Abstract
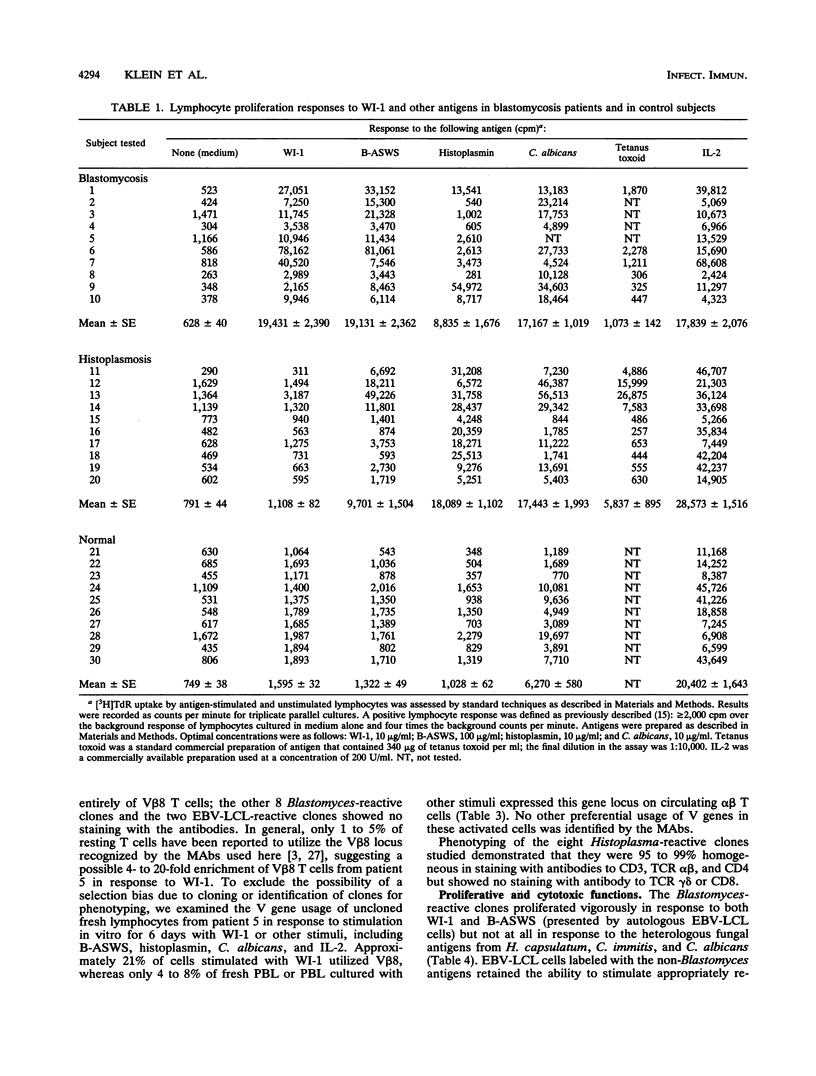
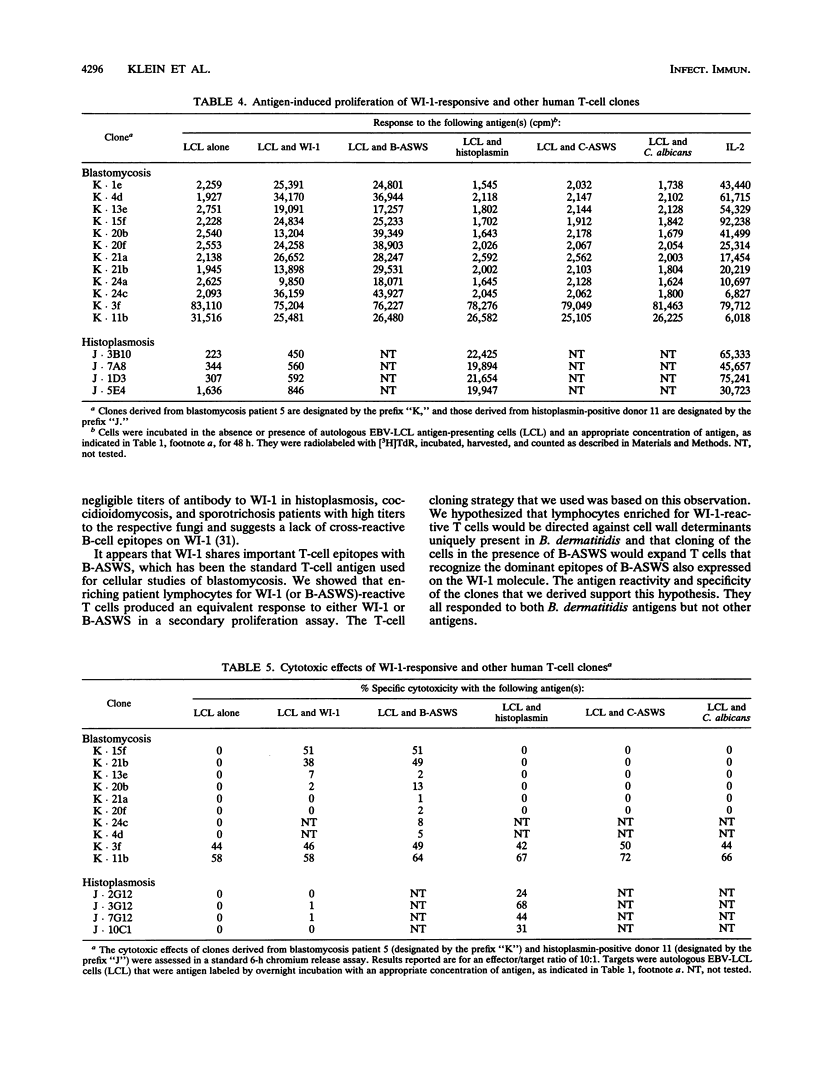
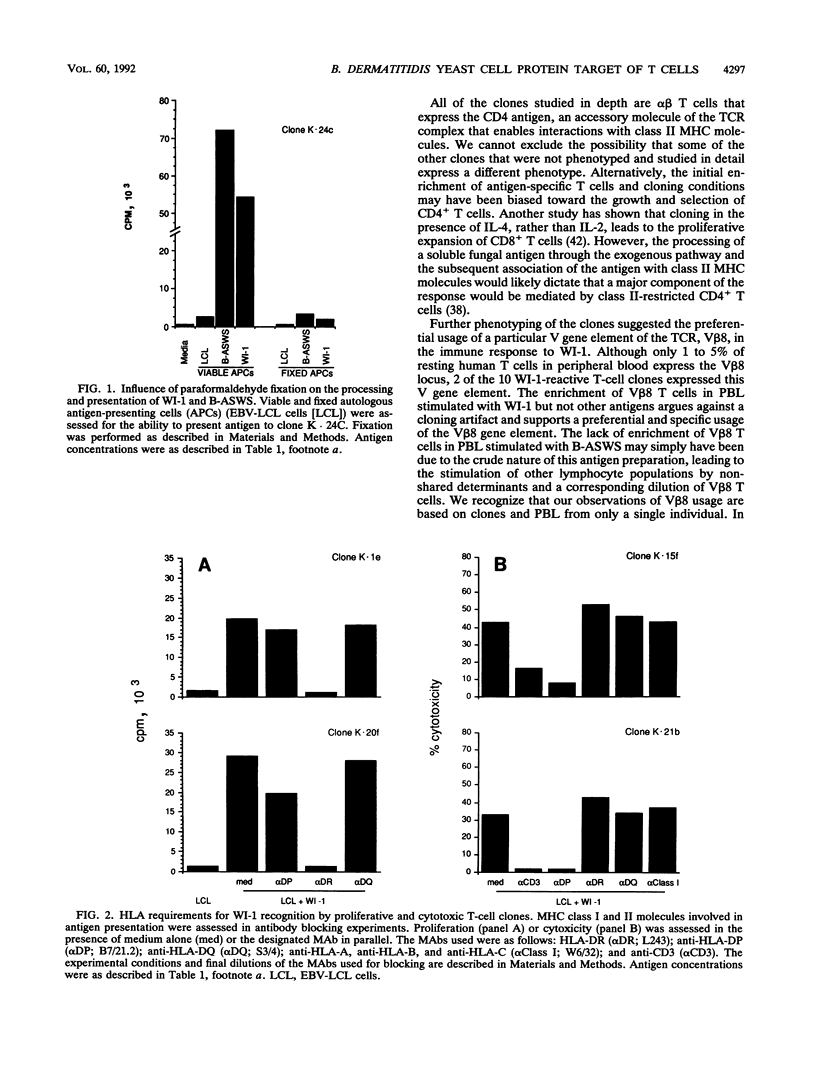
A large body of experimental data has demonstrated the central role of T cells in acquired resistance to the dimorphic fungus Blastomyces dermatitidis. We examined the human T-cell response to WI-1, a 120-kDa B. dermatitidis yeast cell surface protein recently shown to be an immunodominant antigen of the B-cell response in infected humans. Peripheral blood lymphocytes from 10 blastomycosis patients studied proliferated in response to WI-1 (mean, 19,431 cpm) and to the standard, crude cell wall antigen, Blastomyces alkali- and water-soluble antigen (B-ASWS) (mean, 19,131 cpm); lymphocytes from 10 histoplasmosis patients and 10 normal control subjects did not respond to WI-1. WI-1 stimulation of patient lymphocytes and rechallenge with WI-1 or B-ASWS showed that the antigens share immunodominant epitopes. Of 100 WI-1-responsive T-cell clones derived from peripheral blood, 10 were studied in detail to assess the phenotype, function, and ligands recognized. The clones exhibit the CD3+ CD4+ phenotype of helper T cells; 2 of 10 clones (and 21% of antigen-stimulated peripheral blood lymphocytes) use the V beta 8 T-cell receptor gene element to respond to WI-1. All the clones proliferate in response to both WI-1 and B-ASWS but not other fungal antigens, and some mediate potent cytolytic effects on WI-1- and B-ASWS-labeled targets. WI-1 recognition requires antigen processing and presentation of epitopes in association with HLA-DR (to noncytolytic clones) and HLA-DP (to cytolytic clones). From these findings, we conclude that CD4+ T cells with regulatory and cytolytic properties are involved in the development of acquired resistance of B. dermatitidis, that the cells are directed against WI-1, and that the manner of display of WI-1 peptide epitopes in conjunction with major histocompatibility complex class II may influence the profile of the immune response.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adu H. O., Curtis J., Turk J. L. The resistance of C57BL/6 mice to subcutaneous infection with Mycobacterium lepraemurium is dependent on both T cells and other cells of bone marrow origin. Cell Immunol. 1983 Jun;78(2):249–256. doi: 10.1016/0008-8749(83)90279-4. [DOI] [PubMed] [Google Scholar]
