Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is a common hereditary condition that may be diagnosed in utero. Our goal was to evaluate symptoms of ADPKD in children, including left ventricular mass index (LVMI), renal volume, renal function and microalbuminuria in relation to systolic and diastolic blood pressure. Eighty-five children were stratified by blood pressure into three cohorts: hypertensive (95th percentile and over), borderline hypertensive (75–95th percentile) and normotensive (75th percentile and below). There were no differences in gender, age, height, renal function, or microalbuminuria between the groups. Both the hypertensive and borderline hypertensive children had a significantly higher LVMI than normotensive children, with no significant difference between hypertensive and borderline hypertensive groups. There was a significant correlation between renal volume and both systolic and diastolic blood pressures in all subjects. Renal volume in hypertensive children was significantly larger than in the borderline hypertensive group, with no significant difference between normotensive and borderline hypertensive groups. These findings show that an increase in LVMI may be detected earlier than an increase in renal volume in children with ADPKD and borderline hypertension, suggesting that close monitoring of cardiac status is indicated in these children.
Keywords: polycystic kidney disease, children, borderline hypertension, left ventricular mass index
Autosomal dominant polycystic kidney disease (ADPKD) is the most common life-threatening hereditary renal disease, affecting 1 in 400 to 1 in 1000 individuals in the United States.1 ADPKD had previously been known as Adult Polycystic Kidney Disease. It is now clear, however, that affected children and adolescents exhibit several features of ADPKD. Moreover, ADPKD has even been diagnosed in utero.2,3
In most diseases, early intervention is more likely to alter the clinical course as compared to later intervention. Thus, it is important to identify the clinical characteristics of ADPKD at an early age. Previous studies have shown that hypertension is associated with faster progression to end-stage renal disease4 and increased cardiovascular morbidity and mortality in adults with ADPKD.5 The present study was therefore undertaken to evaluate the clinical manifestations of ADPKD in children and adolescents, including left ventricular mass index (LVMI), renal volume and function, and microalbuminuria in relation to systolic and diastolic blood pressure (BP) adjusted for sex, age, and height. A total of 85 children with a mean age of 12.8 years were analyzed according to three levels of BP, namely hypertension (HBP; greater than the 95th percentile for sex, age, and height), borderline hypertension (BBP; 75–95th percentile), and normotension (NBP; less than the 75th percentile).
RESULTS
Demographics
A total of 85 children participated in this study. The characteristics of these subjects are shown in Table 1 according to the three categories of BP including NBP, BBP, and HBP. There were no differences in sex, age, height, or renal function between the groups. Urinary microalbumin excretion was not significantly different between the groups.
Table 1.
The characteristics of normotensive (NBP), borderline hypertensive (BBP), and hypertensive (HBP) subjects
| Parameter | NBP (N=30) | BBP (N=27) | HBP (N=28) | P-value for ANOVA |
|---|---|---|---|---|
| Male/female | 13/17 | 15/12 | 17/11 | NS |
| Age (years) | 12.0±0.8 | 11.8±0.8 | 13.6±0.8 | NS |
| Height (cm) | 151±5 | 151±5 | 160±4 | NS |
| Serum creatinine (mg/100 ml) | 0.66 (0.57-0.70) | 0.69 (0.62-0.77) | 0.74 (0.68-0.81) | NS |
| 24-h creatinine clearance (ml/min/1.73m2) | 135 (127-145) | 127 (117-138) | 130 (120-141) | NS |
| UMA (mg/day) | 31 (19-51) | 22 (14-35) | 23 (16-33) | NS |
| SBP (mm Hg) | 109±2 | 119±2 | 130±3 | <0.0001 |
| DBP (mm Hg) | 64±1 | 68±1 | 72±2 | 0.0005 |
DBP, diastolic blood pressure; NS, not significant; SBP, systolic blood pressure; UMA, urine microalbumin excretion.
Note. SBP: NBP vs BBP (P=0.0122), NBP vs HBP (P<0.0001), BBP vs HBP (P=0.0022); DBP: NBP vs BBP (P=NS), NBP vs HBP (P=0.0003), BBP vs HBP (P=NS). Data presented as mean±s.e. or geometric mean (95% CI).
