Abstract
Background
A period of tumor growth precedes the clinical detection of breast cancer recurrence. We explore immune, endocrine, and behavioral parameters during this period.
Methods
We conducted a phase III clinical trial in which women with surgically treated stage II/III breast cancer (N = 227) were randomized to receive a psychological intervention or assessment-only and then regularly assessed for 10 years. Patients who recurred (R, n = 48) were matched with patients remaining disease-free (DF, n = 48) on demographic and prognostic characteristics, treatment, and duration of disease-free follow-up. Data at three assessment points, occurring, on average, 17, 11, and 4 months before the recurrence was detected clinically, with equivalent time points for the disease-free group, were examined. Mixed-effects models tested for group differences in immune cell counts and function as well as endocrine and behavioral parameters.
Results
In the 17 months prior to recurrence detection, patients exhibited higher white blood cell count, neutrophil, lymphocyte, and natural killer cell counts, relative to DF patients. R patients also showed higher cortisol, worse physical functioning, fatigue, and quality of life. Follow-up analyses showed patients with local recurrences to differ from those with distant recurrence, with the former exhibiting elevated natural killer cell cytotoxicity, lymphocyte proliferative response, fatigue, pain, and emotional distress (depression, anxiety), and the latter exhibiting higher neutrophil, lymphocyte, and natural killer cell counts.
Conclusion
Patients who would recur showed reliable biobehavioral alterations more than a year prior to their diagnosis. This novel observation may contribute to our understanding of the disease relapse processes.
Keywords: Breast neoplasms, Neoplasm metastasis, Leukocytes, Granulocytes, Fatigue, Inflammation
Introduction
Tumor growth is believed to precede a diagnosis of breast cancer recurrence by at least 30 weeks [11, 12, 26]. Observations of signs or symptoms during this time could provide insight into the recurrence process or suggest tests for earlier detection. Potential signs of disease activity include tumor markers, for which reliable tests are in development [9], and hematologic indices, such as lymphocyte and neutrophil counts, which have been correlated with the presence of metastasis at diagnosis [27, 28, 30, 35]. Prior data such as these have been interpreted to suggest that an immune response to tumor cells—either via cytokines released from the tumor, tumor antigenicity, or an inflammatory reaction—may be observable in peripheral blood [7, 25, 31]. Thus, observation of hematologic indices in patients in whom a recurrence is developing, but as yet undetected, could be informative. Although data from patients at diagnosis have been studied, data from the tumor growth period, (i.e., 30 or more weeks before detection) have not been studied. The present study examines this period.
We test the hypothesis that patients who recur evidence different immune, endocrine, or behavioral responses than patients remaining disease-free, prior to the clinical detection of recurrence. Our ongoing randomized trial of a psychological intervention for breast cancer patients provided the opportunity to do so [5]. Patients were accrued and regularly assessed following an initial diagnosis of cancer. Data of primary interest are immune indices (e.g., white blood cell counts and differentials, lymphocyte populations, and functioning); however, data were also available for endocrine and behavioral domains, which may evince subtle changes coinciding with immune change [32]. After 10 years of follow-up, most patients have no evidence of disease, but some (26%) have recurred, making possible examination of patients’ data from the months prior to the recurrence diagnosis. For comparison, patients with recurrence were matched with other patients from the trial who had remained disease-free. In combination, a case-control exploration of precursors to recurrence was possible.
Subjects and methods
Study design and patients
Clinical trial sample
Women (N = 227) with newly diagnosed, surgically treated stage II or III breast cancer (TNM staging system [2, 3]; ICD-9 codes 174.0–174.9) at a university-affiliated National Cancer Institute-designated Comprehensive Cancer Center were accrued between 1994 and 2000. Details of informed consent, accrual, and randomization have been published [5]. This protocol was approved by local institutional review boards and was conducted in accordance with the Helsinki Declaration of 1975. Prior to the beginning of adjuvant therapy, women provided informed consent and completed face-to-face interviews and questionnaires, a nurse completed a health status evaluation, and blood and saliva samples were obtained. Assessments were conducted between 7:30 and 10:30 a.m. to reduce diurnal variability in endocrine and immune responses.
Patients were randomized to assessment-only or psychological intervention with assessment study arms, as described [5]. For those randomized to the intervention, there were significant reductions in emotional distress, improvements in health behaviors, and higher lymphocyte proliferative response at 4 and 12 months [4, 5].
