Abstract
Purpose
Proinflammatory cytokines in degenerative diseases can lead to the loss of normal physiology and the destruction of surrounding tissues. In the present study, the physiological responses of human fetal retinal pigment epithelia (hfRPE) were examined in vitro after polarized activation of proinflammatory cytokine receptors.
Methods
Primary cultures of hfRPE were stimulated with an inflammatory cytokine mixture (ICM): interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ. Western blot analysis and immunofluorescence were used to determine the expression/localization of the cytokine receptors on hfRPE. Polarized secretion of cytokines was measured. A capacitance probe technique was used to measure transepithelial fluid flow (JV) and resistance (RT).
Results
IL-1R1 was mainly localized to the apical membrane and TNFR1 to the basal membrane, whereas IFN-γR1 was detected on both membranes. Activation by apical ICM induced a significant secretion of angiogenic and angiostatic chemokines, mainly across the hfRPE apical membrane. Addition of the ICM to the basal but not the apical bath significantly increased net fluid absorption (JV) across the hfRPE within 20 minutes. Similar increases in JV were produced by a 24-hour exposure to ICM, which significantly decreased total RT.
Conclusions
Chemokine gradients across the RPE can be altered (1) through an ICM-induced change in polarized chemokine secretion and (2) through an increase in ICM-induced net fluid absorption. In vivo, both of these factors could contribute to the development of chemokine gradients that help mediate the progression of inflammation/angiogenesis at the retina/RPE/choroid complex.
The retinal pigment epithelium (RPE) is a monolayer of polarized pigmented cells that form the outer blood-retina barrier.1-3 In the subretinal space (SRS) of the intact eye, the RPE apical membrane is in close physical proximity to the distal ends of the photoreceptors, whereas the basolateral membrane is closely apposed to Bruch’s membrane and the choroidal blood supply. The RPE is remarkable in its ability to transport fluid and to secrete a variety of growth and neurotrophic factors and proinflammatory cytokines and chemokines in a polarized manner.4-9 In vitro, RPE cells have been shown to produce monocyte chemotactic protein (MCP)-1, interleukin (IL)-8, IL-6,10 regulated on activation of normal T-cell expressed and secreted (RANTES),11 interferon (IFN)-γ induced protein (IP) of 10 kDa (IP-10),12 and growth-related oncogene (GRO)-α,13 with or without stimulation by other proinflammatory cytokines. Together, the secretion of proinflammatory cytokines and chemokines14-16 and fluid transport17,18 could act in concert to change the course of inflammatory diseases.
Cytokines are soluble proteinaceous substances (5-20 kDa) produced by a wide variety of cell types that secrete humoral factors and act to modulate the activities of other cells or tissues in normal or pathologic conditions. The functions of cytokines are pleiotropic and redundant. Chemokines are a subfamily of low-molecular-weight cytokines (8-10 kDa) identified by their ability to attract and activate leukocytes. CC and CXC are two structural variants that differ according to variations in a shared cysteine (C) motif. CC chemokines contain adjacent cysteines and are chemoattractants for lymphocytes, monocytes, eosinophils, and basophils, whereas CXC chemokines contain paired cysteines separated by a different amino acid and are chemoattractants for neutrophils. CXC chemokines can be categorized in terms of the “ELR” motif, which has been shown to associate the presence or absence of glutamic acid-leucine-arginine immediately preceding the first cysteine residue of the primary structure of these molecules with proangiogenic activity or its absence with antiangiogenic activity.19 In addition, some CC chemokines are highly angiogenic.20,21
Some chemokines are constitutively expressed in the eye, participate in the normal physiological activities of ocular cells and tissues,10 and undergo a dramatic increase in expression in diseases such as uveitis,15,22 diabetic proliferative retinopathy,23 and age-related macular degeneration (AMD).24 There is growing evidence that the immunologic properties of the RPE play a critical role in the accumulation of drusen and the progression to choroidal neovascularization (CNV) in AMD.25-32
The present study utilizes a previously described hfRPE primary culture model that has the morphology, polarization, and other physiologic properties of intact native tissue.7 A mixture of three proinflammatory cytokines—IL-1β, IFN-γ, and tumor necrosis factor (TNF)-α—was used to stimulate confluent monolayers of hfRPE. These proinflammatory cytokines have been found to be elevated in animal models of experimental autoimmune uveitis (EAU)14 and in patients with uveitis.33,34 Similarly, increased levels of TNF-α and IL-1β are also detected in the vitreous and blood of patients with proliferative diabetic retinopathy (PDR),35 and in those with AMD with CNV.36 Our data show that hfRPE expresses the receptors for all three cytokines and that activation of these receptors can dramatically affect the polarized secretion of cytokines/chemokines, alter transepithelial resistance, and increase net epithelial fluid absorption from the retinal to the choroidal side of the monolayer. These experiments suggest how the RPE in vivo can actively control the inflammatory environment in the SRS and choroid.
Methods
Human Fetal Tissue
The research performed in this study adhered to the tenets of the Declaration of Helsinki and the NIH Institutional Review Board. Human fetal eyes of nominal gestation of 15 to 17 weeks were obtained from Advanced Bioscience Resources (Alameda, CA).
Cell Culture
Primary cell cultures of hfRPE cells were prepared from human fetal eyes as described previously.7 Second-passage cells were seeded in 0.4-μm pore polyester transwells (Transwell; Corning Inc., Corning, NY). Media were changed every 3 days, and the cultures were maintained for 6 weeks before experiments.
Western Blot Analysis and Immunofluorescence
The presence of receptor transcripts for IL-1β, IFN-γ, and TNF-α in hfRPE has been reported previously (Wang F et al. IOVS 2006;47:ARVO E-Abstract 2855). The presence of these receptors was confirmed using Western blot analysis and immunofluorescence. For Western blot analysis, hfRPE cultures were rinsed three times in cold PBS, harvested with a cell scraper, and pelleted at 4°C. The pellet was lysed with RIPA buffer (Sigma-Aldrich, St. Louis, MO) supplemented with complete protease inhibitors for 20 minutes on ice, with periodic vortexing. Cell lysate was centrifuged at 14,000g for 10 minutes, and the supernatant was collected. Extracted protein was quantified with a BCA protein assay (Pierce Biotechnology, Rockford, IL). Twenty micrograms of total protein was loaded into each lane of a 4% to 12% gel (NuPAGE; Invitrogen Corp., Carlsbad, CA) under reducing conditions and transferred to nitrocellulose membranes. The membranes were probed with antibodies against IL-1R1 (Abcam, Cambridge, MA), TNFR1, TNFR2 (Stressgen Bioreagents, Ann Arbor, MI), and IFN-γR α chain (Accurate Chemicals, Westbury, NY) and developed with a gel documentation system (Autochemie; UVP, Upland, CA). The cells grown on transwell inserts were stained for cytokeratin and Na,K-ATPase, localized to the apical membrane, to check the purity and polarity.
For immunofluorescence, primary antibodies against ZO-1 (Invitrogen Corp.), Na,K-ATPase (Abcam), IL-1R1, TNFR1, and IFN-γR α chain (R&D Systems, Minneapolis, MN) were labeled with Zenon antibody labeling technology as per the manufacturer’s instructions (Invitrogen Corp.). Cultures growing on the membrane inserts were placed on ice and washed three times with cold PBS. The cells were fixed for 30 minutes in 4% formaldehyde, washed three times with PBS, and permeabilized for 30 minutes with 0.2% Triton X-100/PBS. The cells were washed three times with PBS and then blocked with a signal enhancer (Image-IT FX; Invitrogen Corp.) for 30 minutes at room temperature. After being washed three times with PBS, the cells were stained with labeled primary antibody at a 1:100 dilution in PBS, washed three times with PBS, and treated with 4% formaldehyde for an additional 15 minutes at room temperature. The cells were washed three times with PBS, mounted on glass slides with antifade reagent with DAPI (Prolong Gold; Invitrogen Corp.), and imaged (Apotome microscope, Axioplan 2; with Axiovision 3.4 software; Carl Zeiss Meditec, Inc., Dublin, CA).
Stimulation with an Inflammatory Cytokine Mixture
IL-1β (10 ng/mL), TNF-α (10 ng/mL), and IFN-γ (100 IU/mL; PeproTech, Rocky Hill, NJ) were prepared in serum-free medium (SFM) and added separately or as a mixture to the cells growing on transwells and assayed for cytokine/chemokine secretion after 24 hours. The inflammatory cytokine mixture (ICM) or its components in Ringer solution were also used in a modified Üssing chamber for fluid transport measurements. The cytokines were added to either the apical or basal compartments or both. For the cytokine/chemokine secretion assay, culture media were removed and washed with serum-free medium (SFM) containing glutamine-penicillin-streptomycin and nonessential amino-acids, incubated overnight, and then replaced by fresh SFM-containing cytokines.