Correlation between LVMI and blood pressure
A significant correlation between LVMI and systolic BP (r=0.45, P<0.0001) for all 85 patients was observed (Figure 1a). Similarly, LVMI correlated significantly with diastolic BP (r=0.29, P<0.007; Figure 1b). Multiple linear regression with age, height, and sex as covariates confirmed a significant predictive value of systolic BP on the log of LVMI (P=0.0004), whereas diastolic BP was not significant after accounting for age, height, and sex (P=0.14).
Figure 1. Correlation between blood pressure and left ventricular mass index (LVMI).
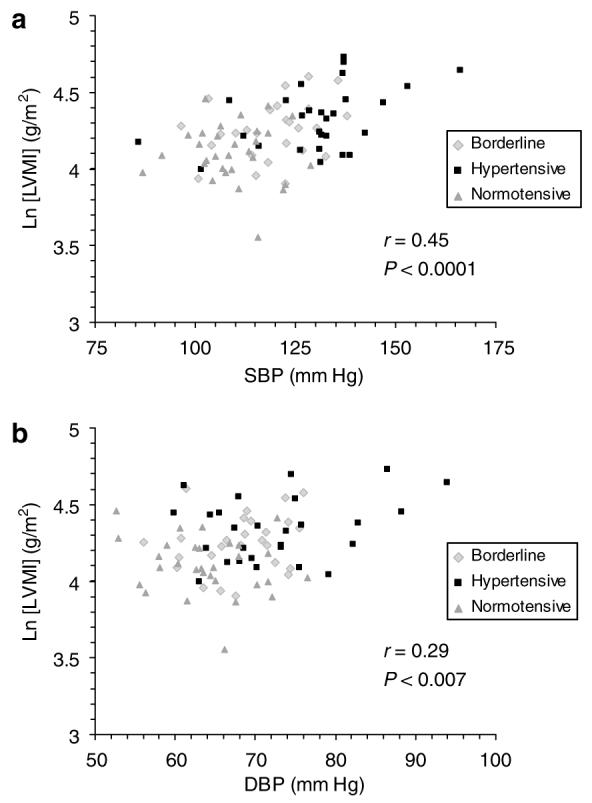
There was a significant correlation between mean systolic blood pressure (SBP) and (LVMI) (a) as well as between mean diastolic blood pressure (DBP) and LVMI (b) in all 85 subjects.
Left ventricular mass index by blood pressure group
The HBP children had significantly higher LVMI than the NBP group (Figure 2). The LVMI in the BBP group was significantly greater than in the NBP children. There was no significant difference in LVMI between the BBP and HBP groups.
Figure 2. Left ventricular mass index (LVMI) was significantly increased in hypertensive (HBP) as compared to borderline hypertensive (BBP) and normotensive (NBP) children with ADPKD.
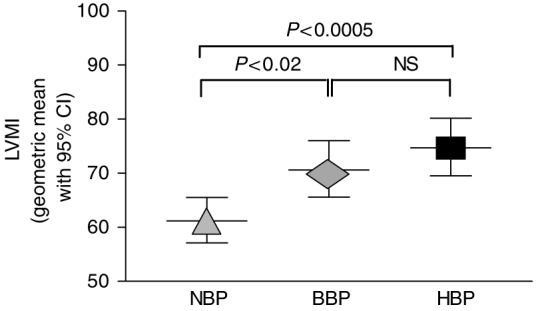
BBP children demonstrated significantly higher LVMI as compared to NBP children with ADPKD. Data presented as geometric mean with 95% confidence intervals (CI), adjusted for age, sex, and height.
Correlation between renal volume and blood pressure
There was a highly significant correlation between the log of renal volume and systolic BP (r=0.70, P<0.0001) for all 85 patients (Figure 3a). A similar correlation also occurred between diastolic BP and renal volume (r=0.52, P<0.0001; Figure 3b). Multiple linear regression with age, height, and sex as covariates confirmed a significant predictive value of systolic BP on the log of renal volume (P=0.0002), whereas diastolic BP was not significant after accounting for age, height, and sex (P=0.2).