Trial follow-up consisted of assessments every 4 months during the first year, every 6 months during years 2–5, and annually during years 6–10. Medical follow-up included annual mammograms and physical examinations every 3 months for the first 2 years and every 6 months thereafter. Suspicious signs or symptoms were pursued through blood assays and scans.
Research design and patients
Recurrence (R) group
Recurrence cases were identified through notification from clinic staff, routine tracking, and/or patients’ own notification and confirmed by examination and follow-up imaging studies. Women diagnosed with a second primary tumor and those recurring within 12 months of the initial diagnosis were excluded. The latter criterion effectively excludes patients who may have had undetected metastatic disease at diagnosis.
To date, 60 of 227 patients (26%) have recurred. Of them, data from 48 (80%) patients were available. Data from ten patients were unavailable, as they recurred more than a year after withdrawing from the trial. Data were available from two other patients but not used since they evidenced recurrence prior to the 12 month follow-up. The disease-free interval for the 48 patients ranged from 13 to 144 months (median = 32.5 months). For each patient, the three assessments immediately prior to the discovery of her recurrence were used, labeled time 1, time 2, and time 3. On average, the time 1 assessment occurred 17.3 months prior to the recurrence diagnosis (SD = 8.7 months), time 2 occurred 11.4 months prior to recurrence diagnosis (SD = 6.7 months), and time 3 occurred 4.5 months prior to recurrence diagnosis (SD = 4.4 months).
Disease-free (DF) group
Each patient with recurrent disease was matched to one patient from the trial who had no evidence of recurrent breast nor second primary diagnosis. Recurrence participants were matched to disease-free participants on study arm (psychological intervention versus assessment-only), menopausal status at original diagnosis (pre/peri versus post), hormone receptor status (positive versus negative), tumor size (<2 cm versus ≥2 cm), nodal status (negative versus 1–3 positive versus ≥4 positive nodes), presence of spouse or significant other, and duration of disease free follow-up. For example, if a woman recurred at 36 months, only disease-free individuals who met the above criteria and who had been followed through 36 months could be selected as matches for that woman. The three assessments prior to the matching time point (in this example, the 18, 24, and 30-month assessments) were used for time 1, time 2, and time 3. In this manner, 48 matches were identified. Exact matches were found for 40 of the 48 recurrence participants; the remaining participants were matched on five of the six variables. Subsequent analyses confirmed that the groups were statistically equivalent on all sociodemographic and prognostic variables, as well as prior cancer treatments received (see Table 1).
Table 1.
Equivalence of recurrence (R) and disease-free (DF) groups in demographic, prognostic, and treatment factors
| Variables | Recurrence (R, n = 48) | Disease-free (DF, n = 48) | P |
|---|---|---|---|
| Sociodemographic | |||
| Age at diagnosis (years) | 51.3 (11.2) | 50.3 (10.7) | 0.66 |
| Menopausal status (% pre/peri) | 25 (52%) | 25 (52%) | 1.00 |
| Body mass index | 28.0 (6.2) | 27.3 (5.5) | 0.55 |
| Race (% Caucasian) | 46 (96%) | 42 (86%) | 0.14 |
| Education (years) | 14.7 (2.7) | 14.5 (2.6) | 0.76 |
| Family Income ($K/year) | 53.2 (41.2) | 62.8 (47.9) | 0.30 |
| Significant other (% yes) | 35 (73%) | 35 (73%) | 1.00 |
| Prognostic | |||
| Stage II versus III (% II) | 43 (90%) | 38 (79%) | 0.16 |
| Tumor size (cm) | 3.3 (1.4) | 3.2 (1.7) | 0.76 |
| Tumor grade | 0.92 | ||
| Well differentiated | 4 (8%) | 3 (6%) | |
| Moderately well differentiated | 19 (40%) | 20 (42%) | |
| Poorly differentiated | 25 (52%) | 25 (52%) | |
| ER/PR receptor status (% positive) | 24 (50%) | 25 (52%) | 0.84 |
