Abstract
Hyperhomocysteinemia is an established risk factor for arterial as well as venous thromboembolism. Individuals with severe hyperhomocysteinemia caused by inherited genetic defects in homocysteine metabolism have an extremely high incidence of vascular thrombosis unless they are treated aggressively with homocysteine-lowering therapy. The clinical value of homocysteine-lowering therapy in individuals with moderate hyperhomocysteinemia, which is very common in populations at risk for vascular disease, is more controversial. Considerable progress in our understanding of the molecular mechanisms underlying the association between hyperhomocysteinemia and vascular thrombotic events has been provided by the development of a variety of murine models. Because levels of homocysteine are regulated by both the methionine and folate cycles, hyperhomocysteinemia can be induced in mice through both genetic and dietary manipulations. Mice deficient in the cystathionine β-synthase (CBS) gene have been exploited widely in many studies investigating the vascular pathophysiology of hyperhomocysteinemia. In this article, we review the established murine models, including the CBS-deficient mouse as well as several newer murine models available for the study of hyperhomocysteinemia. We also summarize the major vascular phenotypes observed in these murine models.
Keywords: Hyperhomocysteinemia, homocysteine, endothelium, thrombosis, murine models
Introduction
Hyperhomocysteinemia is defined as an elevation of plasma total homocysteine (tHcy).1 In humans, plasma levels of tHcy are modulated by diet,2 genetic factors,3-6 certain drugs,7 and renal function.8 Severe hyperhomocysteinemia (plasma tHcy > 100 μmol/L) is classically caused by rare genetic defects in the metabolism of methionine, folate, or vitamin B12,5, 9 but it also can occur in individuals with severe vitamin B12 deficiency due to pernicious anemia.10 Moderate hyperhomocysteinemia (plasma tHcy of 10 to 100 μmol/L) can be caused by renal disease, nutritional deficiencies of folate or vitamin B12, or a common genetic variant in the methylene tetrahydrofolate reductase (MTHFR) gene.1 Moderate hyperhomocysteinemia is highly prevalent in most populations. Depending on the folate content of the diet and the age of the population, it may be found in 5 to 10% of healthy adults and in up to 20−40% of patients with myocardial infarction, stroke or venous thromboembolism. 11, 12, 13
Recent interest in hyperhomocysteinemia has been driven by its association with vascular disease and thrombosis. Premature atherothrombotic disease is a hallmark of severe hyperhomocysteinemia, and dietary intervention to lower severely elevated tHcy levels can prevent life-threatening thrombotic events.14 Like severe hyperhomocysteinemia, moderate hyperhomocysteinemia is also a risk factor for adverse vascular events such as stroke, myocardial infarction, and venous thromboembolism.12, 13 The hypothesis that homocysteine-lowering therapy protects from adverse vascular events in individuals with moderate hyperhomocysteinemia is unproven, however. Several recent clinical trials have suggested that homocysteine-lowering therapy does not protect from secondary vascular events in patients with moderate hyperhomocysteinemia.15-19 Some studies, however, have suggested that population interventions to lower tHcy may produce clinical benefit in a primary prevention setting.20, 21
Several animal models of hyperhomocysteinemia have been developed to examine the vascular pathophysiology of altered homocysteine metabolism. Animal models have been used to investigate mechanisms of thrombosis and vascular dysfunction and to explore the vascular effects of specific genetic or dietary interventions. Several species of animals have been used to investigate vascular structure or function in hyperhomocysteinemia, including baboons,22, 23 monkeys,24 minipigs,25 rabbits,26, 27, and rats.28-30 Murine models of hyperhomocysteinemia are gaining in popularity for vascular studies due to the availability of many transgenic and gene targeted strains and because of improvements in methods for the analysis of vascular structure and function in mice.31-33 The purpose of this review is to summarize the availability and utilization of murine models of hyperhomocysteinemia for the investigation of vascular function.
Homocysteine metabolism
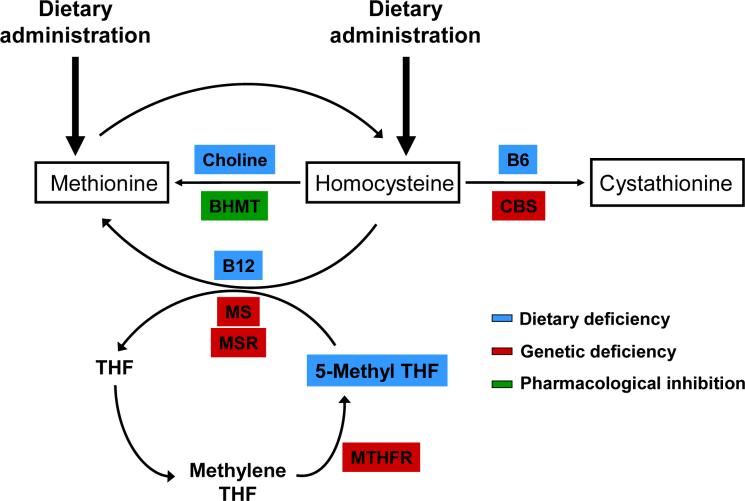
Homocysteine is formed as an intermediate product in the metabolic cycle of methionine (Figure 1). The metabolism of homocysteine is complex because it involves several enzymes and cofactors and because homocysteine is a metabolite in two cyclic pathways (the methionine and folate cycles). After it is formed from methionine, homocysteine can be further metabolized to cystathionine by a vitamin B6-dependent enzyme, cystathionine β-synthase (CBS). Alternatively, homocysteine can be remethylated back to methionine through one of two separate reactions. Betaine homocysteine methyl transferase (BHMT), an enzyme that is found in the liver and kidney, remethylates homocysteine using a methyl group derived from betaine (a derivative of choline). Methionine synthase (MS) is a vitamin B12-dependent enzyme found in most cells that remethylates homocysteine to methionine using a methyl group from 5-methyl tetrahydrofolate. An accessory enzyme, methionine synthase reductase (MSR), is required to maintain the activity of MS. The folate cycle plays a key role in homocysteine remethylation by providing the methyl donor, 5-methyltetrahydrofolate, which is generated by the enzyme MTHFR. It is evident from Figure 1 that homocysteine levels can be manipulated in animals by altering the availability of one or more of the substrates (e.g. methionine, folate, or choline) or cofactors (e.g. vitamin B6 or vitamin B12) or by altering the expression of one of the key enzymes (e.g. CBS, BHMT, MS, MSR, or MTHFR) of these metabolic pathways.
Figure 1.
Approaches to produce hyperhomocysteinemia in mice based on the metabolic pathways of homocysteine. Homocysteine is generated in the cytoplasm as an intermediate metabolite of the methionine cycle. Once formed, homocysteine can be metabolized through one of three pathways. First, homocysteine can be metabolized to cystathionine by cystathionine β-synthase (CBS). This reaction requires vitamin B6 as a cofactor. Second, homocysteine can be remethylated to methionine by methionine synthase (MS) in a reaction that requires vitamin B12. This reaction utilizes a methyl group from 5-methyl tetrahydrofolate (THF) and thus serves to link the methionine cycle with the folate cycle. 5-methyl THF is derived from the activity of methylene tetrahydrofolate reductase (MTHFR). Methionine synthase reductase (MSR) is required to maintain MS in its active conformation. Third, in the liver and kidney homocysteine can be remethylated to methionine by betaine:homocysteine methyltransferase (BHMT). This reaction uses betaine, which is derived from choline, as a methyl donor. Hyperhomocysteinemia can be induced in mice by: 1) dietary administration of methionine or homocysteine, 2) dietary deficiency of folate, vitamin B6, vitamin B12 or choline (blue), 3) genetic deficiency of CBS, MTHFR, MS or MSR, or 4) pharmacological inhibition of BHMT (green).
Murine models of hyperhomocysteinemia
Hyperhomocysteinemia can be produced in mice through dietary modifications, genetic approaches, a combination of dietary and genetic interventions, or by pharmacological approaches.
Diet-induced hyperhomocysteinemia
Several dietary approaches have been developed to induce hyperhomocysteinemia in mice. These include interventions that: 1) increase the metabolic flux through the methionine cycle by adding excess amounts of methionine to the diet, 2) limit the CBS-dependent conversion of homocysteine to cystathionine by decreasing the dietary content of vitamin B6, 3) limit the MS-mediated remethylation of homocysteine by using a diet that is deficient in folate and/or vitamin B12, 4) limit the BHMT-mediated homocysteine remethylation by decreasing the amount of choline in the diet, or 5) adding homocysteine itself to the drinking water. Because deficiency states for folate, vitamin B6, and vitamin B12 are common in human patients with hyperhomocysteinemia and atherothrombotic disease,11 experimental diets that are deficient in these vitamins may have direct relevance to human vascular disease.
