Abstract
We reported previously that Fas-induced hepatic failure in normal mice was attenuated or prevented by exogenous transferrin (Tf), particularly apoTf. Here we show in C57BL6J/129 mice with genetic inactivation of transferrin receptor 2 (TfR2Y245X), that Fas-induced hepatotoxicity (apoptosis; rise in plasma aspartate aminotransferase (AST) levels) was comparable to that in wild-type mice, but was not modified by pretreatment with Tf. Rises in plasma AST were preceded by a decline in serum iron levels. AST elevations and iron declines were more profound in female than in male mice. Female mice also showed higher baseline levels of Bcl-xL in hepatocytes, which declined significantly upon treatment with agonistic anti-Fas antibody. These data confirm the cytoprotective function of Tf, and show a novel property of TfR2. Both apoptotic Fas responses and cytoprotective effects of Tf were associated with significant shifts in plasma iron levels, which quantitatively differed between male and female mice.
Keywords: Hepatocyte apoptosis, Fas signaling, apo-transferrin, transferrin receptor 2, gender effect
INTRODUCTION
Data from several laboratories indicate that the function of transferrin (Tf) is not limited to iron transport [1] but also has potent anti-apoptotic effects [2–4]. Ionized iron has profound effects on cellular redox potential [5], which may be modified bybinding to Tf [6]. The resulting changes, in turn, are expected to alter the activity of various transcription factors and the occurrence of programmed cell death (apoptosis) [7]. We have shown previously that exogenous Tf attenuates or prevents Fas-induced apoptosis in hepatocytes and protects mice against Fas-induced hepatic failure [7,8]. While we expect ApoTf to become saturated with iron upon addition to iron-containing medium or after injection into mice, our studies suggested that administration of ApoTf was more potent than injection of HoloTf. Accordingly, in vitro and ex vivo studies showed that ApoTf resulted in more profound upregulation of anti-apoptotic and downregulation of pro-apoptotic signals than did iron-saturated HoloTf [4,7].
To deliver iron, Tf must be taken up by cells. Unexpectedly, however, an anti-CD71 (Tf receptor 1 [TfR1]) monoclonal antibody (MAB) that prevents iron uptake did not interfere with the anti-apoptotic effects of Tf, suggesting that TfR1 was not directly involved in the protective effect of Tf against Fas-induced apoptosis [8]. The role of Tf receptor 2 (TfR2) in our model [9] has yet to be determined. TfR2 has a lower affinity for holoTf and a more restricted tissue distribution than TfR1, but is prominently expressed on hepatocytes. While TfR2 can deliver iron to cells, the primary function may be connected to hepcidin expression [10]. The stability of cell surface TfR2 is dependent upon the presence of Fe3+ Tf, [9,11]. Here we investigated in murine models the role of TfR2 in the protection of hepatocytes by Tf against Fas-initiated hepatocyte death and the potential impact of different plasma iron levels on the extent of Fas-mediated hepatic injury.
MATERIALS AND METHODS
Reagents
Hamster anti-mouse Fas MAB (clone Jo2, in the NA/LE format [aFas]) was purchased from PharMingen (San Diego, CA); antibodies to Bcl-xL from Cell Signaling Technology (Beverly, MA); rabbit anti-actin antibody from Sigma (St. Louis, MO); secondary goat anti-rabbit IgG-horse-radish peroxidase (HRP) and rabbit anti-mouse IgG-HRP from Pierce (San Francisco, CA); human apo- (ApoTf) and holoTt (FeTf) from Sigma. All Tf preparations were endotoxin “free” as determined by LAL technique at the Biologics Production Facility of the FHCRC. Actinomycin D (ActD) was obtained from Sigma.
Animals
Male and female C57BL6, BALB/c, and SVJ/129 mice, 2–3 months old, were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Heterozygous breeder mice with deletion of TfR2 (TfR2Y245X) (C57BL6J background) were developed in the laboratory of Dr. Robert E. Fleming (St. Louis University School of Medicine, St. Louis, MO) and bred at the FHCRC animal facilities. Mice were used with the approval of the Institutional Animal Care and Use Committee of the FHCRC, in compliance with National Institutes of Health guidelines.
