Abstract
Fish consumption during gestation can provide the fetus with long chain polyunsaturated fatty acids (LCPUFA) and other nutrients essential for growth and development of the brain. However, fish consumption also exposes the fetus to the neurotoxicant, methyl mercury (MeHg). We studied the association between these fetal exposures and early child development in the Seychelles Child Development Nutrition Study (SCDNS). Specifically, we examined a priori models of Ω-3 and Ω-6 LCPUFA measures in maternal serum to test the hypothesis that these LCPUFA families before or after adjusting for prenatal MeHg exposure would reveal associations with child development assessed by the BSID-II at ages 9 and 30 months. There were 229 children with complete outcome and covariate data available for analysis. At 9 months, the PDI was positively associated with total Ω-3 LCPUFA and negatively associated with the ratio of Ω-6/Ω-3 LCPUFA. These associations were stronger in models adjusted for prenatal MeHg exposure. Secondary models suggested that the MeHg effect at 9 months varied by the ratio of Ω-6/Ω-3 LCPUFA. There were no significant associations between LCPUFA measures and the PDI at 30 months. There were significant adverse associations, however, between prenatal MeHg and the 30 month PDI when the LCPUFA measures were included in the regression analysis. The BSID-II Mental Developmental Index (MDI) was not associated with any exposure variable. These data support the potential importance to child development of prenatal availability of Ω-3 LCPUFA present in fish and of LCPUFA in the overall diet. Furthermore, they indicate that the beneficial effects of LCPUFA can obscure the determination of adverse effects of prenatal MeHg exposure in longitudinal observational studies.
Keywords: Long chain polyunsaturated fatty acids, prenatal methyl mercury, Child Development, Fish Consumption, Maternal Nutritional Status, Seychelles Child Development Study (SCDS)
Introduction
Fish consumption during gestation can provide the fetus with long chain polyunsaturated fatty acids (LCPUFA) and other nutrients essential for growth and development of the brain. However, fish consumption also exposes the fetus to the neurotoxicant, methyl mercury (MeHg). The impact of combined LCPUFA and MeHg from fish consumption during pregnancy may lead to contradictory influences on child development outcomes, as suggested in a companion paper by Davidson and colleagues (in review).
All fish contain small amounts of MeHg. The developmental effects of prenatal exposure to MeHg from maternal consumption of a diet high in fish are unclear. Epidemiological studies from New Zealand (Kjellstrom, et al., 1989) and the Faeroe Islands (Grandjean, et al., 1997; Debes, et al., 2006), reported subtle adverse effects on child development outcomes. In contrast, results from the Seychelles Child Development Study have found no consistent neurodevelopmental or neuropathological impairments (Davidson, et al., 1998; Myers, et al., 2003; Lapham, et al., 1995). In fact, enhanced child development was correlated with increasing maternal hair MeHg levels for some endpoints. This apparently anomalous finding might be related to the beneficial effects of nutrient, such as LCPUFA intake from fish.
The LCPUFA, docosahexaenoic acid (DHA, 22:6 Ω-3) and arachidonic acid (AA, 20:4 Ω-6) accumulate preferentially in the developing brain where they are major structural components of brain lipids and play important functional roles in visual and neural processes (Innis, 2005). The Ω-3 LCPUFA, DHA, can affect neurotransmitter metabolism, ion channel activity, signalling pathways and gene expression, while the Ω-6 LCPUFA, AA, plays a role in cell signalling and synaptic transmission via specific eicosanoids and leukotrienes (Innis 2003). Diffusion to the fetus of LCPUFA from circulating maternal triglycerides and free fatty acids is thought to occur via lipoprotein receptors, lipoprotein lipase activity, placental intracellular lipase activities and plasma membrane fatty acid binding protein (Herrara, 2002). Placental transfer of LCPUFA occurs predominantly during the last trimester when brain growth is most rapid.
The endogenous syntheses of DHA and, especially AA, are not trivial in the preterm infant (Carnielli et al, 2007). However, it appears that the fetus cannot synthesize adequate amounts of DHA or AA from their respective essential fatty acid precursors, alpha-linolenic acid (ALA, 18:3 Ω-3) and linoleic acid (LA 18:2 Ω-6) respectively (Carlson, 2001). Data from randomized controlled trials indicate that maternal supplementation with high dose DHA (>1g/d) can significantly increase DHA status in offspring in contrast to maternal supplementation with lower doses of DHA or high doses (>10g/d) of ALA (Decsi and Koletzko, 2005). One randomized controlled trial evaluated children born to women who took cod liver oil rich in the Ω-3 DHA during pregnancy and compared them with children whose mothers had taken maize oil rich in the Ω6 LA (Helland et al, 2003). Children whose mothers received cod liver oil scored higher on the Mental Processing Composite of the Kaufman Assessment Battery for Children (K-ABC) at age four years. Dunstan et al (2006) showed that children aged 2.5 years born to mothers who were supplemented with Ω-3 LCPUFA from week 20 of gestation until delivery had significantly improved eye and hand coordination compared to those whose mothers received placebo (olive oil). Judge and colleagues (2007) studied infants born to mothers who consumed DHA-containing foods during pregnancy and compared them to infants whose mothers consumed placebo (maize oil) containing foods. Those receiving DHA-containing foods scored better for problem-solving, but not for visual recognition memory, at age 9 months
These experimental studies of supplemental DHA, the Ω-3 LCPUFA found in fish, are supported by observational epidemiological studies. The ALSPAC study in the UK reported that children of women who consumed more fish during pregnancy had higher developmental scores at age15 months (Daniels et al, 2004). More recently they reported that high (>340g per week) maternal seafood consumption was associated with improved child development at age 7 years (Hibbeln et al, 2007). They concluded that guidelines from some advisories to limit seafood consumption during pregnancy could possibly be detrimental. In the US, Oken et al (2005) reported that infants whose mothers had higher fish intake in the second trimester had higher percent novelty preference scores on visual recognition memory testing at 6 months of age.