- Boylston A. W., Borst J., Yssel H., Blanchard D., Spits H., de Vries J. E. Properties of a panel of monoclonal antibodies which react with the human T cell antigen receptor on the leukemic line HPB-ALL and a subset of normal peripheral blood T lymphocytes. J Immunol. 1986 Jul 15;137(2):741–744. [PubMed] [Google Scholar]
- Bradsher R. W., Alford R. H. Blastomyces dermatitidis antigen-induced lymphocytes reactivity in human blastomycosis. Infect Immun. 1981 Aug;33(2):485–490. doi: 10.1128/iai.33.2.485-490.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradsher R. W., Balk R. A., Jacobs R. F. Growth inhibition of Blastomyces dermatitidis in alveolar and peripheral macrophages from patients with blastomycosis. Am Rev Respir Dis. 1987 Feb;135(2):412–417. doi: 10.1164/arrd.1987.135.2.412. [DOI] [PubMed] [Google Scholar]
- Bradsher R. W. Development of specific immunity in patients with pulmonary or extrapulmonary blastomycosis. Am Rev Respir Dis. 1984 Mar;129(3):430–434. doi: 10.1164/arrd.1984.129.3.430. [DOI] [PubMed] [Google Scholar]
- Bradsher R. W., Ulmer W. C., Marmer D. J., Townsend J. W., Jacobs R. F. Intracellular growth and phagocytosis of Blastomyces dermatitidis by monocyte-derived macrophages from previously infected and normal subjects. J Infect Dis. 1985 Jan;151(1):57–64. doi: 10.1093/infdis/151.1.57. [DOI] [PubMed] [Google Scholar]
- Bronnimann D. A., Adam R. D., Galgiani J. N., Habib M. P., Petersen E. A., Porter B., Bloom J. W. Coccidioidomycosis in the acquired immunodeficiency syndrome. Ann Intern Med. 1987 Mar;106(3):372–379. doi: 10.7326/0003-4819-106-3-372. [DOI] [PubMed] [Google Scholar]
- Brummer E., Morozumi P. A., Vo P. T., Stevens D. A. Protection against pulmonary blastomycosis: adoptive transfer with T lymphocytes, but not serum, from resistant mice. Cell Immunol. 1982 Nov 1;73(2):349–359. doi: 10.1016/0008-8749(82)90461-0. [DOI] [PubMed] [Google Scholar]
- Brummer E., Morrison C. J., Stevens D. A. Recombinant and natural gamma-interferon activation of macrophages in vitro: different dose requirements for induction of killing activity against phagocytizable and nonphagocytizable fungi. Infect Immun. 1985 Sep;49(3):724–730. doi: 10.1128/iai.49.3.724-730.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casten L. A., Lakey E. K., Jelachich M. L., Margoliash E., Pierce S. K. Anti-immunoglobulin augments the B-cell antigen-presentation function independently of internalization of receptor-antigen complex. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5890–5894. doi: 10.1073/pnas.82.17.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess L., Bock G. N., Mardiney M. R., Jr Restoration of the reactivity of frozen stored human lymphocytes in the mixed lymphocyte reaction and in response to specific antigens. Transplantation. 1972 Dec;14(6):728–733. doi: 10.1097/00007890-197212000-00010. [DOI] [PubMed] [Google Scholar]
- Cox R. A., Arnold D. R. Immunoglobulin E in coccidioidomycosis. J Immunol. 1979 Jul;123(1):194–200. [PubMed] [Google Scholar]
- Cox R. A. Cross-reactivity between antigens of Coccidioides immitis, Histoplasma capsulatum and Blastomyces dermatitidis in lymphocyte transformation assays. Infect Immun. 1979 Sep;25(3):932–938. doi: 10.1128/iai.25.3.932-938.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Larsh H. W. Isolation of skin test-active preparations from yeast-phase cells of Blastomyces dermatitidis. Infect Immun. 1974 Jul;10(1):42–47. doi: 10.1128/iai.10.1.42-47.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Larsh H. W. Yeast- and mycelial-phase antigens of Blastomyces dermatitidis: comparison using disc gel electrophoresis. Infect Immun. 1974 Jul;10(1):48–53. doi: 10.1128/iai.10.1.48-53.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozad G. C., Chang C. T. Cell-mediated immunoprotection in blastomycosis. Infect Immun. 1980 May;28(2):398–403. doi: 10.1128/iai.28.2.398-403.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENTON J. F., McDONOUGH E. S., AJELLO L., AUSHERMAN R. J. Isolation of Blastomyces dermatitidis from soil. Science. 1961 Apr 14;133(3459):1126–1127. doi: 10.1126/science.133.3459.1126. [DOI] [PubMed] [Google Scholar]
- Deighton F., Cox R. A., Hall N. K., Larsh H. W. In vivo and in vitro cell-mediated immune responses to a cell wall antigen of Blastomyces dermatitidis. Infect Immun. 1977 Feb;15(2):429–435. doi: 10.1128/iai.15.2.429-435.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch P., Malkovsky M., Kovats S., Sturm E., Braakman E., Klein B. S., Voss S. D., Morrissey L. W., DeMars R., Welch W. J. Recognition by human V gamma 9/V delta 2 T cells of a GroEL homolog on Daudi Burkitt's lymphoma cells. Science. 1990 Nov 30;250(4985):1269–1273. doi: 10.1126/science.1978758. [DOI] [PubMed] [Google Scholar]
