Abstract
Objective
In utero exposure to drugs of abuse can lead to the Neonatal Abstinence Syndrome (NAS), a condition that is associated with prolonged hospitalization. Buprenorphine is a partial mu opioid agonist used for treatment of adult detoxification and maintenance, but has never been administered to neonates with opioid abstinence. The primary objective of this study was to demonstrate the feasibility and to the extent possible in this sized study, the safety of sublingual buprenorphine in the treatment of NAS. Secondary goals were to evaluate efficacy relative to standard therapy and to characterize buprenorphine pharmacokinetics when sublingually administered.
Methods
We conducted a randomized, open-label, active control study of sublingual buprenorphine for the treatment of opiate withdrawal. Thirteen term infants were allocated to sublingual buprenorphine 13.2–39 mcg/kg/day administered in three divided doses and thirteen to standard of care oral neonatal opium solution (NOS). Dose decisions were made using a modified Finnegan scoring system.
Results
Sublingual buprenorphine was largely effective in controlling NAS. Greater than 98% of plasma concentrations ranged from undetectable to approximately 0.60 ng/ml, which is less than needed to control abstinence symptoms in adults. The ratio of buprenorphine to norbuprenorphine was larger than that seen in adults, suggesting a relative impairment of N-dealkylation. Three infants receiving buprenorphine and one infant receiving standard of care reached protocol-specified maximum doses and required adjuvant therapy with phenobarbital. The mean length of treatment for the NOS group was 32 compared to 22 days for the buprenorphine group. The mean length of stay for the NOS group was 38 days compared to 27 days for the buprenorphine group. Treatment with buprenorphine was well tolerated.
Conclusions
Buprenorphine administered via the sublingual route is feasible and apparently safe, and may represent a novel treatment for NAS.
Keywords (MeSH Unique ID#): Neonatal Abstinence Syndrome (D009357), buprenorphine (D002047), Sublingual Drug Administration (D000286), morphine (D009020)
Introduction
The Neonatal Abstinence Syndrome (NAS) is a complex of signs and symptoms in the postnatal period associated with the sudden withdrawal of maternally transferred opioids. Cardinal manifestations include increased muscle tone, autonomic instability, irritability, poor sucking reflex and impaired weight gain. In epidemiologic studies maternal opioid abuse is common, with toxicological evidence of use in approximately 1% of births. (1) This includes methadone, and increasingly buprenorphine, which is used to treat women with physical dependency to opioid agonists. In aggregate, NAS occurs in 55–94% of infants born to opioid-dependent mothers. (2)
The optimal treatment for NAS has not been established. This is reflected in the considerable heterogeneity in the pharmacologic treatment of NAS among different institutions. (3, 4) Cochrane reviews suggest lack of high quality evidence to support any specific treatment, (5, 6) though expert opinion places opioids as the class of agents that possess the greatest efficacy. (7) Specific opioid agents that are used include morphine sulfate, morphine in the form of neonatal opium solution (NOS) or deodorized tincture of opium, and methadone. Though values vary considerably between institutions, treatment for NAS is associated with long inpatient treatment stays. Administration of morphine or NOS has been reported to have lengths of treatment of 8–79 days, (8–11) though a consensus duration is approximately 30 days. This length of hospitalization is suboptimal due to interference with maternal bonding, potential for nosocomial infection, and resource utilization. There is a need for improved therapeutics that would safely decrease the length of inpatient hospitalization.
Buprenorphine is a partial mu opioid receptor agonist with an extended half-life that has found increasing use in the treatment of adult opiate addiction. (12) The drug has a large first-pass metabolism in adults and is administered via the sublingual route. Buprenorphine has a number of characteristics that would make it an attractive agent in the treatment of NAS. As an agonist/antagonist, buprenorphine has a ceiling effect for respiratory depression. (13) There is a lack of the cardiovascular liability associated with methadone (14) as well as an established safety profile in adults. The long half-life and duration of action prevents the rapid change in receptor occupancy that can precipitate withdrawal symptoms. (15) Finally, there is limited abuse liability, which makes consideration of outpatient treatment for NAS a possibility for carefully screened caregivers. Thus buprenorphine is a candidate to fill the unmet need of improved NAS treatment and certainly as a useful alternate treatment.
There is little experience with the use of buprenorphine in the neonatal or pediatric populations. (16) Published pharmacokinetic parameters are limited to a single investigation in premature infants requiring opioid analgesia. (17) Quantitative drug determination in term infants has been reported only in a single case report involving placental transmission following maternal buprenorphine use. (18) Finally, there is no information about the use of sublingual buprenorphine below the age of 4 years, (19) or indeed, the use of any sublingual medications in the newborn. The primary goal of this trial was to test the hypothesis that buprenorphine administered via the sublingual route in term infants with the NAS is safe, tolerable, and feasible. Secondary goals were to explore buprenorphine efficacy compared to standard of care treatment with NOS based upon a priori endpoints of length of treatment and length of stay. However, this was a preliminary study that was not powered to detect differences in these efficacy endpoints. Another secondary aim was to explore buprenorphine pharmacokinetics within the limits of what can be accomplished in this sized, otherwise healthy neonatal study population.