Neonatal AA status is reported to be less dependent on maternal status than DHA (Otto et al, 1997). This finding fits with the recent experimental confirmation that endogenous AA synthesis from LA in the preterm newborn is significantly higher than DHA synthesis from ALA (Carnielli et al, 2007). Both LA and ALA compete for the same desaturation enzymes in the biosynthetic pathways to AA and DHA respectively. Consequently, the ratio between the relative amounts of Ω-6 and Ω-3 LCPUFA (Ω-6/Ω-3 ratio) might be important in neurodevelopment. The precursor LCPUFA, LA and ALA, are found in varying amounts in different vegetable fats and oils, but preformed AA and DHA are found primarily in animal lipids. Fish is a particularly rich source of DHA and its immediate precursor, eicosapentaenoic acid (EPA, 20:5 Ω-3). Although many studies have indicated a role in neurodevelopment for DHA, we have been unable to find any data linking EPA to neurodevelopment.
Maternal fish consumption brings nutritional benefits, particularly from LCPUFA. However, it also brings possible risk to the developing fetal brain in the form of MeHg, a toxicant known to accumulate in aquatic food chains worldwide (Myers et al, 2003; Clarkson and Strain, 2004). The Food and Agricultural Organization (FAO) of the United Nations estimates that one billion people, most of them living in developing countries, depend on fish as their main protein source (FAO, 2000). This makes fish consumption during pregnancy a subject of scientific interest and public health concern (Cohen et al, 2005; Mozaffarian & Rimm, 2006).
In order to address the risks and benefits of fish consumption, we studied the relationship of maternal LCPUFA and prenatal MeHg exposure to children’s neurodevelopment in a longitudinal observational study in a population consuming large quantities of fish. In a companion paper (Davidson et al, in review), we report on the covariate adjusted effects of two LCPUFA, AA and DHA, and other nutritional and dietary measures on outcome measures. Here we expand our enquiry by focusing on the associations of the Ω-3 and Ω-6 LCPUFA (DHA, AA, EPA, LA, and ALA) with the BSID-II outcomes.
Method
Subjects
We recruited 300 women during their first visit to the ante-natal clinic on Mahé, the main island in Seychelles, during 2001. Inclusion criteria included age over 16 years, native born Seychellois, and residing on Mahé. Six infants were excluded, 4 with major congenital anomalies and 1 set of twins. The study setting and methods are described in detail in the companion paper (Davidson et al., in review). We include only those methods pertinent to the secondary analysis reported here. The study was reviewed and approved by the Seychelles Ethics Board, and the Research Subjects Review Boards at the University of Rochester and Cornell University.
Blood Collection
At 28 weeks gestation and one day after delivery, non-fasting blood samples (30 ml) were taken from mothers. Samples were collected by antecubital venipuncture into evacuated serum tubes. Samples were placed on water ice and allowed to sit for 30 minutes prior to being centrifuged at 2500 rpm for 15 minutes. Aliquots were stored at −80°C until analysis.
Fatty acid analyses
Total lipids were extracted from serum samples using a modified method of Folch et al. (1957). Two internal standards (Sigma Aldrich Co Ltd, Poole, Dorset, UK), heptadecaenoic acid (C17:0) and heneicosanaenoic (C21:0) were added to the samples prior to extraction. Lipid extracts were methylated with boron trifluoride in methanol (Sigma Aldrich Co Ltd) and fatty acid methyl esters were analyzed and quantified using a ThermoFinnegan TRACE MS with Xcalibur software (ThermoFinnegan, Hemel Hempstead, UK). This equipment has a 30m FAMEWAX (Restek Ireland, Belfast, UK) capillary column with an internal diameter of 0.25mm and a 0.25-µm film thickness.
Developmental Assessment
The children were evaluated at four ages (5, 9, 25, and 30 months). The test battery had a total of 16 developmental endpoints, but this secondary study focuses on the primary one, the BSID-II that was administered at ages 9 and 30 months by specially trained evaluators (Bayley, 1993). The BSID-II is a well standardized measure of infant cognition and development yielding two primary endpoints, a Mental Developmental Index (MDI) and a Psychomotor Developmental Index (PDI). The second author (PWD) completed reliabilities on 5% of the BSID-II tests and mean agreements ranged from 92.3 to 98%.
Analysis Plan
Our focus in this analysis was on the Ω-3 and Ω-6 fatty acids and models are not adjusted for other nutrition variables. We compared results with and without adjustment for MeHg. In a companion paper (Davidson et al, in review) we report on the associations of DHA, AA, and other nutritional variables on the BSID-II and the other outcomes measured. In those models we adjusted for 4 additional nutritional measures, TSH, iron, choline, and fish intake. These were not considered here.
All models were adjusted for the same covariates known to be associated with child development as reported by Davidson and colleagues for other analyses of this data set (in review). All analyses for each endpoint followed the same procedures.
For this analysis we considered five basic models. Model 1 adjusted for DHA and AA, the primary LCPUFA associated with neurodevelopment. This model differed from the companion paper in that we did not adjust for the other 4 nutrition variables here. Model 2 adjusted for the sum of DHA and EPA (as a measure of the Ω-3 LCPUFA found in fish), and AA. Model 3 adjusted for Ω-3 (the sum of DHA, EPA, and ALA) and Ω-6 (the sum of AA and LA) LCPUFA. Models 4 and 5 adjusted for ratios of AA to DHA and of Ω-6 to Ω-3 respectively. We also fit secondary models that added MeHg by LCPUFA interactions to models 4 and 5. In these secondary models, LCPUFA were included as indicator variables to distinguish between tertiles of their sample distribution.