Genotyping for the Y245X mutation
Offspring of TfR2Y245X heterozygous pairs were genotyped by polymerase chain reaction (PCR) analysis of genomic tail DNA as described [9,12]. Briefly, the PCR involved 35 cycles of 95°C for 1 min, 65°C for 1 min, and 72°C for 90 sec, using as a forward primer 5’-GTG ACA AGG GGG CAT ATT ATG CAT GGG ATT-3’ and as a reverse primer 3’-TGT TGT GTA GCC CAA GCA GGT CCT GTA CAA-5’. The mutant allele was identified by PCR using oligos at the designated positions. The mutant (homozygous=HO) (922-bp) gives a longer PCR product than the wild type (WT) allele (814-bp). Heterozygous (HT) mice express both alleles.
Experimental in vivo protocol
Sublethal doses of agonistic anti-Fas MAB (aFas, Jo2, 0.08 µg/g of body weight) were given intraperitoneally (i.p.) in a volume of 0.25 mL, and human ApoTf was injected i.p. at doses of 0.1 mg/0.25 mL/mouse at 48, 24, 1 hour before, and 1 hour after aFas injection. Control mice received identical volumes of saline i.p. Blood samples were harvested at 0, 6, 12, 24, 48 hours and 7 days after aFas. Blood samples were obtained without halothane anesthesia via puncture of the cheek pouch to minimize the risk of hemolysis and to avoid potential anesthetic-related hepatotoxicity [13]. Mice were sacrificed on day 7 of the experiment.
Liver histology
Liver tissue was fixed in 10% formalin and embedded in paraffin. Sections (4 µm) were stained with hematoxylin/eosin, or with Prussian blue for determination of iron deposition.
Blood plasma iron measurement
Total iron in blood plasma was measured using QuantiChrom Iron Assay Kit (BioAssay Systems, Hayward, CA) according to the manufacturer’s protocol.
AST determination
Aspartate-aminotransferase (AST) in murine plasma was determined spectrophotometrically at Phoenix Central Laboratory (Everett, WA, USA).
Western blots
For BcL-xL determination in the liver, mice were sacrificed without aFas injection (time 0) or 6 hours after aFas. Liver tissue was collected and lysed with CHAPS buffer (Cell Signaling Technology). The cell extracts were analysed by SDS-PAGE and immunodetected with anti-human Bcl-xL antibodies (Cell Signaling Technology).
Statistics
Student’s t-test was applied for statistical analysis using Graph-PadPrism (version 3) software. Data are presented as means ± SE.
RESULTS
Fas-induced hepatocyte injury is strain and gender dependent
Injection of aFas at sublethal doses, as in previous studies [7,8], induced acute hepatic injury as indicated by a rise in plasma AST levels in mice of all strains studied (Table 1). Without exception, the rise in AST levels was less severe in male than in female mice in response to the same per body weight doses of aFas. C57BL6J mice showed smaller AST increments than BALB/c or SVJ/129 mice. The most pronounced increases in AST levels occurred in female SVJ/129 mice (8934±1833 U/L versus 3912±1280 U/L in female BALB/c, and 660 ±159 U/L in female C57BL6J mice) (Table 1). The time course of AST responses also differed between strains: the peak AST values in female BALB/c, SVJ/129, and C57BL6J mice were observed at 6 hours, 12 hours and 24 hours after aFas injection, respectively (Table 1). These results indicated strain-dependent differences in the kinetics and the magnitudes of the response to Fas-mediated signaling and were consistent with differences in biological activity of the Fas/Fas-Ligand system as also observed by others [15].
Table 1.
AST plasma levels (U/L) in C57BL6, BALB/c, and SVJ/129 mice exposed to sublethal dose of aFas MAB.
| Strain | Gender | Time after aFas injection (hours) | ||||
|---|---|---|---|---|---|---|
| 0 (pre) | 6 | 12 | 24 | 48 | ||
| C57BL6 | Male | 37±5 | 64±7 | 121±17 | 146±14 | 60±2 |
| Female | 52±12 | 85±10 | 275±102 | 660±159* | 74±4* | |
| BALB/c | Male | 50±1 | 216±74 | 689±269 | 127±33 | 51±3 |
| Female | 50±2 | 3912±1280* | 3452±765** | 834±107*** | 62±5 | |
| SVJ/129 | Male | 34±3 | 852±266 | 4088±485 | 736±34 | 41±3 |
| Female | 40±3 | 4876±918** | 8934±1833@ | 1441±222* | 70±2*** | |
p<0.02
p<0.01
p<0.001
p<0.05 for differences between male and female mice. Remaining values were not significantly different
Five mice per group were injected i.p. with agonistic anti-Fas MAB (0.08 µg/g of body weight in 0.25 mL), and blood samples were obtained at 0, 6, 12, 24, and 48 hours after aFas injection for AST determination.
