Abstract
A highly efficient method for the palladium-catalyzed borylation of aryl halides with an inexpensive and atom-economical boron source, pinacol borane, has been developed. This system allows for the conversion of aryl and heteroaryl iodides, bromides and several chlorides, containing a variety of functional groups, to the corresponding pinacol boronate esters. In addition to the increase in substrate scope, this is the first general method where relatively low quantities of catalyst and short reaction times can be employed.
The utility of aryl boronic acids and esters throughout organic synthesis is seen by their use as key intermediates in the preparation of a wide range of synthetic targets.1 Despite their versatility, standard methods for the preparation of these compounds can be harsh and, hence, be incompatible with a variety of functional groups.2 However, various techniques have recently emerged that provide access to aryl boronate esters under milder reaction conditions.3 In particular, palladium-based systems for the conversion of aryl halides to the corresponding carbon-boron bonds have proved to be a powerful synthetic tool. Recently, we reported a highly active catalyst for transforming aryl chlorides into aryl pinacol-derived boronate esters.4 However, this system required the use of an expensive boron source, bis(pinacolato)diboron.5 In addition, the reactions of sterically hindered aryl chlorides were less efficient as they required higher quantities of Pd as well as an increase in the number of equivalents of the expensive boron reagent.
In order to address these issues, we sought to develop a system where pinacol borane, a cheaper and more atom-economical boron source, could be employed in the borylation of aryl halides and concurrently to produce a method applicable to sterically hindered substrates. Although several systems have been developed for the borylation of aryl halides with pinacol borane, these methods have several limitations.6 In general, aryl iodides and bromides are necessary, while the cheaper and more readily available aryl chlorides are unsuitable substrates.7 We are aware of only one report in which aryl chlorides are efficiently transformed to the corresponding boronate esters when using pinacol borane as the boron source.8 However, this method had a limited substrate scope as only para-substituted electron-rich aryl chlorides were efficiently converted to the desired products. In addition, all Pd-catalyzed borylation methods employing pinacol borane rely upon high quantities of palladium catalyst (>3.0 mol%) in order to efficiently process the aryl halides. Herein, we report a highly active catalyst system based upon PdCl2(CH3CN)2 and SPhos (1) as the supporting ligand for the borylation of aryl and heteroaryl iodides and bromides with pinacol borane. This method not only allows for the use of lower amounts of Pd catalyst with shorter reaction times but also proved general for the borylation of a range of aryl, heteroaryl and vinyl chlorides.
We began our work by examining the optimization of the reaction shown in Table 1. We found that a variety of dialkylbiarylphosphine ligands could be employed to afford highly active catalysts. In general, dicyclohexylphosphino biphenyl ligands resulted in higher conversion and yield for this process as compared to the corresponding diphenyl- or di-tert-butylphosphino compounds. For example, a catalyst based upon 8 allowed for an 86% conversion and yield for the borylation of 4-bromoanisole while 7 or 9 resulted in only 53% and 14% conversion of the aryl halide, respectively. In addition, highly active systems were observed when 3 (Table 1, Entry 3) or 6 (Table 1, Entry 6) was employed. The catalyst system derived from PdCl2(CH3CN)2 and 1, however, produced a near quantitative yield of the aryl boronate ester (Table 1, Entry 1).9
Table 1.
Reaction of 4-Bromoanisole with Pinacol Borane.
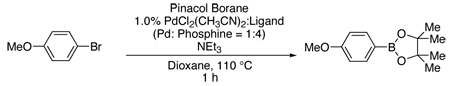 | |||
|---|---|---|---|
| Entry | Ligand | GC Yield (%) | Conversion (%) |
| 1 | 1 | 99 | 100 |
| 2 | 2 | <10 | 28 |
| 3 | 3 | 94 | 100 |
| 4 | 4 | 81 | 87 |
| 5 | 5 | <10 | 15 |
| 6 | 6 | 84 | 100 |
| 7 | 7 | 46 | 53 |
| 8 | 8 | 86 | 86 |
| 9 | 9 | <10 | 14 |
| 10 | 10 | <10 | 16 |
 | |||
Initially, we examined the borylation of aryl iodides. Despite the fact that aryl iodides are more reactive than the corresponding aryl bromides or chlorides, there are no methods, to our knowledge, for their borylation with low catalyst loadings (i.e., <3.0 mol%) or reaction times under one hour. The PdCl2(CH3CN)2/1 combination proved to be highly active in the borylation of 4-iodoanisole producing the desired aryl boronate ester in 94% yield in only 30 minutes (Table 2, Entry 1). The process remained efficient at lower levels of catalyst as the boronate ester was produced in 91% yield after 5 hours when 0.10 mol% Pd was utilized (Table 2, Entry 2).