Each of the five LCPUFA measurements was the geometric mean of the maternal serum value measured at 28-weeks gestation and at delivery as transfer of LCPUFA to the fetus occurs mainly during the third trimester. Approximately 8% of the 28-week values and approximately 20% of the delivery values were not observed. We imputed missing LCPUFA at a single time point as described in Davidson et al., (in review) which also gives further details of the regression analyses.
Results
In this cohort of 229 mother-infant pairs, the mean maternal hair Hg concentration was 5.7 µg/g (SD = 3.7; range 0.2–18.5). Mothers reported consuming an average of 9 meals containing fish per week or an estimated 537 g of fish per week. Table 1 shows the characteristics of the mothers and children studied. Table 2 shows summary statistics for the LCPUFA status of mothers and the BSID outcomes for the children. There were no mothers in this cohort with evidence of clinical nutritional deficiencies. Table 3 gives the correlations between MeHg and each LCPUFA used in these models.
Table 1.
Characteristics of 229 pairs of mothers at recruitment and neonates at delivery
| Mean | SD | Range | |
|---|---|---|---|
| Mothers | |||
| ( 6 Smokers, 222 nonsmokers, 1 NA) | |||
| Age (years) | 27.2 | 5.9 | 16–43 |
| Height*(m) | 1.6 | 0.7 | 1.3–1.8 |
| Weight (kg) | 66.4 | 16.9 | 38–135 |
| Neonates | |||
| (113 males, 116 females) | |||
| Weight (g) | 3239 | 471 | 1870–4450 |
| Length (cm) | 50.7 | 3.3 | 31–62 |
| Gestation age at delivery (weeks) | 38.7 | 1.3 | 34–41 |
one mother was missing height measurement at enrollment
Table 2.
Summary statistics for MeHg, LCPUFA Status among Cohort Mothers ( as measured in maternal serum)* and BSID-II testing on the children
| Variable | N | Mean | SD | Range |
|---|---|---|---|---|
| MeHg (measured in mothers hair in ppm) | 229 | 5.7 | 3.7 | 0.2–18.5 |
| Docosahexaenoic acid mg/ml (DHA) | ||||
| 28 weeks | 216 | 0.19 | 0.06 | 0.07–0.40 |
| Delivery | 183 | 0.16 | 0.06 | 0.06–0.34 |
| mean of 28 weeks and delivery | 229 | 0.17 | 0.05 | 0.07–0.32 |
| Arachidonic acid mg/ml (AA) | ||||
| 28 weeks | 216 | 0.63 | 0.14 | 0.36–1.23 |
| Delivery | 184 | 0.60 | 0.15 | 0.33–1.22 |
| mean of 28 weeks and delivery | 229 | 0.61 | 0.13 | 0.37–1.07 |
| Eicosapentaenoic acid mg/ml (EPA) | ||||
| 28 weeks | 217 | 0.02 | 0.01 | 0.00–0.09 |
| Delivery | 182 | 0.02 | 0.01 | 0.00–0.06 |
| mean of 28 weeks and delivery | 229 | 0.02 | 0.01 | 0.00–0.05 |
| Alpha-Linolenic acid mg/ml (ALA) | ||||
| 28 weeks | 216 | 0.01 | 0.01 | 0.00–0.12 |
| Delivery | 185 | 0.01 | 0.01 | 0.00–0.08 |
| mean of 28 weeks and delivery | 229 | 0.01 | 0.00 | 0.00–0.05 |
| Linoleic acid mg/ml (LA) | ||||
| 28 weeks | 216 | 7.3 | 1.4 | 3.29–11.44 |
| Delivery | 185 | 6.7 | 1.37 | 2.94–10.15 |
| mean of 28 weeks and delivery | 229 | 7.0 | 1.22 | 3.73–9.78 |
| AA/DHA ratio | 229 | 3.7 | 0.86 | 1.75–7.09 |
| Ω 3 (DHA, EPA, ALA mg/ml) | 229 | 0.20 | 0.06 | 0.08–0.36 |
| Ω 6 (AA, LA mg/ml) | 229 | 7.61 | 1.28 | 4.10–10.77 |
| Ω 6/ Ω 3 ratio | 229 | 40.17 | 11.72 | 13.21–90.35 |
| BSID-II MDI 9 months | 229 | 102.7 | 9.8 | 64–150 |
| BSID-II PDI 9 months | 226 | 102.4 | 16.4 | 50–141 |
| BSID-II MDI 30 months | 225 | 86.1 | 9.1 | 56–115 |
| BSID-II PDI 30 months | 228 | 91.4 | 13.7 | 50–123 |
LCPUFAs were measured in maternal blood at 28 weeks and delivery, and the geometric mean of the two measurements of each LCUPFA was used in the analysis.
If a mother had a measurement at only one time point, the missing measurement was estimated as described in the text.
BSID-II = Bayley Scales of Infant Development II
Table 3.