The transwells are constructed with unequal volumes in the apical and basal baths (0.5 and 1.5 mL, respectively) and therefore it seemed possible that local cytokine activity would be differentially affected. This possibility was tested by adding appropriately constructed O-rings into the basal compartment, which reduced basal side volume in three steps from 1.5 to 0.5 mL. There was no change in liquid level between the apical and basal compartments. The cultures were incubated for 24 hours, and supernatant from both compartments were collected and stored at -80°C for subsequent analysis. Chemokine and cytokine levels were assayed with a commercial technology (SearchLight; Pierce Biotechnology). In this technique, proteome arrays are multiplexed using sandwich ELISAs for the quantitative measurement of 2 to 16 cytokines per sample. This chemiluminescent-based ELISA is typically 2- to 10-fold more sensitive than traditional colorimetric methods and has recently been tested against two other commercial assays.37 Although these three systems are not in quantitative agreement, the SearchLight assay technology was found to have the highest sensitivity. The variance of this method was tested by using known amounts of MCP-1 (nominally 20 ng, typically with ±10% variance according to company specs; PeproTech) and IL-8 (nominally 1 ng with ±10% variance; R&D Systems). These cytokines were dissolved in 200 μL of SFM to a final concentration of 100 ± 10 and 1 ± 0.1 ng/mL, respectively, and they compared closely to those obtained from Pierce Biotechnology: 129.2 ± 15 ng/mL for MCP-1 and 1.1 ± 0.1 ng/mL for IL-8 (mean ± SEM; n = 3). In control medium (SFM), the mean cytokine levels (n = 3) varied from 4 × 10-4 (e.g., IL-6, RANTES, and IL-12p70) to 5 × 10-2 ng/mL (e.g., GRO-α). In each sample, we measured the activities of IL-6 and -12 cytokines as well as CC and CXC subfamily chemokines including MCP-1, macrophage inflammation protein (MIP)-1α, MIP-1β, RANTES, MCP-3, MCP-2, macrophage derived cytokine (MDC), GRO-α, IL-8, monokine induced by IFN-γ (MIG), IP-10, and IFN-inducible T-cell α chemoattractant (I-TAC).
Fluid Transport
Cultured hfRPE monolayers were mounted in a modified Üssing chamber, and rates of transepithelial water flow were measured by using a modified capacitance probe technique, as previously described.38-40 Tissue physiology, transport potential, and viability were ascertained by concomitantly measuring transepithelial potential (TEP), tissue resistance (RT), and transepithelial fluid flow (JV). In these experiments, JV, TEP, and RT were recorded for 20 to 30 minutes before addition of the cytokine mixture (ICM in Ringer). ICM was then perfused into one or both bathing solutions on either side of the hfRPE and the recordings continued for another 15 to 60 minutes. In some control experiments, two succeeding cytokines additions were performed to test for reversibility/repeatability of responses. Ringer contained the following (in mM): 100 NaCl, 5 KCl, 23 NaHCO3, 1 MgCl2, 1.8 CaCl2, 0.1 NaH2PO4, 0.4 Na2HPO4, 2 taurine, 35 sucrose, and 5 glucose. The Ringer was initially bubbled with 5% CO2-10% O2-85% N2, to a pH of ∼7.4 and kept inside a CO2 incubator during the experiment. The osmolarity of the control solution was 295 ± 5 mOsm with no measurable change when the cytokine mixture was added.
Statistics
Unless otherwise noted, all experiments were repeated at least three times and data are presented as the mean ± SEM. Statistical comparisons were made using the Student’s t-test (unpaired, two tailed, unless otherwise specified; Excel; Microsoft, Redmond, WA). Differences were considered to be significant if P < 0.05. The data in Table 1 was log transformed to make the distributions being compared symmetrical.41
Table 1.
Effect of ICM on Polarized Secretion of Chemokines and Cytokines by hfRPE
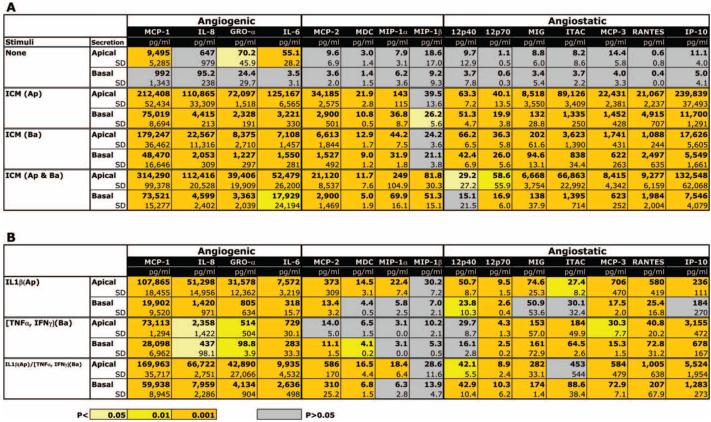 |
Summary of the polarized secretions of simultaneously measured chemokines (n = 12) and cytokines (n = 3) using multiplex sandwich ELISAs. A. Secretion into media with addition of ICM; B. Secretion into media with addition of individual components. The left-hand column defines the stimuli and the site of addition of proinflammatory mediators. These data are reported as actual concentrations and therefore are corrected for volume differences on the apical and basal sides of the membrane insert (Transwell; Corning). Each entry is the mean ± SD of three experiments, and the levels of significance are color coded for ease of comparison.
Results
Expression and Localization of IL-1R, TNF-R, and IFN-γR on hfRPE Plasma Membranes
As a first step in understanding the physiological impact of inflammatory cytokines on hfRPE, we confirmed the presence and location of the corresponding apical and basolateral membrane cognate receptors. IL-1R1, IFN-γR1, and TNFR1, but not TNFR2, expression was confirmed by Western blot in hfRPE. The data in Figure 1 are representative of four primary cultures. IFN-γR1 displayed two bands, close to 100 and 55 kDa, consistent with the observations of Aguet et al.42 Their analysis indicated that the discrepancy of the molecular weight represented by the two bands is very likely due to different levels of glycosylation. The prominent antibody-specific bands close to 55 and 80 kDa, represent TNFR143 and IL-1R1,44 respectively.
Figure 1.
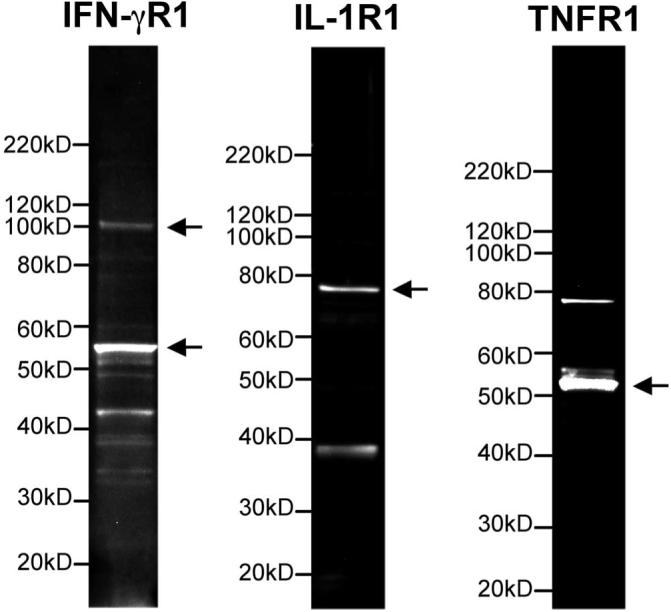
Western blot analysis to identify IFN-γR1, IL-1R1, and TNFR1. IFN-γR1 displayed two bands, close to 100 and 55 kDa. TNFR1, and IFN-γR1 were detected at approximately 55 and 80 kDa, respectively.
As previously reported,7 monolayers of hfRPE cells grown on transwells for 6 weeks exhibit the morphology, pigmentation, polarity, and physiology, typical of native RPE.
Figure 2 shows immunofluorescence of the three cytokine receptors located on hfRPE in maximum intensity projections (MIPs). In all three images, each panel shows two z-sections located on the top and right side of each main panel as well as a high gain image of one of the z-sections to the right. In all images, DAPI (blue) was used to label the nuclei. ZO-1 is labeled red in Figures 2A and 2B and green in 2C. Figure 2A shows IL-1R1 labeled in green, 2B shows TNFR1 labeled in green, and 2C shows IFN-γR1 labeled in red. The overlap of labels results in additional colors (e.g., in panel A, yellow results from overlap of red and green, corresponding to ZO-1 and IL-1R1). Figure 2A shows that IL-1RI (green signal) is located primarily above the ZO-1 (red) and the DAPI (blue) labeled nuclei, indicating a mainly apical location with some staining on the lateral face of the cell. TNFR1 (green signal) shows a mainly basal localization (Fig. 2B). The basal localization of this receptor is indicated by its appearance below ZO-1 (red signal) and in the same plane as the basally located nuclei (DAPI). The IFN-γR1 signal (red) was detected at most of the basolateral cell membranes and on some apical membranes.
Figure 2.