Figure 3. Correlation between blood pressure and renal volume.
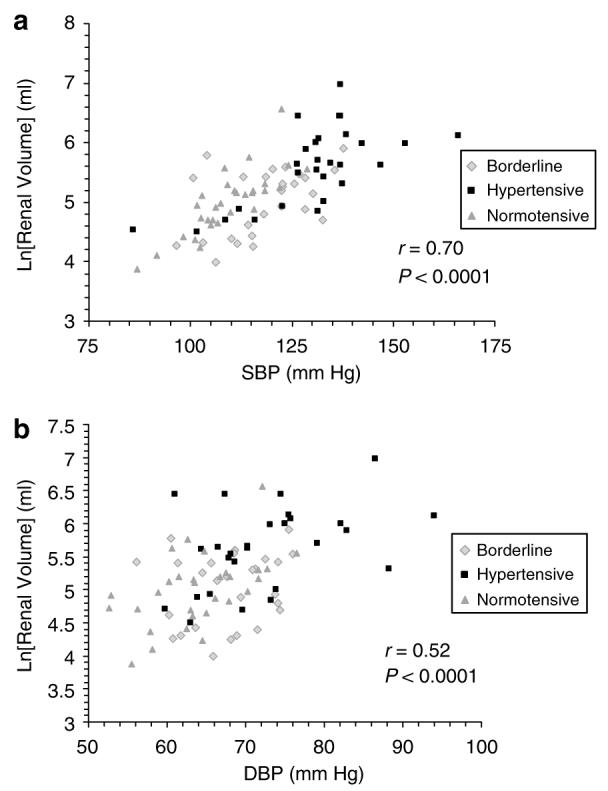
There was a significant correlation between mean systolic blood pressure (SBP) and renal volume (a) as well as between mean diastolic blood pressure (DBP) and renal volume (b) in all 85 subjects.
Renal volume by blood pressure group
Kidney volumes were measured by ultrasonography in the three BP groups (Figure 4). The renal volumes in the HBP children were significantly larger than the renal volumes in the BBP and NBP groups. There was no significant difference in renal volumes between the NBP and BBP groups.
Figure 4. Renal volume was markedly increased in hypertensive (HBP) as compared to borderline hypertensive (BBP) and normotensive (NBP) children with ADPKD.
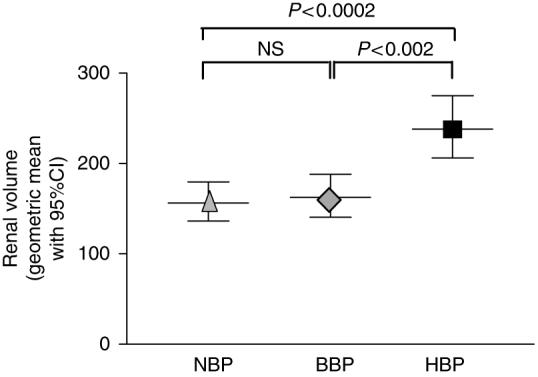
Data presented as geometric mean with 95% confidence intervals (CI), adjusted for age, sex, and height.
DISCUSSION
The purpose of the current study was to relate level of systemic BP to clinical manifestations including LVMI and renal volume in children and young adults with ADPKD. Within this study, the demographics including age, sex, and height were comparable between the three BP groups; this is important due to the normal effects of growth on BP in children. Analysis of all 85 children demonstrated a highly significant correlation between both systolic and diastolic BP and LVMI. It was not surprising that the group with hypertension demonstrated a significant elevation in LVMI. Of particular importance, however, was the finding that among ADPKD children with normal BP, a significant increase in LVMI was present in those with BP within the upper quartile of the normal range as compared to those with lower BP. This elevation was detected before any significant increase in renal volume, suggesting an early and important cardiovascular effect of high normal BP within this population.