Table 2.
Pd-Catalyzed Borylation of Aryl Halides with Pinacol Borane.a
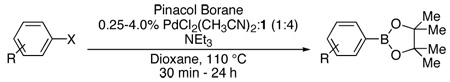 | |||||
|---|---|---|---|---|---|
| Entry | Aryl Halide | Pd (mol%) | Time | Yieldb | |
| 1 | X = I | 1.0 | 30 min | 94 | |
| 2 | X = I | 0.10 | 5 h | 91 | |
| 3 | X = Br | 1.0 | 1 h | 97 | |
| 4 | X = Cl | 3.0 | 24 h | 96c | |
| 5 | 1.0 | 3 h | 85 | ||
| 6 | X = Br | 1.0 | 4 h | 84 | |
| 7 | X = Cl | 3.0 | 24 h | 62c | |
| 8 |  |
1.0 | 5 h | 70 | |
| 9 | 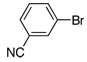 |
1.0 | 3 h | 57 | |
| 10 | 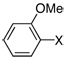 |
X = Br | 2.0 | 4 h | 89 |
| 11 | X = Cl | 4.0 | 24 h | 51c | |
| 12 | 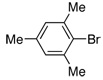 |
2.0 | 4 h | 90 | |
| 13 | 2.0 | 4 h | 68 | ||
| 14 | 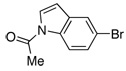 |
2.0 | 4 h | 97 | |
| 15 | 2.0 | 4 h | 74 | ||
| 16 | 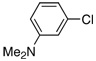 |
3.0 | 24 h | 80c | |
| 17 | 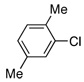 |
3.0 | 24 h | 87c | |
| 18 | 3.0 | 24 h | 59c | ||
| 19 | 3.0 | 24 h | 73c | ||
Reaction Conditions: 1 equiv of aryl or heteroaryl halide, 1.5 equiv of pinacol borane, 3 equiv of NEt3, 1,4-Dioxane (0.60 mL/mmol halide), cat. PdCl2(CH3CN)2, 1:Pd = 4:1.
Isolated yield based upon an average of two runs.
NEt3 (1.00 mL/mmol halide) used as solvent instead of 1,4-Dioxane.
The same catalyst system was highly efficient in the borylation of a range of aryl and heteroaryl bromides. For example, electron-rich aryl bromides, such as 4-bromoanisole (Table 2, Entry 3) and 4-bromo-N,N-dimethylaniline (Table 2, Entry 5), were successfully transformed into the desired pinacol boronate esters in 97% and 85% yield, respectively. Although electron-deficient aryl halides are known to be more challenging substrates for Pd-catalyzed carbon-boron bond-forming processes, 4-bromobenzophenone was smoothly converted to the desired product in five hours of reaction time with 1 mol% Pd (Table 2, Entry 8). Importantly, this method was also applicable to a variety of ortho-substituted aryl bromides as the borylation of the sterically hindered aryl halide, 2-bromomesitylene, resulted in 90% yield of the corresponding aryl boronate ester (Table 2, Entry 12). In addition, a substrate possessing an ortho-functional group, 2-bromoanisole, efficiently furnished the desired product in a high yield (Table 2, Entry 10). The PdCl2(CH3CN)2/1 catalyst system was also suitable in the borylation of a vinyl bromide to produce the alkenyl pinacol boronate in 68% yield (Table 2, Entry 13).10 In addition, heteroaryl bromides were efficiently transformed to the desired products (Table 2, Entries 14–15). This catalyst system offers a general method for the conversion of aryl and vinyl bromides to pinacol boronate ester while maintaining low Pd loadings and minimal reaction times.
