Abstract
Objective
Case series suggest that Nonalcoholic Fatty Liver Disease (NAFLD) is associated with increased all-cause and cardiovascular mortality. The current study compared the survival of subjects with and without suspected NAFLD in a population-based cohort, and placed the finding in the context of previously published case series.
Methods
Primary analysis assessed mortality for NHANES-III participants with and without suspected NAFLD using the National Death Index. Suspected NAFLD was based upon unexplained ALT elevation. The Olmsted County and Cleveland Clinic case series were also used for comparison. Survivals were compared using Proportional Hazards Model and direct age standardization.
Results
The NHANES cohort included 980 with and 6594 subjects without suspected NAFLD. Over a mean of 8.7 years, suspected NAFLD had a hazards ratio of 1.37 (95%CI 0.98–1.91) for all-cause mortality. In the 45–54 age group, suspected NAFLD had significantly higher all-cause (4.40 95%CI 1.27–13.23) and cardiovascular mortality (8.15 , 95%CI 2.00–33.20) after adjusting for conventional cardiovascular risk factors. The age-standardized rate per 10,000 per year was 129 (95%CI 118–140) for the NHANES non-NAFLD cohort, 154 (95%CI 116–198) for the NHANES suspected NAFLD cohort, 214 (95%CI 157–279) for the Olmsted County series, and 426 (95%CI 298–573) for the Cleveland Clinic series.
Conclusion
The magnitude of mortality risk in NAFLD depends on the setting and method of ascertainment. Suspected NAFLD in the 45–54 age group is a strong independent risk factor for cardiovascular death and warrants further cardiovascular risk management guidelines.
Study Highlights
-
What is the current knowledge
NAFLD patients in a community series have 1.34 times the risk of death compared to the general population.
NAFLD patients in referral center series may have even higher all-cause, cardiovascular disease, malignancy, and liver disease mortality.
-
What is new here
Subjects with suspected NAFLD detected in a population-based survey had a hazards ratio of 1.37 for all-cause mortality rate compared to the general population. The excess mortality was mainly due to excess mortality in the age group 45–54.
Suspected NAFLD, especially in the 45–54 year old age group, is a strong independent risk factor for cardiovascular disease death and presents an urgent and pressing need to develop guidelines for cardiovascular risk management in patients with NAFLD.
The magnitude of mortality risk in NAFLD depends on the setting and method of ascertainment.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a spectrum of liver disease that encompasses simple fatty liver, nonalcoholic steatohepatitis (NASH), and cirrhosis. Steatohepatitis is a histologically defined condition characterized by steatosis, inflammatory infiltrates and ballooning degeneration of hepatocytes. NAFLD is the most common liver disease in the United States. As many as one third of adults in the U.S. may have fatty liver based upon epidemiological studies using magnetic resonance(1) or liver biopsy (2). The survival of subjects with NAFLD has been reported using selected case series. In the Olmsted County series of community patients, the generalized mortality ratio was 1.34 times the general Minnesota population. In the Cleveland Clinic series of referral center patients, the all-cause, cardiovascular disease, malignancy, and liver disease mortality rate may have been even higher. However, the community and referral center series cannot be readily compared due to differences in age distribution. The survival in these two case series was compared to the survival in the general population without correction for obesity or metabolic syndrome. Thus, it remains unclear whether NAFLD is an independent risk factor for all-cause and cardiovascular mortality beyond its association with obesity and metabolic syndrome. The survival of subjects with NAFLD detected in population-based screening is unknown. Therefore, the current study aimed to compare the survival of subjects with and without suspected NAFLD in a population-based cohort study, and contrast the finding with those of previously reported NAFLD series from community-based and tertiary care referral center.
METHODS
Study Sample
The primary analysis included participants in the Third National Health and Nutrition Examination Survey (NHANES III). NHANES III was based on a stratified, multistage, probability cluster sampling design to obtain a representative sample of the civilian, non-institutionalized population(3). The baseline survey was conducted in the United States from 1988 to 1994. Subjects were followed until December 2000 for mortality using the National Death Index. The mortality data were initially released in 2004 and subsequently updated to ensure completeness and accuracy in 2007. We included participants who were 35 to 84 year old, ethnically non-Hispanic Black, non-Hispanic White or Mexican American. The primary exposure under investigation was suspected NAFLD which was defined using unexplained serum alanine aminotransferase (ALT) elevation as a surrogate.(4) Given the lack of a single uniform cut-point for ALT elevation we chose to use the widely used gender specific cut point based on the 95th percentile of healthy subjects (ALT > 30 for men, ALT > 19 for woman) (5) to identify cases of suspected NAFLD. Subjects with normal ALT constitute the reference group. After exclusion of other common causes, unexplained ALT elevation was strongly associated with adiposity and other features of the metabolic syndrome, as would be expected for nonalcoholic fatty liver disease(6). In clinical cases series (7–9) and population-based studies (10–12), when appropriate alternative explanations were excluded, 80 to 90% of adults with unexplained ALT elevation had NAFLD as determined by biopsy or imaging. Therefore we excluded 1953 subjects without ALT data and 2301 subjects with identifiable conditions other than NAFLD that might account for the ALT elevation. These conditions included excessive alcohol consumption (>1 alcoholic beverage per day), viral hepatitis (positive hepatitis B surface antigen, positive hepatitis C antibody), iron overload (transferitin saturation > 50%) and use of medications associated with hepatotoxicity (androgens, antivirals medications, antifungals medications, nitrofuratoin, phenytoin, sulfonamides, trazadone or tetracycline). Other chronic liver diseases are uncommon and unlikely to contribute significantly to the cause of ALT elevation a general population(13). We also excluded 6 participants whose mortality status was uncertain. See Figure 1 for inclusion and exclusion flow chart.