The pattern of AST increases in response to Fas signals reflected constitutive iron levels in the mouse strains studied. C57BL6J mice, with low baseline iron levels (140–170 µg/dL), showed less prominent AST rises in both male and female recipients, than did BALB/c and SVJ/129 mice with baseline iron levels of 220–270 µg/dL, and 240–290 µg/dL, respectively. Results in C57BL6J mice are further illustrated in Figure 1. The rise in AST levels (peaking at 24 hours; Figure 1A) was preceded by a decline in plasma iron (Figure 1B) and associated with a decline in Bcl-xL protein [7]. However, the magnitude of the decline was significant only in female mice (which had higher levels of expression at baseline than male mice) (Figure 1C). Early declines (and subsequent increases) in iron levels were also more pronounced in female than in male mice. Female mice showed a decrease in plasma iron from 148±13 µg/dL to 73±8 µg/dL (p<0.01) at 6 hours, followed by an increase to 282±30 µg/dL (p<.02) at 48 hours after aFas injection (Figure 1B). Peak values for plasma iron levels were reached at 48 hours after aFas injection, a time when AST levels had returned to normal values. Such a time course would be consistent with early utilization of iron in Fas signaling and a subsequent release of iron from injured (apoptotic) hepatocytes.
Figure 1. Changes in aspartate-aminotransferase (AST) and total iron (Fe) levels in blood plasma and Bcl-xL expression in liver lysates of C57BL6 mice exposed to agonistic anti-Fas antibody (aFas).
Mice were given i.p. aFas at sublethal doses of 0.08 µg/g body weight. Blood was sampled at 0. 6, 12, 24, 48 hours and 7 days after aFas. Shown are the means ± SE of AST (U/L) (A) and iron (µg/dL) (B). AST levels (at 24 hours) were significantly higher in female than in male mice (p<0.05). Iron levels declined by 6 hours and were increased by 48 hours in aFas-treated mice in comparison to controls (“bleeding”= blood sampling only); however, differences reached significance only in female mice (*p<0.05 and **p<0.002, respectively). C) Expression of Bxl-xL was determined in liver cell lysates obtained from mice sacrificed at 0 or 6 hours after aFas injection (C57BL6 mice; 5 per group) by Western blot and analyzed with Image J. Female mice expressed Bcl-xL at higher levels than male mice and showed significant decreases in Bcl-xL protein following treatment with aFas; very little change was noted in male mice.
Cytoprotection by Tf requires TfR2 and is modulated by iron levels
We have shown that Fas-initiated hepatocyte injury is attenuated or prevented by pretreatment of mice with Tf, particularly with iron-free ApoTf [4,7,8]. Surprisingly, however, blockade of TfR1 by a neutralizing antibody did not prevent the anti-apoptotic effect of Tf [16,17]. We speculated that TfR2 [9,18] might mediate the anti-apoptotic effects of Tf. The availability of mice with genetic inactivation of TfR2 (TfR2Y245X) provided a model in which to determine the role of this receptor for cytoprotection by Tf.
Blood plasma iron levels differed between wild type (WT) mice and mice heterozygous (HT) or homozygous (HO) for the TfR2 deletion (Figure 2A). HO mice had the highest baseline iron levels (217±11 µg/dL in males, and 297±11 µg/dL in females [p<0.001]), heterozygous mice had intermediate levels (180±11 and 199±7 µg/dL, respectively [p=NS]), and WT mice had the lowest iron levels (157±4 and 159±14 µg/dL, respectively [p=NS]). Prominent iron deposition was observed in the livers of HO mice (Figure 2B).
Figure 2. Blood plasma iron levels and hepatic iron deposition in TfR2 mutant mice.
(A) Blood samples were obtained from 2–3 months old mice (five to eleven animals/group), and iron was determined by QuantiChrom Iron Assay Kit. Shown are the means ± SE. (B) Female mice, 2 months old, were killed, liver tissue was fixed in 10% formalin, and paraffin-embedded sections were stained with Prussian Blue (x40). Shown are examples of liver sections from wild type (WT) mice and mice heterozygous (HT) or homozygous (HO) for the TfR2 mutation. Only HO mice showed severe hepatic iron loading [9].