| Nodal involvement (% yes) | 32 (67%) | 32 (67%) | 1.00 |
| Time to follow-up (months) | 27.3 (25.9) | 27.8 (25.8) | 0.93 |
| Treatment received | |||
| Surgery (% segmental mastectomy) | 19 (40%) | 22 (46%) | 0.54 |
| Radiation therapy (% yes) | 24 (50%) | 30 (62%) | 0.22 |
| Hormonal therapy (% yes) | 32 (67%) | 31 (65%) | 0.83 |
| Chemotherapy (% yes) | 41 (85%) | 42 (87%) | 0.76 |
| Psychological intervention (% yes) | 25 (52%) | 26 (54%) | 0.84 |
| Location of recurrent disease | |||
| Local (n = 9) | |||
| Chest wall | 3 | ||
| Breast tissue | 6 | ||
| Regional (n = 2) | |||
| Supraclavicular nodes | 1 | ||
| Internal mammary nodes | 1 | ||
| Distant (n = 37)a | |||
| Lung | 12 | ||
| Liver | 8 | ||
| Bone | 25 | ||
| Pleura | 1 | ||
| Brain | 3 | ||
| Intestine | 1 | ||
| Contralateral breast | 1 | ||
SD standard deviation
aSites of distant disease total more than 37, as some participants recurred at multiple sites
Values are: mean (SD) or N (%). P values indicate group comparisons using analysis of variance or a χ2 test, as appropriate
Measures
Cell counts and function
Quantification of cells
Complete blood cell counts and differentials were obtained. Identification of lymphocyte subsets utilized peripheral blood leukocytes labeled with fluorescent-conjugated monoclonal antibodies, as described [5, 32]. CD3 (total T), CD4 (helper T), CD8 (cytotoxic/suppressor T), and CD56 (NK) cells were measured. Serum albumin was monitored as an indicator of nutritional status.
Natural killer cell cytotoxicity (NKCC)
Natural killer cell cytotoxicity (NKCC) was measured, as it has been found to predict breast cancer incidence [15] and recurrence [20]. We tested NKCC against K562 using a standard chromium release assay, as described [5, 32]. Effector to target (E:T) ratios were 50:1, 25:1, 12.5:1, 6.25:1, and 3.125:1. Cytotoxicity was expressed as lytic units per 107 cells [8].
Lymphocyte proliferative response (LPR)
T-cell proliferative response has been correlated with breast cancer recurrence [36] and survival [21, 37]. LPR to phytohemagluttinin (PHA) and concanavalin A (Con A) was determined, as described [5, 32]. Proliferation was determined via optical density. Serial dilutions of 2.5, 5.0, and 10.0 μg/mL were employed.
Endocrine studies
Salivary cortisol
Cortisol content was determined using the Cortisol Coat-A-Count RIA (Diagnostic Products Corp., Los Angeles, CA, USA). Sensitivity was 0.025 μg/dl. Intra-assay variation was 4.3% and inter-assay variation was 5.2%.
Plasma catecholamines (norepinephrine and epinephrine)
Determinations were made by HPLC with electrochemical detection using standards and chemistry from ChromSystems (Thermo-Alko, Beverly, MA, USA), and C-18 Columns from Waters Corporation (Milford, MA, USA). Intra-assay variation for norepinephrine and epinephrine was 3 and 6%, inter-assay variation was 6 and 13%, and sensitivity was 15 and 6 pg/ml, respectively.
Adrenocorticotropin hormone
We used Immulite 1000 with reagents manufactured specifically for this instrument (Diagnostic Products Corp., Los Angeles, CA, USA). Intra-assay coefficient of variation was 5.6% and inter-assay coefficient of variation was 7.8%. Sensitivity was 9 pg/ml.
Emotional distress and health related quality of life (QoL)
Emotional distress
The 65-item Profile of Mood States (POMS) assessed negative mood on six subscales [22]. Cronbach’s alpha reliability was 0.92.
Quality of life
The 36-item Medical Outcomes Study Short Form-36 (SF-36) measures health-related quality of life on eight subscales [34]. Cronbach’s alpha reliability ranged from 0.83 to 0.94.
Functional status and symptoms
Performance status
The Karnofsky Performance Status (KPS) was used [18].
Symptoms, signs, and toxicities (SS/Tox)
Analytic strategy
Preliminary analyses used analysis of variance (ANOVA) to compare the recurrent (R) and disease-free (DF) groups at the time of original diagnosis on immune, endocrine, and behavioral variables.