Figure 1.
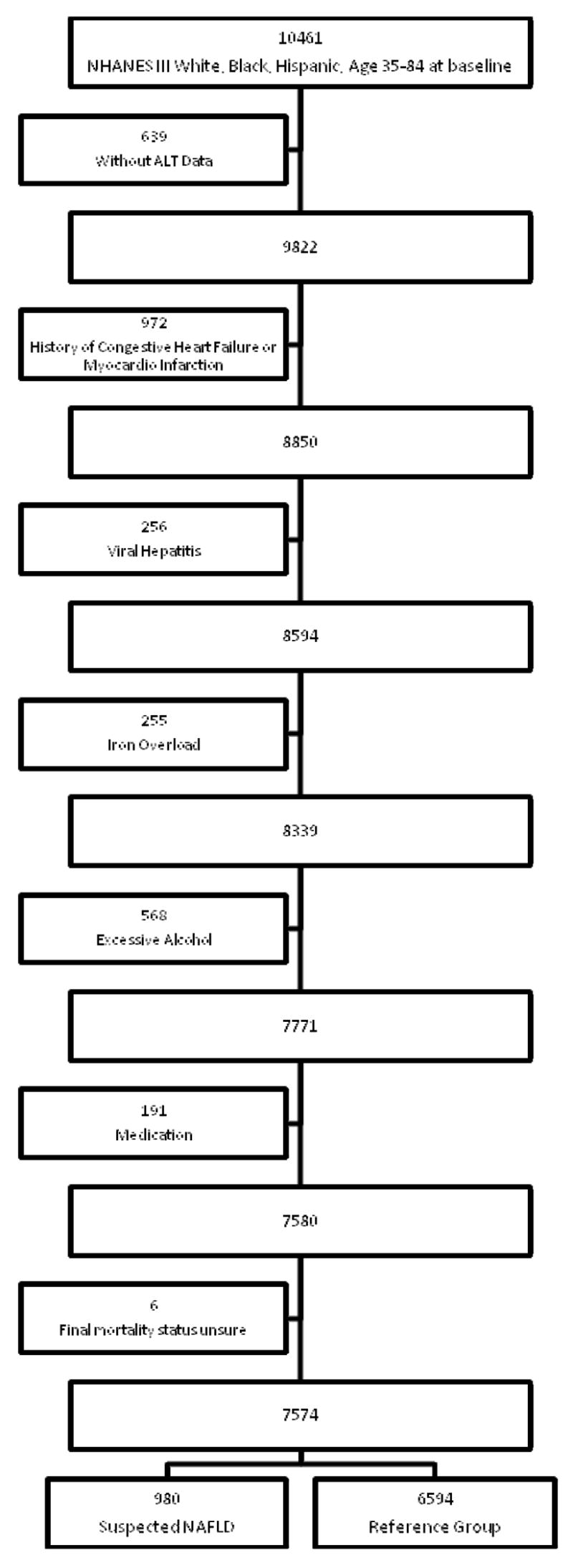
Inclusion and Exclusion Flowchart
Original data were also obtained from the previously published Olmsted County(14) and Cleveland Clinic (15) case series to facilitate comparison among NAFLD diagnosed by different modalities and in different settings. As in the NHANES cohort, we included participants age 35 to 84. The Cleveland Clinic series included 120 patients with NAFLD diagnosed through liver biopsy. Subjects were followed for a mean duration of 8.3 years. The Olmsted County series included 337 residents in Olmsted County who had been diagnosed with NAFLD. Unlike the Cleveland Clinic series, referral patients who did not reside in Olmsted County were excluded. There were 53 patients diagnosed by liver biopsy and 284 diagnosed by imaging (ultrasound or Computed Tomography). We excluded 15 participants whose initial diagnosis was made at postmortem. Subjects were followed for a mean duration of 7.6 years. Further details about these cohorts are available in their initial publications (14, 15).
This study was approved by the National Center for Health Statistics, as well as the institutional review boards for all investigators.
Mortality
The primary outcomes of interest were all-cause and cardiovascular disease mortality. In addition, we examined liver disease mortality and other cause specific mortality. Cardiovascular disease mortality included heart disease (ICD -10 I00–I09,I11,I13,I20–I51) and cerebral vascular disease mortality (ICD-100 I60-I69). Liver disease mortality included death due to chronic liver disease and cirrhosis (ICD-10 K70, K73–K74) as well as malignant neoplasm of liver and intrahepatic bile ducts (ICD-10 C22).