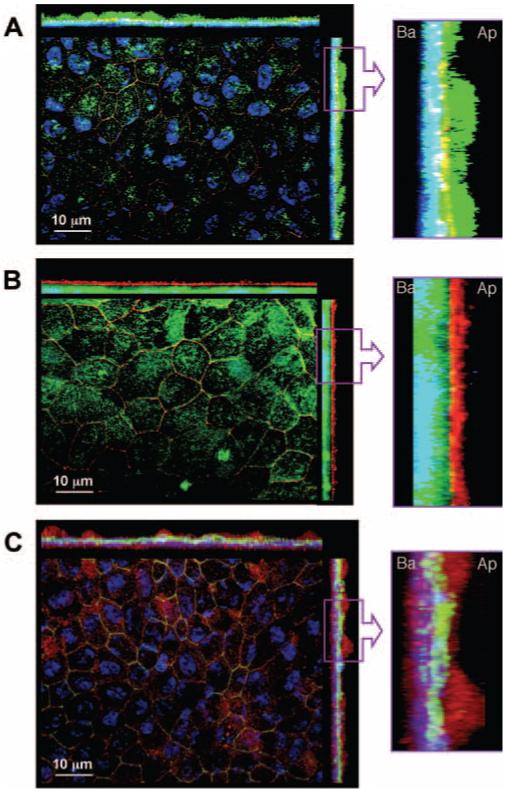
Immunofluorescence staining of hfRPE cultures grown on transwells. In each part of the figure, the left panel is an en face view of the apical membrane shown as a set of superimposed sections (MIP image). For each of the three en face views, the x- and y-axis side views are also shown as MIP images. In each case, the right panel shows a part of the y-axis side view image (labeled area) at higher magnification. ZO-1 highlights cell circumferences in the en face images. In the side view, ZO-1 also marks the boundary between the apical and basolateral membranes. (A) IL-1R1 (green) was mainly localized apically (ZO-1, yellow). (B) TNF-R1 (green) was mainly localized to the basal side (basal ZO-1, red). (C) IFN-γR1 (red) was localized to both the apical and basolateral membranes (ZO-1, green). In all three panels, nuclei of the cells were stained with DAPI (blue).
Polarized Secretion of Cytokines/Chemokines by hfRPE
Table 1 summarizes the polarized secretions of simultaneously measured chemokines (n = 12) and cytokines (n = 3) using multiplex sandwich ELISAs. The left-hand column of this table defines the stimuli and the site of addition of inflammatory mediators. The top two rows of Table 1A show constitutive secretion into the apical or basal bath for unstimulated confluent hfRPE monolayers. Rows 3 to 8 summarize the cytokine secretions to the apical and basal baths after 24 hours’ incubation with ICM (IL-1β, TNF-α, and IFN-γ). Each table entry is the mean ± SD of three experiments, with the levels of significance colored coded for ease of comparison (gray; not significant, P > 0.05; all other colors indicate different levels of statistical significance from P < 0.05 to 0.001). Rows 1 and 2 (apical and basal) summarize two main results: (1) Significant levels of MCP-1, GRO-α, and IL-6 were constitutively secreted into the apical bath; (2) the secretion level of MCP-1 was more than two orders of magnitude greater than that of GRO-α or IL-6.
Rows 3 to 8 (Table 1A) show that addition of ICM to the apical or basal baths produced significant increases in cytokine secretion into both the apical and basal baths for virtually all cytokines tested. Based on the receptor localization data presented in Figure 2, ICM components were added to the apical or basal compartments. A comparison of the middle panels in Tables 1A and 1B shows that ICM components induced far less potent and fewer significant responses (gray bars) than did complete ICM. In Table 1B, a comparison of rows 5 and 6 with rows 1 to 4 shows that concomitant addition of IL-1β to the apical bath and TNF-α and IFN-γ to the basal bath was, in almost all cases, the most effective of the three component stimuli. With only one exception, Table 1B also shows that each component stimuli induced larger changes in the secretion of angiogenic versus angiostatic cytokines/chemokines.19-21
Figure 3 summarizes the mean values in Table 1 as a “heat map” for better visualization and comparison of the patterns of ICM-induced chemokine secretion. The heat map variations in color intensity correspond to the measured secretion levels for different combinations of inflammatory mediators added to the apical or basal baths. In Figure 3A, all cytokine levels were measured using apical samples and in Figure 3B, all levels were obtained from basal-side samples. The magnitude of the responses range over 5 log units and therefore all the data were log10 transformed. The lightest shade of yellow corresponds to secretion levels between 0 and 10 pg/mL, and each increment represents a log unit increase in secretion level.
Figure 3.
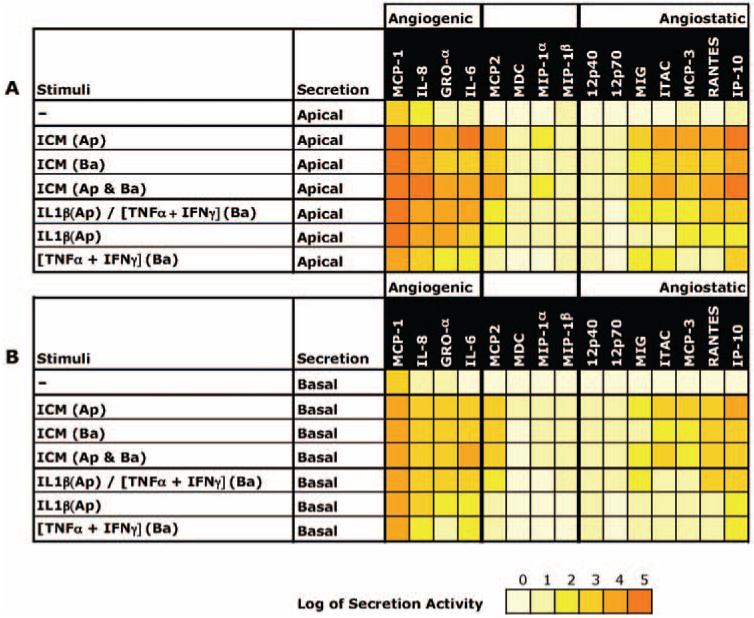
Summary of mean chemokine secretion levels as a heat map. The heat map variations in color intensity correspond to the measured secretion levels for different combinations of inflammatory mediators added to the apical or basal baths. The data were log10 transformed; the lightest shade of yellow corresponds to secretion levels between 0 and 10 pg/mL and each increment represents a log unit increase in secretion level.
The top row in Figure 3A indicates that only MCP-1 was constitutively secreted in large and significant amounts to the apical bath (≈9 ng/mL; Table 1). Figure 3B, top row, shows that the mean secretion of MCP-1 in to the basal bath noticeably exceeded baseline levels but Table 1 (n = 3) shows that this level of secretion did not achieve statistical significance. The subsequent rows in each panel represent the variations in secretion of all the cytokines tested after addition of ICM or its components. In Figures 3A and 3B, the bold vertical lines separate three relatively distinct clusters of cytokine secretion. The heat map patterns shows that (1) relatively little change in secretion was observed for almost all the non-angiogenesis-regulating cytokines (middle panel); (2) any stimuli added to the apical or basal bath induced more apical than basal secretion (i.e., Fig. 3A secretion levels > Fig. 3B levels); (3) addition of ICM to apical or both baths produced the largest changes in cytokine secretion into the apical and basal baths; (4) ICM or its components had their largest effects on MCP-1, IL-8, and GRO-α secretion into the apical or basal baths; and (5) the order of secretion magnitude was angiogenic > angiostatic.
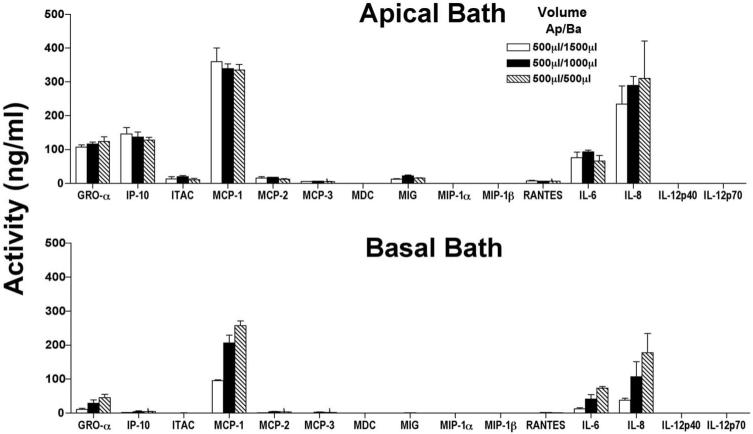
The geometry of the transwell dictates that there were unequal bath volumes on each side of the monolayer, to avoid a hydrostatic pressure difference. In the present experiments (Fig. 3, Table 1) hydrostatic pressure was reduced to 0 by maintaining the apical bath volume at 0.5 mL and basal bath volume at 1.5 mL. The activity measurements in Table 1 and Figure 3 are corrected for this volume difference, but there is still the possibility of a potential artifact that cannot be obviated by a simple volume correction. Autocrine pathways may, for example, cause significant differences in activity and subsequent alterations in receptor mediated stimulation at the apical or basolateral membranes. To examine this possibility, cytokine/chemokine secretion into either bath was measured as a function of basal bath volume between 0.5 and 1.5 mL. In the experiments summarized in Figure 4, ICM was added to both bathing solutions. Cytokine secretion into the apical bath (Fig. 4, top) showed no significant variation in activity as a function of basal bath volume change between 0.5 and 1.5 mL (filled, open, and hatched bars; apical bath volume constant). Figure 4, bottom, shows that the reduction of basal side volume from 1.5 to 0.5 mL increased the level of detectable cytokines (hatched versus solid bars). This suggests that the volume corrections used in Table 1 and Figure 3 provide the correct basal side activity.