We also observed a highly significant association between both systolic and diastolic BP and renal volume. This is quite interesting from several viewpoints. Renal volumes have previously been shown to be larger in hypertensive versus normotensive adults6,7 and children7–10 with ADPKD. The present results support these observations. It has been suggested that renal cyst expansion with attenuation of the renal vasculature may be an important factor in the generation of hypertension in adults with ADPKD.11–13 In contrast, despite a significant difference in BP between normotensive and borderline hypertensive children, there was no detectable difference in renal volume between these two groups. There are at least two potential explanations for these findings. First, it may be that alternative factors are important in the generation of BP in children with ADPKD. Alternatively, it may be that ultrasonography is not sensitive enough to detect differences in renal volume between normotensive and borderline hypertensive children. Recent studies in adults suggest that magnetic resonance imaging provides a reliable assessment of renal structural disease in ADPKD which is superior to that obtained by ultrasound;14 however, we note that in subsequent follow-up in this cohort we were able to demonstrate significant interval change in renal volume within 1 year with repeated ultrasound assessment (unpublished results). Thus we believe our findings to be clinically important.
Although microalbuminuria has been associated with hypertension, increased renal volume, and left ventricular mass (LVM) in adults with ADPKD,15–17 we found no similar association in children with ADPKD.
With advances in renal replacement therapy, cardiovascular disease is now the primary cause of death in adults with ADPKD.5 Thus, the observation that ADPKD children with borderline hypertension had significantly greater LVMI than the normotensive subjects would suggest that any cardiac intervention in ADPKD children should not wait until hypertension is established. A prospective randomized study in hypertensive ADPKD patients with left ventricular hypertrophy demonstrated that treatment with angiotensin converting enzyme inhibitor reversed increased LVM in association with aggressive BP control.18 Early treatment of BP, for example, angiotensin converting enzyme inhibition, may be indicated in children with ADPKD and borderline hypertension.
MATERIALS AND METHODS
This study was supported by the National Institutes of Health and was conducted at the Clinical Translational Research Center at The Children’s Hospital in Denver, Colorado. Subjects were recruited nationally from our ongoing studies of ADPKD, from physician referrals, and from family responses to preliminary information from the PKD Research Foundation. The research protocol was reviewed and approved by the Colorado Multiple Institutional Review Board. Informed consent/assent was obtained from all subjects upon enrollment.
Inclusion criteria included children and adolescents (aged 4–21 years) with ADPKD and normal renal function. Patients were considered to have ADPKD when radiographic imaging demonstrated at least one renal cyst in the setting of a family history of ADPKD or when multiple cysts were present and were clinically consistent with a new diagnosis of ADPKD. Creatinine clearance was estimated from the patient’s height and serum creatinine concentration obtained within 6 months before enrollment using the Schwartz formula.19 Blood pressure norms were determined based on the subject’s sex, height, and age according to guidelines set forth by the National High Blood Pressure Education Program.20–22 HBP was defined as systolic and/or diastolic BP greater than the 95th percentile for sex, age, and height on three or more measurements. BBP was defined as systolic and/or diastolic BP between the 75th and the 95th percentile for sex, age, and height on three or more measurements. NBP was defined as systolic and/or diastolic BP less than the 75th percentile for sex, age, and height on three or more measurements. If a subject was already receiving antihypertensive medication, hypertension was documented by reviewing records or by close monitoring during a 1-week washout period. During this washout period, medications were held as deemed clinically safe by the principal investigators with BP assessed within 2 days of stopping antihypertensive medication. Once hypertension was documented, the subject’s usual antihypertensive regimen was resumed. For the vast majority of subjects, hypertension could be documented from previous records.
Once eligibility was confirmed, each subject was seen for a 2-day visit at the Pediatric Clinical Translational Research Center at The Children’s Hospital in Denver. All subjects underwent a detailed history and physical examination. Blood was drawn for routine serum chemistries and complete blood count. A routine urinalysis was obtained. Two 24-h urine collections were obtained for assessment of creatinine clearance and microalbumin excretion. Abdominal ultrasound and echocardiography were performed as described below.