Cardiovascular Risk Factors
NAFLD shares many risk factors with cardiovascular disease. Therefore, demographic, metabolic and behavioral variables known to be associated with cardiovascular disease were explored. Potential metabolic confounders included systolic blood pressure (SBP), diastolic blood pressure (DBP), diabetes, fasting glucose, insulin sensitivity, total cholesterol, triglyceride, high density lipoprotein (HDL), and waist circumference. Participants were considered to have diabetes if they reported having a diagnosis of diabetes or if they had hemoglobin A1c above 6.5%(16). Diabetes, fasting glucose, insulin sensitivity and triglyceride were only considered in subjects with ≥ 6 hours of fasting prior to phlebotomy. Insulin sensitivity was measured as Quantitative Insulin Sensitivity Check Index (QUICKI = 1/[(log[fasting insulin (lU/mL) + log(fasting glucose [mg/dL])](17). To avoid co-linearity, low density lipoprotein (LDL) was not included in the presence of total cholesterol, triglyceride and HDL; body mass index (BMI) and waist hip ratio were not included in the presence of waist circumference; diabetes, fasting glucose and insulin sensitivity were included in separate models. Behavioral variables included daily alcohol consumption, cigarette use physical activity and HMG-CoA reductase use. Participants were also asked if they considered themselves more active, less active or the same as same age sex peers. Participants were asked about the number of days they drank alcohol and the number of alcoholic drink they consumed on a day that they drank. A drink was defined as 12-oz of beer, 4-oz of wine or one ounce of liquor. Average daily alcohol consumption was calculated as previously reported(18): number of days drinking alcohol per year × average drinks per drinking day/365 days per year. Although excessive alcohol consumption was excluded, alcohol consumption was explored because moderate alcohol consumption has been shown to be cardio-protective.
Statistical Methods
NHANES III was based on a complex, multistage, stratified, clustered probability sampling which should not be treated as a simple random sample. The SUDAAN 9.0 module (Research Triangle Institute, Research Triangle Park, NC) in SAS 9.1 (SAS Institute Inc., Cary, NC) was used to incorporate primary sampling unit (sdppsu6), strata (sdpstra6) and weighting (wtpfhx6) into the analysis(3)
Categorical variables were summarized as frequencies and compared using the Chi-squared test. Continuous variables were reported as means ± standard deviation and compared using two-sample t-test. Direct age standardization based on the 2000 census was used to calculate all-cause and cause specific mortality rates(19).
Cox proportional hazards model was used to compare time to all-cause and cause specific mortality between the NHANES III suspected NAFLD group and reference group. Cox proportional hazards model is more sensitive than direct age standardization to detect a difference in mortality rate because it takes into account the follow-up time and the exact time of death. Proportional hazards assumption was verified using the Schoenfeld residuals. Age, gender and race interactions were tested. The first multivariate model included suspected NAFLD and age. The second multivariate model additionally included gender and race. The third multivariate model additionally included the following risk factors: SBP, DBP, cigarette use, diabetes, total cholesterol, triglyceride, HDL, waist circumference, average daily alcohol use and physical activity. To avoid co-linearity, diabetes, fasting glucose and QUICKI were not included in the same model. Rather, we also substituted fasting glucose and QUICKI for diabetes in the third model. All statistical significance were considered at p-value<0.05.
RESULTS
The NHANES study sample included 980 (12.3%) participants with elevated ALT attributed to suspected NAFLD and 6594 (87.7%) subjects without suspected NAFLD (reference group). Subjects were followed for a mean of 8.7 years (range 0.05 – 11.7 years). There were 95 deaths in those with suspected NAFLD and 1154 deaths in the reference group. Table 1 presents the demographic and metabolic characteristics for those with suspected NAFLD and for the reference group. As compared to the reference group, those with suspected NAFLD were significantly (p<0.0001) younger, more likely to be women, more likely to be Mexican American and less likely to be African American. Subjects with suspected NAFLD were more likely to have metabolic syndrome, insulin resistance and diabetes (p<0.0001).
Table 1.
Demographic and metabolic characteristics of suspected NAFLD and reference group from NHANES cohort
| Suspected NAFLD (n = 1184) | Reference (n = 7014) | |
|---|---|---|
| Categorical variables, n (%) | ||
| Age
35–44 |
389 (41.3%) | 1852 (34.5%) |
| 45–54 | 239 (25.5%) | 1144 (22.0%) |
| 55–64 | 185 (20.1%) | 1290 (18.2%) |
| 65–74 | 129 (10.4%) | 1379 (16.9%) |
| 75–84 | 38 (2.7.0%) | 929 (8.5%) |
| Gender
Male |
354 (37.4%) | 3046 (53.9%) |
| Female | 626 (62.6%) | 3548 (53.9%) |
| Race
Non-Hispanic White |
375 (84.3%) | 3329 (86.3%) |
| Non-Hispanic Black | 169 (7.0%) | 1838 (10.3%) |
| Mexican American | 436 (8.8%) | 1427 (3.4%) |
| Diabetes | 153 (15.2%) | 602 (6.7%) |
| QUICKI < 0.339 | 619 (68.1%) | 2819 (40.3%) |
| Metabolic Syndrome | 441(54.3%) | 1796 (28.8%) |
| Smoking | 185 (18.6%) | 1473 (23.1%) |
| Average Alcohol intake | 0.18 (0.37) | 0.23 (0.42) |
| Physical Activity
More Active |
279 (30.0%) | 2327 (39.4%) |
| Same | 453 (46.1%) | 2902 (43.0%) |
| Less Active | 232 (23.9%) | 1222 (17.6%) |
| Continuous variables, mean (sd) | ||
| Systolic Blood Pressure | 127.2 (17.4) | 126.4 (18.4) |
| Diastolic Blood Pressure | 78.0 (10.6) | 75.6 (9.5) |
| Waist Circumference
Male |
105(14) | 98 (12) |
| Female | 99 (16) | 91 (14) |
| Total Cholesterol | 220 (45) | 214 (41) |
| HDL
Male |
40 (12) | 44 (13) |
| Female | 51 (16) | 56 (15) |
| Triglyceride | 200 (174) | 144 (115) |
| Fasting Glucose | 106 (46) | 99 (32) |
Catagorical variables shown as actual number (weighted frequency). Continuous variables normally distributed shown as mean (standard deviation).