Figure 4.
hfRPE monolayers were stimulated by addition of ICM to both the apical and basal baths for 24 hours (n = 3). Cytokine and chemokine activities were measured in the apical (top) and basal (bottom) baths after the reduction of basal bath volume in three steps from 1500 to 500 μL. The basal volumes were adjusted with O-rings.
Effects of Inflammatory Cytokines on hfRPE Fluid Transport
Figure 5A shows that addition of ICM to the basal bath increased JV by ∼10 μL · cm-2 · h-1 for the first stimulation and by ∼ 3 μL · cm-2 · h-1 for the second stimulation, consistent with an increase in fluid absorption from the retinal to choroidal side of the tissue. Assuming isotonic fluid transport, an increase of 10 μL · cm-2 · h-1 would coincide with an ICM-induced increase in net solute flux of 3 μeq · cm-2 · h-1; or equivalently, 1.5 μeq · cm-2 · h-1 of net Na and Cl flux.5,6,45 The first JV response is reversible, with an activation time of 10 to 20 minutes; the second response is diminished, but still reversible. In these experiments, TEP and RT were used to assess overall transport potential and monolayer integrity, but the chamber design did not allow us to measure relatively fast electrical changes. The apparent changes in TEP and RT were not seen in two other experiments and are probably artifactual, caused by a solution-induced change in the mechanical seal at the tissue perimeter. In three experiments, mean JV more than doubled from 6.7 ± 3.1 to 15.5 ± 5.5 μL · cm-2 · h-1 (P < 0.05; mean ± SEM, n = 3); the mean TEP and RT in control Ringer were 2.2 ± 0.8 mV and 787 ± 98 Ω · cm2, respectively (n = 3). Figure 5B shows that ICM addition to both bathing solutions increased JV by ∼5 μL · cm-2 · h-1, and there were no significant changes in TEP or RT (n = 3). In three experiments, addition of ICM to both bathing solutions increased mean JV by ≈50%, from 12.9 ± 5.0 to 18.4 ± 6.4 μL · cm-2 · h-1 (P < 0.05). In striking contrast, addition of the ICM to the apical bathing solution did not produce a significant change in JV, 12.5 ± 0.9 compared with 11.2 ± 0.9 μL · cm-2 · h-1 (P > 0.1; n = 3).
Figure 5.
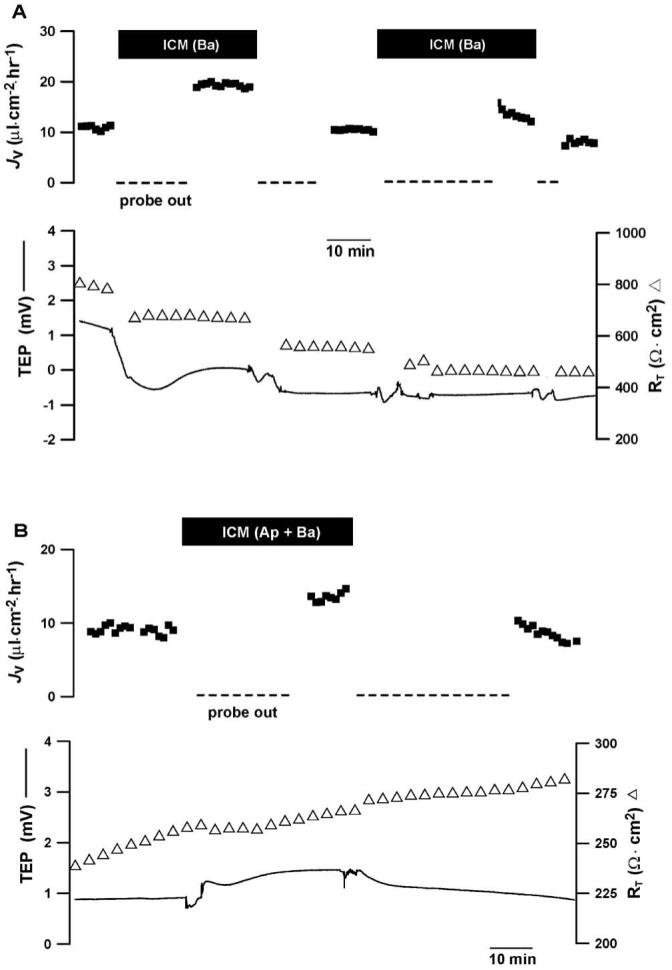
ICM induced changes in hfRPE fluid transport. Top traces: JV plotted as a function of time, with net fluid absorption is indicated by positive values; bottom traces: TEP and RT are plotted as function of time. (A) Addition of ICM to the basal bath increased JV ∼10 μL · cm-2 · h-1 with no significant changes in TEP and RT. (B) Concomitant addition of ICM to apical and basal baths increased JV ∼5 μL · cm-2 · h-1 with no changes in TEP and a slight increase in resistance that was not consistent from experiment to experiment. In four experiments, the mean change in RT was not statistically significant. We used the Student’s t-test to analyze these acute responses to ICM (paired, two tailed).
The ICM-induced changes in JV could be solely due to the observed polarization of the cytokine receptors. To test this hypothesis, we concomitantly added IL-1β to the apical bathing solution and TNF-α, together with IFN-γ, to the solution bathing the basal side. In Figure 6A, JV increased by ∼13 μL · cm-2 · h-1 and then recovered to baseline after washout of cytokines from both bathing solutions. This experiment showed a small cytokine-induced change in RT but a significant change was not observed in six cytokine-treated monolayers (365 ± 97 Ω · cm2 in control versus 368 ± 94 Ω · cm2 in treated monolayers). In six experiments, the ICM combination increased mean JV from 5.4 ± 1.6 to 19 ± 3.6 μL · cm-2 · h-1 (mean ± SEM; P = 0.005). In control experiments, IL-1β was added to the solution bathing the basal membrane, and concomitantly, TNF-α was added together with IFN-γ to the solution bathing the apical membrane. Figure 6B summarizes the data from a representative experiment with a baseline JV of ≈15 μL · cm-2 · h-1. The cocktail produced no change in JV and a small change in TEP. In four such experiments, JV, TEP, and RT were 12.5 ± 1.1 μL · cm-2 · h-1, -0.2± 0.8 mV, 516 ± 70 Ω · cm2, respectively (mean ± SEM) in the absence of this cytokine mixture. In each experiment, subsequent addition of cytokines produced little change. JV, TEP, and RT were 11.2 ± 1.1 μL · cm-2 · h-1, 0.6± 0.5 mV, 364 ± 80 Ω · cm2, respectively (P > 0.1). Therefore, mean steady state JV, TEP, and RT are unaltered by adding cytokines to the sides of the epithelium apparently lacking their cognate receptors.
Figure 6.
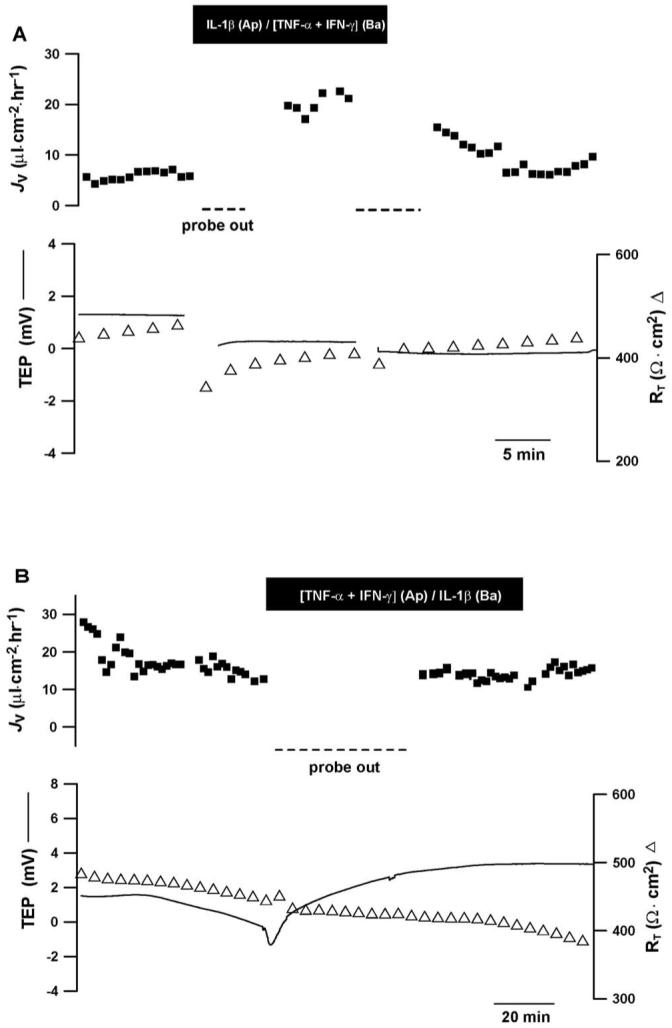
ICM-induced changes in hfRPE fluid transport, formatted as in Figure 5. (A) Addition of IL-1β to the apical and IFN-γ+TNF-α to the basal baths consistent with their primary receptor locations increased JV ∼13 μL · cm-2 · h-1. (B) Addition of these component cytokines to the bath opposite their primary receptor location did not appreciably alter JV, RT, or TEP (n = 4). Student’s t-test used to analyze acute responses to ICM components (paired, two-tailed).