Correlation of Mercury and LCPUFA Measures
| AA | 0.07 |
| DHA | 0.32 |
| DHA + EPA | 0.31 |
| Ω-3 | 0.31 |
| Ω -6 | 0.10 |
| AA/DHA | −0.31 |
| Ω -6/Ω -3 | −0.23 |
The regression coefficients and their associated p-values for the covariate-adjusted relationships between the LCPUFA and the PDI and MDI from the BSID-II (with and without adjustment for MeHg) are shown in Table 4. Models results are given with and without outliers. No model had more than 3 outliers. None of the models had influential points, and no transformations were required. All variance inflation factors were less than 2, indicating that collinearity between model covariates was not an issue. At both 9 and 30 months, the associations with the PDI outcomes were stronger when outliers were excluded and weaker when the models did not adjust for MeHg.
Table 4.
Estimated regression coefficients and p-values for long chain polyunsaturated fatty acids (LCPUFA) and mercury variables against Bayley Scale of Infant Development II: Psychomotor Developmental Index (PDI) at 9 and 30 months of age
| 9 Month PDI | 30 Month PDI | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| all data | excluding outliers | all data | excluding outliers | |||||||||||||
| Hg + LCPUFA | LCPUFA only | Hg + LCPUFA | LCPUFA only | Hg + LCPUFA | LCPUFA only | Hg + LCPUFA | LCPUFA only | |||||||||
| coeff | p-value | coeff | p-value | coeff | p-value | coeff | p-value | coeff | p-value | coeff | p-value | coeff | p-value | coeff | p-value | |
| Model 1 | ||||||||||||||||
| mercury | −0.19 | 0.35 | - | - | −0.18 | 0.33 | - | - | −0.51 | 0.05 | - | - | −0.55 | 0.03 | ||
| DHA | 17.48 | 0.37 | 10.99 | 0.55 | 18.79 | 0.31 | 12.59 | 0.47 | 21.01 | 0.41 | 3.38 | 0.89 | 24.70 | 0.33 | no outliers | |
| AA | 8.18 | 0.24 | 9.35 | 0.17 | 10.10 | 0.13 | 11.17 | 0.09 | −6.03 | 0.51 | −2.96 | 0.74 | −9.13 | 0.31 | ||
| Model 2 | ||||||||||||||||
| mercury | −0.21 | 0.30 | - | - | −0.20 | 0.30 | - | - | −0.53 | 0.04 | - | - | −0.57 | 0.03 | ||
| EPA+DHA | 20.81 | 0.23 | 14.71 | 0.36 | 20.80 | 0.20 | 27.31 | 0.08 | 23.65 | 0.29 | 8.09 | 0.70 | 26.73 | 0.22 | ||
| - | no outliers | |||||||||||||||
| AA | 6.84 | 0.32 | 8.07 | 0.24 | 9.03 | 0.20 | 5.66 | 0.39 | −7.28 | 0.42 | −4.29 | 0.63 | 10.34 | 0.25 | ||
| Model 3 | ||||||||||||||||
| mercury | −0.24 | 0.21 | - | - | −0.20 | 0.25 | - | - | −0.51 | 0.05 | - | - | ||||
| Ω 3 | 33.26 | 0.02 | 28.26 | 0.03 | 43.25 | 0.001 | 39.43 | 0.002 | 18.02 | 0.32 | 7.44 | 0.67 | no outliers | no outliers | ||
| Ω 6 | −0.13 | 0.82 | −0.11 | 0.85 | −0.40 | 0.47 | −0.40 | 0.47 | −0.55 | 0.47 | −0.51 | 0.50 | ||||
| Model 4 | ||||||||||||||||
| mercury | −0.13 | 0.51 | - | - | −0.12 | 0.53 | - | - | −0.48 | 0.06 | - | - | no outliers | no outliers | ||
| AA/DHA | −0.45 | 0.60 | −0.27 | 0.74 | −0.54 | 0.50 | −0.39 | 0.61 | −0.54 | 0.62 | 0.09 | 0.93 | ||||
| Model 5 | ||||||||||||||||
| mercury | −0.20 | 0.28 | - | - | -0.14 | 0.42 | - | - | −0.52 | 0.04 | - | - | no outliers | no outliers | ||
| Ω 6/ Ω 3 | −0.15 | 0.02 | −0.13 | 0.03 | −0.17 | 0.001 | −0.16 | 0.005 | −0.10 | 0.19 | −0.06 | 0.41 | ||||
NS = Not significant
AA = Arachidonic acid
Ω 6 = AA + LA
Ω 3 = DHA + EPA + ALA
DHA = Docosahexaenoic acid
EPA = Eicosapentaenoic acid
Nine Months
In model 1, MeHg, AA, and DHA were not significant predictors of MDI or PDI scores. These results were similar to those reported by Davidson and colleagues (in review) whose analyses included DHA, AA and four other nutrient status indicators. Models 2–5 using various combinations of LCPUFA metrics showed no significant association of prenatal MeHg exposure with the PDI or MDI.
Replacing DHA (used in Model 1) with DHA+EPA (Model 2) had little effect on the model results for PDI or MDI. However, model 3 replacing DHA with Ω-3 LCPUFA and AA with Ω-6 LCPUFA gave results that differed substantially from Model 1 for the PDI, but not for the MDI. Greater Ω-3 LCPUFA values were associated with significantly improved 9-month PDI scores (p = 0.02), but neither Ω-6 LCPUFA nor MeHg were significant predictors in this model. Without adjustment for MeHg, Ω-3 LCPUFA continued to be a significant predictor of the PDI. However, the regression coefficient was somewhat closer to zero, indicating a weaker association.
Models 4 and 5 each adjusted for a single LCPUFA index, namely the AA/DHA ratio (Model 4) and the Ω-6/Ω-3 ratio (Model 5). In model 4 the AA/DHA ratio was not a significant predictor of the PDI. However, in model 5 larger values of the Ω-6/Ω-3 ratio were associated with significantly lower PDI scores (p=.02). Without adjustment for MeHg, the Ω-6/Ω-3 ratio remained a significant predictor of the PDI (p=.03).