As shown in Figure 3, treatment with aFas resulted in alterations of plasma iron levels as also observed in other strains (see above). Male mice showed a modest decline in iron levels at 6 – 12 hours; female mice also experienced a decline by 6 hours, but showed a significant rise at 12 hours. As expected, pretreatment with ApoTf prevented the decline in iron levels in WT male and female mice (no significant difference compared to controls). However, no protective effect of ApoTf was apparent in HT or HO TfR2Y245X mice. As with other strains, AST levels increased in male and female mice of all genotypes in response to aFas. AST elevations peaked at 12–24 hours (500±28 U/L, 515±69 U/L, and 604±41 U/L in female WT, HT, and HO mice, respectively). Differences between female and male mice were significant for HO mice at 12 [p<0.001] and 24 hours [p<0.05]), and were of the same order of magnitude as in female C57BL6J mice (660±159 U/L), the strain from which the TfR2Y245X mice were derived (Table 1, Figure 3, panels A-F). Similarly, the rise in plasma AST levels (highest in HO mice, which also had the highest iron levels) was preceded by a decline in plasma iron. However, while pretreatment with ApoTf prevented the rise in serum AST levels in WT mice, AST levels in HT and HO mice were not affected by ApoTf. The fact that Tf failed to provide protection even in HT mice (without significant hepatic iron loading) indicated that liver iron by itself was not responsible for the observed effects in HO mice. These data are consistent with a pivotal role of TfR2 for the cytoprotective effects of Tf. A lack of protection in HT mice was likely due to haploinsufficiency of TfR2.
Figure 3. Serum aspartate aminotransferase (AST) and plasma iron (Fe) levels in wild type (WT) mice and in mice heterozygous (HT) or homozygous (HO) for the TfR2 mutation.
Mice were injected i.p. with sublethal doses of anti-Fas MAB (aFas, Jo2, 0.08 µg/g body weight) and treated with 0.25 mL saline or human ApoTf, 0.1 mg/0.25 mL/mouse at 48, 24, 1 hour before, and 1 hour after aFas injection. Additional mice received no treatment (“bleeding” = blood sampling only). Blood for AST and Fe determination was collected at 0, 6, 12, 24, 48 hours and 7 days after aFas. Results are given as mean ± SE, plotted over time. A) wild type (WT) female mice (*p<0.02, **p<0.01 versus control); B) WT male mice (*p<0.05, **p<0.01 versus control); C) heterozygous (HT) female mice; D) HT male mice; E) homozygous (HO) female mice; F) HO male mice. AST levels remained in the normal range after injection of saline or Tf without aFas, and changes in iron levels in mice injected with Tf only paralleled those seen in controls (not shown). G) Mice were treated as in A–F, but iron levels were followed for 7 days (168 hours). Fluctuations were more extensive in female than in male mice, (two male WT “bleeding” controls died due to technical problems). Arrows indicate time of injection of aFas.
*p<0.01 – saline [109±2 µg/dL] versus ApoTf [194±8 µg/dL],
**p<0.01 – saline [68±2 µg/dL] versus ApoTf [118±9 µg/dL].
DISCUSSION
We have shown that Fas-mediated hepatocyte injury and fatal hepatic failure due to hepatocyte apoptosis are prevented or attenuated by exogenous Tf, and that this cytoprotective effect of Tf is modulated by iron [7,8]. The cytoprotective effect of Tf was consistently greater with ApoTf than with FeTf. Blockade of TfR1 by a specific MAB [19], did not interfere with the protective effect of Tf [8], fitting with observations by others who showed that modulation of apoptotic signals by Tf did not require TfR1 [3]. TfR2 is a second, evolutionally more primitive receptor for Tf, apparently with several functions [11,18], which [20] is highly expressed on hepatocytes [9]. Here we show that TfR2 is required for the Tf-mediated protection of hepatocytes against Fas-induced injury. A proposed model is shown in Figure 4. Homozygous TfR2 mutant mice derived no protection from exogenous Tf against Fas-mediated injury, and even heterozygous mice failed to benefit from pretreatment with Tf. The results also show that additional parameters, in particular the genetic background of mice, their plasma iron levels, and the gender of the treated mouse determined the responses to Fas injury and the extent of protection by Tf.