The proinflammatory cytokines act acutely to alter JV (Figs. 5, 6). However, the cytokine secretion experiments summarized in Table 1 and Figure 3 measure a chronic (24 hour) effect of ICM induced cytokine secretion. For a closer comparison of these experiments, we first assessed the constancy of monolayer resistance over 24 hours using the EVOM resistance meter (World Precision Instruments, Sarasota, FL). RT was measured at t = 0 and t = 24 hours in a total of 10 transwells (five controls and five others to be treated with ICM on both sides of the well). These data, summarized in Figure 7, shows that at t = 0, these two groups had no significant difference in mean RT (328 ± 90 compared with 402 ± 81 Ω · cm2; n = 5, P = 0.86). The control group resistance at 0 and 24 hours was unchanged (328 ± 90 compared with 381 ± 87 Ω · cm2; n = 5; P = 0.85). In contrast, addition of ICM for 24 hours to both sides of the cell migration wells significantly decreased RT from 402 ± 81 Ω · cm2 to 169 ± 25 Ω · cm2 (n = 5; P = 0.02). After 24 hours, the ICM-treated wells were significantly decreased in RT compared with the 24-hour matched control wells (381 ± 87 compared with 169 ± 25 Ω · cm2 (n = 5; P = 0.04). These matched wells were then transferred to a modified Üssing chamber for measurement of JV, TEP, and RT.
Figure 7.
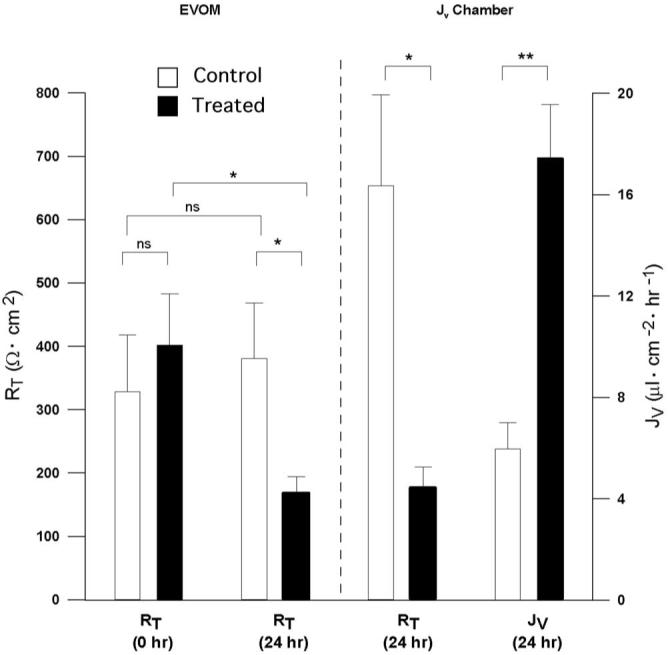
Twenty-four-hour ICM-induced changes in hfRPE fluid transport and RT. RT and JV summary for hfRPE monolayers treated with ICM in both apical and basal bath for 24 hours and hfRPE control monolayers. RT decrease was statistically significant for ICM-treated filters at t = 0 versus t = 24 hours (*P < 0.05). A statistically significant increase in JV was observed in control versus 24-hour ICM-treated filters (**P < 0.01). NS, nonsignificant.
The results of the Üssing chamber experiments are summarized in Figure 7 (right) and show that the addition of ICM to both sides of the hfRPE monolayer for 24 hours more than doubled JV, from 6.0 ± 1.0 to 17.0 ± 2.0 μL · cm-2 · h-1 (P < 0.002; n = 5). Concomitantly, the mean RT decreased from 654 ± 143 to 176 ± 34 Ω · cm2 (P = 0.02; n = 5), whereas the mean TEP (0.3 ± 0.5 mV) was unchanged over this period (P > 0.5).
Therefore, the acute and 24-hour experiments with ICM are comparable in their effects on JV and TEP, but not in their effects on RT.
We also added ICM only to the apical bath of the hfRPE monolayer for 24 hours. In contrast to the addition of ICM to both sides, apical ICM produced no significant change in JV (9.0 ± 1.4 μL · cm-2 · h-1 vs 9.1 ± 2.6 μL · cm-2 · h-1 (n = 6; P > 0.4), but there was still a significant decrease in mean RT from 312 to 112 Ω · cm2 (n = 6; P = 0.004).
Discussion
In the present experiments, we used a model of hfRPE that closely mimics many of the physiological characteristics of adult RPE.7,46,47 In vivo, the secretion of cytokines/chemokines by the RPE plays a critically important role in the maintenance of an immune specialized environment that is part of the innate immune system and is required for neuronal homeostasis in the retina.48-52
Constitutive Secretion of Chemokines by hfRPE: Protective Effects
In the absence of stimuli, hfRPE constitutively secreted physiologically significant quantities of MCP-1 preferentially into the apical bath, and recent evidence from animal models suggests a critical role of this angiogenic chemokine in the development of AMD.29-31 Other angiogenic chemokines (GRO-α, IL-8, IL-6) were also secreted constitutively by the hfRPE into the SRS, but at much lower levels (Table 1). The latter two chemokines are also part of the downregulatory immune environment of the retina/RPE interface51 and could participate in the activation of microglia, along with MCP-1.53,54 As in the CNS,55 MCP-1 may also protect retinal neurons from glutamate toxicity. The constitutive and induced secretion of this and other chemoattractants (GRO-α, IL-6, or IL-8 in Table 1) to the apical bath may also provide signaling in vivo to attract leukocytes across the RPE to protect the RPE/retina complex (Economopoulou M, et al. IOVS 2007;48:ARVO E-Abstract 4925).29,56 In addition, the secretion and accumulation of MCP-1 in the SRS may require the formation of active dimers or tetramers57 to provide a mechanism of chronic protection.
ICM-Induced Changes in RPE Secretion of Cytokines/Chemokines: A Model for Damage
Compared with constitutive secretion by the RPE, addition of ICM to the apical bath produced large, physiological changes in the secretion of both angiogenic and angiostatic cytokines/chemokines. Analysis of the data summarized in Table 1 and Figure 3 show that, in most cases, the rate of cytokine secretion into the apical bath significantly exceeded that secreted into the basal bath, whether ICM was added to the apical or basal or both baths or IL-1β, TNF-α, or IFN-γ were added, according to the location of their respective cognate receptors (compare Fig. 3A with 3B). Another general conclusion apparent from the data summarized in Figure 3 is that angiogenic cytokines were secreted at a greater rate than were angiostatic cytokines (compare left- and right-hand panels). It is possible that the immunocytochemistry data did not detect small amounts of IL-1R on the basolateral side of the tissue or small amounts of TNFR on the apical side of the tissue. The possible interactions of these receptors and their signaling pathways are currently under study in our laboratory (Li R, et al., manuscript in preparation).
Our observation that stimulated hfRPE cells secrete exceptionally high amounts of MCP-1 onto the apical and basal sides of the cells suggests an important role for this chemokine in diseases that have an inflammatory component, such as CNV.24 Recently, it has been shown that the MCP-1 released from astrocytes and Müller cells after retinal detachment may be an important determinant of photoreceptor degeneration.54,58 However, in the absence of a pathophysiologic stimulus, MCP-1 may be protective (see Constitutive Secretion of Chemokines by hfRPE: Protective Effects). There is strong and accumulating evidence that drusen biogenesis and AMD are closely associated with local and chronic inflammation between the RPE basement membrane and Bruch’s membrane.25,59 Dissipation of this protective chemical gradient with age could lead to sub-RPE lipid accumulation, the formation of large drusen, and the progression to CNV.60
Polarized secretion of IL-6 and IL-8 was also significantly induced by ICM. IL-8 is a potent chemoattractant for neutrophils and basophils.61 Both IL-6 and IL-8 are highly angiogenic.62,63 The preferential secretion of these molecules from the apical side induced by ICM suggests that they play important roles in inflammation and neovascularization in the retina and SRS.12,50,52,59 We were also surprised to observe that I-TAC and MIG were secreted into the apical bath in substantial amounts (Table 1). These chemokines belong to the CXC family and are generally thought to be angiostatic and involved in the regulation of chemotaxis, cell growth, and T-cell activation.64 Relatively little is known, however, about the mechanisms by which these particular angiostatic chemokines exert their effects, and their physiological roles in the eye have not been explored. In addition, IP-10, MCP- 3, and RANTES, were also preferably secreted into the apical bath by hfRPE when stimulated by apical ICM. To our knowledge, the polarized secretion of these angiostatic chemokines has not been previously observed in the eye. We also observed the polarized secretion of GRO-α and MCP-2, both chemoattractants for neutrophils and monocytes, respectively.65 GRO-α along with other CXCR2 ligands (e.g., IL-8) has been implicated in the pathogenesis of age-related diseases such as Alzheimer’s.66
Inflammatory Cytokine Stimulates Fluid Absorption across the RPE Monolayer
In vivo, an increase of fluid absorption from the SRS could help regulate the chemical composition of that extracellular space (including cytokines/chemokines). Based on the immunocytochemistry and fluid transport data, acute and after 24 hours (Figs. 2, 5, 6, 7), we can conclude that TNF-α and possibly IFN-γ are the prime determinants of the ICM-induced increase in JV, whereas IL-1β and IFN-γ may be the prime determinants of the ICM-induced RT changes. This interpretation of the JV data is uncertain, because the IFN-γR1 subunit is located on both sides of the cell, whereas IFN-γR2 is located mainly on the basolateral side (data not shown). The detection of IFNR-γR1 on both sides of the cell, and the apical or both-sided ICM-induced changes in RT, suggest that this subunit is important for controlling transepithelial resistance. We also observed that cytokine secretion induced by apical ICM significantly exceeded that produced by the addition of IL-1 alone into the apical bath (Table 1). TNFR seems clearly localized to the basal side of the cell, which suggests that apical IFNR-γR1 and IL-1R can act synergistically to mediate cytokine secretion. These complex mechanisms are undoubtedly important in understanding the immune system-RPE interaction and required further study.