Thirty months
In model 1, prenatal MeHg was associated with a significantly lower PDI score (p=0.05), but not a lower MDI score. The MeHg effect in this model was significant only when adjustment was made for DHA and AA, a finding similar to that found by Davidson and colleagues (in review). Neither AA nor DHA were significant predictors of either outcome in this model. In Models 2–5, prenatal MeHg was a significant (p<0.05) or borderline significant (p=0.06) predictor of the PDI, but there was no association with the MDI. This finding is consistent with Model 1. In models 2– 5 none of the LCPUFA was significant predictors of the PDI or MDI. The sign of the regression coefficients for Ω-3 and Ω-6 LCPUFA (model 3), AA/DHA ratio (model 4) and Ω-3/Ω-6 ratio (model 5) were the same as for the 9-month PDI. Compared to the 9-month results, the magnitude of the regression coefficients were generally, but not always, closer to zero suggesting a weaker association.
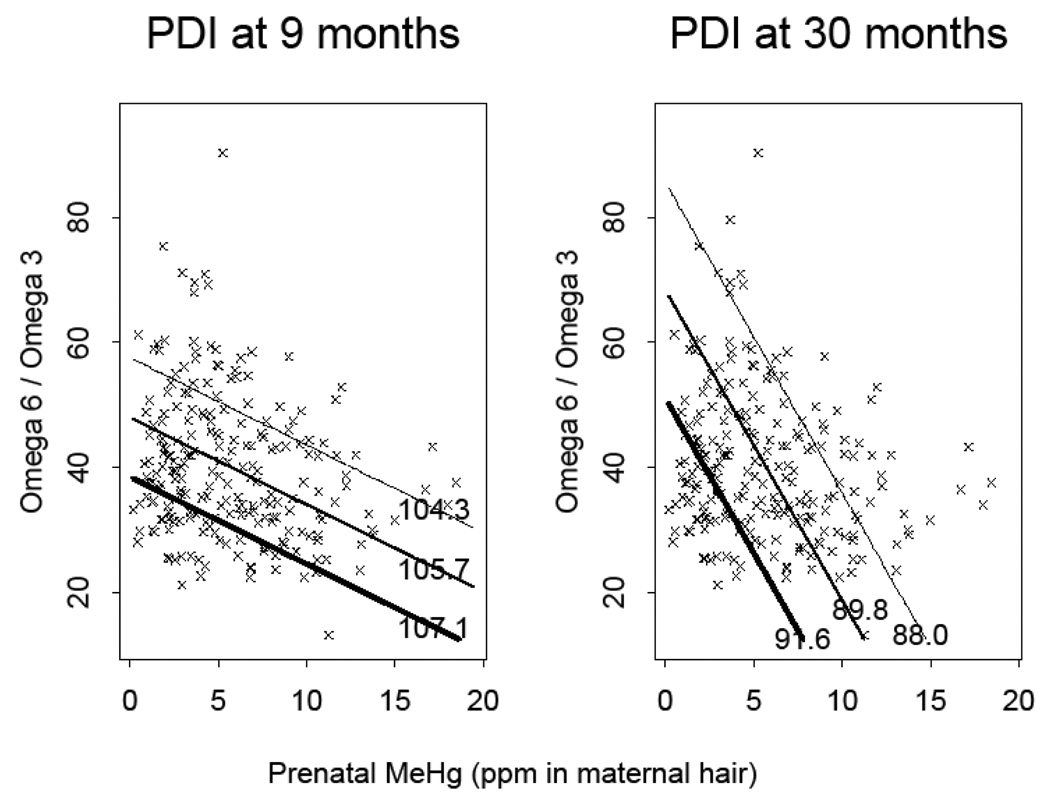
Figure 1 depicts key relationships from model 5. The crosses shown in both panels are the observed values of the Ω-6/Ω-3 ratio (y-axis) and the observed values of MeHg (x-axis). These two variables are negatively correlated (r=−0.23). Also shown are contours of estimated PDI, at 9 months (left panel) and at 30 months (right panel), shown as labeled diagonal lines. The centermost (and outer) lines show the mean (and +/− 2 times the standard error) estimated PDI values. Thicker lines correspond to larger values of estimated PDI, as labeled in the plot interior. Each plot can be thought of as a 3-dimensional plot of the observed Ω-6/Ω-3 ratio, the observed MeHg, and the estimated PDI. The estimated values of the PDI are depicted as contours of constant values. The value of the estimated PDI increases when moving from one contour line to another contour line with a larger value.
Figure 1.
Joint Effects of Prenatal MeHg and the Ω-6/Ω-3 Ratio on Estimated PDI at 9 months (left panel) and at 30 months (right panel). The points are observed values of MeHg, and the Ω-6/Ω-3 ratio. The solid lines are contours of constant PDI as estimated from the Model 5 regression at 9 months (left panel) or 30 months (right panel). Thicker lines correspond to larger values of estimated PDI. The center-most solid diagonal line in each plot is the contour corresponding to the mean PDI at the corresponding age, and shows the values of MeHg and the Ω-6/Ω-3 ratio at which the estimated PDI is predicted to be constant at its mean value. The other solid lines correspond to constant values of PDI, for PDI at its mean plus or minus twice its standard error from the model.
