;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Laane K, Theobald DE, Pena Y, Bruce CC, Huszar AC, Wojcieszek M, Everitt BJ, Robbins TW. Enduring deficits in sustained visual attention during withdrawal of intravenous methylenedioxymethamphetamine self-administration in rats: results from a comparative study with d-amphetamine and methamphetamine. Neuropsychopharmacology. 2007b;32:1195–1206. doi: 10.1038/sj.npp.1301220. [DOI] [PubMed] [Google Scholar]
- Davidson ES, Finch JF, Schenk S. Variability in subjective responses to cocaine: initial experiences of college students. Addict Behav. 1993;18:445–453. doi: 10.1016/0306-4603(93)90062-e. [DOI] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Cunningham KA. The effects of the 5-hydroxytryptamine(1A) agonist 8-hydroxy-2-(di-n-propylamino)tetralin on spontaneous activity, cocaine-induced hyperactivity and behavioral sensitization: a microanalysis of locomotor activity. J Pharmacol Exp Ther. 2000;292:610–617. [PubMed] [Google Scholar]
- de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27:813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Dellu-Hagedorn F. Relationship between impulsivity, hyperactivity and working memory: a differential analysis in the rat. Behav Brain Funct. 2006;2:10. doi: 10.1186/1744-9081-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats VI: the effects of ethanol and selective serotonergic drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1999;146:413–421. doi: 10.1007/pl00005486. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MT, Ghods-Sharifi S. Dopaminergic and Glutamatergic Regulation of Effort- and Delay-Based Decision Making. Neuropsychopharmacology. 2007:1–14. doi: 10.1038/sj.npp.1301565. [DOI] [PubMed] [Google Scholar]
- Gulley JM. Individual differences in novelty- and cocaine-induced locomotor activity as predictors of food-reinforced operant behavior in two outbred rat strains. Pharmacol Biochem Behav. 2007;86:749–757. doi: 10.1016/j.pbb.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Hoover BR, Larson GA, Zahniser NR. Individual differences in cocaine-induced locomotor activity in rats: behavioral characteristics, cocaine pharmacokinetics, and the dopamine transporter. Neuropsychopharmacology. 2003;28:2089–2101. doi: 10.1038/sj.npp.1300279. [DOI] [PubMed] [Google Scholar]
- Haertzen CA, Kocher TR, Miyasato K. Reinforcements from the first drug experience can predict later drug habits and/or addiction: results with coffee, cigarettes, alcohol, barbiturates, minor and major tranquilizers, stimulants, marijuana, hallucinogens, heroin, opiates and cocaine. Drug Alcohol Depend. 1983;11:147–165. doi: 10.1016/0376-8716(83)90076-5. [DOI] [PubMed] [Google Scholar]
- Heil SH, Johnson MW, Higgins ST, Bickel WK. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addict Behav. 2006;31:1290–1294. doi: 10.1016/j.addbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Herges S, Taylor DA. Involvement of serotonin in the modulation of cocaine-induced locomotor activity in the rat. Pharmacol Biochem Behav. 1998;59:595–611. doi: 10.1016/s0091-3057(97)00473-5. [DOI] [PubMed] [Google Scholar]
- Ho MY, Velazquez-Martinez DN, Bradshaw CM, Szabadi E. 5-Hydroxytryptamine and interval timing behaviour. Pharmacol Biochem Behav. 2002;71:773–785. doi: 10.1016/s0091-3057(01)00672-4. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99:461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Lambert NM, McLeod M, Schenk S. Subjective responses to initial experience with cocaine: an exploration of the incentive-sensitization theory of drug abuse. Addiction. 2006;101:713–725. doi: 10.1111/j.1360-0443.2006.01408.x. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Bornovalova MA, Reynolds EK, Daughters SB, Curtin JJ. Risk factors in the relationship between gender and crack/cocaine. Exp Clin Psychopharmacol. 2007;15:165–175. doi: 10.1037/1064-1297.15.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Cunningham KA. Serotonin2C receptors (5-HT2C R) control expression of cocaine-induced conditioned hyperactivity. Drug Alcohol Depend. 2006;81:275–282. doi: 10.1016/j.drugalcdep.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Mobini S, Chiang TJ, Ho MY, Bradshaw CM, Szabadi E. Effects of central 5-hydroxytryptamine depletion on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology (Berl) 2000;152:390–397. doi: 10.1007/s002130000542. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Oderinde V, Mathias CW, Harper RA, Swann AC. Increased impulsivity in cocaine dependent subjects independent of antisocial personality disorder and aggression. Drug Alcohol Depend. 2002;68:105–111. doi: 10.1016/s0376-8716(02)00106-0. [DOI] [PubMed] [Google Scholar]