The nutritional requirements for the laboratory mouse as recommended by the National Research Council (NRC) include 5 g/Kg methionine, 0.5 mg/Kg folic acid, 10 μg/Kg vitamin B12, and 7 mg/Kg of vitamin B6.34 In comparison with the NRC guidelines, typical commercial mouse chows contain slightly less methionine (3−5 g/Kg) and excess amounts of B vitamins (2−8 mg/Kg folate, 25−90 μg/Kg vitamin B12, and 7−17 mg/Kg vitamin B6). When used as control diets, these commercial chows produce plasma tHcy levels of 3−6 μmol/L (Table 1). Most studies of hyperhomocysteinemia in mice have used control and experimental diets with casein and/or soy-based dietary formulations, but some authors have advocated the use of amino acid defined diets to avoid lot-to-lot variability in the content of methionine and other amino acids.35 Most studies have used ad libitum feeding protocols, but some have advocated the use of pair feeding,36 to ensure that all of the mice have similar food intake.
Table 1.
Formulations of experimental mouse diets and plasma tHcy levels in different strains of mice.
|
Diet |
Methionine (g/Kg) |
Folate (mg/Kg) |
Vitamin B12 (μg/Kg) |
Vitamin B6 mg/Kg |
Choline (mmol/Kg) |
Mouse strain |
Plasma tHcy (μmol/L) |
Reference* |
|---|---|---|---|---|---|---|---|---|
| Standard chow | 4.0 | 7.5 | 91 | 17 | 21 | C57BL/6 | 4−5 | 39-41, 48 |
| BALB/c | 3 | 43 | ||||||
| Apoe−/− | 6 | 77 | ||||||
| 3.3 | 2.0 | 25 | 7 | 10 | Apoe−/− | 5 | 36 | |
| 5.2 | 2.0 | 25 | 7 | 10 | C57BL/6 | 5 | 53 | |
| |
3.7 |
3.2 |
55 |
15 |
21 |
C57BL/6 |
4 |
38 |
| High methionine | 12 | 2.0 | 25 | 7 | 10 | Apoe−/− | 18 | 37 |
| 20 | 3.2 | 55 | 15 | 21 | C57BL/6 | 40 | 38 | |
| 24.6 | 1.5 | 25 | 7 | 14 | C57BL/6 | 240−334 | 44, 45 | |
| 4.0 + 0.5% methionine in drinking water | 7.5 | 91 | 17 | 21 | C57BL/6 | 8−23 | 39-42 | |
| BALB/c | 4 | 43 | ||||||
| |
4.0 + 0.5% methionine in drinking water |
1.5 |
91 |
17 |
21 |
C57BL/6 |
60 |
44 |
| Low folate, vitamin B12 and/or vitamin B6 | 4.1 | 0.2 | 25 | 8.5 | 8 | C57BL/6 | 8 | 58 |
| BALB/c | 7 | 43 | ||||||
| 4.3 | 0.9 | 5.3 | 3.7 | 8 | Apoe−/− | 10 | 57 | |
| |
3.3 |
0.1 |
1.8 |
0.15 |
10 |
Apoe−/− |
244 |
36 |
| High methionine with low folate, vitamin B12 and/or vitamin B6 | 8.2 | 0.2 | 25 | 8.5 | 8 | C57BL/6 | 12−37 | 69, 70, 88 |
| BALB/c | 9 | 43 | ||||||
| Apoe−/− | 15 | 77 | ||||||
| 7.7 | 0.1 | 1.8 | 7 | 10 | Apoe−/− | 87 | 36 | |
| |
13.7 |
0.5 |
6.4 |
9 |
8 |
Apoe−/− |
54 |
50 |
| Low choline |
3.0 |
2.6 |
33 |
9 |
5 |
C57BL/6 |
15 |
35 |
| D,L-homocysteine (0.9 g/L) in drinking water | 4.3 | 0.5 | 6.4 | 9 | 8 | Apoe−/− | 52 | 57 |
| |
3.7 |
3.2 |
55 |
15 |
21 |
Apoe−/− |
33 |
56 |
| L-homocystine (0.9 g/L) in drinking water | 4.3 | 0.5 | 6.4 | 9 | 8 | Apoe−/− | 16 | 57 |
In several cases, the primary authors were contacted to obtain the complete formulation of the diets used in their studies.
High methionine diets
Several methods have been employed to manipulate the dietary intake of methionine in mice (Table 1). One common approach is to formulate an experimental diet with an increased amount of L-methionine to increase the total methionine content of the diet. Moderate hyperhomocysteinemia (plasma tHcy levels of 18−60 μmol/L) can be achieved by increasing the total methionine content up to 12 to 20 g/Kg37, 38 or by adding 0.5% L-methionine to the drinking water (Table 1).39-44 Severe hyperhomocysteinemia (plasma tHcy levels greater than 200 μmol/L) can be obtained by increasing the total methionine content even higher (Table 1),44, 45 but a dietary content of methionine greater than 20 g/Kg may produce untoward effects on growth and other toxic effects. In a study of apolipoprotein E-deficient (Apoe−/−) mice, a dietary methionine content of 22 g/Kg resulted in weight loss and a dietary methionine content of 44 g/Kg was found to cause severe growth retardation and early death.46 Massive methionine excess also can be fatal in humans.47
Diets deficient in folate, vitamin B12, and/or vitamin B6
Another common dietary approach to induce hyperhomocysteinemia in mice is to restrict the dietary intake of the B vitamins involved in homocysteine metabolism (folate, vitamin B12, and/or vitamin B6). Many folate-deficient diets also contain an antimicrobial agent such as sulfathiazole to inhibit the growth of intestinal bacteria that produce folates.36, 43, 48, 49 Depending on the degree of B vitamin restriction, these diets can produce either a mild degree of hyperhomocysteinemia (plasma tHcy levels of 8−10 μmol/L) or very severe hyperhomocysteinemia (plasma tHcy levels greater than 200 μmol/L) (Table 1). As with extremely high levels of methionine supplementation, severe restriction of B vitamins can produce weight loss36 and other non-specific effects. Because folate deficiency may lead to neural tube defects and developmental delay,50-52 folate-deficient diets should be used cautiously in pregnant or very young mice. To avoid the toxicity of diets that contain extremely high amounts of methionine or extremely low amounts of B vitamins, several investigators have formulated diets that contain modestly elevated amounts of methionine (8−14 g/Kg) in combination with moderate restriction of folate, vitamin B12, and/or vitamin B6. Such diets can be used to elevate plasma tHcy to levels of 10−90 μmol/L (Table 1).
Choline-deficient diets
Choline is another extremely important dietary constituent that has a major influence on homocysteine metabolism. Choline serves as a dietary source of betaine, the methyl donor for homocysteine remethylation catalyzed by BHMT (Figure 1). Therefore, the amount of choline in the diet can greatly impact the plasma tHcy level. It often can be difficult to compare the choline content of diets that are formulated with different chemical sources of choline, such as choline chloride or choline bitartrate, especially when the molar concentration of choline is not provided. The NRC nutritional requirement for choline (as free base) is 7.9 mmol/Kg,34 which is equivalent to about 2 g choline bitartrate/Kg or 1.1 g choline chloride/Kg of diet. Most commercially-available mouse chows contain 8−21 mmol choline/Kg, which meets the NRC requirement (Table 1). A dietary content of choline below 8 mmol/Kg usually produces elevation of plasma tHcy, even when the dietary content of methionine is not excessive and the diet contains sufficient amounts of folate, vitamin B12, and vitamin B6 (Table 1).35 Even when the dietary content of choline is within the NRC recommended range, choline may become a major determinant of plasma tHcy levels when the BHMT remethylation pathway is metabolically stressed, for example by enzymatic defects in CBS or MTHFR or by diets that contain excess amounts of methionine or are deficient in folate or vitamin B12. Choline has been demonstrated to be a limiting nutrient after oral methionine loading in mice.53 Conversely, choline-rich diets have been used to lower plasma tHcy and improve the viability of mice with severely impaired transsulfuration defects.54 A similar approach is to use betaine-rich diets to increase BHMT-mediated remethylation of homocysteine in mice with transsulfuration defects due to CBS deficiency.35 Betaine-rich diets also have been used to improve the viability of mice with MTHFR deficiency.55
Dietary administration of homocysteine
Lastly, plasma tHcy levels in mice can be manipulated by adding homocysteine itself to the drinking water (Table 1). The addition of D,L-homocysteine (0.9 g/L) to the drinking water of Apoe−/− mice has been reported to elevate the plasma tHcy concentration to 33 to 52 μmol/L.56, 57
The addition of 0.9 g/L homocystine (the disulfide derivative of L-homocysteine) produced a lesser elevation of plasma tHcy (16 μmol/L)57 (Table 1). In another study,46 the long-term (up to 12 months) treatment of Apoe−/− mice with 1.8 g/L D,L-homocysteine elevated the plasma tHcy concentration to 146 μmol/L.
