Abstract
Background and Purpose
Studies on adult stroke patients have demonstrated functional changes in cortical excitability, metabolic rate, or blood flow after motor therapy, measures that can fluctuate rapidly over time. This study evaluated whether evidence could also be found for structural brain changes during an efficacious rehabilitation program.
Methods
Chronic stroke patients were randomly assigned to receive either constraint-induced movement therapy (n=16) or a comparison therapy (n=20). Longitudinal voxel-based morphometry was performed on structural MRI scans obtained immediately before and after patients received therapy.
Results
The group receiving constraint-induced movement therapy exhibited far greater improvement in use of the more affected arm in the life situation than the comparison therapy group. Structural brain changes paralleled these improvements in spontaneous use of the more impaired arm for activities of daily living. There were profuse increases in gray matter in sensory and motor areas both contralateral and ipsilateral to the affected arm that were bilaterally symmetrical, as well as bilaterally in the hippocampus. In contrast, the comparison therapy group failed to show gray matter increases. Importantly, the magnitude of the observed gray matter increases was significantly correlated with amount of improvement in real-world arm use.
Conclusions
These findings suggest that a previously overlooked type of brain plasticity, structural remodeling of the human brain, is harnessed by constraint-induced movement therapy for a condition once thought to be refractory to treatment: motor deficit in chronic stroke patients.
Keywords: constraint-induced movement therapy, hemiplegia, imaging, motor activity, MRI, stroke rehabilitation, voxel-based morphometry
Merzenich et al1 and other investigators2 showed in animals that altering behaviorally relevant afferent input to the central nervous system can produce plastic changes in the function and organization of the brain. Sustained increased use of a body part by an animal leads to an increase in the brain's cortical representation of that body part,3 whereas decreased input reduces the representational zone of that body part, as occurs after amputation of a digit1 or somatosensory deafferentation of an entire forelimb in monkeys.4 Similar phenomena have been demonstrated in humans after both increased use5 and decreased use resulting from upper extremity amputation6 or stroke7 using functional imaging or mapping techniques.
A neurorehabilitation technique termed Constraint-Induced Movement therapy (CI therapy) was developed in this laboratory from basic research with monkeys.8 This treatment has been shown to substantially increase the amount of use of an affected upper extremity after stroke9–12 and also greatly alter the size of the regional brain activity or activation pattern associated with the more affected arm.7,13–15 Until now, neuroanatomical evaluations of treatment changes in humans have relied solely on functional brain mapping or imaging techniques such as focal transcranial magnetic stimulation,7 positron emission tomography,15 and functional MRI,14 which assess alterations in brain physiology that may change rapidly.
Recently, investigators using voxel-based morphometry (please see online supplement I at http://www.stroke.ahajournals.org) have provided evidence of structural neuroplasticity (ie, increases or decreases in amount of gray matter) resulting from increases or decreases in afferent input to the undamaged central nervous system. In accord with functional neuroimaging studies, limb amputation is associated with decreased thalamic gray matter, a structural brain change presumably reflecting the loss of sensory input from a specific body part.16 Conversely, increased purposive activity, such as frequent use of street and traffic patterns by veteran London cab drivers17 and learning to juggle,18 yields gray matter increases in these healthy individuals. We hypothesized that structural neuroplasticity could also be harnessed in damaged human brains to influence rehabilitation outcomes among a group of patients with chronic stroke receiving CI therapy.
Subjects and Methods
Participants
Forty-nine patients with chronic stroke aged 64.5±11.9 years with mild to moderate upper extremity hemiparesis were recruited for this study; 26 were male, 20 exhibited right-side hemiparesis, and 41 were right-hand-dominant before stroke. Patients were informed that they would be participating in a project to test the importance of different components of CI therapy and would thus be randomly assigned to receive different variants of the therapy. Some received all the components of CI therapy, including the transfer package (described later), whereas others received a comparison therapy that had all the components of CI therapy except for the transfer package. There were no significant differences in patient demographics between groups.