Methods
Study Design
This was a single site, randomized, open label trial conducted between April 2005 and January 2008. Twenty-six neonates were randomized to treatment with either sublingual buprenorphine or NOS in a 1:1 ratio. NAS was graded using a modified Finnegan scale, which is the standard instrument at Thomas Jefferson University Hospital. (20, 21) Initiation of treatment was based on any consecutive 3 scores adding up to ≥ 24. Inclusion criteria included ≥ 37 weeks gestation, exposure to opioids in utero, and demonstration of signs and symptoms of NAS requiring treatment. Exclusion criteria were major congenital malformations and/or intrauterine growth retardation, (22) medical illness requiring intensification of medical therapy, concomitant maternal benzodiazepine or severe alcohol abuse, maternal use of alcohol or of benzodiazepines in the 30 days prior to enrollment (as determined by self-report or intake urine drug screen), concomitant neonatal use of cytochrome P450 (CYP) 3A inhibitors or inducers prior to initiation of NAS treatment, seizure activity or other neurologic abnormality, breast feeding or inability of mother to give informed consent due to co-morbid psychiatric diagnosis. Informed consent was obtained for each patient prior to any study procedures. The study protocol conformed to the ethical guidelines of the Declaration of Helsinki as reflected in approval by the Institutional Review Board of Thomas Jefferson University. Computer-generated randomization was performed by the Hospital Investigational Drug Service.
Study Treatments
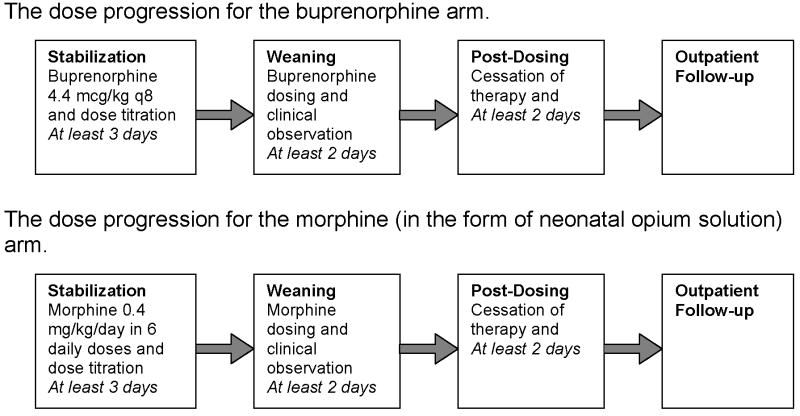
Patients randomized to sublingual buprenorphine initially received 13.2 mcg/kg/day in 3 divided doses. Buprenorphine solution was prepared by mixing buprenorphine for injection (Buprenex, Reckitt Benckiser, Richmond, VA) in 100% ethanol USP (30% final concentration) and simple syrup (85 gm sucrose/100 ml). Final buprenorphine concentration was 0.06 mg/mL. The solution was administered under the tongue followed by insertion of a pacifier to reduce swallowing. Nursing staff were instructed in sublingual technique by means of inservice training with observed administration. Initial volumes were ~0.2 ml and volumes >0.5 ml were administered in two aliquots separated by 2 minutes. Dose escalation was a 20% increase for Finnegan scale scores greater than 24 total on either 2 or 3 measures or a single score of 12. Patients with inadequate control could be administered a rescue dose of 50% of the previous dose, after which the subsequent dose would be advanced 20%. After at least 3 days of dose stabilization, patients could begin weaning for scores less than 8. Weaning was at intervals of 10% until cessation of dosing at or near the initial dose. If NAS was not controlled with maximally specified dose of 39 mcg/kg/day, patients were administered phenobarbital with a goal serum concentration of 20–30 mg/dl (Figure 1). This protocol was designed based on estimated buprenorphine pharmacokinetics and pharmacodynamics, and thus took into account differences relative to morphine in terms of starting dose and weaning protocol.
Figure 1.
Study Flow
Standard of care treatment at Thomas Jefferson University Hospital consisted of an initial dose of morphine 0.4 mg/kg/day (in the form of NOS) in 6 divided doses with dose escalation of 10%/day for Finnegan scale scores greater than 24 total on either 2 or 3 measures or a single score of 12. The final alcohol concentration of NOS was 0.19%. Adjunctive treatment with phenobarbital was initiated when the dose of NOS reached 1 mg/kg/day. Infants were weaned from NOS once they demonstrated control of their NAS as measured by the Finnegan scale for 48 hours. Daily dose was weaned by 10% every 24 hours as tolerated. If an infant required rescue therapy the equivalent of one extra dose of NOS was given. Regardless of treatment allocation to buprenorphine or NOS, all patients were observed for at least two days following the cessation of dosing. Dosing for all patients was based upon birth weight. In both buprenorphine and NOS groups, use of adjunct phenobarbital was considered a treatment failure, but not an adverse event.