The categorical variables Diabetes, QUICKI <0.339 and Metabolic Syndrome was only considered in subjects with ≥ 6 hours of fasting prior to phlebotomy. Therefore the denominator was 849 for the suspected NAFLD group and 5779 for the reference group. The mean total cholesterol, triglyceride and fasting glucose was also calculated from fasting subjects only.
The NHANES participants was weighted differently to adjust for the probability of cluster sampling and oversampling of Hispanics and African Americans. Therefore the actual number does not match the weighted percentage.
The directly age-standardized rate for all-cause and cause specific mortality for the suspected NAFLD group and the reference group are presented in table 2. The mortality rates were compared using Cox proportional hazards model adjusting for age (Table 2). Suspected NAFLD had a hazards ratio of 1.37 (95% CI 0.98 –1.91, p=0.067) for all cause mortality. None of the cause specific mortalities were significantly different. A significant age interaction was noted in the survival analysis for both all-cause mortality (p=0.01) and cardiovascular mortality (p=0.006), Figure 2 and 3 show the Kaplan-Meier all-cause survival and cardiovascular disease survival curves for subjects with and without suspected NAFLD stratified by age. In the age group 35–44, mortality was very low. In the age group 45–54, suspected NAFLD was associated with higher all-cause and cardiovascular mortality. In the age group 55–84, all-cause mortality and cardiovascular mortality were higher than the in younger age groups but suspected NAFLD was not associated with higher mortality compared to age match peers. Further analysis of all-cause mortality and cardiovascular mortality stratified age as 45–54 and 55–84. The age group 35–44 was dropped from further analysis due to low mortality (1.33% and 0.49%).
Table 2.
Number of deaths and age-standardized mortality rate per 10,000* all-cause mortality and cause specific mortality; NHANES III
| Suspected NAFLD Group | Reference Group | Hazards Ratio(95%CI)** | |||
|---|---|---|---|---|---|
| # of Deaths | Age-standardized Rate per10,000 (95%CI)* | # of Deaths | Age-standardized Rate per10,000 (95%CI)* | ||
| All-cause Mortality | 95 | 1340 (1005–1719) | 1154 | 1120 (1030–1214) | 1.37 (0.98 –1.91) |
| Cardiovascular Disease | 26 | 372 (206–580) | 417 | 387 (341–436) | 1.17 (0.69 – 1.98) |
| Malignant Neoplasm | 27 | 346 (198–528) | 308 | 324 (276–377) | 1.14 (0.71 – 1.82) |
| Chronic Lower Respiratory Disease | 7 | 129 (33–269) | 58 | 76 (53–103) | 1.20 (0.39 – 3.67) |
| Liver Disease | 5 | 39 (2–104) | 17 | 11 (5–20) | 4.15 (0.55 – 31.11) |
| Diabetes Mellitus | 5 | 112 (23–245) | 40 | 26 (15–40) | 3.31 (0.98 – 11.22) |
| Accidents | 3 | 14 (2–32) | 27 | 23 (12–36) | 0.79 (0.19 – 3.22) |
| Influenza and Pneumonia | 3 | 58 (8–137) | 33 | 29 (17–42) | 1.33 (0.34 – 5.24) |
| Alzheimer’s Disease | 0 | 19 | 20 (10–32) | ||
| Nephritis, Nephrotic Syndrome and Nephrosis | 1 | 5 (0–14) | 12 | 9 (4–15) | 1.44 (0.20 – 10.49) |
| Septicemia | 2 | 5 (0–12) | 17 | 16 (7–27) | 0.79 (0.18 – 3.41) |
directly standardized to the 2000 U.S. population, ages 35–84 years
from proportional hazards model adjusting for age.
Figure 2.
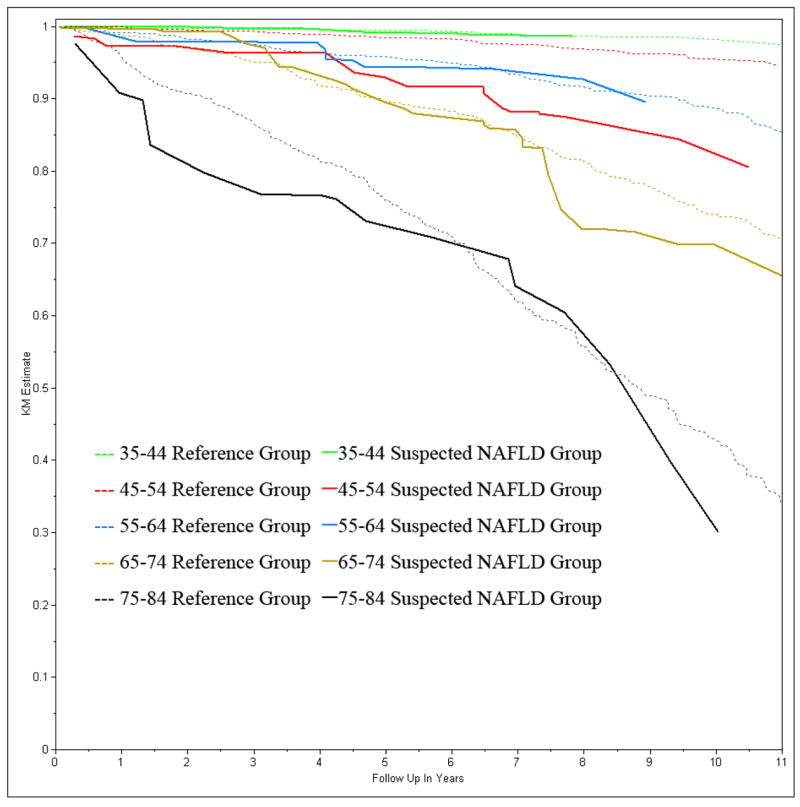
All-cause Survival Stratified by Age Groups and suspected NAFLD status; NHANES III
Figure 3.