Limitations of dietary models
A major limitation of all of the dietary models of hyperhomocysteinemia is that elevation of homocysteine is almost always accompanied by alterations in other metabolites that may influence vascular pathophysiology. For example, folate-deficient diets may deplete tissue folate pools independently of their effects on plasma tHcy levels.58 Similarly, methionine-rich diets may affect tissue levels of S-adenosylmethionine (SAM), potentially altering SAM-dependent methylation of DNA, phospholipids, and histones. Both folate deficiency and methionine excess have been proposed to have adverse effects on vascular function that may be independent of elevated homocysteine.36, 59
Genetic models of hyperhomocysteinemia
To avoid potential confounding effects of dietary interventions that may be independent of altered homocysteine metabolism, several genetic models of hyperhomocysteinemia have been developed. These include mice with targeted deletions of the genes encoding CBS, MTHFR, and MS, as well as transgenic mice that express the human CBS gene. These genetic models provide the opportunity to study the effects of hyperhomocysteinemia in the absence of dietary interventions.
Genetic models of altered CBS expression
In the mid 1990s, the first genetic model of hyperhomocysteinemia in mice was developed through the targeted disruption of the Cbs gene, which encodes murine CBS.60 These mice are predisposed to hyperhomocysteinemia because they have an impaired ability to convert homocysteine to cystathionine (Figure 1). This mouse line was initially developed as a model of hereditary homocystinuria. Mice homozygous for the targeted Cbs gene (Cbs−/− mice) have markedly elevated plasma tHcy levels (over 200 μmol/L) that are very similar to those in human patients with homozygous CBS gene mutations.5
Cbs−/− mice do not exhibit all of the phenotypic features of human CBS deficiency, however. For example, Cbs−/− mice have only a modest elevation of plasma methionine, they do not develop ocular or skeletal abnormalities, and they tend to develop more severe hepatic steatosis than humans with homozygous CBS mutations. Unlike human subjects with hereditary homocystinuria, Cbs−/− mice survive poorly beyond three to five weeks of age.60, 61 This difference may reflect the fact that most human patients with homozygous CBS gene mutations have a small amount of residual CBS activity whereas Cbs−/− mice have a complete absence of CBS activity. The poor survival of Cbs−/− mice is associated with generalized growth retardation rather than any specific developmental defect. The yield of adult Cbs−/− mice can be increased by the use of diets that are supplemented with choline54 and by an aggressive approach to animal husbandry that includes delayed weaning and selected removal of littermate mice that may compete for maternal care.61 Even with these aggressive approaches, however, a high rate of growth retardation and early death has been seen in most studies of Cbs−/− mice. 54,61 Therefore, most investigations of vascular endpoints have utilized mice heterozygous for the disrupted Cbs gene (Cbs +/− mice). When fed a typical control diet, Cbs+/− mice have a mild elevation of plasma tHcy levels (5−9 μmol/L, compared with <5 μmol/L in wild-type Cbs+/+ mice). Even this mild degree of elevation of tHcy has been found to produce vascular pathophysiological effects in some studies.39, 62, 63
To overcome the poor survival of Cbs−/− mice and provide a more useful murine model of severe hyperhomocysteinemia, Wang and colleagues45 generated a transgenic mouse line that expresses the human CBS transgene under the control of a zinc-inducible metallothionein promoter. When these human CBS transgenic mice were crossbred with Cbs−/− mice and fed a diet supplemented with zinc, the human CBS transgene was found to rescue Cbs−/− mice from early lethality. The resultant adult human CBS transgenic Cbs−/− mice had moderate hyperhomocysteinemia (plasma tHcy levels of 25−90 μmol/L).45 When excess zinc was removed from the diet after weaning, these mice developed extremely high levels of plasma tHcy (up to 231 μmol/L) with no apparent decrement in survival.45 The same group has generated another human CBS transgenic mouse line with a missense mutation (I278T) in the human CBS transgene corresponding to a common mutation in human CBS-deficient subjects.64 The human CBS I278T transgenic mouse also rescues Cbs−/− mice from early lethality, and, in either the presence or absence of excess zinc, produces levels of plasma tHcy greater than 250 μmol/L in human CBS I278T transgenic Cbs−/−mice.
These new transgenic mouse lines should provide a good resource to examine the vascular phenotype of a purely genetic, rather than dietary, model of severe hyperhomocysteinemia. One limitation of these transgenic models is that expression of the human CBS transgene requires exposure to relatively high levels of zinc (e.g. 25 mmol/L in the drinking water), which may raise concerns about potential confounding metabolic or enzymatic influences of zinc. As with any transgenic model, another limitation is the possibility that transgene-insertion effects could theoretically influence the vascular phenotype.
Genetic models of MTHFR deficiency
In 2001, mice deficient in MTHFR were generated through the targeted disruption of its gene, Mthfr.65 These mice have an impaired ability to convert homocysteine to methionine (Figure 1). When fed a control diet, mice homozygous for the targeted disruption of the Mthfr gene (Mthfr−/− mice) have moderate hyperhomocysteinemia (plasma tHcy ∼30 μmol/L). Despite having only a moderate elevation of plasma tHcy, Mthfr−/− mice suffer from decreased survival within the first five weeks of age and develop motor and gait abnormalities due to cerebellar hypoplasia. Dietary supplementation with betaine throughout pregnancy and during lactation helps to decrease the early mortality of Mthfr−/− mice from 83% to 26%.55 Betaine supplementation also partially reverses the abnormal cerebellar developmental in Mthfr−/− mice. Mice heterozygous for Mthfr deficiency (Mthfr+/− mice) survive normally but have only a small increase in plasma tHcy, to about 5 μmol/L compared with about 3 μmol/L in wild type Mthfr+/+ littermates.65 Mthfr+/− mice have been utilized in several studies investigating the vascular effects of altered homocysteine remethylation.43, 66
Genetic models of MS deficiency
Another mouse model with a genetic defect in homocysteine remethylation is the MS-deficient mouse. This mouse line was generated by targeted disruption of the Mtr67 gene, which encodes murine MS. These mice have a limited capacity to convert homocysteine to methionine (Figure 1). Unlike homozygous disruption of the Cbs or Mthfr genes, homozygous disruption of the Mtr gene produces complete embryonic lethality. This suggests that MS activity is absolutely required for early embryonic development. The embryonic lethality of Mtr−/− mice is likely caused by a “methyl folate trap,” in which folates accumulate as 5-methyl tetrahydrofolate and are not available for thymidylate or purine synthesis or other folate-dependent one carbon reactions. Dietary supplementation with folic acid, methionine, choline, or betaine does not rescue the lethality of Mtr−/− embryos.67
Mice with heterozygous disruption of the Mtr gene (Mtr+/− mice) survive normally without gross anatomical abnormalities or growth retardation. Levels of plasma tHcy are mildly elevated in Mtr+/− mice (5−10 μmol/L) compared with wild-type (Mtr+/+) littermates (generally less than 5 μmol/L) when they are fed a control diet.58, 67 Some,67 but not all58 studies have found that plasma tHcy levels are higher in female than in male Mtr+/− mice. Like Mthfr+/− mice, Mtr+/− mice have been utilized to investigate the vascular effects of altered homocysteine remethylation.58
Other genetic models of hyperhomocysteinemia
An alternative murine model of defective homocysteine remethylation was recently developed by Elmore et al.68 Using a gene-trap vector insertion method, these investigators generated a mouse line that is deficient in MSR, the enzyme that catalyzes the reductive reactivation of MS (Figure 1). MSR is essential to maintain the functional activity of MS, which is susceptible to oxidation during its normal reaction cycle. Mice homozygous for the gene trap mutation (Mtrrgt/gt mice) have normal viability and development. Mtrrgt/gt mice are not completely deficient in MSR activity, but instead demonstrate a variable degree of MSR expression (from <1% to 37% of wild-type levels) in different tissues. They have a moderate increase in plasma tHcy levels (18 μmol/L vs. 5 μmol/L in wild-type Mtrr+/+ mice). They also have a 30% decrease in plasma methionine levels. The vascular phenotype of these mice has not yet been investigated.