Fukuda et al.,17 showed that TNF-α stimulated alveolar fluid clearance, apparently as a result of increased Na uptake by the alveolar epithelial cells. TNF-α was also found to activate 5-nitro 2-(3-phenylpropylamino) benzoate (NPPB)-sensitive Cl channels and Ba-sensitive K channels on rat liver cell line.67 These two channels are involved in basal Cl and K secretion in human RPE. It is clear that fluid transport across RPE can be driven by Na influx and basal Cl and K secretion.4-6 IFN-γ was also reported to upregulate the response to UTP, eliciting an intracellular Ca spike and activating Ca-dependent Cl channels in airway epithelial cells.68 Similar ionic mechanisms may also mediate fluid transport in RPE.45
We hypothesize that in inflammatory conditions in vivo, TNF-α or IFN-γ would cause fluid removal from the SRS and therefore increase the activity of any cytokines/chemokines that are present and preferentially secreted by the RPE to the SRS. In addition to the chemokines, molecules such as glutamate, Ca, K, and Na in the SRS could also be concentrated, which would alter photoreceptor function (e.g., dark current) or lead to cell death.69-71 If the fluid transported from the SRS is allowed to accumulate around Bruch’s membrane, this extra volume would cause a decrease of chemical activity in the extracellular space just outside the basement membrane. The transfer of volume from the SRS to the choroid per se would increase chemokine gradients across the RPE and drive inflammatory cells toward the SRS.
The acute JV responses (Figs. 5, 6) seemed puzzling because the ICM-induced changes are thought to be mediated by de novo synthesis of transcription factors and other molecules in the signal transduction pathways of IFN-γ, IL-1β, or TNF-α over a period of hours. These changes in JV occur in tens of minutes, suggesting that one or more of the ICM components are acting on plasma membrane transporters/receptors or second messengers coupled to active solute-driven fluid transport. Consistent with this finding, several recent studies have shown that lipopolysaccharide (LPS) activation of TLR4 plasma membrane receptors can lead to synthesis NF-κβ precursors in less than 20 minutes.72,73
The secretion data (Table 1, Fig. 3) were obtained after 24 hours of incubation with ICM. This chronic exposure significantly altered the pattern of cytokine/chemokine secretion to the apical/basal baths, and one would also expect significant changes in hfRPE physiology. This expectation is confirmed by the data summarized in Figure 7, which show that ICM significantly decreased RT and increased JV. It is intriguing that both acute and chronic ICM exposure increased JV, whereas RT was only affected by chronic exposure. The ICM-induced changes in JV were due to a direct effect of ICM (acute JV experiments) with a possible contribution from other cytokines, measured or unmeasured, secreted over the 24-hour period. In vivo, the reduction in RT would be consistent with the opening of a pathway (paracellular or cellular) that allows immune system cells to reach retinal sites of inflammatory injury.74-77 In addition, the transmigration of these cells across the RPE from choroid to retina may be significantly enhanced, as indicated earlier, by an increase in chemokine gradients after dehydration of the SRS.
Physiological Implications
The elevation of ICM or its separate components has been observed in a variety of eye diseases—for example, uveitis, PDR, and AMD.22-24,36,78,79 The initial infiltration of immune cells such as macrophages, neutrophils, or T cells secrete proinflammatory cytokines that trigger an even wider secretory response from the RPE, thus amplifying the inflammatory process. In addition, significant physiologic responses of the RPE may be accompanied by similar responses from other retinal cells, such as microglia.53
In PDR, elevated TNF-α and IL-1β were detected in both serum (103.8 and 12.8 pg/mL, respectively) and vitreous (160.7 and 34.1 pg/mL, respectively).35 In addition, elevated MCP-1/CCL2 (2171 pg/mL), IL-8/CXCL8 (173.5 pg/mL),23,80 and IP-10 (11.7 ng/mL)23 were measured in the vitreous. The RPE was implicated as a possible source of the accumulated chemokines in the vitreous as might be predicted from the data in Table 1, taking into account dilution effects. Elevated TNF-α and IL-1β in the vitreous or choroidal blood could stimulate RPE from the apical or basolateral sides to secret large amounts of these chemokines. This chemokine accumulation in the retina may account for the aggregation of leukocytes in retinal vessels, causing vessel blockage, and eventual retinal neovascularization.81
RPE cells are considered a major source of chemotactic molecules in AMD.25-27,51,82 We demonstrated in earlier work that these cells constitutively secrete VEGF in a polarized fashion.7 A possible connection between the proinflammatory stimulus and VEGF was tested in the present studies by measuring the constitutive secretion of VEGF into the apical and basal baths. Constitutive secretion was not significantly altered after 24 hours of ICM stimulation on both sides of the epithelium (data not shown). In AMD the degeneration of RPE cells could alter the uptake of oxidized photoreceptors or affect drusen formation that triggers the release of various cytokines/chemokines, such as IL-8, IL-6, and MCP-1.82,83 In vivo, the polarized constitutive secretion of MCP-1 and IL-8 into the apical bath would create a chemokine gradient across the RPE that drives monocytes, neutrophils, and T-cells from the choroidal blood vessels toward the RPE basement membrane and then across the RPE monolayer to the SRS. These immune cells would serve to reduce the debris that normally occurs on either side of the RPE after normal metabolic stress and aging.29-31 The accumulation of immunologically active drusen25,28 with age can trigger local inflammatory responses that activate immune cells to release further proinflammatory cytokines such as ICM. In addition, the ICM-stimulated release of IL-6 provides a proinflammatory signal that has been shown to promote CNV.50 Therefore, acute inflammatory alterations in RPE physiology (Figs. 5, 6) are sustained and augmented (Fig. 7), probably by activation of basolateral membrane receptors for TNF-α and IFN-γ, and in vivo may lead to chronic inflammation, CNV, retinal degeneration, and progression to blindness.
Acknowledgments
The authors thank Igal Gery for helpful advice and George Reed for advice on statistics.
Supported by the NEI/NIH Intramural Program.