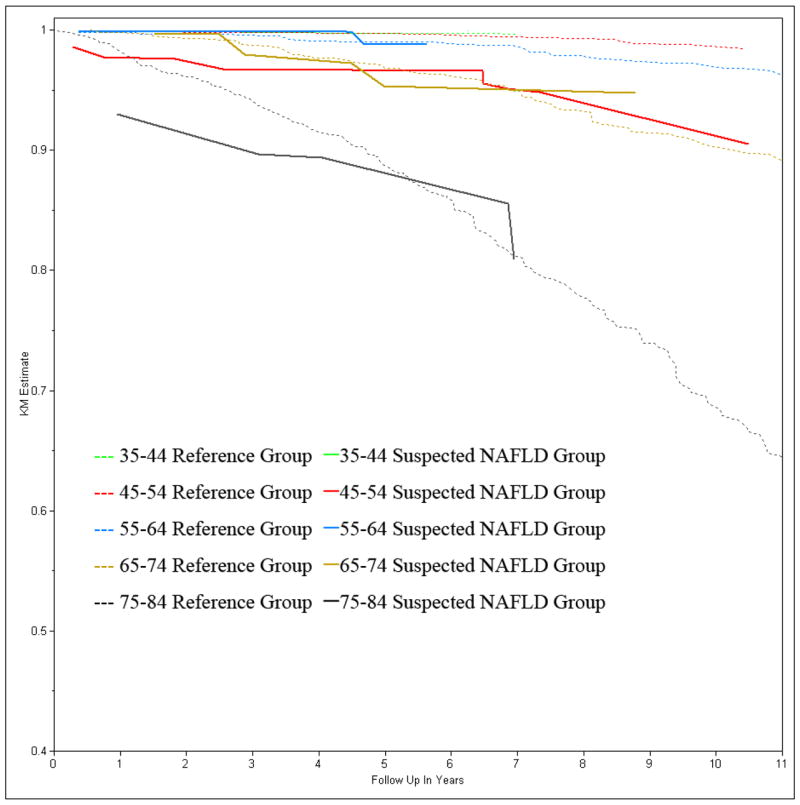
Cardiovascular Disease Survival Stratified by Age Groups and suspected NAFLD status: NHANES III
In the 45–54 age group there were 239 subjects in the suspected NAFLD group and 1144 subjects in the reference group. Among the suspected NAFLD group, there were 19 deaths, of which 9 were due to cardiovascular disease. Among the reference group, there were 58 deaths of which 15 were due to cardiovascular disease. As shown in Table 3, suspected NAFLD was associated with higher all-cause and cardiovascular mortality in model 1 which corrected for age, model 2 which additionally corrected for gender and race differences, and in multivariate model 3 which additionally corrected for metabolic and lifestyle characteristics. The hazards ratio was 4.10 (95% CI 1.27 – 13.23) for all-cause mortality and 8.15 (95% CI 2.00 – 33.20) for cardiovascular mortality in model 3. Substituting diabetes with fasting glucose or QUICKI in Model 3 did not significantly change the hazards ratio.
Table 3.
Multivariate analysis of the risk of death from all cause mortality and cardiovascular disease mortality comparing subjects with and without suspected NAFLD among two age groups; NHANES III
| Cardiovascular Disease Mortality HR (95% confidence interval) | All Cause Mortality HR (95% confidence interval) | |
|---|---|---|
| Baseline Age 45–54 | ||
| Model 1 | 6.53 (2.16 – 19.77) | 4.03 (1.76 – 9.21) |
| Model 2 | 7.92 (2.54 – 24.74) | 4.33 (1.83 – 10.21) |
| Model 3 | 8.43 (2.43 – 22.72) | 4.14 (1.26 – 13.58) |
| Baseline Age 55–84 | ||
| Model 1 | 0.66 (0.29 – 1.48) | 1.03 (0.69 – 1.54) |
| Model 2 | 0.76 (0.34 – 1.72) | 1.18 (0.0.77 – 1.80) |
| Model 3 | 0.79 (0.28 – 2.19) | 1.26 (0.76 – 2.06) |
Model 1 Corrected for age
Model 2 Corrected for age, gender, race
Model 3 Corrected for age, gender, race, SBP, DBP, waist circumference, total cholesterol, HDL cholesterol, triglyceride, smoking, CRP, daily alcohol, physical activity, diabetes, HMG-CoA reductase inhibitor use
In the 55–84 age group there were 352 subjects in the suspected NAFLD group and 3598 subjects in the reference group. Among those with suspected NAFLD, there were 62 deaths, of which 16 were due to cardiovascular disease. Among the reference group there were 1047 deaths, of which 395 were due to cardiovascular disease. As shown in table 3, suspected NAFLD was not associated with increased mortality in this age group.