Combined genetic and dietary models of hyperhomocysteinemia
Dietary interventions can be combined with genetic models to generate mice with a wide range of plasma tHcy levels (Table 2). This approach has been used extensively with Cbs+/− mice to examine the vascular phenotype of hyperhomocysteinemia.40, 41, 69, 70 Cbs+/− mice are particularly susceptible to elevation of plasma tHcy when they are fed diets that contain high amounts of methionine (Table 2). Dietary interventions, including high methionine and low folate diets, also have been used with Mthfr+/−43 and Mtr+/-58 mice to examine the effects of varying plasma tHcy concentrations on vascular function. In contrast to Cbs+/− mice, Mthfr+/− mice have smaller elevations of plasma tHcy in response to high methionine diets but greater elevations in response to low folate diets (Table 2). These findings in murine models are consistent with clinical observations in humans with transsulfuration defects (such as CBS deficiency), who are more susceptible to methionine-induced hyperhomocysteinemia than subjects with remethylation defects (such as MTHFR deficiency).71, 72
Table 2.
Plasma tHcy levels (μmol/L) in Cbs+/− or Mthfr+/− mice fed different diets.
Pharmacological inhibition of BHMT
BHMT deficient mice are not yet available, but Collinsova and colleagues73 used a pharmacological inhibitor to limit the enzymatic activity of BHMT in a mouse model. Intraperitoneal injection of S-(δ-carboxylbutyl)-DL-homocysteine, an S-alkylated homocysteine derivative, produced a transient 60−90% reduction in hepatic BHMT activity that was associated with a 2−3 fold increase in plasma tHcy levels (up to 12 μmol/L). Repeated injections of the inhibitor caused a further increase in plasma tHcy concentration after three days (∼ 18 μmol/L).
Other factors that influence plasma tHcy levels in murine models
In mice, as in humans, the plasma concentration of tHcy is influenced by many factors in addition to diet and specific genetic defects in homocysteine metabolic enzymes. These factors include sex, age, genetic strain or background, the duration of dietary treatment, and the timing and methods of sample collection.
Influence of sex and age
In humans, plasma tHcy levels are higher in males than females and tend to increase with age in both genders.74, 75 Most studies examining the vascular effects of hyperhomocysteinemia in murine models have reported similar levels of plasma tHcy in male and female mice. Some recent studies, however, have found that plasma tHcy levels are higher in female than male mice.67, 76 This sexual dimorphism in plasma tHcy levels appears to be caused by higher expression of CBS in the kidney of male mice than female mice.76 The influence of age on plasma tHcy has not been studied systematically in mice. However, in a recent study we found that male Apoe−/− mice fed a high methionine/low folate diet exhibited an age-dependent decrease in plasma tHcy levels.77
Genetic background
As with most murine models,78 the phenotype of hyperhomocysteinemic mice potentially can be influenced by strain differences, genetic drift, and subtle variations in the genetic background of inbred lines of mice. These genetic background effects can confound experimental studies of vascular function. We have found that mice on a C57BL/6 background have a relatively higher sensitivity to diet-induced hyperhomocysteinemia compared with mice on a BALB/c background fed the same diet (Table 1). The overall influence of genetic background on plasma tHcy levels has not been examined systematically, however. Approaches that can be taken to minimize the potential confounding effects of genetic background include the utilization of inbred mouse lines that have been maintained by backcrossing to a defined genetic background for multiple generations and experimental designs with littermate comparison groups.
Duration of diet
When mice are fed diets that are low in folate or high in methionine, plasma tHcy levels generally increase relatively quickly (within 2 weeks) and remain elevated as long as the diet is continued. Various durations of dietary feeding, ranging from one to twelve months, have been used to study the vascular effects of hyperhomocysteinemia.36-38, 40, 41, 46, 48, 69, 70, 79 It is important to remember that an extended period of dietary feeding may lead to secondary metabolic effects. For example, we have observed an initial increase, followed by a decline, in plasma tHcy in mice fed a high methionine/low folate diet for up to one year. The fall in plasma tHcy after 8−12 months of dietary feeding was associated with hepatic steatosis and increased plasma levels of methionine. We speculate that these secondary metabolic effects may be caused by decreased expression of hepatic methionine adenosyltransferase (MAT), which catalyzes the activation of methionine with ATP to produce S-adenosyl methionine.
Sample collection
The plasma tHcy concentration also may be influenced by the timing and method of sample collection. Blood is often collected into EDTA or another anticoagulant and is kept on ice prior to centrifugation. Separated plasma is flash frozen and stored at −80°C until it is analyzed for tHcy by HPLC or immunological assays. Homocysteine also can be determined in samples of serum using similar assays; it is not known if there are significant differences between serum tHcy and plasma tHcy levels in mice. Most assays for tHcy measure the total amount of homocysteine and its disulfide derivatives, which include the disulfide homocystine, homocysteine-cysteine mixed disulfides, and S-homocysteinylated albumin.1 Hemolyzed samples may have higher levels of tHcy due to red cell lysis. In some studies, blood samples are collected from mice after surgical procedures that may involve the infusion of saline or drugs and/or prolonged anesthesia.39, 40, 43, 58, 69 Such procedures may have unintended effects on plasma tHcy levels. Depending on the methionine content of the diet, the plasma concentration of tHcy may exhibit a large diurnal variation.80 Velez-Carrasco et al. demonstrated that the addition of methionine to the diet produced a much greater increase in non-fasting than fasting plasma tHcy levels.80
Vascular phenotypes of hyperhomocysteinemic mice
Many of the murine models described above have been utilized to study the vascular pathophysiology of hyperhomocysteinemia (Table 3). Because of the poor growth and survival of mice with homozygous defects in the Cbs, Mthfr, or Mtr genes, most investigators have chosen to study mice with heterozygous Cbs or Mthfr gene defects, often in combination with dietary approaches to produce hyperhomocysteinemia. Despite the considerable variability in the diets and genotypes used to produce hyperhomocysteinemia, several consistent vascular phenotypic effects have been observed in these studies.32, 81
Table 3.
Vascular phenotypes of hyperhomocysteinemic mice.
|
Diet |
Phenotype |
Vascular bed |
Mouse strain |
Plasma tHcy (μmol/L) |
Reference |
|---|---|---|---|---|---|
| Standard chow | Endothelial dysfunction | Mesenteric arterioles, aorta | Cbs+/− | 5−9 | 62, 63 |
| Cremasteric artery, aorta | Cbs−/− | 277 | 61 | ||
| Mesenteric arterioles | Mthfr+/− | 8 | 66 | ||
| Cerebral arterioles | Mtr+/− | 5 | 58 | ||
| Hypertrophy or remodeling | Aorta | Cbs+/− | 28 | 83 | |
| Cerebral arterioles | Cbs+/− | 8 | 39 | ||
| |
Accelerated atherosclerosis |
Aortic arch |
Apoe−/− Cbs−/− |
210 |
86 |
| High methionine | Endothelial dysfunction | Cerebral arterioles | Cbs+/+ | 8 | 40 |
| Cerebral arterioles | Cbs+/− | 20 | 40 | ||
| Aorta | Cbs+/− | 24 | 41 | ||
| Hypertrophy | Cerebral arterioles | Cbs+/+ | 17 | 39 | |
| Cerebral arterioles | Cbs+/− | 21 | 39 | ||
| Accelerated atherosclerosis | Aortic arch | Apoe−/− | 5−54 | 36, 46, 57 | |
| Aortic arch | Apoe−/− Cbs+/− | 14 | 86 | ||
| Increased stiffness | Aorta | Cbs+/+ | 41 | 82 | |
| Increased permeability | Carotid artery | Cbs+/+ | 41 | 82 | |
| Decreased re-endothelialization | Carotid artery | Cbs+/− | 140 | 38 | |
| |
Increased neointima formation |
Carotid artery |
Cbs+/− |
140 |
38 |
| Low folate, vitamin B12 and/or vitamin B6 | Endothelial dysfunction | Aorta | Cbs+/− | 25 | 48 |
| Cerebral arterioles | Mtr+/+ | 8 | 58 | ||
| Cerebral arterioles | Mtr+/− | 10 | 58 | ||
| Increased stiffness | Aorta | Cbs+/+ | 8 | 49 | |
| Aorta | Cbs+/− | 16 | 49 | ||
| Increased permeability | Carotid artery | Cbs+/+ | 8 | 49 | |
| |
|
Carotid artery |
Cbs+/− |
16 |
49 |
| High methionine with low folate, vitamin B12, and/or vitamin B6 | Endothelial dysfunction | Cerebral arterioles | Mthfr+/+ | 9 | 43 |
| Cerebral arterioles | Mthfr+/− | 17 | 43 | ||
| Accelerated thrombosis | Carotid artery | Cbs+/+ | 14 | 69 | |
| Carotid artery | Cbs+/− | 43 | 69 | ||
| Accelerated atherosclerosis | Aortic arch | Apoe−/− | 47−88 | 36, 37 | |
| |
Altered blood brain barrier |
Cerebral vessels |
Cbs+/− |
98 |
89 |
| D,L-homocysteine in drinking water | Accelerated Atherosclerosis | Aortic arch | Apoe−/− | 16−52 | 46, 56, 57, 85 |
The most commonly observed vascular abnormality in murine models of hyperhomocysteinemia is endothelial vasomotor dysfunction due to impaired bioavailability of endothelium-derived nitric oxide.81 Endothelial vasomotor dysfunction has been observed in large arteries, such as the aorta and carotid artery, as well as in smaller vessels, such as mesenteric, cremasteric, and cerebral arterioles. Susceptibility to endothelial impairment may vary with the degree of hyperhomocysteinemia. Smaller arterioles appear to be susceptible to endothelial dysfunction in the presence of mild hyperhomocysteinemia, whereas large arteries tend to exhibit functional abnormalities only in the presence of higher concentrations of plasma tHcy.39-42, 48, 58, 62, 63 Investigators interested in studying endothelial function in large conduit arteries such as the aorta or carotid artery generally should utilize a dietary or genetic model that produces plasma tHcy levels of 20 μmol/L or higher (Table 1). On the other hand, studies of endothelial function in small resistance vessels, such as mesenteric or cerebral arterioles, can be readily performed using murine models that produce plasma tHcy concentrations of 10 to 20 μmol/L.