Sixteen CI therapy and 20 comparison therapy patients (7 and 15 male, respectively) aged 38 to 87 years (mean, 63.3±12.0) who, on average, experienced stroke onset 3.6±3.6 years previously received volumetric T1-weighted MRI during the week before therapy and again the week after completion of therapy. Medical constraints such as aneurysm clips, obesity, or claustrophobia prevented the other individuals from receiving scans. These excluded patients did not differ significantly on any demographic or outcome measures other than side of deficit; 2 of 13 excluded patients versus 50% of included patients had right hemiparesis. However, side of stroke has not been found to make a significant difference in CI therapy outcome.10,12
Patients who were currently undergoing pharmacological treatments for their motor disability (eg, botulinum toxin) or who had previously been treated with CI therapy were excluded before enrollment. All patients were treated one-on-one by physical or occupational therapists experienced in the administration of CI therapy. The study was performed at the University of Alabama at Birmingham, whose Institutional Review Board for human research approved this research. All patients provided signed informed consent.
Procedures
Patients randomized to CI therapy received intensive in-laboratory training of the more impaired arm on functional tasks for 3 hours daily for 10 consecutive weekdays, restraint of the less-impaired arm for a target 90% of waking hours, and a number of behavioral techniques termed the “transfer package” lasting an additional 0.5 hours in the laboratory. The transfer package, designed to facilitate transfer of therapeutic gains to real-world activities, included daily monitoring of life situation use of the more affected arm in several ways and problem-solving with a therapist to overcome perceived barriers to using the extremity. Details of the treatment protocol may be found elsewhere.19,20 Comparison therapy patients received only the in-laboratory training component of CI therapy. The Quality of Movement scale of the Motor Activity Log (MAL) and the Wolf Motor Function test were administered before and after the therapy course to assess treatment efficacy. The MAL is an instrument with an established high reliability and validity21 that obtains information on how well and how often activities of daily living were performed by a patient's impaired arm in the home environment and is a useful index of spontaneous real-world motor ability. The Wolf Motor Function Test is a validated and reliable objective measure of in-laboratory motor ability involving movements made on request.22,23
MRI Analysis
We used longitudinal voxel-based morphometry in SPM5 (Well-come Department of Cognitive Neurology) running under Matlab 7.1 (MathWorks) to compare changes in gray matter resulting from CI therapy versus comparison therapy (online supplement II). Images were equated for deficit side by flipping left-to-right the brains of subjects with left arm hemiparesis. Subjects with lesions occupying >1% of sensory and motor cortices were excluded from analyses of these brain regions because their inclusion would have confounded statistical conclusion validity. Eleven subjects had relatively large infarctions (49±35 cm3) in the sensory and motor cortices contralateral to their motor deficit and were thus excluded. Additionally, 1 of these subjects also had a magnetic artifact in the motor cortex ipsilateral to the deficit and was therefore excluded from analyses of both contralateral and ipsilateral sensory and motor cortices.
Focal within-group changes in gray matter were identified using paired t tests at individual 2-mm isometric voxels (ie, voxel-wise statistics). More spatially diffuse changes in gray matter were quantified by testing for increases over a significant number of adjacent voxels (ie, cluster-wise statistics). For both levels of analysis, a priori regions of interest were defined and analyzed separately; this enabled excluding subjects with infarcts in each of these regions. Regions of interest for this study included bilateral primary sensory and motor cortices and the premotor and supplementary motor areas, because localized functional changes have been demonstrated there in response to CI therapy,7,13–15 and the hippocampus because there is considerable evidence of structural changes in this region after ischemia in other brain areas, exercise, and learning.17,24–26 Regions of interest were drawn in MRIcro while consulting standard stereotaxic atlases and defined liberally to account for intersubject variability in these infarct-affected brains. Correction for family-wise error (FWE) used nonparametric permutation procedures in the SnPM5b program for both levels of analysis (online supplement III).27
Follow-up mixed-model repeated measures ANOVAs were used to examine between-group differences in average gray matter change per voxel (online supplement IV). Analyses were restricted to brain regions that had exhibited significant gray matter increases in the previous within-group analyses: a sensorimotor cluster contralateral to the hemiparetic arm, a sensorimotor cluster ipsilateral to the hemiparetic arm, and bilateral hippocampi. The hippocampi were integrated during follow-up analyses rather than analyzing contralateral and ipsilateral clusters separately because there were no significant differences in gray matter change between contralateral and ipsilateral hippocampi. A follow-up regression analysis was also conducted for each brain region, again using average gray matter change per voxel as the dependent variable, to determine whether magnitude of gray matter increase was related to amount of improvement in real world arm use as measured by the MAL.