Primary analyses used mixed-effects modeling to test the effects of group (R versus DF), time (months), and the group × time interaction. Number of months prior to the recurrence diagnosis (or the equivalent for the DF group) was used as the time factor. The following were considered for control: baseline level of the dependent variable, study arm, tumor grade, age, body mass index, concurrent treatment (chemotherapy, radiation, and/or hormonal therapy), concurrent menopausal status, and, for analysis of immune cell function, relevant cell counts (NK, CD4+ T cells). Using a backward elimination procedure, any covariates significant at P < 0.20 remained in the final models. The Bayseian Information Criterion was used to determine whether to include the time effect. An alpha level of 0.05, two-sided, was used. We used the Statistical Package for the Social Sciences (SPSS v.14; [1]). Follow-up mixed-model analyses tested for differential patterns of data between patients with local versus distant disease.
Results
Descriptive and preliminary analyses
Table 2 provides descriptive data. Using data obtained at the time of initial diagnosis, the groups were equivalent on most hematologic (P > 0.080) and health indices (P > 0.101). However, R patients had higher baseline basophil counts (P = 0.035). For the behavioral data, R patients reported greater distress on two POMS subscales: fatigue and confusion (P < 0.036). Regardless of the significance of baseline group differences, baseline values were entered as controls for all the main analyses.
Table 2.
Mean and standard deviations of examined variables for the recurrence and disease-free groups
| Variables | Recurrence (R) | Disease-free (DF) | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline M (SD) | Time 1 M (SD) | Time 2 M (SD) | Time 3 M (SD) | Baseline M (SD) | Time 1 M (SD) | Time 2 M (SD) | Time 3 M (SD) | |
| Cell counts | ||||||||
| White blood cell count (k/μl) | 6.27 (1.96) | 5.45 (2.01) | 5.55 (1.90) | 5.78 (2.01) | 6.09 (1.75) | 5.18 (2.64) | 4.83 (2.36) | 4.62 (1.62) |
| Eosinophil count (k/μl) | 0.17 (0.12) | 0.16 (0.11) | 0.16 (0.09) | 0.15 (0.10) | 0.19 (0.16) | 0.14 (0.11) | 0.17 (0.11) | 0.13 (0.09) |
| Basophil count (k/μl)a | 0.05 (0.04) | 0.03 (0.02) | 0.03 (0.02) | 0.03 (0.02) | 0.03 (0.03) | 0.03 (0.02) | 0.03 (0.02) | 0.03 (0.02) |
| Monocyte count (k/μl) | 0.60 (0.66) | 0.43 (0.17) | 0.46 (0.21) | 0.44 (0.20) | 0.47 (0.19) | 0.43 (0.14) | 0.42 (0.12) | 0.39 (0.14) |
| Neutrophil count (k/μl) | 3.94 (1.57) | 3.37 (1.26) | 3.34 (1.25) | 3.65 (1.38) | 3.87 (1.70) | 3.18 (1.41) | 2.70 (0.73) | 2.61 (0.93) |
| Lymphocyte count (k/μl) | 1.81 (0.67) | 1.60 (0.71) | 1.61 (0.65) | 1.59 (0.59) | 1.71 (0.50) | 1.27 (0.51) | 1.34 (0.48) | 1.32 (0.44) |
| CD3+ T cell count (k/μl) | 1.38 (0.57) | 1.18 (0.56) | 1.22 (0.56) | 1.17 (0.54) | 1.29 (0.42) | 0.95 (0.42) | 0.98 (0.41) | 0.96 (0.41) |