In addition to abnormal vascular function, hyperhomocysteinemic mice also develop structural alterations of the vessel wall. Several studies have demonstrated vascular hypertrophy, remodeling, altered vascular mechanics, or increased stiffness of arteries or arterioles in mice with hyperhomocysteinemia.39, 49, 82, 83 Increased neointima formation in response to arterial injury also has been observed.38 Accelerated thrombosis of the carotid artery has been detected using the rose bengal photochemical thrombosis method33 in Cbs+/+, Cbs+/−, and Apoe−/− mice fed a high methionine/low folate diet.69, 77
Several groups have utilized murine models to investigate the effects of hyperhomocysteinemia on the development and progression of atherosclerosis. None of the murine models of hyperhomocysteinemia described above has been observed to spontaneously produce advanced atherosclerotic lesions in the absence of hyperlipidemia. However, hyperhomocysteinemia has been observed to potentiate the development of atherosclerosis in susceptible strains of mice, such as hyperlipidemic Apoe−/− mice37 or C57BL/6 mice fed an atherogenic diet containing cholate.84 Hofmann et al. reported that Apoe−/− mice fed a hyperhomocysteinemic diet developed atherosclerotic lesions in the aortic sinus that were of greater size and complexity than those seen in Apoe−/− mice fed normal chow.37 Several other investigators, using a variety of dietary and genetic approaches to induce hyperhomocysteinemia in Apoe−/− mice, have confirmed and extended the observations of Hofmann.46, 56, 57, 85, 86 An interesting study by Troen et al suggested that a high dietary intake of methionine may be atherogenic in Apoe−/− mice, even in the absence of significant hyperhomocysteinemia.36
Taken together, the vascular phenotypes observed in multiple murine models of hyperhomocysteinemia strongly suggest that hyperhomocysteinemia, whether induced by dietary or genetic approaches, is a causative factor in the development of atherothrombotic vascular disease. It still remains an open question, however, whether it is homocysteine itself or a related metabolic factor that is the key etiological agent.
Perspectives
It is apparent from Tables 1, 2 and 3 that a large number of murine models of hyperhomocysteinemia have been developed and are now available for the study of vascular endpoints. These murine models are particularly valuable in light of the inconclusive results emerging from the homocysteine-lowering trials in human subjects.15, 17, 20, 21, 87 Among the available models, Cbs gene-targeted mice have been studied most extensively and have provided a great deal of new information about the vascular phenotypes of hyperhomocysteinemia.
A limitation of all of the murine models of hyperhomocysteinemia is that elevation of homocysteine, whether induced by dietary or genetic means, may be accompanied by alterations in other metabolites that may influence vascular pathophysiology. Even in the absence of dietary modification, all of the currently available genetic models of hyperhomocysteinemia produce significant alterations in folate pools, SAM levels, and/or other homocysteine-related metabolites.31 A future challenge, with important clinical implications, will be to design experimental approaches to distinguish between the direct and indirect vascular effects of hyperhomocysteinemia. Such studies may be facilitated by new murine models, such as the human CBS transgenic mouse,45 that utilize inducible promoters to conditionally express CBS or other transgenes. An alternative approach would be to develop murine models with an endothelium- or vascular muscle-specific knockout or knockdown of Cbs, Mthfr, or other methionine or folate cycle genes. One gene of particular interest is BHMT (Figure 1). This gene is expressed only in the liver and kidney, which are the two major organs that regulate plasma tHcy levels. The value of such murine models in defining the effects of hyperhomocysteinemia on vascular disease susceptibility should increase as better techniques become available for the quantitative assessment of vascular phenotypes in mice.
Acknowledgements
Supported by the Office of Research and Development, U.S. Department of Veterans Affairs, National Institutes of Health grants HL63943 and NS24621 to S.R.L, and a Beginning Grant-in-Aid Award from the American Heart Association to S.D.
Footnotes
Publisher's Disclaimer: This is an un-copyedited author manuscript that was accepted for publication in Arteriosclerosis, Thrombosis, and Vascular Biology, copyright The American Heart Association. This may not be duplicated or reproduced, other than for personal use or within the “Fair Use of Copyrighted Materials” (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at Arteriosclerosis, Thrombosis, and Vascular Biology. The American Heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
References
- 1.Mudd SH, Finkelstein JD, Refsum H, Ueland PM, Malinow MR, Lentz SR, Jacobsen DW, Brattstrom L, Wilcken B, Wilcken DE, Blom HJ, Stabler SP, Allen RH, Selhub J, Rosenberg IH. Homocysteine and its disulfide derivatives: a suggested consensus terminology. Arterioscler Thromb Vasc Biol. 2000;20:1704–1706. doi: 10.1161/01.atv.20.7.1704. [DOI] [PubMed] [Google Scholar]
- 2.Selhub J, Jacques PF, Wilson PW, Rush D, Rosenberg IH. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA. 1993;270:2693–2698. doi: 10.1001/jama.1993.03510220049033. [DOI] [PubMed] [Google Scholar]
- 3.Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG. MTHFR 677C-->T polymorphism and risk of coronary heart disease: a meta-analysis. JAMA. 2002;288:2023–2031. doi: 10.1001/jama.288.16.2023. [DOI] [PubMed] [Google Scholar]
- 4.Kruger WD, Wang L, Jhee KH, Singh RH, Elsas LJ., 2nd Cystathionine β-synthase deficiency in Georgia (USA): correlation of clinical and biochemical phenotype with genotype. Hum Mutat. 2003;22:434–441. doi: 10.1002/humu.10290. [DOI] [PubMed] [Google Scholar]
- 5.Mudd SH, Skovby F, Levy HL, Pettigrew KD, Wilcken B, Pyeritz RE, Andria G, Boers GH, Bromberg IL, Cerone R, et al. The natural history of homocystinuria due to cystathionine β-synthase deficiency. Am J Hum Genet. 1985;37:1–31. [PMC free article] [PubMed] [Google Scholar]
- 6.Watkins D, Ru M, Hwang HY, Kim CD, Murray A, Philip NS, Kim W, Legakis H, Wai T, Hilton JF, Ge B, Dore C, Hosack A, Wilson A, Gravel RA, Shane B, Hudson TJ, Rosenblatt DS. Hyperhomocysteinemia due to methionine synthase deficiency, cblG: structure of the MTR gene, genotype diversity, and recognition of a common mutation, P1173L. Am J Hum Genet. 2002;71:143–153. doi: 10.1086/341354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dierkes J, Westphal S. Effect of drugs on homocysteine concentrations. Semin Vasc Med. 2005;5:124–139. doi: 10.1055/s-2005-872398. [DOI] [PubMed] [Google Scholar]
- 8.van Guldener C, Stehouwer CD. Homocysteine and methionine metabolism in renal failure. Semin Vasc Med. 2005;5:201–208. doi: 10.1055/s-2005-872405. [DOI] [PubMed] [Google Scholar]
- 9.Tonetti C, Saudubray JM, Echenne B, Landrieu P, Giraudier S, Zittoun J. Relations between molecular and biological abnormalities in 11 families from siblings affected with methylenetetrahydrofolate reductase deficiency. Eur J Pediatr. 2003;162:466–475. doi: 10.1007/s00431-003-1196-9. [DOI] [PubMed] [Google Scholar]