Results
Clinical Results
Mixed repeated measures ANOVAs on MAL scores demonstrated that the CI therapy group showed far greater use of the more affected arm in the life situation than the comparison therapy group (F(1,32)=26.0; P<0.0001; Table). Follow-up analyses of simple main effects revealed that CI therapy recipients showed a significant improvement in real-world arm use (t(15)=9.36; P<0.0001; d′=2.34) that was 2.88-times greater than that seen in comparison therapy patients, although the comparison group also made a clinically significant improvement on the MAL28 (t(19)=4.54; P<0.001; d′=1.02; Figure 1a). Mixed repeated measures ANOVA revealed that both groups improved on log-squared Wolf Motor Function Test performance time scores12,29 (F(1,32)=7.1; P=0.012). No interaction effect was observed, indicating that the 2 therapies were equally effective at yielding significant improvements on this standardized, laboratory-based measure of motor ability, despite the large posttreatment differences between groups in amount of use of their arm for daily activities in the life situation. Thus, the transfer package enabled the CI therapy group to be highly successful in transferring what they had learned in the laboratory to the life situation, whereas the comparison group, which did not receive the transfer package, showed only a small amount of real world improvement.
Table. Clinical Outcomes Data for CI Therapy and Comparison Therapy Patients.
| Pretreatment | Posttreatment | Treatment Change | d′* | |
|---|---|---|---|---|
| CI therapy | ||||
| MAL | 1.23±0.76 | 3.00±0.90 | 1.77† | 2.34 |
| WMFT performance time | 1.04±1.04 | 0.90±1.02 | −0.14‡ | 0.44 |
| Comparison therapy | ||||
| MAL | 1.09±0.77 | 1.70±1.04 | 0.61† | 1.02 |
| WMFT performance time | 1.30±1.23 | 1.16±1.09 | −0.14‡ | 0.45 |
Cohen d′ is a within-subjects measure of effect size. It is the mean change divided by the SD of the change. A value of 0.57 is considered large in the meta-analysis literature.
A significant difference (P<0.05) between groups is marked with this symbol.
A negative change in performance time represents an improvement.
WMFT indicates Wolf Motor Function test.
Figure 1.
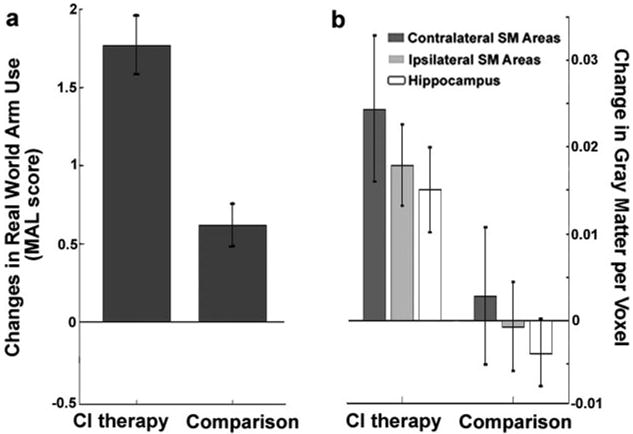
Structural brain changes parallel changes in real-world arm use. The change in real-world arm use (a), as measured by the Quality of Movement scale of the MAL, was significantly greater in the CI therapy group compared to the comparison group (F(1,32)=26.0; P<0.0001). Data shown are mean changes for the group receiving CI therapy (n=16) and the comparison group (n=20) with standard error bars. Similarly, the CI therapy group (b) showed larger increases in gray matter in contralateral sensory motor (SM) areas (PFWE<0.002), ipsilateral SM areas (PFWE=0.023), and bilateral hippocampus (PFWE ipsilateral=0.033 and PFWE contralateral<0.005). Data shown are mean changes for each region of interest with standard error bars.