- Gately M. K., Mayer M. M., Henney C. S. Effect of anti-lymphotoxin on cell-mediated cytotoxicity. Evidence for two pathways, one involving lymphotoxin and the other requiring intimate contact between the plasma membranes of killer and target cells. Cell Immunol. 1976 Nov;27(1):82–93. doi: 10.1016/0008-8749(76)90156-8. [DOI] [PubMed] [Google Scholar]
- Hall N. K., Deighton F., Larsh H. W. Use of an alkali-soluble water-soluble extract of Blastomyces dermatitidis yeast-phase cell walls and isoelectrically focused components in peripheral lymphocyte transformations. Infect Immun. 1978 Feb;19(2):411–415. doi: 10.1128/iai.19.2.411-415.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hank J. A., Sondel P. M. Recognition of autologous lymphoblastoid cells by cloned human T cells: inhibition of cytotoxicity by anti-HLA antibody but not by antibody to Epstein-Barr virus induced cell surface antigen. Hum Immunol. 1984 Apr;9(4):211–219. doi: 10.1016/0198-8859(84)90026-0. [DOI] [PubMed] [Google Scholar]
- Kappler J., Kotzin B., Herron L., Gelfand E. W., Bigler R. D., Boylston A., Carrel S., Posnett D. N., Choi Y., Marrack P. V beta-specific stimulation of human T cells by staphylococcal toxins. Science. 1989 May 19;244(4906):811–813. doi: 10.1126/science.2524876. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., De Libero G. Listeria monocytogenes-reactive T lymphocyte clones with cytolytic activity against infected target cells. J Exp Med. 1986 Jul 1;164(1):363–368. doi: 10.1084/jem.164.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., Väth U., Müller I. Effective protection against Listeria monocytogenes and delayed-type hypersensitivity to listerial antigens depend on cooperation between specific L3T4+ and Lyt 2+ T cells. Infect Immun. 1985 Apr;48(1):263–266. doi: 10.1128/iai.48.1.263-266.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein B. S., Bradsher R. W., Vergeront J. M., Davis J. P. Development of long-term specific cellular immunity after acute Blastomyces dermatitidis infection: assessments following a large point-source outbreak in Wisconsin. J Infect Dis. 1990 Jan;161(1):97–101. doi: 10.1093/infdis/161.1.97. [DOI] [PubMed] [Google Scholar]