Pharmacokinetics
Pharmacokinetic samples were drawn at pre-dose, post-dose, and mid-interval times in neonates randomized to buprenorphine. Pre- and post-dose samples were drawn 15–45 minutes before or after a dose. Capillary blood was obtained via heelstick. There was a protocol-specified maximum blood volume of 12 ml per patient. Buprenorphine and its primary metabolite norbuprenorphine were measured essentially as described by Moody et al. (23) with the transition of norbuprenorphine as the acetonitrile adduct (and its internal standard) modified to m/z 455 (459) to 414 (417) at ≈ −25eV. (24) This method was modified to accommodate the lower volume infant blood samples. Instead of the normal 1 mL aliquot used for plasma from adults, a 0.1 mL aliquot was used. To control for the smaller aliquot size, quality control samples were also run as 0.1 ml aliquots at concentrations of 0.25, 2.0 and 7.5 ng/ml. In any run at least 2/3rds of all quality controls had to be within ±20% of target and at least one of two, or two of three at any concentration, had to meet that acceptance criteria. The mean results for buprenorphine controls (N=13, expressed as percent of target) were 100.3, 95.3 and 98.5% at 0.25, 2.0 and 7.5 ng/ml, respectively. Those for norbuprenorphine were 94.3, 94.8, and 94.0%. The limits of quantification (LOQ) were 0.1 ng/ml for both analytes in most samples. The LOQ was 0.2 ng/ml in the 3% of the samples which had a small volume.
Statistics
This was a pilot study to examine the first use of sublingual administration of buprenorphine in neonates. In light of limited a priori knowledge of buprenorphine behavior in the neonatal population, sample size was not based upon a formal power calculation. Group comparisons for continuous variables were made using the Student’s t-test or the Wilcoxon Rank Sum test where appropriate. Statistical analysis was completed with JMP 5.1.2, (SAS Institute Inc.).
Results
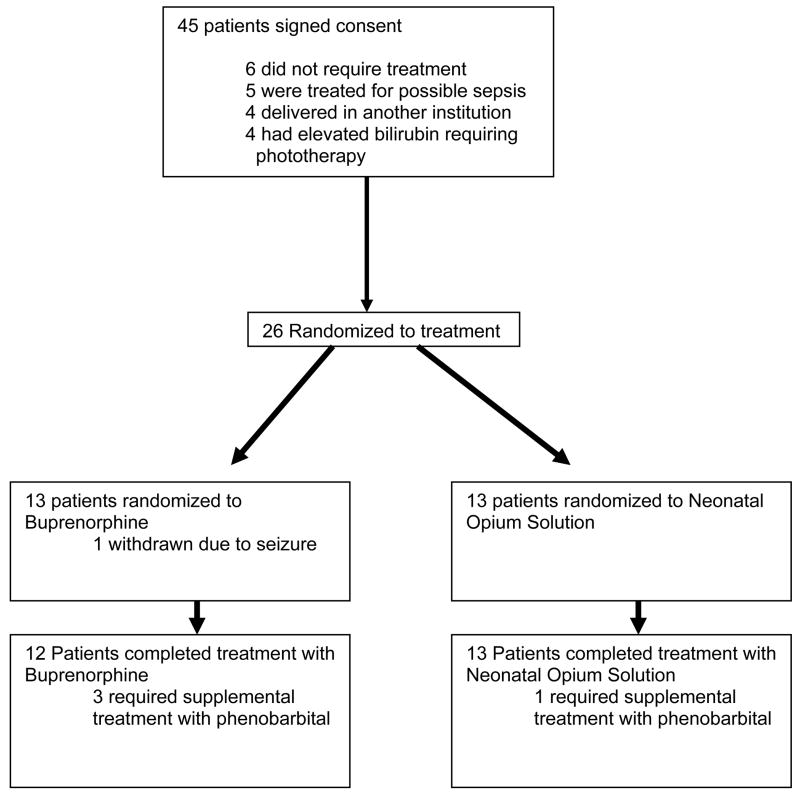
A total of 26 patients received treatment with either buprenorphine or NOS during the conduct of this trial. The buprenorphine and NOS groups were similar with regards to their gestational ages, birth weights and APGAR scores. (Table 1) All mothers were maintained on methadone. One subject randomized to buprenorphine did not complete a course of treatment. Enrollment is presented in Figure 2.
Table 1. Characteristics of Patients in the trial.
| Neonatal opium solution | Buprenorphine | P value | ||
|---|---|---|---|---|
| N | 13 | 13 | ||
| Sex | Male | 7 | 10 | |
| Female | 6 | 3 | ||
| Race | White, non Hispanic | 13 | 11 | |
| White, Hispanic | 0 | 2 | ||
| Onset of treatment mean days after birth (SD) || | 2.8 (1.6) | 2.5 (1.1) | 0.4 | |
| Gestational Age (weeks) || | 38.7 | 39.3 | 0.9 | |
| Birthweight (gm) | 3020 | 2985 | 0.97 | |
| 1-Minute APGAR Score* || | 8.2 | 7.3 | 0.2 | |
| 5-Minute APGAR Score* || | 8.8 | 8.5 | 0.3 |
Mean
One child in the buprenorphine group was born out of the hospital and did not have apgar scores recorded
Figure 2.