Anatomic Results
Structural brain changes paralleled changes in amount of use of the impaired extremity for activities of daily living (Figure 1b). Cluster-wise analysis showed that the CI therapy group exhibited profuse increases in gray matter in sensory and motor areas both contralateral (PFWE=0.002; cluster size=11 478 voxels) and ipsilateral (PFWE=0.023; cluster size=4199 voxels) to the affected arm, as well as in bilateral hippocampi (PFWE ipsilateral=0.033; cluster size=269 voxels; and PFWE contralateral=0.005; cluster size=533 voxels). The large clusters of voxels showing gray matter increases in sensory and motor areas were bilaterally symmetrical and, when cross-referenced with standard brain atlases, encompassed the hand/arm regions of primary sensory and motor cortices as well as the anterior supplementary motor area and portions of the premotor area. Voxel-wise analysis showed significant changes in individual voxels in the anterior supplementary motor area contralateral to the motor deficit (PFWE< 0.05). In contrast, the comparison therapy group did not exhibit gray matter increases and, as noted previously, showed relatively small improvements in real-world arm use. Cortical surface-rendered images of the results for the CI therapy and comparison therapy subjects are presented in Figure 2. Follow-up mixed-model repeated measures ANOVAs on the total gray matter change within each region of interest (online supplement IV) showed that the increase in gray matter from pretreatment to posttreatment differed significantly between groups for the ipsilateral sensorimotor cluster (P=0.041) and the hippocampus (P=0.006) and was marginally significant for the contralateral cluster (P=0.087), providing additional evidence that CI therapy patients who receive the transfer package show significantly greater increases in gray matter than comparison patients not given the transfer package (Figure 1b). Of importance, the magnitude of gray matter increase within each sensorimotor cluster and hippocampus was significantly correlated with improvement on the MAL (rs>0.45; Pcontralateral=0.024; Pipsilateral=0.003; Phippocampus=0.005; Figure 3). Gray matter increases within these brain areas were not significantly correlated with age, infarct volume, or cortical involvement of the infarct.
Figure 2.
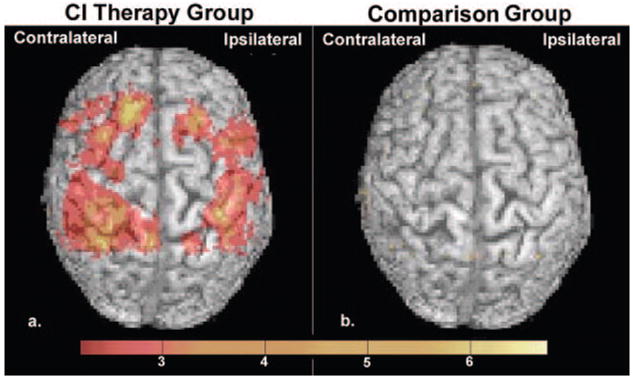
Cortical surface-rendered images of gray matter change. Gray matter increases displayed on a standard brain for the (a) CI therapy group and for the (b) comparison group. Surface rendering was performed with a depth of 20 mm. Color bar values indicate t statistics ranging from 2.2 to 6.7.
Figure 3.