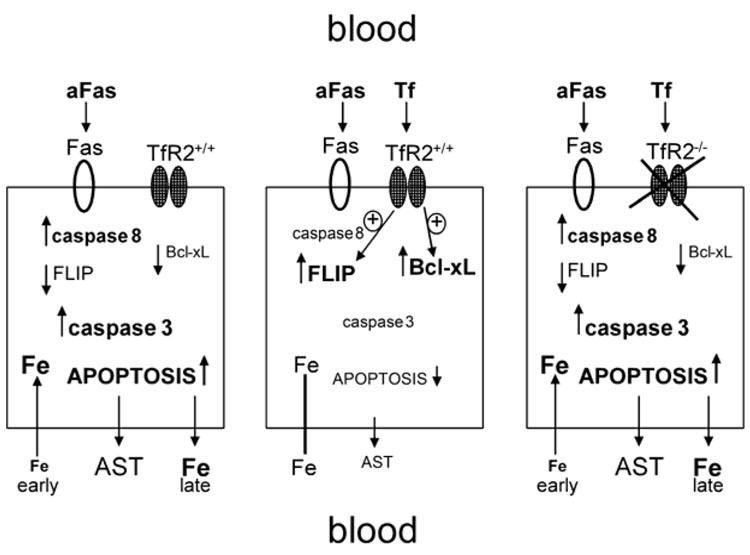
Figure 4. Proposed model.
Left panel depicts events in an unmodified hepatocyte exposed to agonistic anti-Fas antibody (aFas). The middle panel shows an unmodified hepatocyte exposed to aFas in the presence of exogenous transferrin (Tf). The arrow-less line between intracellular iron (Fe) and extracelluar Fe indicates absence of significant unidirectional shifts. The right panel shows a hepatocyte lacking functional transferrin receptor 2 (TfR2) exposed to aFas in the presence of exogenous Tf; in contrast to the middle panel, no protection is provided by Tf (see text for additional abbreviations).
Hepatic injury as determined by AST elevations in serum was most severe in SVJ/129 mice, and peak AST elevations were reached earlier in BALB/c and SVJ/129 mice than in C57BL6 mice, consistent with strain-specific responses reported by others [15]. Importantly, however, the overall patterns of response were similar, including responses in wild type C57BLJ6 mice, which provided the background for the TfR2Y245X mutant mice. The association of AST elevations with plasma iron levels, however, was unexpected. Fas-induced AST elevations were less severe and more delayed in mice with low iron, and were more severe and occurred earlier in mice with higher baseline iron, suggesting the possibility that an iron gradient between hepatocytes and blood plasma was relevant for the extent of hepatic injury [21].
Furthermore, female mice consistently showed higher AST elevations and more severe fluctuations in plasma iron levels after aFas exposure than did male mice. While there was a decline in plasma iron levels early after aFas exposure in both female and male mice, female, but not male mice experienced significant increases in plasma iron with a delay after aFas treatment. Conceivably, this delayed rise in plasma iron was related to the extent of hepatocyte injury, which, as reflected by hepatocyte apoptosis [8] and AST elevations, was greater in female mice. Female mice have been shown to possess higher iron stores (and, e.g., higher levels of mRNA for the iron regulatory peptide hepcidin [22]) than male mice. Further, in response to aFas, there was a significant decrease in the expression of the anti-apoptotic molecule Bcl-xL in female but not in male mice (Figure 2C), providing additional, albeit circumstantial evidence for an effect of iron levels on apoptotic responses. Significant gender differences have also been reported by others. For example, Naugler et al. [23] showed a higher incidence of carcinogen induced liver cancer in male than in female mice, associated with differences in MyD88-dependent interleukin-6 production, which was inhibited by estrogen. The exact mechanism that accounts for the gender differences in the present model remains to be determined. However, involvement of pro-inflammatory cytokines such as interleukin-6 is not unlikely as this cytokine also regulates the expression of hepcidin [24].