Despite the economic advantages of employing aryl chlorides in Pd-catalyzed borylation chemistry, there remains only one report, to our knowledge, of the successful combination of an aryl chloride with pinacol borane.8 However, as previously stated, this method was only applicable to para-substituted electron-rich aryl chlorides (i.e., 4-chloroanisole and 4-chloro-N-methylaniline). We were pleased to discover that the PdCl2(CH3CN)2/1 catalyst system was efficient in the reaction of similar substrates as 4-chloroanisole was successfully converted to the pinacol boronate ester in greater than 95% yield (Table 2, Entry 4).11 Furthermore, this method was also general for a wide range of aryl chlorides as an electron-neutral aryl halide, 4-n-butylchlorobenzene, resulted in a 62% yield of the desired product (Table 2, Entry 7). Although ortho-substituted aryl chlorides have proven to be challenging substrates for a variety of systems, the PdCl2(CH3CN)2/1 catalyst also remained applicable in these couplings as modest to good yields resulted in both cases (Table 2, Entries 11 and 17). In addition, this method represents the only process employing pinacol borane as the boron source by which a heteroaryl chloride (Table 2, Entry 18) or vinyl chloride (Table 2, Entry 19) has been successfully converted to the corresponding pinacol boronate ester. Despite the success of the catalyst system for a variety of aryl chlorides, electron-poor substrates still remain problematic. In general, reactions of these substrates resulted in incomplete conversion of the aryl chloride as well as an increase in reduced arene byproduct.
In summary, we have demonstrated the utility of the PdCl2(CH3CN)2/1 catalyst system in the borylation of a variety of aryl and heteroaryl halides. The reactions of aryl iodides and bromides can be conducted with relatively low Pd loadings and short reaction times while still maintaining a wide substrate scope. In addition, the method represents the first general system for the borylation of aryl and heteroaryl chlorides whereby a range of substrates can be converted to the desired pinacol boronate esters using pinacol borane.
Experimental Section
General Procedure for the Pd-Catalyzed Borylation of Aryl Iodides and Bromides with Pinacol Borane
An oven-dried resealable Schlenk tube possessing a Teflon screw valve was charged with PdCl2(CH3CN)2 (1.0–2.0%) and 1 (4.0–8.0%). The Schlenk tube was capped with a rubber septum and then evacuated and backfilled with argon (this sequence was carried out a total of two times). 1,4-Dioxane (0.30 mL) was added via syringe, through the septum, followed by the addition of the aryl halide (0.50 mmol), NEt3 (0.209 mL, 152 mg, 1.50 mmol) and pinacol borane (0.109 mL, 96 mg, 0.75 mmol) in a like manner (aryl halides that were solids were added with the other solid reagents). The septum was then replaced with a Teflon screw valve and the Schlenk tube was sealed. The reaction mixture was heated to 110 °C until the aryl halide had been completely consumed as determined by gas chromatography and was then allowed to cool to room temperature. The reaction solution was filtered through a thin pad of celite (eluting with ethyl acetate) and the eluent was concentrated under reduced pressure. The crude material so obtained was purified via flash chromatography on silica gel.
General Procedure for the Pd-Catalyzed Borylation of Aryl Chlorides with Pinacol Borane
An oven-dried resealable Schlenk tube possessing a Teflon screw valve was charged with PdCl2(CH3CN)2 (3.0–4.0%) and SPhos (12.0–16.0%). The Schlenk tube was capped with a rubber septum and then evacuated and backfilled with argon (this sequence was carried out a total of two times). NEt3 (0.500 mL) was added via syringe, through the septum, followed by the addition of the aryl chloride (0.50 mmol) and pinacol borane (0.109 mL, 96.1 mg, 0.75 mmol) in a like manner (aryl halides that were solids were added with the other solid reagents). The septum was then replaced with a Teflon screw valve and the Schlenk tube was sealed. The reaction mixture was heated to 110 °C until the aryl halide had been completely consumed as determined by gas chromatography and was then allowed to cool to room temperature. The reaction solution was filtered through a thin pad of celite (eluting with ethyl acetate) and the eluent was concentrated under reduced pressure. The crude material so obtained was purified via flash chromatography on silica gel.