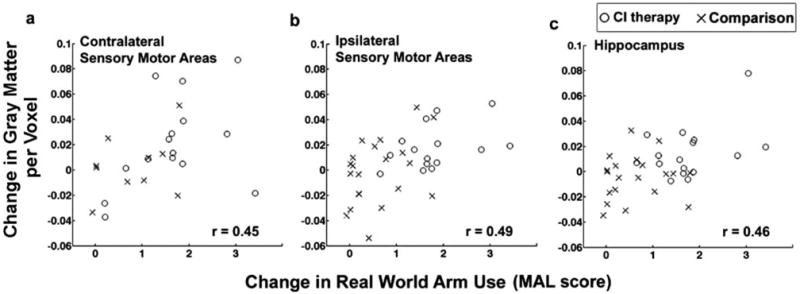
Relationship between the magnitude of gray matter increase and the amount of change in real world arm use. The increase in gray matter in the (a) contralateral sensory and motor areas, (b) ipsilateral sensory and motor areas, and (c) hippocampus is significantly correlated with improvements on the Quality of Movement scale of the MAL (rs≥0.45, P≤0.024). CI therapy patients are represented with an “O” (n=12, 15, and 16, respectively) and comparison patients with an “X” (n=13, 20, and 20, respectively).
Discussion
Three different analyses provided converging evidence that the group receiving CI therapy showed profuse changes in gray matter in sensory and motor areas of the brain and hippocampus, accompanied by large improvements in spontaneous real-world arm function. The group receiving the comparison therapy showed much smaller improvements in real-world arm use and did not exhibit gray matter increases despite equivalent in-laboratory motor training. One possible explanation for these findings is that the cerebral structural changes are sensitive to the behavioral relevance of motor tasks, such as use of the more affected arm in the activities of daily living at home encouraged by the transfer package. Jenkins et al3 showed in monkeys that repetitive behaviorally relevant sensory stimulation resulted in plastic expansion of the cortical representations of the stimulated digits. Equal amounts of sensory stimulation that was not behaviorally relevant did not significantly alter these representation zones. Perhaps a similar phenomenon explains the overall lack of structural gray matter changes in comparison group stroke patients whose therapy did not incorporate the transfer package, which appears to substantially increase use of the impaired arm in the life situation. This interpretation is consistent with the direct relationship between improvements in spontaneous real-world arm use and the magnitude of morphological brain change.
The results demonstrate that not only does CI therapy produce functional changes in the brains of stroke patients involving increases in the differential excitability, metabolic activity, and oxygen consumption of sensorimotor regions of the brain but also it induces correlated morphometric changes in these areas. The data do not allow determining whether the 2 processes are causally related or whether the 2 reflect the operation of a common underlying process.
Increases were also observed in the gray matter of the hippocampus, which may have included the adjacent subventricular zone. The hippocampus is known to be involved in learning and memory and these 2 processes may be associated with the improved limb use that occurs with CI therapy. Evidence also indicates that stem cells are located at this site in the adult mammalian brain24,30 and simulated stroke in animals can increase the quantity of these cells.24 One might speculate that the increases in gray matter observed in the hippocampal region or sensory and motor areas of the brain are mediated in part by increased production of neuronal or glial stem cells that might migrate to an infarcted area and participate in its repair.31 Alternatively or in addition, gray matter increases may result from rehabilitation-induced increases in dendritic arborization and synaptic density,32 and possibly gliosis or angiogenesis. Notably, the gray matter increases that we observed occurred over the course of just 2 weeks of therapy, emphasizing the rapid time course in which structural neuroplastic changes can take place.
The results also lend new insight to previous reports of the occurrence of plastic changes that are functional in nature in the motor cortex that innervates the less affected arm.13,14 Plastic recruitment of brain areas that were previously nonparticipating or less involved in the movement of the affected arm appear to be associated with the improvement in movement produced by CI therapy after stroke and possibly other types of neurological injury. The present results suggest that these functional brain changes, therefore, may be supported by the regional structural changes reported here.
Currently, one can only speculate as to the nature and function of the observed structural brain changes. Voxel-based morphometry does not have sufficient resolution to identify the microscopic mechanisms underlying rehabilitation-induced gray matter changes. The robustness of the observed brain changes needs to be established by replicating this study on stroke patients with more severe hemiparesis and on other clinical populations with which CI therapy has proven effective. Furthermore, it still remains to be determined whether these structural changes are retained over time. Our data does not allow determining whether structural brain changes are a cause or an effect of the observed behavioral changes, whether these 2 phenomena interact, or whether both are perpetuated and maintained by other processes. Finally, the potential for differential impacts of CI therapy on neuroplasticity in mature versus developing nervous systems remains unexplored.