- Muller CP, Carey RJ, Salloum JB, Huston JP. Serotonin1A-receptor agonism attenuates the cocaine-induced increase in serotonin levels in the hippocampus and nucleus accumbens but potentiates hyperlocomotion: an in vivo microdialysis study. Neuropharmacology. 2003;44:592–603. doi: 10.1016/s0028-3908(03)00046-7. [DOI] [PubMed] [Google Scholar]
- Paine TA, Dringenberg HC, Olmstead MC. Effects of chronic cocaine on impulsivity: relation to cortical serotonin mechanisms. Behav Brain Res. 2003;147:135–147. doi: 10.1016/s0166-4328(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Poulos CX, Parker JL, Le AD. Dexfenfluramine and 8-OH-DPAT modulate impulsivity in a delay-of-reward paradigm: implications for a correspondence with alcohol consumption. Behav Pharmacol. 1996;7:395–399. doi: 10.1097/00008877-199608000-00011. [DOI] [PubMed] [Google Scholar]
- Przegalinski E, Filip M. Stimulation of serotonin (5-HT)1A receptors attenuates the locomotor, but not the discriminative, effects of amphetamine and cocaine in rats. Behav Pharmacol. 1997;8:699–706. doi: 10.1097/00008877-199712000-00004. [DOI] [PubMed] [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behav Pharmacol. 2006;17:651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Takahashi Y, Gugsa N, Bissonette GB, Schoenbaum G. Previous cocaine exposure makes rats hypersensitive to both delay and reward magnitude. J Neurosci. 2007;27:245–250. doi: 10.1523/JNEUROSCI.4080-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. Acute cocaine differentially alters accumbens and striatal dopamine clearance in low and high cocaine locomotor responders: behavioral and electrochemical recordings in freely moving rats. J Pharmacol Exp Ther. 2002;302:1201–1211. doi: 10.1124/jpet.102.035816. [DOI] [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. Individual differences in cocaine-induced locomotor sensitization in low and high cocaine locomotor-responding rats are associated with differential inhibition of dopamine clearance in nucleus accumbens. J Pharmacol Exp Ther. 2003;305:180–190. doi: 10.1124/jpet.102.047258. [DOI] [PubMed] [Google Scholar]
- Schafer J, Brown SA. Marijuana and cocaine effect expectancies and drug use patterns. J Consult Clin Psychol. 1991;59:558–565. doi: 10.1037//0022-006x.59.4.558. [DOI] [PubMed] [Google Scholar]
- Schenk S, Snow S, Horger BA. Pre-exposure to amphetamine but not nicotine sensitizes rats to the motor activating effect of cocaine. Psychopharmacology (Berl) 1991;103:62–66. doi: 10.1007/BF02244075. [DOI] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B. Cocaine exposure causes long-term increases in impulsive choice. Behav Neurosci. 2007;121:543–549. doi: 10.1037/0735-7044.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel EC, Cunningham KA. The relationship between the locomotor response to a novel environment and behavioral disinhibition in rats. Drug Alcohol Depend. 2008;92:69–78. doi: 10.1016/j.drugalcdep.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Robinson TE. Subthalamic nucleus lesions increase impulsive action and decrease impulsive choice - mediation by enhanced incentive motivation? Eur J Neurosci. 2006;24:2345–2354. doi: 10.1111/j.1460-9568.2006.05117.x. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, van Koten R, Schoffelmeer AN, Vanderschuren LJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia AJ, Perales JC, Perez-Garcia M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addict Behav. 2007;32:950–966. doi: 10.1016/j.addbeh.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacology (Berl) 2003;170:320–331. doi: 10.1007/s00213-003-1546-3. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006a;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Cardinal RN, Robbins TW. Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cereb Cortex. 2006b;16:106–114. doi: 10.1093/cercor/bhi088. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacology. 2005;30:669–682. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]
- Wogar MA, Bradshaw CM, Szabadi E. Effect of lesions of the ascending 5-hydroxytryptaminergic pathways on choice between delayed reinforcers. Psychopharmacology (Berl) 1993;111:239–243. doi: 10.1007/BF02245530. [DOI] [PubMed] [Google Scholar]