These plots suggest that as MeHg increased, a lower Ω-6/Ω-3 ratio was required to keep the predicted PDI at the same level. Conversely, as the Ω-6/Ω-3 ratio increased, the plots indicate that a smaller value of MeHg was required to keep the estimated PDI at the same level. These suggested relationships held at both 9 months and 30 months despite the fact that at 9 months the Ω-6/Ω-3 ratio was significant but MeHg was not, and at 30 months MeHg was significant but the Ω-6/Ω-3 ratio was not.
These models and figures assume that there is no interaction between MeHg and LCPUFA. In our secondary analyses we included interactions between MeHg and LCPUFA tertiles corresponding to Models 4 and 5. There was no evidence of interactions at 30 months (p > 0.50 for all interaction terms), but there was at 9 months. In the expanded version of Model 4, the MeHg* Ω-3 interaction was of borderline significance (p=0.09), whereas the MeHg* Ω-6 interaction was not (p=0.72). In the expanded version of Model 5, the MeHg* Ω-6/Ω-3 ratio was significant (p=0.03). In this model, as the tertiles of the Ω-6/Ω-3 ratio increased, the MeHg effect became more negative. For subjects in the highest Ω-6/Ω-3 tertile, the MeHg slope was −1.26 (p=0.004).
Discussion
We found that maternal serum Ω-3 LCPUFA as measured during the last trimester was positively associated with the psychomotor development measured at 9 months of age As maternal values for Ω-3 increased, the PDI scores improved. Psychomotor development was also inversely related to the Ω-6/Ω-3 ratio. As the Ω-6/Ω-3 ratio increased the PDI scores declined. There were no significant associations with the MDI at 9 or 30 months or with the PDI at the 30 month evaluation. The associations we found were strongest when prenatal MeHg exposure was included in the analyses.
An association of Ω-3 LCPUFA with improved development is consistent with experimental findings that have reported improved development when mothers were supplemented with Ω-3 LCPUFA (Helland et al., 2003; Dunstan et al., 2006; Judge et al., 2007; Colombo et al, 2004) or when preterm infants were supplemented with DHA (O’Connor et al, 2001). Cohen et al (2005) have estimated that increasing maternal DHA intake by 100 mg/day during pregnancy would increase a child’s IQ by 0.13 points. In the current study we did not find any associations with the cognitive index (MDI) of the BSID-II, but we did find a significant association with the PDI, a test of psychomotor skills. The PDI is considered to be the gold standard for measuring both fine and gross motor development of children aged less than four years, but it is less related to cognitive processes (such as attention, memory, inhibition, or higher-order functions) in which LCPUFA have been implicated (Cheatham et al, 2006). Why the associations in this study appeared only with the PDI is not clear.
In the current study, DHA or DHA and EPA combined (LCPUFA that predominantly come from maternal intakes of fish and other seafood) were not associated with the PDI. Maternal serum total Ω-3 LCPUFA (in model 3 that included total Ω-6 LCPUFA), however, showed an association. These findings might be explained if ALA, the precursor of DHA and EPA contributed substantially to overall Ω-3 LCPUFA requirements in pregnancy. Such a possibility, if substantiated by further studies, would be a matter of public health importance as the dietary sources of ALA are more plentiful than DHA and EPA. Although de Groot (2004) found that maternal supplementation with ALA and LA did not increase neonatal DHA and AA status, their study did suggest that functional DHA status was improved.
The negative association we found between the maternal Ω-6/Ω-3 LCPUFA ratio and the PDI outcome at 9 months suggests that Ω-6 LCPUFA may attenuate the positive effects of the Ω-3 LCPUFA. The Ω-6/Ω-3 LCPUFA ratio has been implicated in numerous brain related functions (Yehuda, 2003). A high dietary Ω-6/Ω-3 LCPUFA ratio, which in part determines the tissue ratio of these LCPUFA, has been purported to promote the pathogenesis of many chronic diseases (Simopoulos, 2006). Other authors (Hibbeln et al, 2006) have indicated that high intakes of LA, the major Ω-6 LCPUFA in the diet, may increase Ω-3 LCPUFA requirements needed to prevent such diseases. Our results also suggested that the combined impact of MeHg and the Ω-6/Ω-3 LCPUFA ratio on the PDI changed from 9 to 30 months; the effect of MeHg on the PDI at 9 months, but not at 30 months, depends on the Ω-6/Ω-3 ratio. These effects have not previously been reported and deserve further study.
We anticipated that nutritional factors might modify the neurotoxic action of MeHg in high fish-eating populations (Clarkson & Strain, 2003) and postulated that not adjusting for nutritional confounders may have masked detrimental effects of MeHg on neurodevelopment. Other authors have reported improved performance (Oken et al., 2005; Daniels et al, 2004; Hibbeln et al., 2006) associated with dietary measures of fish consumption rather than biological measures of nutritional exposure.
Our findings support the view that because of confounding by the adverse effect of MeHg, the beneficial effect of Ω-3 LCPUFA and other nutritional factors from fish is likely to be underestimated. Associations between maternal measures of LCPUFA and outcome were strengthened when the confounding factor of prenatal exposure to MeHg was adjusted for in the regression models. Our findings are also pertinent to the ongoing debate on the risks and benefits of fish consumption during pregnancy and support the importance of LCPUFA in the diet. Other investigators have reviewed the data on fish consumption and reached similar conclusions (Cohen et al, 2005; Mozaffarian & Rimm, 2006).
Our study has a number of strengths. The subjects were enrolled early in pregnancy, followed sequentially, and a number of biological parameters were measured at several time points. However, the current analyses focused on only the Ω-3 and Ω-6 LCPUFA and the four primary endpoints on the BSID-II at 9 and 30 months. Further analyses with other nutritional confounders might have produced different results (Strain et al, 2004; Georgieff, 2007). In addition the study population was not as large as originally planned and may be underpowered. Although we analyzed the data based on known biological relationships, the large number of analyses may have resulted in chance findings.