Despite the need to elucidate these issues, this study shows that a rehabilitation intervention can result in structural reorganization in damaged human brains and that the magnitude of this structural change is directly proportional to the amount of clinical improvement. The present study suggests that evaluating the neural mechanisms of structural brain change and the patient characteristics or pharmacological factors that may influence the cellular processes underlying these brain changes will be a promising avenue of future research.
Supplemental Material
Acknowledgments
The authors thank J. Ogorek and A. Timberlake for their assistance with data processing.
Sources of Funding: This work was supported by grant HD34273 from the National Institutes of Health and approved by the University of Alabama at Birmingham Institutional Review Board.
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is an un-copyedited author manuscript that was accepted for publication in Stroke, copyright The American Heart Association. This may not be duplicated or reproduced, other than for personal use or within the “Fair Use of Copyrighted Materials” (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at Stroke. The American Heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
References
- 1.Merzenich MM, Nelson RJ, Stryker MP, Cynader MS, Schoppmann A, Zook JM. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984;224:591–605. doi: 10.1002/cne.902240408. [DOI] [PubMed] [Google Scholar]
- 2.Kaas JH, Merzenich MM, Killackey HP. The reorganization of somatosensory cortex following peripheral nerve damage in adult and developing mammals. Annu Rev Neurosci. 1983;6:325–356. doi: 10.1146/annurev.ne.06.030183.001545. [DOI] [PubMed] [Google Scholar]
- 3.Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guic-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol. 1990;63:82–104. doi: 10.1152/jn.1990.63.1.82. [DOI] [PubMed] [Google Scholar]
- 4.Pons TP, Garraghty PE, Ommaya AK, Kaas JH, Taub E, Mishkin M. Massive cortical reorganization after sensory deafferentation in adult macaques. Science. 1991;252:1857–1860. doi: 10.1126/science.1843843. [DOI] [PubMed] [Google Scholar]
- 5.Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E. Increased cortical representation of the fingers of the left hand in string players. Science. 1995;270:305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- 6.Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375:482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- 7.Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- 8.Taub E. Somatosensory deafferentation research with monkeys: implications for rehabilitation medicine. In: Ince LP, editor. Behavioral psychology in rehabilitation medicine: clinical applications. New York: Williams & Wilkins; 1980. pp. 371–401. [Google Scholar]
- 9.Taub E, Miller NE, Novack TA, Cook EW, III, Fleming WC, Nepomuceno CS, Connell JS, Crago JE. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347–354. [PubMed] [Google Scholar]
- 10.Taub E, Uswatte G, King DK, Morris D, Crago J, Chatterjee A. A placebo controlled trial of Constraint-Induced Movement therapy for upper extremity after stroke. Stroke. 2006;37:1045–1049. doi: 10.1161/01.STR.0000206463.66461.97. [DOI] [PubMed] [Google Scholar]
- 11.Taub E. Harnessing brain plasticity through behavioral techniques to produce new treatments in neurorehabilitation. Am Psychol. 2004;59:692–704. doi: 10.1037/0003-066X.59.8.692. [DOI] [PubMed] [Google Scholar]
- 12.Wolf SL, Winstein C, Miller JP, Taub E, Uswatte G, Morris DM, Giuliani C, Light KE, Nichols-Larsen D EXCITE Investigators. Effect of Constraint Induced Movement Therapy on Upper Extremity Function 3 to 9 Months after Stroke: The EXCITE Randomized Clinical Trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 13.Kopp B, Kunkel A, Mühlnickel W, Villringer K, Taub E, Flor H. Plasticity in the motor system related to therapy-induced improvement of movement after stroke. Neuroreport. 1999;10:807–810. doi: 10.1097/00001756-199903170-00026. [DOI] [PubMed] [Google Scholar]