- 10.Limal N, Scheuermaier K, Tazi Z, Sene D, Piette JC, Cacoub P. Hyperhomocysteinaemia, thrombosis and pernicious anaemia. Thromb Haemost. 2006;96:233–235. [PubMed] [Google Scholar]
- 11.Lentz SR, Haynes WG. Homocysteine: is it a clinically important cardiovascular risk factor? Cleve Clin J Med. 2004;71:729–734. doi: 10.3949/ccjm.71.9.729. [DOI] [PubMed] [Google Scholar]
- 12.Homocysteine studies Collaboration Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288:2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 13.Den Heijer M, Lewington S, Clarke R. Homocysteine, MTHFR and risk of venous thrombosis: a meta-analysis of published epidemiological studies. J Thromb Haemost. 2005;3:292–299. doi: 10.1111/j.1538-7836.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 14.Yap S, Boers GH, Wilcken B, Wilcken DE, Brenton DP, Lee PJ, Walter JH, Howard PM, Naughten ER. Vascular outcome in patients with homocystinuria due to cystathionine β-synthase deficiency treated chronically: a multicenter observational study. Arterioscler Thromb Vasc Biol. 2001;21:2080–2085. doi: 10.1161/hq1201.100225. [DOI] [PubMed] [Google Scholar]
- 15.Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, Wang H, Nordrehaug JE, Arnesen E, Rasmussen K. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 16.den Heijer M, Willems HP, Blom HJ, Gerrits WB, Cattaneo M, Eichinger S, Rosendaal FR, Bos GM. Homocysteine lowering by B vitamins and the secondary prevention of deep vein thrombosis and pulmonary embolism: A randomized, placebo-controlled, double-blind trial. Blood. 2007;109:139–144. doi: 10.1182/blood-2006-04-014654. [DOI] [PubMed] [Google Scholar]
- 17.Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, McQueen MJ, Probstfield J, Fodor G, Held C, Genest J., Jr. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–1577. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 18.Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, Gaziano JM. Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: a randomized controlled trial. JAMA. 2007;298:1163–1170. doi: 10.1001/jama.298.10.1163. [DOI] [PubMed] [Google Scholar]
- 19.Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, Howard VJ, Sides EG, Wang CH, Stampfer M. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565–575. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Qin X, Demirtas H, Li J, Mao G, Huo Y, Sun N, Liu L, Xu X. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet. 2007;369:1876–1882. doi: 10.1016/S0140-6736(07)60854-X. [DOI] [PubMed] [Google Scholar]
- 21.Yang Q, Botto LD, Erickson JD, Berry RJ, Sambell C, Johansen H, Friedman JM. Improvement in stroke mortality in Canada and the United States, 1990 to 2002. Circulation. 2006;113:1335–1343. doi: 10.1161/CIRCULATIONAHA.105.570846. [DOI] [PubMed] [Google Scholar]
- 22.Harker LA, Ross R, Slichter SJ, Scott CR. Homocystine-induced arteriosclerosis. The role of endothelial cell injury and platelet response in its genesis. J Clin Invest. 1976;58:731–741. doi: 10.1172/JCI108520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harker LA, Slichter SJ, Scott CR, Ross R. Homocystinemia. Vascular injury and arterial thrombosis. N Engl J Med. 1974;291:537–543. doi: 10.1056/NEJM197409122911101. [DOI] [PubMed] [Google Scholar]
- 24.Lentz SR, Sobey CG, Piegors DJ, Bhopatkar MY, Faraci FM, Malinow MR, Heistad DD. Vascular dysfunction in monkeys with diet-induced hyperhomocyst(e)inemia. J Clin Invest. 1996;98:24–29. doi: 10.1172/JCI118771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rolland PH, Friggi A, Barlatier A, Piquet P, Latrille V, Faye MM, Guillou J, Charpiot P, Bodard H, Ghiringhelli O, et al. Hyperhomocysteinemia-induced vascular damage in the minipig. Captopril-hydrochlorothiazide combination prevents elastic alterations. Circulation. 1995;91:1161–1174. doi: 10.1161/01.cir.91.4.1161. [DOI] [PubMed] [Google Scholar]
- 26.Lang D, Kredan MB, Moat SJ, Hussain SA, Powell CA, Bellamy MF, Powers HJ, Lewis MJ. Homocysteine-induced inhibition of endothelium-dependent relaxation in rabbit aorta: role for superoxide anions. Arterioscler Thromb Vasc Biol. 2000;20:422–427. doi: 10.1161/01.atv.20.2.422. [DOI] [PubMed] [Google Scholar]
- 27.Sauls DL, Lockhart E, Warren ME, Lenkowski A, Wilhelm SE, Hoffman M. Modification of fibrinogen by homocysteine thiolactone increases resistance to fibrinolysis: a potential mechanism of the thrombotic tendency in hyperhomocysteinemia. Biochemistry (Mosc) 2006;45:2480–2487. doi: 10.1021/bi052076j. [DOI] [PubMed] [Google Scholar]
- 28.Bagi Z, Ungvari Z, Koller A. Xanthine oxidase-derived reactive oxygen species convert flow-induced arteriolar dilation to constriction in hyperhomocysteinemia: possible role of peroxynitrite. Arterioscler Thromb Vasc Biol. 2002;22:28–33. doi: 10.1161/hq0102.101127. [DOI] [PubMed] [Google Scholar]
- 29.Morita H, Kurihara H, Yoshida S, Saito Y, Shindo T, Oh-Hashi Y, Kurihara Y, Yazaki Y, Nagai R. Diet-induced hyperhomocysteinemia exacerbates neointima formation in rat carotid arteries after balloon injury. Circulation. 2001;103:133–139. doi: 10.1161/01.cir.103.1.133. [DOI] [PubMed] [Google Scholar]
- 30.Symons JD, Rutledge JC, Simonsen U, Pattathu RA. Vascular dysfunction produced by hyperhomocysteinemia is more severe in the presence of low folate. Am J Physiol Heart Circ Physiol. 2006;290:H181–191. doi: 10.1152/ajpheart.00765.2005. [DOI] [PubMed] [Google Scholar]
- 31.Elmore CL, Matthews RG. The many flavors of hyperhomocyst(e)inemia: insights from transgenic and inhibitor-based mouse models of disrupted one-carbon metabolism. Antioxid Redox Signal. 2007;9:1911–1921. doi: 10.1089/ars.2007.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lentz SR. Mechanisms of homocysteine-induced atherothrombosis. J Thromb Haemost. 2005;3:1646–1654. doi: 10.1111/j.1538-7836.2005.01364.x. [DOI] [PubMed] [Google Scholar]
- 33.Westrick RJ, Winn ME, Eitzman DT. Murine models of vascular thrombosis (Eitzman series). Arterioscler Thromb Vasc Biol. 2007;27:2079–2093. doi: 10.1161/ATVBAHA.107.142810. [DOI] [PubMed] [Google Scholar]
- 34.Subcommittee on Animal Nutrition, Board on Agriculture, National Research Council. Nutrient requirements of laboratory animals. Fourth rev ed National Academy Press; Washington, DC: 1995. Nutrient requirement of the mouse. pp. 80–102. [Google Scholar]
- 35.Schwahn BC, Wendel U, Lussier-Cacan S, Mar MH, Zeisel SH, Leclerc D, Castro C, Garrow TA, Rozen R. Effects of betaine in a murine model of mild cystathionine β-synthase deficiency. Metabolism. 2004;53:594–599. doi: 10.1016/j.metabol.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 36.Troen AM, Lutgens E, Smith DE, Rosenberg IH, Selhub J. The atherogenic effect of excess methionine intake. Proc Natl Acad Sci U S A. 2003;100:15089–15094. doi: 10.1073/pnas.2436385100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hofmann MA, Lalla E, Lu Y, Gleason MR, Wolf BM, Tanji N, Ferran LJ, Jr., Kohl B, Rao V, Kisiel W, Stern DM, Schmidt AM. Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J Clin Invest. 2001;107:675–683. doi: 10.1172/JCI10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan H, Jiang X, Yang F, Li Z, Liao D, Trial J, Magera MJ, Durante W, Yang X, Wang H. Hyperhomocysteinemia inhibits post-injury reendothelialization in mice. Cardiovasc Res. 2006;69:253–262. doi: 10.1016/j.cardiores.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baumbach GL, Sigmund CD, Bottiglieri T, Lentz SR. Structure of cerebral arterioles in cystathionine β-synthase-deficient mice. Circ Res. 2002;91:931–937. doi: 10.1161/01.res.0000041408.64867.1d. [DOI] [PubMed] [Google Scholar]