Footnotes
Disclosure: G. Shi, None; A. Maminishkis, None; T. Banzon, None; S. Jalickee, None; R. Li, None; J. Hammer, None; S.S. Miller, None
References
- 1.Marmor MF, Wolfensberger TJ. The Retinal Pigment Epithelium: Function and Disease. Oxford University Press; New York: 1998. [Google Scholar]
- 2.Rizzolo LJ. Development and role of tight junctions in the retinal pigment epithelium. Int Rev Cytol. 2007;258:195–234. doi: 10.1016/S0074-7696(07)58004-6. [DOI] [PubMed] [Google Scholar]
- 3.Marmorstein AD. The polarity of the retinal pigment epithelium. Traffic. 2001;2:867–872. doi: 10.1034/j.1600-0854.2001.21202.x. [DOI] [PubMed] [Google Scholar]
- 4.Miller SS, Steinberg RH, Oakley B., 2nd The electrogenic sodium pump of the frog retinal pigment epithelium. J Membr Biol. 1978;44:259–279. doi: 10.1007/BF01944224. [DOI] [PubMed] [Google Scholar]
- 5.Hughes BA, Miller SS, Farber DB. Adenylate cyclase stimulation alters transport in frog retinal pigment epithelium. Am J Physiol. 1987;252:C385–C395. doi: 10.1152/ajpcell.1987.252.4.C385. [DOI] [PubMed] [Google Scholar]
- 6.Edelman JL, Lin H, Miller SS. Acidification stimulates chloride and fluid absorption across frog retinal pigment epithelium. Am J Physiol. 1994;266:C946–C956. doi: 10.1152/ajpcell.1994.266.4.C946. [DOI] [PubMed] [Google Scholar]
- 7.Maminishkis A, Chen S, Jalickee S, et al. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 2006;47:3612–3624. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holtkamp GM, Van Rossem M, de Vos AF, Willekens B, Peek R, Kijlstra A. Polarized secretion of IL-6 and IL-8 by human retinal pigment epithelial cells. Clin Exp Immunol. 1998;112:34–43. doi: 10.1046/j.1365-2249.1998.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holtkamp GM, De Vos AF, Peek R, Kijlsta A. Analysis of the secretion pattern of monocyte chemotactic protein-1 (MCP-1) and transforming growth factor-beta 2 (TGF-beta2) by human retinal pigment epithelial cells. Clin Exp Immunol. 1999;118:35–40. doi: 10.1046/j.1365-2249.1999.01016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holtkamp GM, Kijlstra A, Peek R, de Vos AF. Retinal pigment epithelium-immune system interactions: cytokine production and cytokine-induced changes. Prog Retin Eye Res. 2001;20:29–48. doi: 10.1016/s1350-9462(00)00017-3. [DOI] [PubMed] [Google Scholar]
- 11.Momma Y, Nagineni CN, Chin MS, Srinivasan K, Detrick B, Hooks JJ. Differential expression of chemokines by human retinal pigment epithelial cells infected with cytomegalovirus. Invest Ophthalmol Vis Sci. 2003;44:2026–2033. doi: 10.1167/iovs.02-0980. [DOI] [PubMed] [Google Scholar]
- 12.Elner SG, Delmonte D, Bian ZM, Lukacs NW, Elner VM. Differential expression of retinal pigment epithelium (RPE) IP-10 and interleukin-8. Exp Eye Res. 2006;83:374–379. doi: 10.1016/j.exer.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Crane IJ, Wallace CA, McKillop-Smith S, Forrester JV. Control of chemokine production at the blood-retina barrier. Immunology. 2000;101:426–433. doi: 10.1046/j.1365-2567.2000.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foxman EF, Zhang M, Hurst SD, et al. inflammatory mediators in uveitis: differential induction of cytokines and chemokines in Th1-versus Th2-mediated ocular inflammation. J Immunol. 2002;168:2483–2492. doi: 10.4049/jimmunol.168.5.2483. [DOI] [PubMed] [Google Scholar]
- 15.van Kooij B, Rothova A, Rijkers GT, de Groot-Mijnes JD. Distinct cytokine and chemokine profiles in the aqueous of patients with uveitis and cystoid macular edema. Am J Ophthalmol. 2006;142:192–194. doi: 10.1016/j.ajo.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 16.Takase H, Yu CR, Ham DI, et al. inflammatory processes triggered by TCR engagement or by local cytokine expression: differences in profiles of gene expression and infiltrating cell populations. J Leukoc Biol. 2006;80:538–545. doi: 10.1189/jlb.1205719. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda N, Jayr C, Lazrak A, et al. Mechanisms of TNF-alpha stimulation of amiloride-sensitive sodium transport across alveolar epithelium. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1258–L1265. doi: 10.1152/ajplung.2001.280.6.L1258. [DOI] [PubMed] [Google Scholar]
- 18.Elia N, Tapponnier M, Matthay MA, et al. Functional identification of the alveolar edema reabsorption activity of murine tumor necrosis factor-alpha. Am J Respir Crit Care Med. 2003;168:1043–1050. doi: 10.1164/rccm.200206-618OC. [DOI] [PubMed] [Google Scholar]
- 19.Benelli R, Lorusso G, Albini A, Noonan DM. Cytokines and chemokines as regulators of angiogenesis in health and disease. Curr Pharm Des. 2006;12:3101–3115. doi: 10.2174/138161206777947461. [DOI] [PubMed] [Google Scholar]
- 20.Bernardini G, Ribatti D, Spinetti G, et al. Analysis of the role of chemokines in angiogenesis. J Immunol Methods. 2003;273:83–101. doi: 10.1016/s0022-1759(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 21.Hedin KE. Chemokines: new, key players in the pathobiology of pancreatic cancer. Int J Gastrointest Cancer. 2002;31:23–29. doi: 10.1385/IJGC:31:1-3:23. [DOI] [PubMed] [Google Scholar]
- 22.Verma MJ, Lloyd A, Rager H, et al. Chemokines in acute anterior uveitis. Curr Eye Res. 1997;16:1202–1208. doi: 10.1076/ceyr.16.12.1202.5034. [DOI] [PubMed] [Google Scholar]
- 23.Elner SG, Strieter R, Bian ZM, et al. Interferon-induced protein 10 and interleukin 8. C-X-C chemokines present in proliferative diabetic retinopathy. Arch Ophthalmol. 1998;116:1597–1601. doi: 10.1001/archopht.116.12.1597. [DOI] [PubMed] [Google Scholar]
- 24.Grossniklaus HE, Ling JX, Wallace TM, et al. Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol Vis. 2002;8:119–126. [PubMed] [Google Scholar]
- 25.Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 26.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 27.Hageman GS, Hancox LS, Taiber AJ, et al. Extended haplotypes in the complement factor H (CFH) and CFH-related (CFHR) family of genes protect against age-related macular degeneration: characterization, ethnic distribution and evolutionary implications. Ann Med. 2006;38:592–604. [PMC free article] [PubMed] [Google Scholar]
- 28.Crabb JW, Miyagi M, Gu X, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ambati J, Anand A, Fernandez S, et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9:1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- 30.Tuo J, Bojanowski CM, Zhou M, et al. Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48:3827–3836. doi: 10.1167/iovs.07-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Combadiere C, Feumi C, Raoul W, et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007;117:2920–2928. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curnow SJ, Falciani F, Durrani OM, et al. Multiplex bead immuno-assay analysis of aqueous humor reveals distinct cytokine profiles in uveitis. Invest Ophthalmol Vis Sci. 2005;46:4251–4259. doi: 10.1167/iovs.05-0444. [DOI] [PubMed] [Google Scholar]
- 34.Takase H, Futagami Y, Yoshida T, et al. Cytokine profile in aqueous humor and sera of patients with infectious or noninfectious uveitis. Invest Ophthalmol Vis Sci. 2006;47:1557–1561. doi: 10.1167/iovs.05-0836. [DOI] [PubMed] [Google Scholar]
- 35.Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye. 2006;20:1366–1369. doi: 10.1038/sj.eye.6702138. [DOI] [PubMed] [Google Scholar]
- 36.Cousins SW, Espinosa-Heidmann DG, Csaky KG. Monocyte activation in patients with age-related macular degeneration: a biomarker of risk for choroidal neovascularization? Arch Ophthalmol. 2004;122:1013–1018. doi: 10.1001/archopht.122.7.1013. [DOI] [PubMed] [Google Scholar]
- 37.Lash GE, Scaife PJ, Innes BA, et al. Comparison of three multiplex cytokine analysis systems: Luminex, SearchLight and FAST Quant. J Immunol Methods. 2006;309:205–208. doi: 10.1016/j.jim.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Jiang Y, Grotberg JB. Bolus contaminant dispersion in oscillatory tube flow with conductive walls. J Biomech Eng. 1993;115:424–431. doi: 10.1115/1.2895507. [DOI] [PubMed] [Google Scholar]
- 39.Maminishkis A, Jalickee S, Blaug SA, et al. The P2Y(2) receptor agonist INS37217 stimulates RPE fluid transport in vitro and retinal reattachment in rat. Invest Ophthalmol Vis Sci. 2002;43:3555–3566. [PubMed] [Google Scholar]
- 40.Edelman JL, Miller SS. Epinephrine stimulates fluid absorption across bovine retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1991;32:3033–3040. [PubMed] [Google Scholar]
- 41.Snedecor GW, Cochran WG. Statistical Methods. 8th ed. Iowa State University Press; Ames, IA: 1989. [Google Scholar]
- 42.Aguet M, Dembic Z, Merlin G. Molecular cloning and expression of the human interferon-gamma receptor. Cell. 1988;55:273–280. doi: 10.1016/0092-8674(88)90050-5. [DOI] [PubMed] [Google Scholar]