- 14.Schaechter JD, Kraft E, Hilliard TS, Dijkhuizen RM, Benner T, Finklestein SP, Rosen BR, Cramer SC. Motor recovery and cortical reorganization after constraint-induced movement therapy in stroke patients: a preliminary study. Neurorehabil Neural Repair. 2002;16:326–338. doi: 10.1177/154596830201600403. [DOI] [PubMed] [Google Scholar]
- 15.Wittenberg GF, Chen R, Ishii K, Bushara KO, Taub E, Gerber LH, Hallett M, Cohen LG. Constraint-Induced therapy in stroke: magnetic-stimulation motor maps and cerebral activation. Neurorehabil Neural Repair. 2003;17:48–57. doi: 10.1177/0888439002250456. [DOI] [PubMed] [Google Scholar]
- 16.Draganski B, Moser T, Lummel N, Ganssbauer S, Bogdahn U, Haas F, May A. Decrease of thalamic gray matter following limb amputation. Neuroimage. 2006;31:951–957. doi: 10.1016/j.neuroimage.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- 19.Taub E, Uswatte G, Mark VW, Morris DM. The learned nonuse phenomenon: implications for rehabilitation. Eura Medicophys. 2006;42:241–256. [PubMed] [Google Scholar]
- 20.Morris DM, Taub E, Mark VW. Constraint-induced movement therapy: characterizing the intervention protocol. Eura Medicophys. 2006;42:257–268. [PubMed] [Google Scholar]
- 21.Uswatte G, Taub E, Morris D, Light K, Thompson PA. The Motor Activity Log-28: assessing daily use of the hemiparetic arm after stroke. Neurology. 2006;67:1189–1194. doi: 10.1212/01.wnl.0000238164.90657.c2. [DOI] [PubMed] [Google Scholar]
- 22.Morris DM, Uswatte G, Crago JE, Cook EW, III, Taub E. The reliability of the wolf motor function test for assessing upper extremity function after stroke. Arch Phys Med Rehabil. 2001;82:750–755. doi: 10.1053/apmr.2001.23183. [DOI] [PubMed] [Google Scholar]
- 23.Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf Motor Function Test as outcome measure for research in patients after stroke. Stroke. 2001;32:1635–1639. doi: 10.1161/01.str.32.7.1635. [DOI] [PubMed] [Google Scholar]
- 24.Yamashima T, Tonchev AB, Vachkov IH, Popivanova BK, Seki T, Sawamoto K, Okano H. Vascular adventitia generates neuronal progenitors in the monkey hippocampus after ischemia. Hippocampus. 2004;14:861–875. doi: 10.1002/hipo.20001. [DOI] [PubMed] [Google Scholar]
- 25.Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 26.Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16:250–260. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- 27.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van der Lee J, Beckerman H, Knol D, de Vet H, Bouter L. Clinimetric properties of the Motor Activity Log for the assessment of arm use in hemiparetic patients. Stroke. 2004;35:1–5. doi: 10.1161/01.STR.0000126900.24964.7e. [DOI] [PubMed] [Google Scholar]
- 29.Wolf SL, Thompson P, Morris D, Rose DK, Winstein C, Taub E, Giuliani C, Pearson SL. The EXCITE trial: attributes of the Wolf Motor Function Test in patients with subacute stroke. Neurorehabil Neural Repair. 2005;19:194–205. doi: 10.1177/1545968305276663. [DOI] [PubMed] [Google Scholar]
- 30.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 31.Kolb B, Morshead C, Gonzalez C, Kim M, Gregg C, Shingo T, Weiss S. Growth factor-stimulated generation of new cortical tissue and functional recovery after stroke damage to the motor cortex of rats. J Cereb Blood Flow Metab. 2007;27:983–997. doi: 10.1038/sj.jcbfm.9600402. [DOI] [PubMed] [Google Scholar]
- 32.Briones TL, Suh E, Jozsa L, Woods J. Behaviorally induced synaptogenesis and dendritic growth in the hippocampal region following transient global cerebral ischemia are accompanied by improvement in spatial learning. Exp Neurol. 2006;198:530–538. doi: 10.1016/j.expneurol.2005.12.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