- 40.Dayal S, Arning E, Bottiglieri T, Boger RH, Sigmund CD, Faraci FM, Lentz SR. Cerebral vascular dysfunction mediated by superoxide in hyperhomocysteinemic mice. Stroke. 2004;35:1957–1962. doi: 10.1161/01.STR.0000131749.81508.18. [DOI] [PubMed] [Google Scholar]
- 41.Dayal S, Bottiglieri T, Arning E, Maeda N, Malinow MR, Sigmund CD, Heistad DD, Faraci FM, Lentz SR. Endothelial dysfunction and elevation of S-adenosylhomocysteine in cystathionine β-synthase-deficient mice. Circ Res. 2001;88:1203–1209. doi: 10.1161/hh1101.092180. [DOI] [PubMed] [Google Scholar]
- 42.Dayal S, Brown KL, Weydert CJ, Oberley LW, Arning E, Bottiglieri T, Faraci FM, Lentz SR. Deficiency of glutathione peroxidase-1 sensitizes hyperhomocysteinemic mice to endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2002;22:1996–2002. doi: 10.1161/01.atv.0000041629.92741.dc. [DOI] [PubMed] [Google Scholar]
- 43.Devlin AM, Arning E, Bottiglieri T, Faraci FM, Rozen R, Lentz SR. Effect of Mthfr genotype on diet-induced hyperhomocysteinemia and vascular function in mice. Blood. 2004;103:2624–2629. doi: 10.1182/blood-2003-09-3078. [DOI] [PubMed] [Google Scholar]
- 44.Werstuck GH, Lentz SR, Dayal S, Hossain GS, Sood SK, Shi YY, Zhou J, Maeda N, Krisans SK, Malinow MR, Austin RC. Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J Clin Invest. 2001;107:1263–1273. doi: 10.1172/JCI11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L, Jhee KH, Hua X, DiBello PM, Jacobsen DW, Kruger WD. Modulation of cystathionine β-synthase level regulates total serum homocysteine in mice. Circ Res. 2004;94:1318–1324. doi: 10.1161/01.RES.0000129182.46440.4a. [DOI] [PubMed] [Google Scholar]
- 46.Zhou J, Moller J, Danielsen CC, Bentzon J, Ravn HB, Austin RC, Falk E. Dietary supplementation with methionine and homocysteine promotes early atherosclerosis but not plaque rupture in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1470–1476. doi: 10.1161/hq0901.096582. [DOI] [PubMed] [Google Scholar]
- 47.Cottington EM, LaMantia C, Stabler SP, Allen RH, Tangerman A, Wagner C, Zeisel SH, Mudd SH. Adverse event associated with methionine loading test: a case report. Arterioscler Thromb Vasc Biol. 2002;22:1046–1050. doi: 10.1161/01.atv.0000020400.25088.a7. [DOI] [PubMed] [Google Scholar]
- 48.Lentz SR, Erger RA, Dayal S, Maeda N, Malinow MR, Heistad DD, Faraci FM. Folate dependence of hyperhomocysteinemia and vascular dysfunction in cystathionine β-synthase-deficient mice. Am J Physiol Heart Circ Physiol. 2000;279:H970–975. doi: 10.1152/ajpheart.2000.279.3.H970. [DOI] [PubMed] [Google Scholar]
- 49.Symons JD, Zaid UB, Athanassious CN, Mullick AE, Lentz SR, Rutledge JC. Influence of folate on arterial permeability and stiffness in the absence or presence of hyperhomocysteinemia. Arterioscler Thromb Vasc Biol. 2006;26:814–818. doi: 10.1161/01.ATV.0000204408.01416.16. [DOI] [PubMed] [Google Scholar]
- 50.Gospe SM, Jr., Gietzen DW, Summers PJ, Lunetta JM, Miller JW, Selhub J, Ellis WG, Clifford AJ. Behavioral and neurochemical changes in folate-deficient mice. Physiol Behav. 1995;58:935–941. doi: 10.1016/0031-9384(95)00156-d. [DOI] [PubMed] [Google Scholar]
- 51.Li D, Pickell L, Liu Y, Rozen R. Impact of methylenetetrahydrofolate reductase deficiency and low dietary folate on the development of neural tube defects in splotch mice. Birth Defects Res A Clin Mol Teratol. 2006;76:55–59. doi: 10.1002/bdra.20223. [DOI] [PubMed] [Google Scholar]
- 52.Li D, Rozen R. Maternal folate deficiency affects proliferation, but not apoptosis, in embryonic mouse heart. J Nutr. 2006;136:1774–1778. doi: 10.1093/jn/136.7.1774. [DOI] [PubMed] [Google Scholar]
- 53.da Costa KA, Gaffney CE, Fischer LM, Zeisel SH. Choline deficiency in mice and humans is associated with increased plasma homocysteine concentration after a methionine load. Am J Clin Nutr. 2005;81:440–444. doi: 10.1093/ajcn.81.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robert K, Nehme J, Bourdon E, Pivert G, Friguet B, Delcayre C, Delabar JM, Janel N. Cystathionine β-synthase deficiency promotes oxidative stress, fibrosis, and steatosis in mice liver. Gastroenterology. 2005;128:1405–1415. doi: 10.1053/j.gastro.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 55.Schwahn BC, Laryea MD, Chen Z, Melnyk S, Pogribny I, Garrow T, James SJ, Rozen R. Betaine rescue of an animal model with methylenetetrahydrofolate reductase deficiency. Biochem J. 2004;382:831–840. doi: 10.1042/BJ20030822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thampi P, Stewart BW, Joseph L, Melnyk SB, Hennings LJ, Nagarajan S. Dietary homocysteine promotes atherosclerosis in apoE-deficient mice by inducing scavenger receptors expression. Atherosclerosis. 2008;197:620–629. doi: 10.1016/j.atherosclerosis.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 57.Zhou J, Moller J, Ritskes-Hoitinga M, Larsen ML, Austin RC, Falk E. Effects of vitamin supplementation and hyperhomocysteinemia on atherosclerosis in apoE-deficient mice. Atherosclerosis. 2003;168:255–262. doi: 10.1016/s0021-9150(03)00138-2. [DOI] [PubMed] [Google Scholar]
- 58.Dayal S, Devlin AM, McCaw RB, Liu ML, Arning E, Bottiglieri T, Shane B, Faraci FM, Lentz SR. Cerebral vascular dysfunction in methionine synthase-deficient mice. Circulation. 2005;112:737–744. doi: 10.1161/CIRCULATIONAHA.104.529248. [DOI] [PubMed] [Google Scholar]
- 59.Doshi SN, McDowell IF, Moat SJ, Payne N, Durrant HJ, Lewis MJ, Goodfellow J. Folic acid improves endothelial function in coronary artery disease via mechanisms largely independent of homocysteine lowering. Circulation. 2002;105:22–26. doi: 10.1161/hc0102.101388. [DOI] [PubMed] [Google Scholar]
- 60.Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow MR, Maeda N. Mice deficient in cystathionine β-synthase: animal models for mild and severe homocyst(e)inemia. Proc Natl Acad Sci U S A. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang X, Yang F, Tan H, Liao D, Bryan RM, Jr., Randhawa JK, Rumbaut RE, Durante W, Schafer AI, Yang X, Wang H. Hyperhomocystinemia impairs endothelial function and eNOS activity via PKC activation. Arterioscler Thromb Vasc Biol. 2005;25:2515–2521. doi: 10.1161/01.ATV.0000189559.87328.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eberhardt RT, Forgione MA, Cap A, Leopold JA, Rudd MA, Trolliet M, Heydrick S, Stark R, Klings ES, Moldovan NI, Yaghoubi M, Goldschmidt-Clermont PJ, Farber HW, Cohen R, Loscalzo J. Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia. J Clin Invest. 2000;106:483–491. doi: 10.1172/JCI8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weiss N, Heydrick S, Zhang YY, Bierl C, Cap A, Loscalzo J. Cellular redox state and endothelial dysfunction in mildly hyperhomocysteinemic cystathionine β-synthase-deficient mice. Arterioscler Thromb Vasc Biol. 2002;22:34–41. doi: 10.1161/hq1201.100456. [DOI] [PubMed] [Google Scholar]