Directly age-standardized mortality rates from the case series and the NHANES III cohort are shown in Table 4. Cases identified by liver biopsy from Olmsted County and the Cleveland Clinic, had similar all-cause mortality rates (474 and 426 per 10K per year), while cases from Olmsted County identified by imaging had all-cause mortality rates similar to the suspected NAFLD group from the NHANES cohort (156 and 154 per 10K per year). Cardiovascular disease, cancer and liver disease mortality rates were substantially higher for the Cleveland Clinic series (identified by biopsy) than both the NHANES cohort reference group and suspected NAFLD group. Liver disease mortality for the Olmsted County cases identified by imaging was also higher than for the NHANES cohort reference group.
Table 4.
Age-standardized mortality* by presence of NAFLD in three studies
| NHANES III Population Based Cohort | Olmsted County Case Series | Cleveland Clinic Case Series | |||
|---|---|---|---|---|---|
| Reference (n=6594) | Suspected NAFLD (n=980) | Imaging (n=284) | Biopsy (n=53) | Biopsy (n=120) | |
| All-cause Mortality | 129 (118 – 140) | 154 (116 – 198) | 156 (102 – 221) | 474 (284 – 705) | 426 (298 – 573) |
| Cardiovascular Disease | 44 (39 – 50) | 43 (24 – 67) | 56 (26 – 95) | 19 (0 – 52) | 87 (32 – 161) |
| Malignant Neoplasm | 37 (32 – 43) | 40 (23 – 61) | 10 (2–22) | 259 (122 – 438) | 112 (50 – 195) |
| Liver Disease | 1 (1 – 2) | 5 (0 – 12) | 26 (7 – 53) | 35 (4 – 84) | 62 (27 – 107) |
directly standardized to the 2000 U.S. population, ages 35–84 years
DISCUSSION
This prospective cohort study showed that subjects with suspected NAFLD detected in a population-based survey had a hazards ratio of 1.37 for all-cause mortality rate compared to the general population, however this was of marginal statistical significance. All-cause and cardiovascular mortality was substantially increased in those aged 45–54 years, but not among other age groups. This increased risk was not accounted for by conventional cardiovascular risk factors or by metabolic syndrome features. NAFLD ascertained by imaging in a community setting was associated with a similar mortality rate as suspected NAFLD detected using unexplained ALT elevation in a population-based survey. Patients referred to hepatologists with biopsy proven NAFLD, however, had substantially higher all-cause, cardiovascular, malignant neoplasm, and liver disease mortality rates.
In comparing the NHANES suspected NAFLD cohort to the other two case series, we found that they had a similar pattern of survival as the patients from the community diagnosed by an imaging modality. The patients with liver biopsy proven NAFLD in the Olmsted County and Cleveland Clinic case series had a substantially higher mortality rate. Although the use of ALT and imaging are susceptible to error, misclassification bias alone is unlikely to account for such a substantial difference in survival. Among people with NAFLD, those who were referred to hepatologists may have a more advanced liver disease than those detected in the community or population bases screening but were not referred. While patients with NASH are at greater risk for cirrhosis and liver related death, it remains unclear whether patients with NASH have lower overall survival than patients with bland or simple steatosis(15, 20). Patients with diabetes and metabolic syndrome may be preferentially referred to hepatologist for liver biopsy. Unlike the Cox proportional hazards model where multivariate analysis adjusted for metabolic risk factors, comparing mortality using direct age-standardization does not adjust for metabolic profile. It is likely that the substantially higher mortality in biopsy proven NAFLD may in part be due to patients’ adverse metabolic profile. Therefore, the magnitude of risk for all cause mortality in NAFLD depends on the setting and method of ascertainment.
Historically, there has been controversy over the significance of NAFLD as a disease(21). NAFLD is the most common chronic liver disease in the United States and leads to end stage liver disease in a subset of patients. For people with NAFLD, liver disease ranked 2nd to 3rd (14, 15) as the most common cause of death. The directly age-standardized mortality rate for liver-related death in the Cleveland Clinic referral series (62 per 10,000 per yr) and the Olmsted County community series (26 and 35 per 10,000 per yr) and the NHANES cohort suspected NAFLD group (5 per 10,000 per yr) were higher than the NHANES cohort reference group (1 per 10,000 per year), although the confidence interval of two NHANES groups overlapped. Most importantly, the all-cause mortality rates for subjects with NAFLD were higher than the NHANES cohort reference group. Together, these data confirm studies from community-based and tertiary care cohorts that NAFLD is a serious clinco-pathological entity independently associated with increased mortality and not a benign incidental finding.
There have been three major studies on the survival of subjects with NAFLD. In addition to the Cleveland Clinic (15)and Olmsted County series, the Swedish hospital cohort (20) included 129 patients with biopsy proven NAFLD referred to two hospitals in Sweden. Subjects were followed for a mean of 13.7 years. There were 68 patients with bland steatosis and 71 patients with steatohepatitis. Compared to the general population, patients with steatohepatitis had higher all-cause mortality, cardiovascular mortality and liver disease mortality but patients with bland steatosis were not at increased risk for death. In the Cleveland Clinic cohort,(15) patients with NASH had a higher but not statistically significant mortality as compared to patients with simple steatosis. However, all patients with fibrosis on liver biopsy were labeled as NASH in the Cleveland(15) and the Swedish(20) series; thus, none of these studies allowed to determine whether it was presence of fibrosis per se or necroinflammatory injury that was associated with a higher mortality. Therefore, the extent to which histology or individual histological features predict overall and cardiovascular mortality remains unclear
NAFLD is closely related to metabolic syndrome and therefore shares many risk factors with cardiovascular disease(22, 23). Studies have shown that patients with NAFLD may have impaired endothelial function (24), increased carotid intimal-medial thickness (25–27) and increased prevalence of ischemic heart disease based on resting electrocardiogram findings (28). In a prospective nested case control study of patients with diabetes(29), the presence of NAFLD was associated with 1.8 times the odds of having a cardiovascular event within 5 years. The association was attenuated but not abolished by correction for metabolic syndrome. In a large Korean prospective study of 35–59 year old government and private school employees(30), elevated ALT without exclusion of other liver diseases or correction for confounders, was associated with increased risk of cardiovascular disease death in men. Despite these findings, NAFLD has not been widely considered a risk factor for cardiovascular disease. There is an urgent and pressing need to develop guidelines for cardiovascular risk management in patients with NAFLD.