This study was not designed to consider the influence of postnatal exposures to either MeHg or nutrients. Further, previous work has indicated that prenatal and postnatal MeHg levels are not correlated (r=0.08, Myers, et al., 2003). This issue is discussed in greater detail by Davidson and colleagues (in review).
The effects of LCPUFA are largely prenatal. Randomized controlled trials have indicated the benefits of LCPUFA taken by mothers during pregnancy (Helland et al., 2003; Dunstan et al., 2006; Judge et al., 2007; Colombo et al, 2004). In contrast, the evidence for postnatal effects of LCPUFA supplementation on infant development is contradictory at best. The three largest randomized controlled trials were undertaken in the UK (Lucas et al, 1999), in four centers in the USA (Auestad et al, 2003) and in The Netherlands (Bouwestra et al, 2005), and involved some 879 term infants. Those studies used a variety of neural and visual endpoints, and showed no advantage of supplementation over standard formula.
In conclusion, we found significant associations between different measures of LCPUFA and child development measured by the BSID-II. Although no inferences can be drawn on causality from this study, the significant associations are consistent with experimental findings. These data highlight the potential importance to child development of the prenatal availability of Ω-3 LCPUFA from fish and the overall diet and also indicate possible attenuation of such effects by both Ω-6 LCPUFA and MeHg. We found potential adverse associations between MeHg and outcome that were uncovered only when LCPUFA were included in the regression analyses. The data indicate that confounding of adverse effects of MeHg by nutrients, or conversely, confounding of beneficial effects of LCPUFA by MeHg, should be considered when evaluating data from observational studies.
Acknowledgements
This study was supported exclusively by grants 5-R01-ES010219 and 2-T32-ES007271 from the US National Institute of Environmental Health Sciences, National Institutes of Health, and by the Government of Seychelles. No author had any conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auestad N, Scott DT, Janowsky JS, et al. Visual, cognitive, and language assessments at 39 months: a follow-up study of children fed formulas containing long-chain polyunsaturated fatty acids to 1 year of age. Pediatrics. 2003;112:E177–E183. doi: 10.1542/peds.112.3.e177. [DOI] [PubMed] [Google Scholar]
- Bouwestra H, Dijck-Brouwer DA, Boehm G, et al. Long-chain polyunsaturated fatty acids and neurological developmental outcome at 18 months in healthy term infants. Acta Paediatr. 2005;94:26–32. doi: 10.1111/j.1651-2227.2005.tb01783.x. [DOI] [PubMed] [Google Scholar]
- Carnielli VP, Simonata M, Verlato G, et al. Synthesis of long-chain polyunsaturated fatty acids in preterm infants fed formula with pong chain polyunsaturated fatty acids. Am J Clin Nutr. 2007;86:1323–1330. doi: 10.1093/ajcn/86.5.1323. [DOI] [PubMed] [Google Scholar]
- Carlson SE. Docosahexaenoic acid and arachidonic acid in infant development. Semin Neonatal. 2001;6:437–449. doi: 10.1053/siny.2001.0093. [DOI] [PubMed] [Google Scholar]
- Cheatham CL, Colombo J, Carlson SE. N-3 fatty acids and cognitive and visual acuity development: methodologic and conceptual considerations. Am J Clin Nutr. 2006;83:1458S–1466S. doi: 10.1093/ajcn/83.6.1458S. [DOI] [PubMed] [Google Scholar]
- Clarkson TW, Strain JJ. Methyl mercury: loaves versus fishes. Seychelles Med Dent J. 2004;7:61–66. doi: 10.1016/j.neuro.2020.09.018. [DOI] [PubMed] [Google Scholar]
- Cohen JT, Bellinger DC, Connor WE, et al. A quantitative risk-benefit analysis of changes in population fish consumption. Am J Prev Med. 2005a;29:325–334. doi: 10.1016/j.amepre.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Cohen JT, Bellinger DC, Connor WE, Shaywitz BA. A quantitative analysis of prenatal intake of n-3 polyunsaturated fatty acids and cognitive development. Am J Prev Med. 2005b;29:366–374. doi: 10.1016/j.amepre.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Colombo J, Kannass KN, Shaddy DJ, et al. Maternal DHA and the development of attention in infancy and toddlerhood. Child Dev. 2004;75:1254–1267. doi: 10.1111/j.1467-8624.2004.00737.x. [DOI] [PubMed] [Google Scholar]
- Daniels JL, Longnecker MP, Rowland AS, Golding J. Fish intake during pregnancy and early cognitive development of offspring. Epidemiology. 2004;15:394–402. doi: 10.1097/01.ede.0000129514.46451.ce. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Strain JJ, Myers GJ, Thurston SW, Bonham MP, Shamlaye CF, Stokes-Riner A, Wallace JMW, Robson PJ, Duffy EM, Georger LA, Sloane-Reeves J, Cernichiari E, Canfield RL, Cox C, Huang L-S, Janciuras J, Clarkson TW. Neurodevelopmental Effects of Maternal Nutritional Status and Exposure to Methylmercury from Eating Fish during Pregnancy. NeuroToxicol. doi: 10.1016/j.neuro.2008.06.001. In Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PW, Myers GJ, Cox C, et al. Effects of prenatal and postnatal methyl mercury exposure from fish consumption on neurodevelopment: outcomes at 66 months of age in the Seychelles Child Development Study. JAMA. 1998;280:701–707. doi: 10.1001/jama.280.8.701. [DOI] [PubMed] [Google Scholar]