On the first day of the visit, the dominant arm for BP measurement was determined after 5 min of quiet sitting, and all subsequent BP measurements were taken in the dominant arm with a cuff appropriate for the size of the subject’s arm after 5 min of quiet sitting. A total of 12 sitting BP measurements were obtained during the study period using a programmable oscillometric BP monitor (Dinamap 1846 SX; Critikon).
Standard two-dimensional and Doppler echocardiogram was performed with the patient in a supine position using an Accuson 128 XP/5 ultrasound. Neither the echocardiographer nor the cardiologist reading the echocardiogram was aware of the subject’s BP status. LVM was determined by the formula: LVM = 0.80 (1.04×(LVDd+PWT+IVSd)3-(LVDd)3+0.6, where LVDd = left ventricular diameter in diastole, PWT = posterior wall thickness in diastole, and IVSd = ventricular septal thickness in diastole.23 LVM was then corrected by body surface area and reported as LVMI corrected for body surface area in g/m2.
Renal volumes were measured by performing standard abdominal ultrasonography. Neither the ultrasound technician nor the radiologist was aware of the subject’s BP status. Volumes were calculated as the volume of a modified ellipse. Cyst number was recorded as the actual number when less than 15 or as greater than 15 cysts per kidney.
Statistical analysis
Statistical analysis was performed using SAS 9.1. Outcome variables were analyzed for the distributional assumption of normality. Unadjusted baseline characteristics were compared between groups using the ANCOVA. Natural log transformations were applied to renal volume, LVM, and microalbumin excretion. ANCOVA was used to compare groups after adjusting for age, height, and sex. These data were reported as geometric mean and 95% confidence intervals, adjusted for age, sex, and height. The Tukey—Kramer P-value adjustment was used to determine which groups differed. Pearson correlations and multiple linear regression were used to show the relationships among variables in the population.
ACKNOWLEDGMENTS
This research was supported by Grant R01 DK058793 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (NIH); Grant MO1 RR00069 from the General Clinical Research Centers Program of the National Center for Research Resources, NIH; and the Zell Family Foundation.
Footnotes
DISCLOSURE All authors declared no competing interests.
REFERENCES
- 1.Ecder T, Fick-Brosnahan G, Schrier RW. Polycystic kidney disease. In: Schrier RW, editor. Diseases of the Kidney and Urinary Tract. Lippincott Williams and Wilkins; Philadelphia: 2007. pp. 502–539. [Google Scholar]
- 2.Pretorius DH, Lee ME, Manco-Johnson ML, et al. Diagnosis of autosomal dominant polycystic kidney disease in utero and in the young infant. J Ultrasound Med. 1987;6:249–255. doi: 10.7863/jum.1987.6.5.249. [DOI] [PubMed] [Google Scholar]
- 3.MacDermot KD, Saggar-Malik AK, Economides DL, et al. Prenatal diagnosis of autosomal dominant polycystic kidney disease (PKD1) presenting in utero and prognosis for very early onset disease. J Med Genet. 1998;35:13–16. doi: 10.1136/jmg.35.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabow PA, Johnson AM, Kaehny WD, et al. Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int. 1992;41:1311–1319. doi: 10.1038/ki.1992.195. [DOI] [PubMed] [Google Scholar]
- 5.Fick GM, Johnson AM, Hammond WS, et al. Causes of death in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1995;5:2048–2056. doi: 10.1681/ASN.V5122048. [DOI] [PubMed] [Google Scholar]
- 6.Gabow PA, Chapman AB, Johnson AM, et al. Renal structure and hypertension in autosomal dominant polycystic kidney disease. Kidney Int. 1990;38:1177–1180. doi: 10.1038/ki.1990.330. [DOI] [PubMed] [Google Scholar]