The major limitation of the current study was the use of ALT to classify suspected NAFLD. Currently there are no population based data in the United States with liver imaging and long-term follow-up. Future studies would be enhanced by the inclusion the use of liver imaging for the assessment of hepatic steatosis. Importantly, the use of NHANES made the current findings more generalizable to the United States population. The use of a cohort with and without suspected NAFLD allowed adjustment for metabolic characteristics and detection of age interaction which has not been reported in previous studies. In contrast, studies based upon liver biopsy have inherent issues of referral bias. Thus the use of unexplained ALT elevation as a surrogate of NAFLD although imperfect was appropriate for a large population based study. Moreover, measures were employed to optimize the predictive value of unexplained ALT elevation. In the Dallas Heart Study, without excluding alternative diagnoses for elevated ALT, the positive and negative predictive values for ALT were 51% and 72%.(1) However, in population-based studies from Israel(10), Japan(11) and Taiwan(12), after applying exclusions for infections and toxins the positive predictive value improved to 60 – 90% and the negative predictive value improved to 70 to 90%. Notably the current study used strict exclusion criteria to exclude excessive alcohol, viral hepatitis, iron overload and hepatotoxic medications. Thus the majority of participants in the current study were accurately classified as having or not having suspected NAFLD. Although misclassification bias existed, such error would be expected to falsely diminish the size of association that would otherwise have been observed. The most important limitation of using ALT as a surrogate, however, was the inability to distinguish between bland steatosis versus steatohepatitis, which may prognosticate different all-cause and cardiovascular mortality.
Our finding of increased all-cause mortality and cardiovascular disease in the 45 – 54 age group was based on the discovery of an age interaction term. Further studies are required to confirm this finding. The possibility remains that there may be a mortality difference in the 35–44 age group but relatively low mortality rate for this age group would require an even larger sample size. Based upon the natural history of NAFLD, the 45–54 age group may have a combination of greater disease severity and longer duration at a young enough age to produce maximal excess mortality. As one assesses an older age group there is a higher background mortality which limits the ability to detect small effect sizes on survival. In those subjects who had biopsy proven disease there was the greatest negative effect on survival. Expressing the mortality rate as a directly standardized rate allows future cohorts to be compared to NHANES, and the Cleveland Clinic and Olmsted County case series.
Conclusion
Suspected NAFLD, especially in the 45–54 year-old age group, was a strong independent risk factor for cardiovascular disease death and presents an urgent and pressing need to develop guidelines for cardiovascular risk management in patients with NAFLD. Although suspected NAFLD had marginal elevation of all-cause mortality when all age groups are considered, 45–55 is the common age when patients present with unexplained ALT elevation and suspected NAFLD. The magnitude of mortality risk in NAFLD depends on the setting and method of ascertainment.
Acknowledgments
Grant Support: This study was supported in part by NIH NRSA grant T32 DK07202 and by M01 RR000827 from the National Center for Research Resources of the National Institutes of Health for the General Clinical Research Center at UCSD. The funders did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Funding/Support: This study was supported in part by NIH NRSA grant T32 DK07202 and by M01 RR000827 from the National Center for Research Resources of the National Institutes of Health for the General Clinical Research Center at UCSD. The funders did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Abbreviations
- ALT
serum alanine aminotransferase
- BMI
body mass index
- DBP
diastolic blood pressure
- HDL
high density lipoprotein
- LDL
low density lipoprotein
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NHANES III
Third National Health and Nutrition Examination Survey
- SBP
systolic blood pressure
- QUICKI
quantitative insulin sensitivity check index
Footnotes
Potential Financial Conflicts of Interest: The authors have no financial conflicts of interest.
Author Contribution: Jeffrey B. Schwimmer, MD accepted full responsibility for the conduct of the study, had access to the data and had control of the decision to publish.
Study Concept and Design: Deborah L. Wingard, PhD; Jeffrey B. Schwimmer, MD
Acquisition of data: Paul Angulo, MD; Zobair M. Younossi MD, MPH
Statistical Analysis: Winston Dunn, MD; Ronghui Xu, PhD; Christopher Rogers, PhD:
Drafting of the manuscript: Winston Dunn, MD
Critical revision of the manuscript: Ronghui Xu, PhD; Deborah L. Wingard, PhD; Paul Angulo MD; Zobair M. Younossi MD, MPH; Jeffrey B. Schwimmer, MD.
All authors have approved the final draft.