The mechanism of the early decline in plasma iron levels following exposure to aFas is not immediately apparent. However, iron is required for the initial steps of Fas-mediated apoptosis [21,25], and the observed decline in iron was possibly related to iron import into cells. As discussed above, the ensuing apoptosis would then lead to release of iron from apoptotic hepatocytes and a rise in plasma iron. Furthermore, we observed in other studies (unpublished data) that cross linking of Fas by Fas-ligand resulted in downregulation of hepcidin mRNA in the liver of mice, which, in principle, should lead to an increase in iron uptake or iron release from macrophages. The observed opposing effect of ApoTf is in agreement with previously published data [7,8], which showed that exposure of hepatocytes to Tf, particularly in conjunction with pro-apoptotic stimuli, directly upregulated anti-apoptotic molecules such as Flice Inhibitory Protein (FLIP) and Bcl-xL, and led to decreased cytochrome c release [26].
Hepatoprotective effects similar to those observed with ApoTf have been reported for hemoxygenase-1 (HO-1), the rate limiting enzyme in heme metabolism [27], and a substantial literature documents effects of HO-1 on both apoptosis and iron homeostasis. [21,27–32] Overexpression and induction of HO-1 have been shown to prevent graft-versus-host disease of the liver, at least in part by counteracting apoptotic hepatic cell death [27]. However, while we observed in ancillary studies that HO-1 was upregulated in hepatocytes in response to FeTf, there was no upregulation in response to ApoTf (data not shown). Such an outcome suggested that increased iron delivery by FeTf upregulated HO-1 [21]. This finding was of interest in view of the observed differences between ApoTf and FeTf: as ApoTf binds iron upon addition to the system, this chelating function may contribute to the cytoprotective effect mediated by ApoTf.
In summary, we have shown that the extent of Fas-mediated apoptosis in hepatocytes is dependent upon baseline iron levels and involves alterations of iron homeostasis. The cytoprotective/anti-apoptotic effects of Tf require expression of functional TfR2. The greater extent of Fas-mediated hepatotoxicity in female mice may at least in part be due to greater reduction in Bcl-xL levels in response to Fas in female mice. Thus, the study provides novel data on the function of TfR2 and may serve as rationale for the use of ApoTf and, possibly, other iron-chelating agents as cytoprotective strategies. Such an approach might be effective in patients who receive high-dose chemotherapy which is associated with liver toxicity or in patients after allogeneic hematopoietic cell transplantation where hepatic graft-versus-host disease (with hepatocyte apoptosis being a prominent feature) is a frequent complication.
ACKNOWLEDGEMENTS
We thank David Hockenbery MD and Andrew Mhyre PhD for critical comments, the technicians of the animal facilities for excellent services, and Bonnie Larson and Helen Crawford for help with manuscript preparation.
This work was supported in part by NIH grants HL036444, HL082941, DK063016, DK056465, CA023226 and CA074131, Bethesda, MD.
Grant support: NIH grants HL036444, HL082941, DK063016, DK056465, CA023226 and CA074131.
Footnotes
Conflict of interest. The authors declare that they have no conflict of interest.
REFERENCES
- 1.Pierpaoli W, Lesnikov V, Lesnikova M, Arrighi S. Donor-derived plasma transferrin facilitates the engraftment of xenogeneic (rat) bone marrow in irradiated mice. Bone Marrow Transplant. 1996;18:203–207. [published erratum appears in Bone Marrow Transplant 1996 Dec;18(6):1201] [PubMed] [Google Scholar]
- 2.Weinzimer SA, Gibson TB, Collett-Solberg PF, Khare A, Liu B, Cohen P. Transferrin is an insulin-like growth factor-binding protein-3 binding protein. J Clin Endocrinol Metab. 2001;86:1806–1813. doi: 10.1210/jcem.86.4.7380. [DOI] [PubMed] [Google Scholar]