- Debes F, Budtz-Jørgensen E, Weihe P, et al. Impact of prenatal methylmercury exposure on neurobehavioral function at the age of 14 years. Neurotoxicol Teratol. 2006;28:363–375. doi: 10.1016/j.ntt.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decsi T, Koletzko B. N-3 fatty acids and pregnancy outcomes. Curr Opin Clin Nutr Metab Care 2005; 8: 161-6Innis SM. Essential fatty acid transfer and fetal development. Placenta. 2005;26:S69–S75. doi: 10.1097/00075197-200503000-00009. [DOI] [PubMed] [Google Scholar]
- de Groot RHM, Homstra G, van Houwelingen AC, Roumen F. Effect of α-linolenic acid supplementation during pregnancy on maternal and neonatal polyunsaturated fatty acid status and pregnancy outcome. Am J Clin Nutr. 2004;79:251–260. doi: 10.1093/ajcn/79.2.251. [DOI] [PubMed] [Google Scholar]
- Dunstan JA, Simmer K, Dixon G, Prescott SL. Cognitive assessment of children at age 2.5 years after maternal fish oil supplementation in pregnancy: a randomized controlled trial. Archives of Diseases in Childhood, Fetal Neonatal Ed. 2006 Dec 21; doi: 10.1136/adc.2006.099085. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2000. Geneva: FAO; 2000. [Google Scholar]
- Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr. 2007;85 suppl:614S–620S. doi: 10.1093/ajcn/85.2.614S. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White R, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997;19:417–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very long chain n-3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics. 2003;111:E39–E44. doi: 10.1542/peds.111.1.e39. [DOI] [PubMed] [Google Scholar]
- Herrera E. Implications of dietary fatty acids during pregnancy on placental, fetal and postnatal development – A review. Placenta. 2002;23:S9–S19. doi: 10.1053/plac.2002.0771. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR, Nieminen LRG, Blasbalg TL, Riggs JA, Lands EM. Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. Am J Clin Nutr. 2006;83:1483S–1493S. doi: 10.1093/ajcn/83.6.1483S. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, Golding J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007;269:578–585. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- Innis SM. Prenatal biochemistry and physiology of long-chain polyunsaturated fatty acids. J Pediatr. 2003;143:S1–S8. doi: 10.1067/s0022-3476(03)00396-2. [DOI] [PubMed] [Google Scholar]
- Innis SM. Essential fatty acid transfer and fetal development. Placenta. 2005 Suppl A:S70–S75. doi: 10.1016/j.placenta.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Judge MP, Harel O, Lammi-Keefe CJ. Maternal consumption of a docosahexaenoic acid-containing functional food during pregnancy: benefit for infant performance on problem-solving but not on recognition memory tasks at age 9 months. Am J Clin Nutr. 2007;85:1572–1577. doi: 10.1093/ajcn/85.6.1572. [DOI] [PubMed] [Google Scholar]
- Kjellstrom T, Kennedy P, Wallis S, et al. National Swedish Environmental board Report 3642. Sweden: Solna; 1989. Physical and mental development of children with prenatal exposure to mercury from fish. Stage 2. Interviews and psychological tests at age 6. [Google Scholar]
- Lapham LW, Cernichiari E, Cox C, et al. An analysis of autopsy brain tissue from infants prenatally exposed to methylmercury. NeuroToxicol. 1995;16:689–704. [PubMed] [Google Scholar]
- Lucas A, Stafford M, Morley R, et al. Efficacy and safety of long-chain polyunsaturated fatty acid supplementation of infant-formula milk: a randomized trial. Lancet. 1999;354:1948–1954. doi: 10.1016/S0140-6736(99)02314-4. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Rimm EB. Fish intake, contaminants and human health: evaluating the risks and benefits. JAMA. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Cox C, et al. Prenatal methyl-mercury exposure from ocean fish consumption in the Seychelles Child Development Study. Lancet. 2003;361:1686–1692. doi: 10.1016/S0140-6736(03)13371-5. [DOI] [PubMed] [Google Scholar]
- Oken E, Wright RO, Kleinman KP, et al. Maternal fish consumption, hair mercury and infant cognition in a US cohort. Environ Health Perspect. 2005;113:1376–1380. doi: 10.1289/ehp.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto SJ, van Houwelingen AC, Antal M, et al. Maternal and neonatal essential fatty acid status in phospholipids: An international comparative study. Eur J Clin Nutr. 1997;51:232–242. doi: 10.1038/sj.ejcn.1600390. [DOI] [PubMed] [Google Scholar]
- O’Connor DL, Hall R, Adamkin D, et al. Growth and development in preterm infants fed long-chain polyunsaturated fatty acids: a prospective, randomized controlled trial. Pediatrics. 2001;108:359–371. doi: 10.1542/peds.108.2.359. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic disease. Biomed Pharmacother. 2006;60:502–507. doi: 10.1016/j.biopha.2006.07.080. [DOI] [PubMed] [Google Scholar]
- Strain JJ, Bonham MP, Duffy EM, et al. Nutrition and neurodevelopment: the search for candidate nutrients in the Seychelles Child Development Nutrition Study. Seychelles Med Dent J. 2004;7:77–83. doi: 10.1016/j.neuro.2020.09.021. [DOI] [PubMed] [Google Scholar]
- Yehuda S. Omega-6/omega-3 ratio and brain-related functions. In: Simopoulos AP, Cleland LG, editors. Omega-6/omega-3 essential fatty acid ratio: the scientific evidence. World rev nutr diet. vol 92. Karger: Basel; 2003. pp. 37–56. [DOI] [PubMed] [Google Scholar]