References
- 1.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 2.Ryan CK, Johnson LA, Germin BI, et al. One hundred consecutive hepatic biopsies in the workup of living donors for right lobe liver transplantation. Liver Transpl. 2002;8:1114–22. doi: 10.1053/jlts.2002.36740. [DOI] [PubMed] [Google Scholar]
- 3.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Vital Health Stat. 1994. National Center for Health Statistics. [PubMed] [Google Scholar]
- 4.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures. Vital Health Stat 1. 1994:1–407. [PubMed] [Google Scholar]
- 5.Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 6.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–7. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 7.Daniel S, Ben-Menachem T, Vasudevan G, et al. Prospective evaluation of unexplained chronic liver transaminase abnormalities in asymptomatic and symptomatic patients. Am J Gastroenterol. 1999;94:3010–4. doi: 10.1111/j.1572-0241.1999.01451.x. [DOI] [PubMed] [Google Scholar]
- 8.Skelly MM, James PD, Ryder SD. Findings on liver biopsy to investigate abnormal liver function tests in the absence of diagnostic serology. J Hepatol. 2001;35:195–9. doi: 10.1016/s0168-8278(01)00094-0. [DOI] [PubMed] [Google Scholar]
- 9.Ratziu V, Giral P, Charlotte F, et al. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117–23. doi: 10.1016/s0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- 10.Zelber-Sagi S, Nitzan-Kaluski D, Halpern Z, et al. Prevalence of primary non-alcoholic fatty liver disease in a population-based study and its association with biochemical and anthropometric measures. Liver Int. 2006;26:856–63. doi: 10.1111/j.1478-3231.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- 11.Jimba S, Nakagami T, Takahashi M, et al. Prevalence of non-alcoholic fatty liver disease and its association with impaired glucose metabolism in Japanese adults. Diabet Med. 2005;22:1141–5. doi: 10.1111/j.1464-5491.2005.01582.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen CH, Huang MH, Yang JC, et al. Prevalence and risk factors of nonalcoholic fatty liver disease in an adult population of taiwan: metabolic significance of nonalcoholic fatty liver disease in nonobese adults. J Clin Gastroenterol. 2006;40:745–52. doi: 10.1097/00004836-200609000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Yu AS, Keeffe EB. Elevated AST or ALT to nonalcoholic fatty liver disease: accurate predictor of disease prevalence? Am J Gastroenterol. 2003;98:955–6. doi: 10.1111/j.1572-0241.2003.07485.x. [DOI] [PubMed] [Google Scholar]
- 14.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–21. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–9. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 16.Woerle HJ, Pimenta WP, Meyer C, et al. Diagnostic and therapeutic implications of relationships between fasting, 2-hour postchallenge plasma glucose and hemoglobin a1c values. Arch Intern Med. 2004;164:1627–32. doi: 10.1001/archinte.164.15.1627. [DOI] [PubMed] [Google Scholar]
- 17.Hrebicek J, Janout V, Malincikova J, et al. Detection of insulin resistance by simple quantitative insulin sensitivity check index QUICKI for epidemiological assessment and prevention. J Clin Endocrinol Metab. 2002;87:144–7. doi: 10.1210/jcem.87.1.8292. [DOI] [PubMed] [Google Scholar]
- 18.Ruhl CE, Everhart JE. Joint effects of body weight and alcohol on elevated serum alanine aminotransferase in the United States population. Clin Gastroenterol Hepatol. 2005;3:1260–8. doi: 10.1016/s1542-3565(05)00743-3. [DOI] [PubMed] [Google Scholar]
- 19.Anderson RN, Rosenberg HM. Age standardization of death rates: implementation of the year 2000 standard. Natl Vital Stat Rep. 1998;47:1–16. 20. [PubMed] [Google Scholar]
- 20.Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–73. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 21.Tarantino G. Is NAFLD an incidentaloma? Gastroenterology. 2006;130:1014–5. doi: 10.1053/j.gastro.2006.01.077. [DOI] [PubMed] [Google Scholar]
- 22.Ioannou GN, Weiss NS, Boyko EJ, et al. Elevated serum alanine aminotransferase activity and calculated risk of coronary heart disease in the United States. Hepatology. 2006;43:1145–51. doi: 10.1002/hep.21171. [DOI] [PubMed] [Google Scholar]
- 23.Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124:71–9. doi: 10.1053/gast.2003.50004. [DOI] [PubMed] [Google Scholar]
- 24.Villanova N, Moscatiello S, Ramilli S, et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42:473–80. doi: 10.1002/hep.20781. [DOI] [PubMed] [Google Scholar]
- 25.Targher G, Bertolini L, Padovani R, et al. Relation of nonalcoholic hepatic steatosis to early carotid atherosclerosis in healthy men: role of visceral fat accumulation. Diabetes Care. 2004;27:2498–500. doi: 10.2337/diacare.27.10.2498. [DOI] [PubMed] [Google Scholar]
- 26.Brea A, Mosquera D, Martin E, et al. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: a case-control study. Arterioscler Thromb Vasc Biol. 2005;25:1045–50. doi: 10.1161/01.ATV.0000160613.57985.18. [DOI] [PubMed] [Google Scholar]
- 27.Volzke H, Robinson DM, Kleine V, et al. Hepatic steatosis is associated with an increased risk of carotid atherosclerosis. World J Gastroenterol. 2005;11:1848–53. doi: 10.3748/wjg.v11.i12.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin YC, Lo HM, Chen JD. Sonographic fatty liver, overweight and ischemic heart disease. World J Gastroenterol. 2005;11:4838–42. doi: 10.3748/wjg.v11.i31.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Targher G, Bertolini L, Poli F, et al. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541–6. doi: 10.2337/diabetes.54.12.3541. [DOI] [PubMed] [Google Scholar]
- 30.Kim HC, Nam CM, Jee SH, et al. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. Bmj. 2004;328:983. doi: 10.1136/bmj.38050.593634.63. [DOI] [PMC free article] [PubMed] [Google Scholar]