- 3.Fassl S, Leisser C, Huettenbrenner S, Maier S, Rosenberger G, Strasser S, Grusch M, Fuhrmann G, Leuhuber K, Polgar D, Stani J, Tichy B, Nowotny C, Krupitza G. Transferrin ensures survival of ovarian carcinoma cells when apoptosis is induced by TNFalpha, FasL, TRAIL, or Myc. Oncogene. 2003;22:8343–8355. doi: 10.1038/sj.onc.1207047. [DOI] [PubMed] [Google Scholar]
- 4.Lesnikov V, Lesnikova M, Shulman HM, Arrighi S, Pierpaoli W, Deeg HJ. In vivo cytoprotective and immunomodulatory effects of transferrin. Research Advances in Blood. 2001;1:169–178. [Google Scholar]
- 5.Aracena P, Aguirre P, Munoz P, Nunez MT. Iron and glutathione at the crossroad of redox metabolism in neurons. Biological Research. 2006;39:157–165. doi: 10.4067/s0716-97602006000100017. [DOI] [PubMed] [Google Scholar]
- 6.Orino K, Watanabe K. Molecular, physiological and clinical aspects of the iron storage protein ferritin. The Veterinary Journal. doi: 10.1016/j.tvjl.2007.07.006. in press. [DOI] [PubMed] [Google Scholar]
- 7.Lesnikov VA, Abbasi N, Lesnikova MP, Lazaro CA, Campbell JS, Fausto N, Deeg HJ. Protection of human and murine hepatocytes against Fas-induced death by transferrin and iron. Apoptosis. 2006;11:79–87. doi: 10.1007/s10495-005-3086-2. [DOI] [PubMed] [Google Scholar]
- 8.Lesnikov VA, Lesnikova MP, Shulman HM, Wilson H-M, Hockenberry DM, Kocher M, Pierpaoli W, Deeg HJ. Prevention of Fas-mediated hepatic failure by transferrin. Lab Invest. 2004;84:342–352. doi: 10.1038/labinvest.3700035. [DOI] [PubMed] [Google Scholar]
- 9.Fleming RE, Ahmann JR, Migas MC, Waheed A, Koeffler HP, Kawabata H, Britton RS, Bacon BR, Sly WS. Targeted mutagenesis of the murine transferrin receptor-2 gene produces hemochromatosis. Proc Natl Acad Sci USA. 2002;99:10653–10658. doi: 10.1073/pnas.162360699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Domenico I, McVey WD, Kaplan J. Regulation of iron acquisition and storage: consequences for iron-linked disorders (Review) Nat Rev Mol Cell Biol. 2008;9:72–81. doi: 10.1038/nrm2295. [DOI] [PubMed] [Google Scholar]
- 11.Kawabata H, Germain RS, Ikezoe T, Tong X, Green EM, Gombart AF, Koeffler HP. Regulation of expression of murine transferrin receptor 2. Blood. 2001;98:1949–1954. doi: 10.1182/blood.v98.6.1949. [DOI] [PubMed] [Google Scholar]
- 12.Fleming RE, Migas MC, Holden CC, Waheed A, Britton RS, Tomatsu S, Bacon BR, Sly WS. Transferrin receptor 2: continued expression in mouse liver in the face of iron overload and in hereditary hemochromatosis. Proc Natl Acad Sci USA. 2000;97:2214–2219. doi: 10.1073/pnas.040548097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golde WT, Gollobin P, Rodriguez LL. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Animal. 2005;34:39–43. doi: 10.1038/laban1005-39. [DOI] [PubMed] [Google Scholar]
- 14.Wu JC, Merlino G, Fausto N. Establishment and characterization of differentiated, nontransformed hepatocyte cell lines derived from mice transgenic for transforming growth factor alpha. Proc Natl Acad Sci USA. 1994;91:674–678. doi: 10.1073/pnas.91.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kayagaki N, Yamaguchi N, Nagao F, Matsuo S, Maeda H, Okumura K, Yagita H. Polymorphism of murine Fas ligand that affects the biological activity. Proc Natl Acad Sci USA. 1997;94:3914–3919. doi: 10.1073/pnas.94.8.3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lesnikov V, Lesnikova M, Deeg HJ. Pro-apoptotic and anti-apoptotic effects of transferrin and transferrin-derived glycans on hematopoietic cells and lymphocytes. Exp Hematol. 2001;29:477–489. doi: 10.1016/s0301-472x(00)00687-1. [DOI] [PubMed] [Google Scholar]
- 17.Lesnikova M, Lesnikov V, Arrighi S, Kistler G, Pierpaoli W, Deeg HJ. Upregulation of interleukin-10 and inhibition of alloantigen responses by transferrin and transferrin-derived glycans. Journal of Hematotherapy and Stem Cell Research. 2000;9:381–392. doi: 10.1089/15258160050079498. [DOI] [PubMed] [Google Scholar]