Disposition of Enrolled Patients
Pharmacokinetics
A total of 202 samples were analyzed. With the exception of three outliers, the maximum buprenorphine concentrations were 0.60 ng/ml. One outlier of 3.69 ng/ml buprenorphine occurred in an infant at the initial dose of 13.2 mcg/ml. The outlier values of 1.80 and 0.85 ng/ml occurred in one subject at the maximum protocol specified dose of 39 mcg/kg. The sample with 3.69 ng/ml of buprenorphine had a norbuprenorphine concentration of 0.83 ng/ml; all other samples had less than 0.33 ng/ml norbuprenorphine. A significant portion of samples were below the limit of quantification (35.6% for buprenorphine and 68.9% for norbuprenorphine). Low values occurred even during collection times temporally close to a dose, at higher doses, and during periods of adequate control of abstinence symptoms, suggesting possible uncoupling of plasma and peripheral compartment buprenorphine concentrations. Overall, there was a high degree of intra-subject variability. The ratio of buprenorphine to norbuprenorphine in the 51 samples where both were detected ranged from 0.47 to 8.1. Ratios were generally higher at peak compared to trough draws.
Adverse Events
The second patient randomized to buprenorphine developed generalized seizures 78 hours after the initial dose. The infant had 4 up titrations prior to this event, but no alteration in dose in the 16 hours immediately preceding the event and had demonstrated improved symptomatic control over this time period with Finnegan scores between 6 and 8. Buprenorphine was halted and treatment initiated with phenobarbital and NOS. Post event evaluation revealed normal serum hematology, chemistry, C-reactive protein, and lumbar puncture indices, and negative cultures. An interictal EEG was negative and MRI of the brain revealed a small amount of dependent subdural hemorrhage within the posterior fossa likely related to the birthing process and deemed unlikely to be symptomatic, with no parenchymal abnormalities. Lack of maternal exposure to benzodiazepines was reconfirmed. This child’s total length of stay was 28 days. At one-year follow up, the child was developmentally normal and seizure-free. A causal link of under-treatment of withdrawal or a dose dependent effect of buprenorphine was not immediately apparent to the investigators. Independent review was performed and it was recommended that the trial be resumed using the established protocol. Another child receiving buprenorphine developed a mild fungal paronychia judged to be unrelated to study drug.
Efficacy
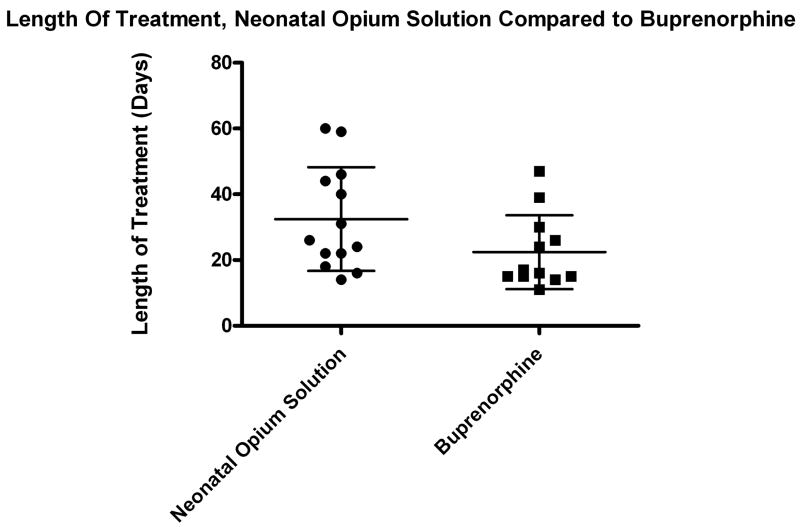
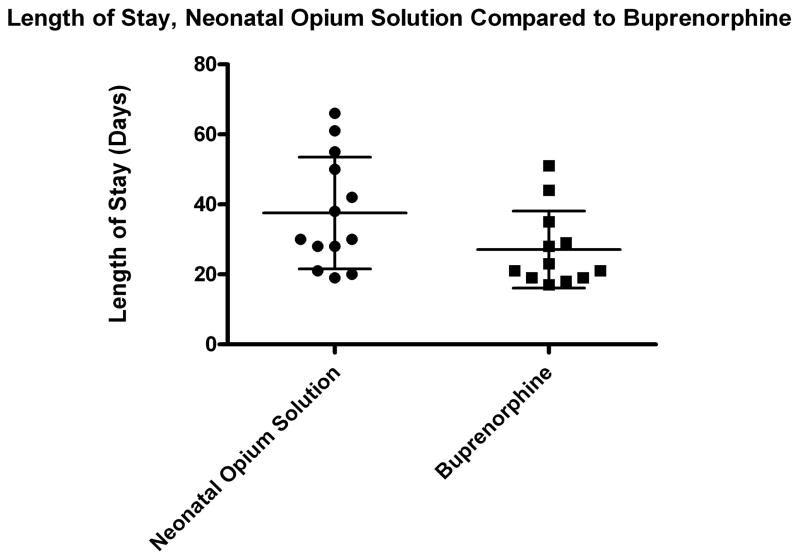
Despite a trend toward lower values in the buprenorphine-treated group, the clinical outcome variables of length of treatment and length of stay were not significantly different between the two groups (Table 2). The patient discontinued from the buprenorphine arm was not included in the efficacy analysis. The length of stay for this infant was 28 days. The mean length of treatment for the NOS group was 32 days (n=13, range 14–60 days, SD 16 days) compared to 22 days (n=12, range 11–47 days, SD 11 days) for the buprenorphine group, p-value=0.077 (Figure 3). The mean length of stay for the NOS group was 38 days (n=13, range 19–66 days, SD 16 days) compared to 27 days (n=12, range 17–51 days, SD 11 days) for the buprenorphine group, p-value=0.068. There was significant heterogeneity of response between patients. (Figure 4) Supplemental treatment with phenobarbital was required in three patients in the buprenorphine arm who reached the maximum dose of 39 mcg/kg/day and one patient in the NOS group.