- 43.Tartaglia LA, Goeddel DV. Two TNF receptors. Immunol Today. 1992;13:151–153. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- 44.Dower SK, Kronheim SR, Hopp TP, et al. The cell surface receptors for interleukin-1 alpha and interleukin-1 beta are identical. Nature. 1986;324:266–268. doi: 10.1038/324266a0. [DOI] [PubMed] [Google Scholar]
- 45.Peterson WM, Meggyesy C, Yu K, Miller SS. Extracellular ATP activates calcium signaling, ion, and fluid transport in retinal pigment epithelium. J Neurosci. 1997;17:2324–2337. doi: 10.1523/JNEUROSCI.17-07-02324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Voloboueva LA, Killilea DW, Atamna H, Ames BN. N-tert-butyl hydroxylamine, a mitochondrial antioxidant, protects human retinal pigment epithelial cells from iron overload: relevance to macular degeneration. FASEB J. 2007;21:4077–4086. doi: 10.1096/fj.07-8396com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li R, Maminishkis A, Wang FE, Miller SS. PDGF-C and -D Induced proliferation/migration of human RPE is abolished by inflammatory cytokines. Invest Ophthalmol Vis Sci. 2007;48:5722–5732. doi: 10.1167/iovs.07-0327. [DOI] [PubMed] [Google Scholar]
- 48.Canataroglu H, Varinli I, Ozcan AA, Canataroglu A, Doran F, Varinli S. Interleukin (IL)-6, interleukin (IL)-8 levels and cellular composition of the vitreous humor in proliferative diabetic retinopathy, proliferative vitreoretinopathy, and traumatic proliferative vitreoretinopathy. Ocul Immunol inflamm. 2005;13:375–381. doi: 10.1080/09273940490518900. [DOI] [PubMed] [Google Scholar]
- 49.Fahey JV, Schaefer TM, Channon JY, Wira CR. Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Hum Reprod. 2005;20:1439–1446. doi: 10.1093/humrep/deh806. [DOI] [PubMed] [Google Scholar]
- 50.Izumi-Nagai K, Nagai N, Ozawa Y, et al. Interleukin-6 receptor-mediated activation of signal transducer and activator of transcription-3 (STAT3) promotes choroidal neovascularization. Am J Pathol. 2007;170:2149–2158. doi: 10.2353/ajpath.2007.061018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nussenblatt RB, Ferris F., 3rd Age-related macular degeneration and the immune response: implications for therapy. Am J Ophthalmol. 2007;144:618–626. doi: 10.1016/j.ajo.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanda A, Abecasis G, Swaroop A. Inflammation in the pathogenesis of age-related macular degeneration. Br J Ophthalmol. 2008 Apr;92(4):448–450. doi: 10.1136/bjo.2007.131581. [DOI] [PubMed] [Google Scholar]
- 53.Langmann T. Microglia activation in retinal degeneration. J Leukoc Biol. 2007;81:1345–1351. doi: 10.1189/jlb.0207114. [DOI] [PubMed] [Google Scholar]
- 54.Nakazawa T, Hisatomi T, Nakazawa C, et al. Monocyte chemoattractant protein 1 mediates retinal detachment-induced photoreceptor apoptosis. Proc Natl Acad Sci U S A. 2007;104:2425–2430. doi: 10.1073/pnas.0608167104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eugenin EA, D’Aversa TG, Lopez L, Calderon TM, Berman JW. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem. 2003;85:1299–1311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- 56.Forrester JV. Macrophages eyed in macular degeneration. Nat Med. 2003;9:1350–1351. doi: 10.1038/nm1103-1350. [DOI] [PubMed] [Google Scholar]
- 57.Proudfoot AE, Handel TM, Johnson Z, et al. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc Natl Acad Sci U S A. 2003;100:1885–1890. doi: 10.1073/pnas.0334864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakazawa T, Matsubara A, Noda K, et al. Characterization of cytokine responses to retinal detachment in rats. Mol Vis. 2006;12:867–878. [PubMed] [Google Scholar]
- 59.Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration: emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38:450–471. doi: 10.1080/07853890600946724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Handa JT. New molecular histopathologic insights into the pathogenesis of age-related macular degeneration. Int Ophthalmol Clin. 2007;47:15–50. doi: 10.1097/IIO.0b013e31802bd546. [DOI] [PubMed] [Google Scholar]
- 61.Paccaud JP, Schifferli JA, Baggiolini M. NAP-1/IL-8 induces up-regulation of CR1 receptors in human neutrophil leukocytes. Biochem Biophys Res Commun. 1990;166:187–192. doi: 10.1016/0006-291x(90)91929-m. [DOI] [PubMed] [Google Scholar]
- 62.Motro B, Itin A, Sachs L, Keshet E. Pattern of interleukin 6 gene expression in vivo suggests a role for this cytokine in angiogenesis. Proc Natl Acad Sci U S A. 1990;87:3092–3096. doi: 10.1073/pnas.87.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petzelbauer P, Watson CA, Pfau SE, Pober JS. IL-8 and angiogenesis: evidence that human endothelial cells lack receptors and do not respond to IL-8 in vitro. Cytokine. 1995;7:267–272. doi: 10.1006/cyto.1995.0031. [DOI] [PubMed] [Google Scholar]
- 64.Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol. 1997;61:246–257. [PubMed] [Google Scholar]
- 65.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 66.Xia M, Hyman BT. GROalpha/KC, a chemokine receptor CXCR2 ligand, can be a potent trigger for neuronal ERK1/2 and PI-3 kinase pathways and for tau hyperphosphorylation-a role in Alzheimer’s disease? J Neuroimmunol. 2002;122:55–64. doi: 10.1016/s0165-5728(01)00463-5. [DOI] [PubMed] [Google Scholar]
- 67.Nietsch HH, Roe MW, Fiekers JF, Moore AL, Lidofsky SD. Activation of potassium and chloride channels by tumor necrosis factor alpha: role in liver cell death. J Biol Chem. 2000;275:20556–20561. doi: 10.1074/jbc.M002535200. [DOI] [PubMed] [Google Scholar]
- 68.Galietta LJ, Folli C, Caci E, et al. Effect of inflammatory stimuli on airway ion transport. Proc Am Thorac Soc. 2004;1:62–65. doi: 10.1513/pats.2306017. [DOI] [PubMed] [Google Scholar]
- 69.Zeng HY, Zhu XA, Zhang C, Yang LP, Wu LM, Tso MO. Identification of sequential events and factors associated with microglial activation, migration, and cytotoxicity in retinal degeneration in rd mice. Invest Ophthalmol Vis Sci. 2005;46:2992–2999. doi: 10.1167/iovs.05-0118. [DOI] [PubMed] [Google Scholar]
- 70.Olney JW. The toxic effects of glutamate and related compounds in the retina and the brain. Retina. 1982;2:341–359. [PubMed] [Google Scholar]
- 71.David P, Lusky M, Teichberg VI. Involvement of excitatory neuro-transmitters in the damage produced in chick embryo retinas by anoxia and extracellular high potassium. Exp Eye Res. 1988;46:657–662. doi: 10.1016/s0014-4835(88)80054-x. [DOI] [PubMed] [Google Scholar]
- 72.Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-kappaB activation. Science. 2005;309:1854–1857. doi: 10.1126/science.1112304. [DOI] [PubMed] [Google Scholar]
- 73.Werner SL, Barken D, Hoffmann A. Stimulus specificity of gene expression programs determined by temporal control of IKK activity. Science. 2005;309:1857–1861. doi: 10.1126/science.1113319. [DOI] [PubMed] [Google Scholar]
- 74.Stamatovic SM, Keep RF, Kunkel SL, Andjelkovic AV. Potential role of MCP-1 in endothelial cell tight junction ‘opening’: signaling via Rho and Rho kinase. J Cell Sci. 2003;116:4615–4628. doi: 10.1242/jcs.00755. [DOI] [PubMed] [Google Scholar]
- 75.Xu H, Manivannan A, Dawson R, et al. Differentiation to the CCR2+ inflammatory phenotype in vivo is a constitutive, time-limited property of blood monocytes and is independent of local inflammatory mediators. J Immunol. 2005;175:915–6923. doi: 10.4049/jimmunol.175.10.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 77.Zen K, Parkos CA. Leukocyte-epithelial interactions. Curr Opin Cell Biol. 2003;15:557–564. doi: 10.1016/s0955-0674(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 78.Elner SG, Elner VM, Jaffe GJ, Stuart A, Kunkel SL, Strieter RM. Cytokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Curr Eye Res. 1995;14:1045–1053. doi: 10.3109/02713689508998529. [DOI] [PubMed] [Google Scholar]
- 79.Yuuki T, Kanda T, Kimura Y, et al. inflammatory cytokines in vitreous fluid and serum of patients with diabetic vitreoretinopathy. J Diabetes Complications. 2001;15:257–259. doi: 10.1016/s1056-8727(01)00155-6. [DOI] [PubMed] [Google Scholar]
- 80.Hernandez C, Segura RM, Fonollosa A, Carrasco E, Francisco G, Simo R. Interleukin-8, monocyte chemoattractant protein-1 and IL-10 in the vitreous fluid of patients with proliferative diabetic retinopathy. Diabet Med. 2005;22:719–722. doi: 10.1111/j.1464-5491.2005.01538.x. [DOI] [PubMed] [Google Scholar]
- 81.Miyamoto K, Khosrof S, Bursell SE, et al. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A. 1999;96:10836–10841. doi: 10.1073/pnas.96.19.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kijlstra A, La Heij E, Hendrikse F. Immunological factors in the pathogenesis and treatment of age-related macular degeneration. Ocul Immunol Inflamm. 2005;13:3–11. doi: 10.1080/09273940590909185. [DOI] [PubMed] [Google Scholar]
- 83.Higgins GT, Wang JH, Dockery P, Cleary PE, Redmond HP. Induction of angiogenic cytokine expression in cultured RPE by ingestion of oxidized photoreceptor outer segments. Invest Ophthalmol Vis Sci. 2003;44:1775–1782. doi: 10.1167/iovs.02-0742. [DOI] [PubMed] [Google Scholar]