- 64.Wang L, Chen X, Tang B, Hua X, Klein-Szanto A, Kruger WD. Expression of mutant human cystathionine β-synthase rescues neonatal lethality but not homocystinuria in a mouse model. Hum Mol Genet. 2005;14:2201–2208. doi: 10.1093/hmg/ddi224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Z, Karaplis AC, Ackerman SL, Pogribny IP, Melnyk S, Lussier-Cacan S, Chen MF, Pai A, John SW, Smith RS, Bottiglieri T, Bagley P, Selhub J, Rudnicki MA, James SJ, Rozen R. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum Mol Genet. 2001;10:433–443. doi: 10.1093/hmg/10.5.433. [DOI] [PubMed] [Google Scholar]
- 66.Virdis A, Iglarz M, Neves MF, Amiri F, Touyz RM, Rozen R, Schiffrin EL. Effect of hyperhomocystinemia and hypertension on endothelial function in methylenetetrahydrofolate reductase-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1352–1357. doi: 10.1161/01.ATV.0000083297.47245.DA. [DOI] [PubMed] [Google Scholar]
- 67.Swanson DA, Liu ML, Baker PJ, Garrett L, Stitzel M, Wu J, Harris M, Banerjee R, Shane B, Brody LC. Targeted disruption of the methionine synthase gene in mice. Mol Cell Biol. 2001;21:1058–1065. doi: 10.1128/MCB.21.4.1058-1065.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elmore CL, Wu X, Leclerc D, Watson ED, Bottiglieri T, Krupenko NI, Krupenko SA, Cross JC, Rozen R, Gravel RA, Matthews RG. Metabolic derangement of methionine and folate metabolism in mice deficient in methionine synthase reductase. Mol Genet Metab. 2007;91:85–97. doi: 10.1016/j.ymgme.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dayal S, Wilson KM, Leo L, Arning E, Bottiglieri T, Lentz SR. Enhanced susceptibility to arterial thrombosis in a murine model of hyperhomocysteinemia. Blood. 2006;108:2237–2243. doi: 10.1182/blood-2006-02-005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lentz SR, Piegors DJ, Fernandez JA, Erger RA, Arning E, Malinow MR, Griffin JH, Bottiglieri T, Haynes WG, Heistad DD. Effect of hyperhomocysteinemia on protein C activation and activity. Blood. 2002;100:2108–2112. doi: 10.1182/blood-2002-03-0727. [DOI] [PubMed] [Google Scholar]
- 71.Hanson NQ, Aras O, Yang F, Tsai MY. C677T and A1298C polymorphisms of the methylenetetrahydrofolate reductase gene: incidence and effect of combined genotypes on plasma fasting and post-methionine load homocysteine in vascular disease. Clin Chem. 2001;47:661–666. [PubMed] [Google Scholar]
- 72.Tsai MY, Garg U, Key NS, Hanson NQ, Suh A, Schwichtenberg K. Molecular and biochemical approaches in the identification of heterozygotes for homocystinuria. Atherosclerosis. 1996;122:69–77. doi: 10.1016/0021-9150(95)05748-x. [DOI] [PubMed] [Google Scholar]
- 73.Collinsova M, Strakova J, Jiracek J, Garrow TA. Inhibition of betaine-homocysteine S-methyltransferase causes hyperhomocysteinemia in mice. J Nutr. 2006;136:1493–1497. doi: 10.1093/jn/136.6.1493. [DOI] [PubMed] [Google Scholar]
- 74.Andersson A, Brattstrom L, Israelsson B, Isaksson A, Hamfelt A, Hultberg B. Plasma homocysteine before and after methionine loading with regard to age, gender, and menopausal status. Eur J Clin Invest. 1992;22:79–87. doi: 10.1111/j.1365-2362.1992.tb01940.x. [DOI] [PubMed] [Google Scholar]
- 75.Lussier-Cacan S, Xhignesse M, Piolot A, Selhub J, Davignon J, Genest J., Jr. Plasma total homocysteine in healthy subjects: sex-specific relation with biological traits. Am J Clin Nutr. 1996;64:587–593. doi: 10.1093/ajcn/64.4.587. [DOI] [PubMed] [Google Scholar]
- 76.Vitvitsky V, Prudova A, Stabler S, Dayal S, Lentz SR, Banerjee R. Testosterone regulation of renal cystathionine β-synthase: implications for sex-dependent differences in plasma homocysteine levels. Am J Physiol Renal Physiol. 2007;293:F594–600. doi: 10.1152/ajprenal.00171.2007. [DOI] [PubMed] [Google Scholar]
- 77.Wilson KM, McCaw RB, Leo L, Arning E, Lhotak S, Bottiglieri T, Austin RC, Lentz SR. Prothrombotic effects of hyperhomocysteinemia and hypercholesterolemia in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:233–240. doi: 10.1161/01.ATV.0000251607.96118.af. [DOI] [PubMed] [Google Scholar]
- 78.Stevens JC, Banks GT, Festing MF, Fisher EM. Quiet mutations in inbred strains of mice. Trends Mol Med. 2007;13:512–519. doi: 10.1016/j.molmed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 79.Devlin AM, Bottiglieri T, Domann FE, Lentz SR. Tissue-specific changes in H19 methylation and expression in mice with hyperhomocysteinemia. J Biol Chem. 2005;280:25506–25511. doi: 10.1074/jbc.M504815200. [DOI] [PubMed] [Google Scholar]
- 80.Velez-Carrasco W, Merkel M, Twiss CO, Smith JD. Dietary methionine effects on plasma homocysteine and HDL metabolism in mice. J Nutr Biochem. 2008;19:362–370. doi: 10.1016/j.jnutbio.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dayal S, Lentz SR. Role of redox reactions in the vascular phenotype of hyperhomocysteinemic animals. Antioxid Redox Signal. 2007;9:1899–1909. doi: 10.1089/ars.2007.1806. [DOI] [PubMed] [Google Scholar]
- 82.Mullick AE, Zaid UB, Athanassious CN, Lentz SR, Rutledge JC, Symons JD. Hyperhomocysteinemia increases arterial permeability and stiffness in mice. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1349–1354. doi: 10.1152/ajpregu.00335.2006. [DOI] [PubMed] [Google Scholar]
- 83.Ovechkin AV, Tyagi N, Sen U, Lominadze D, Steed MM, Moshal KS, Tyagi SC. 3-Deazaadenosine mitigates arterial remodeling and hypertension in hyperhomocysteinemic mice. Am J Physiol Lung Cell Mol Physiol. 2006;291:L905–911. doi: 10.1152/ajplung.00543.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou J, Werstuck GH, Lhotak S, Shi YY, Tedesco V, Trigatti B, Dickhout J, Majors AK, Dibello PM, Jacobsen DW, Austin RC. Hyperhomocysteinemia induced by methionine supplementation does not independently cause atherosclerosis in C57BL/6J mice. FASEB J. 2008 doi: 10.1096/fj.07-105353. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dai J, Li W, Chang L, Zhang Z, Tang C, Wang N, Zhu Y, Wang X. Role of redox factor-1 in hyperhomocysteinemia-accelerated atherosclerosis. Free Radic Biol Med. 2006;41:1566–1577. doi: 10.1016/j.freeradbiomed.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 86.Wang H, Jiang X, Yang F, Gaubatz JW, Ma L, Magera MJ, Yang X, Berger PB, Durante W, Pownall HJ, Schafer AI. Hyperhomocysteinemia accelerates atherosclerosis in cystathionine β-synthase and apolipoprotein E double knock-out mice with and without dietary perturbation. Blood. 2003;101:3901–3907. doi: 10.1182/blood-2002-08-2606. [DOI] [PubMed] [Google Scholar]
- 87.Loscalzo J. Homocysteine trials--clear outcomes for complex reasons. N Engl J Med. 2006;354:1629–1632. doi: 10.1056/NEJMe068060. [DOI] [PubMed] [Google Scholar]
- 88.Vitvitsky V, Dayal S, Stabler S, Zhou Y, Wang H, Lentz SR, Banerjee R. Perturbations in homocysteine-linked redox homeostasis in a murine model for hyperhomocysteinemia. Am J Physiol Regul Integr Comp Physiol. 2004;287:R39–46. doi: 10.1152/ajpregu.00036.2004. [DOI] [PubMed] [Google Scholar]
- 89.Kamath AF, Chauhan AK, Kisucka J, Dole VS, Loscalzo J, Handy DE, Wagner DD. Elevated levels of homocysteine compromise blood-brain barrier integrity in mice. Blood. 2006;107:591–593. doi: 10.1182/blood-2005-06-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]