- Klein B. S., Jones J. M. Isolation, purification, and radiolabeling of a novel 120-kD surface protein on Blastomyces dermatitidis yeasts to detect antibody in infected patients. J Clin Invest. 1990 Jan;85(1):152–161. doi: 10.1172/JCI114406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein B. S., Vergeront J. M., Weeks R. J., Kumar U. N., Mathai G., Varkey B., Kaufman L., Bradsher R. W., Stoebig J. F., Davis J. P. Isolation of Blastomyces dermatitidis in soil associated with a large outbreak of blastomycosis in Wisconsin. N Engl J Med. 1986 Feb 27;314(9):529–534. doi: 10.1056/NEJM198602273140901. [DOI] [PubMed] [Google Scholar]
- Lancaster M. V., Sprouse R. F. Isolation of a purified skin test antigen from Blastomyces dermatitidis yeast-phase cell wall. Infect Immun. 1976 Sep;14(3):623–625. doi: 10.1128/iai.14.3.623-625.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. R., Cerottini J. C., Ryser J. E., Maryanski J. L., Taswell C., Widmer M. B., Brunner K. T. Quantitation and cloning of cytolytic T lymphocytes and their precursors. Immunol Rev. 1980;51:93–123. doi: 10.1111/j.1600-065x.1980.tb00318.x. [DOI] [PubMed] [Google Scholar]
- Maecker H. T., Levy R. Prevalence of antigen receptor variants in human T cell lines and tumors. J Immunol. 1989 Feb 15;142(4):1395–1404. [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- Moller D. R., Konishi K., Kirby M., Balbi B., Crystal R. G. Bias toward use of a specific T cell receptor beta-chain variable region in a subgroup of individuals with sarcoidosis. J Clin Invest. 1988 Oct;82(4):1183–1191. doi: 10.1172/JCI113715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison L. A., Lukacher A. E., Braciale V. L., Fan D. P., Braciale T. J. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med. 1986 Apr 1;163(4):903–921. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Adoptive protection of the Mycobacterium tuberculosis-infected lung. Dissociation between cells that passively transfer protective immunity and those that transfer delayed-type hypersensitivity to tuberculin. Cell Immunol. 1984 Mar;84(1):113–120. doi: 10.1016/0008-8749(84)90082-0. [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., Elferink D. G., Hermans J., de Vries R. R. HLA class II restriction repertoire of antigen-specific T cells. I. The main restriction determinants for antigen presentation are associated with HLA-D/DR and not with DP and DQ. Hum Immunol. 