- 18.Kawabata H, Yang R, Hirama T, Vuong PT, Kawano S, Gombart AF, Koeffler HP. Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family. J Biol Chem. 1999;274:20826–20832. doi: 10.1074/jbc.274.30.20826. [DOI] [PubMed] [Google Scholar]
- 19.Trowbridge IS, Lopez F. Monoclonal antibody to transferrin receptor blocks transferrin binding and inhibits human tumor cell growth in vitro. Proc Natl Acad Sci USA. 1982;79:1175–1179. doi: 10.1073/pnas.79.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawabata H, Tong X, Kawanami T, Wano Y, Hirose Y, Sugai S, Koeffler HP. Analyses for binding of the transferrin family of proteins to the transferrin receptor 2. Br J Haematol. 2004;127:464–473. doi: 10.1111/j.1365-2141.2004.05224.x. [DOI] [PubMed] [Google Scholar]
- 21.Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, Tysoe SA, Wolosker H, Baranano DE, Dore S, Poss KD, Snyder SH. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nature Cell Biology. 1999;1:152–157. doi: 10.1038/11072. [DOI] [PubMed] [Google Scholar]
- 22.Courselaud B, Troadec MB, Fruchon S, Ilyin G, Borot N, Leroyer P, Coppin H, Brissot P, Roth MP, Loreal O. Strain and gender modulate hepatic hepcidin 1 and 2 mRNA expression in mice. Blood Cells Molecules & Diseases. 2004;32:283–289. doi: 10.1016/j.bcmd.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [see comment] [DOI] [PubMed] [Google Scholar]
- 24.Truksa J, Peng H, Lee P, Beutler E. Different regulatory elements are required for response of hepcidin to interleukin-6 and bone morphogenetic proteins 4 and 9. Br J Haematol. 2007;139:138–147. doi: 10.1111/j.1365-2141.2007.06728.x. [DOI] [PubMed] [Google Scholar]
- 25.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 26.Kim TH, Zhao Y, Barber MJ, Kuharsky DK, Yin XM. Bid-induced cytochrome c release is mediated by a pathway independent of mitochondrial permeability transition pore and Bax. J Biol Chem. 2000;275:39474–39481. doi: 10.1074/jbc.M003370200. [DOI] [PubMed] [Google Scholar]
- 27.Gerbitz A, Ewing P, Wilke A, Schubert T, Eissner G, Dietl B, Andreesen R, Cooke KR, Holler E. Induction of heme oxygenase-1 before conditioning results in improved survival and reduced graft-versus-host disease after experimental allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2004;10:461–472. doi: 10.1016/j.bbmt.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 28.McDaid J, Yamashita K, Chora A, Ollinger R, Strom TB, Li XC, Bach FH, Soares MP. Heme oxygenase-1 modulates the allo-immune response by promoting activation-induced cell death of T cells. FASEB J. 2005;19:458–460. doi: 10.1096/fj.04-2217fje. [DOI] [PubMed] [Google Scholar]
- 29.Pae HO, Choi BM, Oh GS, Lee MS, Ryu DG, Rhew HY, Kim YM, Chung HT. Roles of heme oxygenase-1 in the antiproliferative and antiapoptotic effects of nitric oxide on Jurkat T cells. Mol Pharmacol. 2004;66:122–128. doi: 10.1124/mol.66.1.122. [DOI] [PubMed] [Google Scholar]
- 30.Choi BM, Pae HO, Jeong YR, Oh GS, Jun CD, Kim BR, Kim YM, Chung HT. Overexpression of heme oxygenase (HO)-1 renders Jurkat T cells resistant to fas-mediated apoptosis: involvement of iron released by HO-1. Free Radical Biology & Medicine. 2004;36:858–871. doi: 10.1016/j.freeradbiomed.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Lee SK, Park DY, Lee HJ, Lee J, Choi MK, Jeon BH, Jun CD, Lee SK, Kim EC. Functional interaction between nitric oxide-induced iron homeostasis and heme oxygenase-1 in immortalized and malignant oral keratinocytes. Cancer Lett. 2007;249:283–293. doi: 10.1016/j.canlet.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Watts RN, Ponka P, Richardson DR. Effects of nitrogen monoxide and carbon monoxide on molecular and cellular iron metabolism: mirror-image effector molecules that target iron (Review) Biochem J. 2003;369:429–440. doi: 10.1042/BJ20021302. [erratum appears in Biochem J. 2003 Mar 15;730(Pt 3):1111] [DOI] [PMC free article] [PubMed] [Google Scholar]