Table 2. Outcomes of Treatment with Buprenorphine and Neonatal Opium Solution.
Data from the patient withdrawn from treatment due to seizure is not included. This infant had a length of stay of 28 days.
| Buprenorphine | Neonatal Opium Solution | p value* | |
|---|---|---|---|
| N | 12 | 13 | |
| Length of Treatment (days) | 22 | 32 | 0.077 |
| Length of Stay (days) | 27 | 38 | 0.068 |
Wilcoxon rank sum
Figure 3. Length of Treatment.
Length of treatment for infants treated with Neonatal Opium Solution (circles, n=13) and sublingual Buprenorphine (squares, n=12). One patient withdrawn from the buprenorphine group who had a length of treatment of 4 days of buprenorphine is not included. Small bars represent standard deviation and the large bar represents mean.
Figure 4. Length of Stay.
Length of stay for infants treated with Neonatal Opium Solution (circles, n=13) and sublingual Buprenorphine (squares, n=12).. One patient withdrawn from the buprenorphine group who had a length of stay of 28 days is not included. Small bars represent standard deviation and the large bar represents mean.
Discussion
This study is the first reported use of buprenorphine for the treatment of NAS. The feasibility of using sublingually administered buprenorphine for this indication was demonstrated. Administration was technically easy, and a decreased interval of dosing was convenient for nursing staff. With regard to safety, buprenorphine has a theoretical potential to precipitate withdrawal due to its partial agonist/antagonist activity. With the exception of a seizure in the second child treated, treatment with buprenorphine was uneventful. The etiology of the seizure in the second child treated with buprenorphine remains cryptic. Undertreatment of NAS due to underdosing is unlikely, given improved control of NAS symptoms in the day preceding the event. Buprenorphine-induced toxicity is also unlikely due to lack of reports of seizure induction in adults and a post-dose drug concentration of 0.35 ng/ml in this patient. While future investigations with the use of buprenorphine in this population will need to remain vigilant for this adverse event, it is possible that this seizure will be amongst the 10% classified as idiopathic at this age. (25)
A secondary endpoint of the study was an examination of efficacy compared to the standard of care treatment at our institution. Buprenorphine use was associated with a 31% reduction in length of treatment and a 29% reduction in the length of stay. However, the variance seen in both treatment arms was large and the two treatments were not statistically different. A mechanism to explain this suggestion of improved efficacy is not immediately apparent, but a longer half-life and greater affinity for the mu opioid receptor are plausible explanations. It should be noted that this feasibility study employed an unblinded design. Though the same nursing and physician teams made assessments of Finnegan scoring in both treatment arms, there was no blinding of staff or parents. We believe treatment allocation did not influence assessment of abstinence symptoms; however, occult bias in scoring or in weaning decisions cannot be definitively excluded. There was a higher percentage of males in the buprenorphine (10/13) compared to the NOS (7/13) groups. This imbalance is not expected to have biased treatment toward the buprenorphine group, as a recent investigation suggested that male neonates may have more severe NAS than females. (26)
The case report of Marquet describes a buprenorphine serum concentration of 1.9 ng/ml in a newborn of 20 hours age whose mother was maintained on 4 mg/day of buprenorphine. (18) Dose selection for this clinical trial employed a monoexponential pharmacokinetic model with a target steady state concentration of 2 ng/mL. With the exception of three outliers, actual concentrations observed during the trial were <0.6 ng/ml, with many samples below the limit of quantification of 0.1 ng/ml. It is notable that in 9 of 12 completed patients there was good control of withdrawal symptoms at these concentrations. This is surprising, as amelioration of adult signs of withdrawal is estimated to occur at 0.7 ng/ml and above. (27) This differential pharmacodynamic response based upon age may represent an additional safety margin for concentration-dependent adverse events associated with opioid agonists.