- 7.Chapman AB, Guay-Woodford LM, Grantham JJ, et al. Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Kidney Int. 2003;64:1035–1045. doi: 10.1046/j.1523-1755.2003.00185.x. [DOI] [PubMed] [Google Scholar]
- 8.Seeman T, Dusek J, Vondrichova H, et al. Ambulatory blood pressure correlates with renal volume and number of renal cysts in children with autosomal dominant polycystic kidney disease. Blood Press Monit. 2003;8:107–110. doi: 10.1097/01.mbp.0000085762.28312.4a. [DOI] [PubMed] [Google Scholar]
- 9.Fick-Brosnahan GM, Tran ZV, Johnson AM, et al. Progression of autosomal dominant polycystic kidney disease in children. Kidney Int. 2001;5:1654–1662. doi: 10.1046/j.1523-1755.2001.0590051654.x. [DOI] [PubMed] [Google Scholar]
- 10.Seeman T, Sikut M, Konrad M, et al. Blood pressure and renal function in autosomal dominant polycystic kidney disease. Pediatr Nephrol. 1997;11:592–596. doi: 10.1007/s004670050343. [DOI] [PubMed] [Google Scholar]
- 11.Bell PE, Hossack KF, Gabow PA, et al. Hypertension in autosomal dominant polycystic kidney disease. Kidney Int. 1988;34:683–690. doi: 10.1038/ki.1988.233. [DOI] [PubMed] [Google Scholar]
- 12.Chapman AB, Johnson A, Gabow PA, et al. The renin-angiotensin aldosterone system and autosomal dominant polycystic kidney disease. N Engl J Med. 1990;323:1091–1096. doi: 10.1056/NEJM199010183231602. [DOI] [PubMed] [Google Scholar]
- 13.Chapman AB, Schrier RW. Pathogenesis of hypertension in autosomal dominant polycystic kidney disease. Semin Nephrol. 1991;11:653–660. [PubMed] [Google Scholar]
- 14.O’Neill WC, Robbin ML, Bae KT, et al. Sonographic assessment of the severity and progression of autosomal dominant polycystic kidney disease: the Consortium of Renal Imaging Studies in Polycystic Kidney Disease (CRISP) Am J Kidney Dis. 2005;46:1058–1064. doi: 10.1053/j.ajkd.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Vea A, Gutierrez C, Bardaji A, et al. Microalbuminuria in normotensive patients with autosomal-dominant polycystic kidney disease. Scand J Urol Nephrol. 1998;32:356–359. doi: 10.1080/003655998750015331. [DOI] [PubMed] [Google Scholar]
- 16.Ecder T, Chapman AB, Brosnahan GM, et al. Effect of antihypertensive therapy on renal function and urinary albumin excretion in hypertensive patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2000;35:427–432. doi: 10.1016/s0272-6386(00)70195-8. [DOI] [PubMed] [Google Scholar]
- 17.Wong H, Vivian L, Weiler G, et al. Patients with autosomal dominant polycystic kidney disease hyperfiltrate early in their disease. Am J Kidney Dis. 2004;43:624–628. doi: 10.1053/j.ajkd.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 18.Schrier R, McFann K, Johnson A, et al. Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal-dominant polycystic kidney disease: results of a seven-year prospective randomized study. J Am Soc Nephrol. 2002;13:1733–1739. doi: 10.1097/01.asn.0000018407.60002.b9. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz GJ, Haycock GB, Edelmann CM, Jr, et al. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;2:259–263. [PubMed] [Google Scholar]
- 20.Blumenthal S. Report of task-force on blood-pressure control in children—prepared by national heart lung and blood institutes task-force on blood pressure control in children. Pediatrics. 1977;59:797–820. [PubMed] [Google Scholar]
- 21.Report of the 2nd Task-Force on Blood-Pressure Control in Children—1987. Pediatrics. 1987;79:1–25. [PubMed] [Google Scholar]
- 22.Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: a working group report from the National High Blood Pressure Education Program. National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents. Pediatrics. 1996;98(4 Pt 1):649–658. [PubMed] [Google Scholar]
- 23.Ivy DD, Shaffer EM, Johnson AM, et al. Cardiovascular abnormalities in children with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 5:2032–2036. doi: 10.1681/ASN.V5122032. [DOI] [PubMed] [Google Scholar]