Supplementary Material
Supporting Information Available: Experiment procedures and spectral data for all new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgements
We thank the National Institutes of Health (GM 46059) for funding this work. We are grateful to Merck, Amgen and Boehringer Ingelheim for additional support. The Varian NMR instruments used in this work were purchased with funds from the National Science Foundation (CHE 9808061 and DBI 9729592).
References
- 1.For a review on the applications of aryl boronic acids and esters, see Kotha S, Lahiri K, Kashinath D. Tetrahedron. 2002;58:9633–9695.
- 2.Hall DG. Structure, Properties, and Preparation of Boronic Acid Derivatives. In: Hall DG, editor. Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine. Weinham: VCH; 2005. pp. 1–99. [Google Scholar]
- 3.For a review on transition metal-catalyzed carbon-boron bond formation, see Ishiyama T, Miyaura N. Chem. Rec. 2004;3:271–280. doi: 10.1002/tcr.10068.
- 4.Billingsley K, Barder TE, Buchwald SL. Angew. Chem., Int. Ed. 2007;46:5359–5363. doi: 10.1002/anie.200701551. [DOI] [PubMed] [Google Scholar]
- 5.For other systems employing bis(pinacolato)diboron in Pd-catalyzed borylations, see: Fürstner A, Seidel G. Org. Lett. 2002;4:541–543. doi: 10.1021/ol0171463. Ishiyama T, Ishida K, Miyaura N. Tetrahedron. 2001;57:9813–9815. Ishiyama T, Itoh Y, Kitano Y, Miyaura N. Tetrahedron Lett. 1997;38:3447–3450. Giroux A, Han Y, Prasit P. Tetrahedron Lett. 1997;38:3841–3844.
- 6.For other systems employing pinacol borane in Pd-catalyzed borylations of aryl iodies and bromides, see: Baudoin O, Guénard D, Guéritte F. J. Org. Chem. 2000;65:9268–9271. doi: 10.1021/jo005663d. Murata M, Oyama T, Watanabe S, Masuda Y. J. Org. Chem. 2000;65:164–168. doi: 10.1021/jo991337q. For a system employing pinacol borane in Pd-catalyzed borylations of aryl bromides, see: Broutin P-E, Cerna I, Campaniello M, Leroux F, Colobert F. Org. Lett. 2004;6:4419–4422. doi: 10.1021/ol048303b.
- 7.For a review on Pd-catalyzed coupling reactions of aryl chlorides, see: Littke AF, Fu GC. Angew. Chem., Int. Ed. 2002;41:4176–4211. doi: 10.1002/1521-3773(20021115)41:22<4176::AID-ANIE4176>3.0.CO;2-U.
- 8.Murata M, Sambommatsu T, Watanabe S, Masuda Y. Synlett. 2006;12:1867–1870. [Google Scholar]
- 9.Reactions were conducted at a bath temperature of 110 °C. However, the borylation of aryl iodides and bromides can be conducted at 80 °C, but longer reaction times are necessary. In addition, NBu3 can be substituted for NEt3 for the borylation of aryl iodides and bromides, but the reactions of aryl chlorides proved to be less efficient with NBu3.
- 10.For a method for the borylation of alkenyl iodides and triflates, see: Murata M, Oyama T, Watanabe S, Masuda Y. Synthesis. 2000;6:778–780.
- 11.Although a solvent mixture of 1,4-dioxane and triethylamine was effective for the borylation of aryl iodides and bromides, the reactions of aryl chlorides did not proceed to completion after 24 h under these conditions. However, if the reaction was conducted in just triethylamine as the solvent, then complete conversion of the aryl chloride was observed in all cases.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Available: Experiment procedures and spectral data for all new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.