Given the necessity of a limited sampling regimen in those otherwise healthy newborns and large proportion of samples below the limits of quantification, formal pharmacokinetic parameters could not be generated. While the study population as a whole had measured buprenorphine and norbuprenorphine concentrations that remained within a relatively narrow range, there was significant dose-to-dose intra-subject variability that could not be explained solely by developmental ontogeny of metabolic enzymes. It is more likely that the variability noted was a reflection of variability of extent of sublingual dosing. Variability did not decrease as the study progressed. It is anticipated that some of the dose was swallowed, and that the amount swallowed and metabolized pre-systemically would vary from dose to dose. Morphine pharmacokinetics in neonates are also quite variable, (28) and ultimately clinical efficacy rather than fully characterized pharmacokinetic parameters will primarily drive dose selection. The ratio of buprenorphine to norbuprenorphine was between 0.47–8.1 in samples that had both analytes. These are higher than those calculated from the data presented by Huang et al. of 0.165 to 1.40. (29) That and the finding that norbuprenorphine was not present in two thirds of the samples suggest impaired N-dealkylation of buprenorphine in the newborn.
The need for phenobarbital rescue in three children randomized to buprenorphine suggests that the maximal dose used in the trial may not be high enough for the control of NAS. This theory is supported by the plasma concentrations observed and the inherent safety margin in adults, as well as that observed in this trial. An investigation using higher doses of buprenorphine is presently underway. The use of 30% ethanol solution was mandated by the Food and Drug Administration, and a future goal will be the reduction of ethanol administered. Benzodiazepine use was an exclusion criteria, which limits the generalizability of results to all infants exposed in utero to opioids. The rationale for this exclusion was anecdotal evidence of decreased therapeutic index of buprenorphine in adults who also abused benzodiazapines. Benzodiazepines cross into the placenta, (30, 31) though maternal confounders have made it difficult to estimate adverse effects specific to in utero exposure of benzodiazepine. (32) Having established a safety parameter, future investigations should also include neonates born to poly-drug abusing mothers. Breastfeeding was also an exclusion criteria. However, thought should be given to revisiting this exclusion, particularly in buprenorphine-maintained mothers, given suggestions of improved neonatal outcomes compared to formula fed infants. (33). As the abuse potential of buprenorphine is less than that of morphine and the dosing interval is longer, it is possible that buprenorphine may facilitate the treatment of NAS in care settings of lower acuity. Home administration in highly selected patients with visiting nursing care could be explored.
Research of optimal drug use in pregnant females and their infants has traditionally lagged that in other populations. (34) The present study could constitute the first major advance in decades in the treatment of NAS, particularly in view of the increasing use of buprenorphine in the maintenance of opioid-dependent pregnant females, particularly in Europe. (35) Incidence of NAS in the offspring of buprenorphine maintained mothers appears similar to that of mothers maintained on methadone. (36, 37) The use of buprenorphine for the treatment of these children is attractive.
In conclusion, we have demonstrated in this pilot study that the treatment of NAS with sublingual buprenorphine is feasible and has an acceptable safety margin. Confirmation of safety will require further study. A seizure occurred in one subject in the buprenorphine arm, but a direct causal relation could not be established. Intra-dose variability of buprenorphine is high, but control of symptoms occurred at serum concentrations lower than the 0.7 ng/ml estimated as the threshold needed to ameliorate adult abstinence. There is a suggestion of improved efficacy in terms of length of stay and length of treatment, but will need to be confirmed in a larger double blind, properly-powered clinical trial.
Acknowledgments
The project described was supported by the Commonwealth of Pennsylvania Tobacco Fund and Grant Number R21DA018207 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health. Dr. LaRusso is supported by NIH Postdoctoral training grant T32 GM08562. No authors declare a conflict of interest.
References The authors would like to thank Drs Ed Johnson and Ashwin A. Patkar for their scientific input.