1985 Jun;13(2):105–116. doi: 10.1016/0198-8859(85)90017-5. [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., Mutis T. Specific killing of cytotoxic T cells and antigen-presenting cells by CD4+ cytotoxic T cell clones. A novel potentially immunoregulatory T-T cell interaction in man. J Exp Med. 1990 Jun 1;171(6):2011–2024. doi: 10.1084/jem.171.6.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliard X., Malefijt R. W., de Vries J. E., Spits H. Interleukin-4 mediates CD8 induction on human CD4+ T-cell clones. Nature. 1988 Oct 13;335(6191):642–644. doi: 10.1038/335642a0. [DOI] [PubMed] [Google Scholar]
- Pavlov H., Hogarth M., McKenzie I. F., Cheers C. In vivo and in vitro effects of monoclonal antibody to Ly antigens on immunity to infection. Cell Immunol. 1982 Jul 15;71(1):127–138. doi: 10.1016/0008-8749(82)90502-0. [DOI] [PubMed] [Google Scholar]
- Rollwagen F. M., Dasch G. A., Jerrells T. R. Mechanisms of immunity to rickettsial infection: characterization of a cytotoxic effector cell. J Immunol. 1986 Feb 15;136(4):1418–1421. [PubMed] [Google Scholar]
- Sarosi G. A., Hammerman K. J., Tosh F. E., Kronenberg R. S. Clinical features of acute pulmonary blastomycosis. N Engl J Med. 1974 Mar 7;290(10):540–543. doi: 10.1056/NEJM197403072901004. [DOI] [PubMed] [Google Scholar]
- Scillian J. J., Cozad G. C., Spencer H. D. Passive transfer of delayed hypersensitivity to Blastomyces dermatitidis between mice. Infect Immun. 1974 Oct;10(4):705–711. doi: 10.1128/iai.10.4.705-711.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimonkevitz R., Colon S., Kappler J. W., Marrack P., Grey H. M. Antigen recognition by H-2-restricted T cells. II. A tryptic ovalbumin peptide that substitutes for processed antigen. J Immunol. 1984 Oct;133(4):2067–2074. [PubMed] [Google Scholar]
- Sondel P. M., O'Brien C., Porter L., Schlossman S. F., Chess L. Cell-mediated destruction of human leukemic cells by MHC identical lymphocytes: requirement for a proliferative trigger in vitro. J Immunol. 1976 Dec;117(6):2197–2203. [PubMed] [Google Scholar]
- Spencer H. D., Cozad G. C. Role of delayed hypersensitivity in blastomycosis of mice. Infect Immun. 1973 Mar;7(3):329–334. doi: 10.1128/iai.7.3.329-334.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheat L. J., Slama T. G., Zeckel M. L. Histoplasmosis in the acquired immune deficiency syndrome. Am J Med. 1985 Feb;78(2):203–210. doi: 10.1016/0002-9343(85)90427-9. [DOI] [PubMed] [Google Scholar]