Abbreviations
- NAS
neonatal abstinence syndrome
- NOS
neonatal opium solution
- SD
standard deviation
- LOQ
limits of quantification
- CYP
cytochrome
Footnotes
ClinicalTrials.gov Identifier # NCT00521248
References
- 1.Vega WA, Kolody B, Hwang J, Noble A. Prevalence and magnitude of perinatal substance exposures in California. N Engl J Med. 1993;16;329(12):850–4. doi: 10.1056/NEJM199309163291207. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics Committee on Drugs. Neonatal drug withdrawal. Pediatrics. 1998;101(6):1079–88. [PubMed] [Google Scholar]
- 3.Nandakumar N, Sankar VS. What is the best evidence based management of neonatal abstinence syndrome? Arch Dis Child Fetal Neonatal Ed. 2006;91(6):F463. doi: 10.1136/adc.2006.095166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarkar S, Donn SM. Management of neonatal abstinence syndrome in neonatal intensive care units: A national survey. J Perinatol. 2006;1;26(1):15–7. doi: 10.1038/sj.jp.7211427. [DOI] [PubMed] [Google Scholar]
- 5.Osborn DA, Jeffery HE, Cole M. Opiate treatment for opiate withdrawal in newborn infants. Cochrane Database Syst Rev. 2005;(3):CD002059. doi: 10.1002/14651858.CD002059.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Osborn DA, Jeffery HE, Cole MJ. Sedatives for opiate withdrawal in newborn infants. Cochrane Database Syst Rev. 2005;(3):CD002053. doi: 10.1002/14651858.CD002053.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Johnson K, Gerada C, Greenough A. Treatment of neonatal abstinence syndrome. Arch Dis Child Fetal Neonatal Ed. 2003;88(1):F2–5. doi: 10.1136/fn.88.1.F2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lainwala S, Brown ER, Weinschenk NP, Blackwell MT, Hagadorn JI. A retrospective study of length of hospital stay in infants treated for neonatal abstinence syndrome with methadone versus oral morphine preparations. Adv Neonatal Care. 2005;5(5):265–72. doi: 10.1016/j.adnc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Langenfeld S, Birkenfeld L, Herkenrath P, Muller C, Hellmich M, Theisohn M. Therapy of the neonatal abstinence syndrome with tincture of opium or morphine drops. Drug Alcohol Depend. 2005;77(1):31–6. doi: 10.1016/j.drugalcdep.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Coyle MG, Ferguson A, Lagasse L, Oh W, Lester B. Diluted tincture of opium (DTO) and phenobarbital versus DTO alone for neonatal opiate withdrawal in term infants. J Pediatr. 2002;140(5):561–4. doi: 10.1067/mpd.2002.123099. [DOI] [PubMed] [Google Scholar]
- 11.Jackson L, Ting A, McKay S, Galea P, Skeoch C. A randomised controlled trial of morphine versus phenobarbitone for neonatal abstinence syndrome. Arch Dis Child Fetal Neonatal Ed. 2004;89(4):F300–4. doi: 10.1136/adc.2003.033555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krantz MJ, Mehler PS. Treating opioid dependence. growing implications for primary care. Arch Intern Med. 2004;164(3):277–88. doi: 10.1001/archinte.164.3.277. [DOI] [PubMed] [Google Scholar]
- 13.Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: Ceiling effects at high doses. Clin Pharmacol Ther. 1994;55(5):569–80. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- 14.Krantz MJ, Lewkowiez L, Hays H, Woodroffe MA, Robertson AD, Mehler PS. Torsade de pointes associated with very-high-dose methadone. Ann Intern Med. 2002;137(6):501–4. doi: 10.7326/0003-4819-137-6-200209170-00010. [DOI] [PubMed] [Google Scholar]
- 15.Greenwald M, Johanson CE, Bueller J, Chang Y, Moody DE, Kilbourn M, et al. Buprenorphine duration of action: Mu-opioid receptor availability and pharmacokinetic and behavioral indices. Biol Psychiatry. 2007;61(1):101–10. doi: 10.1016/j.biopsych.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 16.Michel E, Zernikow B. Buprenorphine in children. A clinical and pharmacological review. Schmerz. 2006;20(1):40–50. doi: 10.1007/s00482-005-0456-1. [DOI] [PubMed] [Google Scholar]
- 17.Barrett DA, Simpson J, Rutter N, Kurihara-Bergstrom T, Shaw PN, Davis SS. The pharmacokinetics and physiological effects of buprenorphine infusion in premature neonates. Br J Clin Pharmacol. 1993;36(3):215–9. doi: 10.1111/j.1365-2125.1993.tb04220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marquet P, Chevrel J, Lavignasse P, Merle L, Lachatre G. Buprenorphine withdrawal syndrome in a newborn. Clin Pharmacol Ther. 1997;62(5):569–71. doi: 10.1016/S0009-9236(97)90053-9. [DOI] [PubMed] [Google Scholar]
- 19.Maunuksela EL, Korpela R, Olkkola KT. Comparison of buprenorphine with morphine in the treatment of postoperative pain in children. Anesth Analg. 1988;67(3):233–9. [PubMed] [Google Scholar]
- 20.Finnegan LP, Kaltenbach K. Neonatal abstinence syndrome. In: Hoekelman RA, Friedman SB, Nelson N, Seidel HM, editors. Primary Pediatric Care. 2. Mosby; St. Louis: 1992. pp. 1367–78. [Google Scholar]
- 21.Jones HE, Johnson RE, Jasinski DR, O'Grady KE, Chisholm CA, Choo RE, et al. Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: Effects on the neonatal abstinence syndrome. Drug Alcohol Depend. 2005;79(1):1–10. doi: 10.1016/j.drugalcdep.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Mamelle N, Cochet V, Claris O. Definition of fetal growth restriction according to constitutional growth potential. Biol Neonate. 2001;80(4):277–85. doi: 10.1159/000047157. [DOI] [PubMed] [Google Scholar]
- 23.Moody DE, Slawson MH, Strain EC, Laycock JD, Spanbauer AC, Foltz RL. A liquid chromatographic-electrospray ionization-tandem mass spectrometric method for determination of buprenorphine, its metabolite, norbuprenorphine, and a coformulant, naloxone, that is suitable for in vivo and in vitro metabolism studies. Anal Biochem. 2002;306(1):31–9. doi: 10.1006/abio.2002.5673. [DOI] [PubMed] [Google Scholar]
- 24.Chang Y, Moody DE, McCance-Katz EF. Novel metabolites of buprenorphine detected in human liver microsomes and human urine. Drug Metab Dispos. 2006;34(3):440–8. doi: 10.1124/dmd.105.006148. [DOI] [PubMed] [Google Scholar]
- 25.Mizrahi EM, Kellaway P. Diagnosis and management of neonatal seizures. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- 26.Jansson LM, Dipietro JA, Elko A, Velez M. Maternal vagal tone change in response to methadone is associated with neonatal abstinence syndrome severity in exposed neonates. J Matern Fetal Neonatal Med. 2007;20(9):677–85. doi: 10.1080/14767050701490327. [DOI] [PubMed] [Google Scholar]
- 27.Kuhlman JJ, Jr, Levine B, Johnson RE, Fudala PJ, Cone EJ. Relationship of plasma buprenorphine and norbuprenorphine to withdrawal symptoms during dose induction, maintenance and withdrawal from sublingual buprenorphine. Addiction. 1998;93(4):549–59. doi: 10.1046/j.1360-0443.1998.93454910.x. [DOI] [PubMed] [Google Scholar]
- 28.Kart T, Christrup LL, Rasmussen M. Recommended use of morphine in neonates, infants and children based on a literature review: Part 1--pharmacokinetics. Paediatr Anaesth. 1997;7(1):5–11. doi: 10.1046/j.1460-9592.1997.d01-30.x. [DOI] [PubMed] [Google Scholar]
- 29.Huang W, Moody DE, McCance-Katz EF. The in vivo glucuronidation of buprenorphine and norbuprenorphine determined by liquid chromatography-electrospray ionization-tandem mass spectrometry. Ther Drug Monit. 2006;28(2):245–51. doi: 10.1097/01.ftd.0000197094.92559.b4. [DOI] [PubMed] [Google Scholar]
- 30.McGrath C, Buist A, Norman TR. Treatment of anxiety during pregnancy: Effects of psychotropic drug treatment on the developing fetus. Drug Saf. 1999;20(2):171–86. doi: 10.2165/00002018-199920020-00006. [DOI] [PubMed] [Google Scholar]
- 31.Myllynen P, Vahakangas K. An examination of whether human placental perfusion allows accurate prediction of placental drug transport: Studies with diazepam. J Pharmacol Toxicol Methods. 2002;48(3):131–8. doi: 10.1016/S1056-8719(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 32.Wikner BN, Stiller CO, Kallen B, Asker C. Use of benzodiazepines and benzodiazepine receptor agonists during pregnancy: Maternal characteristics. Pharmacoepidemiol Drug Saf. 2007;16(9):988–94. doi: 10.1002/pds.1391. [DOI] [PubMed] [Google Scholar]
- 33.Abdel-Latif ME, Pinner J, Clews S, Cooke F, Lui K, Oei J. Effects of breast milk on the severity and outcome of neonatal abstinence syndrome among infants of drug-dependent mothers. Pediatrics. 2006;117(6):e1163–9. doi: 10.1542/peds.2005-1561. [DOI] [PubMed] [Google Scholar]
- 34.Chambers CD, Polifka JE, Friedman JM. Drug safety in pregnant women and their babies: Ignorance not bliss. Clin Pharmacol Ther. 2008;83(1):181–3. doi: 10.1038/sj.clpt.6100448. [DOI] [PubMed] [Google Scholar]
- 35.Fischer G, Ortner R, Rohrmeister K, Jagsch R, Baewert A, Langer M, et al. Methadone versus buprenorphine in pregnant addicts: A double-blind, double-dummy comparison study. Addiction. 2006;101(2):275–81. doi: 10.1111/j.1360-0443.2006.01321.x. [DOI] [PubMed] [Google Scholar]
- 36.Kahila H, Saisto T, Kivitie-Kallio S, Haukkamaa M, Halmesmaki E. A prospective study on buprenorphine use during pregnancy: Effects on maternal and neonatal outcome. Acta Obstet Gynecol Scand. 2007;86(2):185–90. doi: 10.1080/00016340601110770. [DOI] [PubMed] [Google Scholar]
- 37.Johnson RE, Jones HE, Fischer G. Use of buprenorphine in pregnancy: Patient management and effects on the neonate. Drug Alcohol Depend. 2003;21;70(2 Suppl):S87–S101. doi: 10.1016/s0376-8716(03)00062-0. [DOI] [PubMed] [Google Scholar]