| CD4+ T cell count (k/μl) | 0.95 (0.43) | 0.80 (0.43) | 0.83 (0.44) | 0.82 (0.39) | 0.89 (0.29) | 0.64 (0.33) | 0.68 (0.33) | 0.68 (0.28) |
| CD8+ T cell count (k/μl) | 0.47 (0.23) | 0.47 (0.27) | 0.49 (0.28) | 0.46 (0.28) | 0.42 (0.19) | 0.39 (0.17) | 0.38 (0.19) | 0.36 (0.19) |
| CD56+ NK cell count (k/μl) | 0.21 (0.13) | 0.23 (0.16) | 0.26 (0.19) | 0.28 (0.22) | 0.21 (0.13) | 0.21 (0.11) | 0.20 (0.09) | 0.20 (0.09) |
| Cell function | ||||||||
| NKCC (lytic units per 107 cells) | 5.30 (1.03) | 5.86 (1.25) | 5.98 (1.27) | 5.83 (1.13) | 5.23 (0.99) | 5.79 (0.87) | 5.54 (0.93) | 5.84 (1.09) |
| Con A (optical density) | 0.19 (0.13) | 0.19 (0.20) | 0.18 (0.16) | 0.19 (0.14) | 0.21 (0.14) | 0.19 (0.16) | 0.17 (0.12) | 0.19 (0.15) |
| PHA (optical density) | 0.32 (0.18) | 0.27 (0.22) | 0.26 (0.18) | 0.29 (0.19) | 0.31 (0.14) | 0.25 (0.15) | 0.25 (0.15) | 0.28 (0.17) |
| Endocrine studies | ||||||||
| Salivary cortisol | 0.19 (0.09) | 0.29 (0.12) | 0.27 (0.19) | 0.27 (0.13) | 0.17 (0.10) | 0.19 (0.09) | 0.24 (0.19) | 0.24 (0.18) |
| ACTH | 18.5 (9.1) | 15.7 (7.0) | 14.1 (7.9) | 16.4 (11.4) | 24.5 (12.2) | 17.6 (9.7) | 18.1 (9.9) | 22.1 (15.2) |
| Epinephrine | 26.0 (14.0) | 23.9 (14.1) | 23.7 (12.6) | 31.2 (14.8) | 28.0 (17.6) | 22.3 (12.4) | 28.1 (17.3) | 29.4 (18.4) |
| Norepinephrine | 324 (104) | 369 (159) | 362 (149) | 409 (206) | 297 (103) | 371 (166) | 358 (154) | 372 (178) |
| Emotional distress | ||||||||
| Anxiety | 10.63 (5.51) | 6.17 (4.60) | 6.58 (4.25) | 6.36 (4.09) | 8.94 (5.05) | 5.64 (4.00) | 6.27 (4.71) | 5.56 (4.70) |
| Depression | 8.38 (6.49) | 4.48 (4.83) | 5.60 (5.47) | 4.07 (4.91) | 6.23 (4.73) | 3.26 (3.57) | 4.07 (5.09) | 3.36 (3.19) |
| Anger | 5.79 (4.25) | 4.79 (4.83) | 5.69 (4.43) | 4.82 (4.09) | 4.79 (4.05) | 3.90 (4.10) | 5.27 (6.24) | 3.40 (3.03) |
| Vigor | 10.0 (4.87) | 12.4 (5.25) | 12.3 (4.96) | 12.2 (4.87) | 11.9 (5.70) | 13.1 (5.26) | 12.3 (5.14) | 13.6 (4.68) |
| Fatiguea | 8.69 (5.12) | 7.70 (5.01) | 7.62 (5.63) | 7.52 (5.15) | 6.65 (4.24) | 5.98 (4.26) | 5.96 (4.37) | 5.18 (4.36) |
| Confusiona | 7.71 (4.22) | 4.45 (2.74) | 4.87 (3.21) | 4.20 (2.91) | 5.83 (3.77) | 3.90 (2.56) | 4.40 (3.39) | 3.64 (2.69) |
| Quality of life | ||||||||
| Physical functioning | 74.9 (18.7) | 76.2 (22.6) | 76.9 (22.3) | 74.6 (23.8) | 81.6 (14.6) | 84.6 (18.0) | 86.7 (15.0) | 86.3 (16.2) |
| Role functioning: physical | 13.5 (25.8) | 66.9 (36.1) | 64.4 (39.7) | 65.3 (36.7) | 9.4 (21.7) | 81.5 (32.2) | 72.2 (42.4) | 78.3 (36.0) |
| Bodily pain | 45.3 (20.9) | 69.8 (24.3) | 70.0 (23.1) | 67.1 (22.9) | 44.5 (22.5) | 78.0 (18.5) | 70.7 (24.4) | 73.8 (17.7) |
| General health perceptions | 67.3 (20.3) | 67.7 (22.9) | 71.1 (21.5) | 70.6 (21.3) | 73.1 (17.3) | 75.5 (19.1) | 74.0 (18.2) | 72.2 (19.0) |
| Vitality | 39.3 (18.3) | 52.3 (24.1) | 51.8 (21.7) | 51.3 (23.3) | 46.3 (23.6) | 56.0 (18.2) | 57.2 (18.8) | 63.0 (20.0) |
| Social functioning | 64.4 (27.0) | 84.3 (19.8) | 82.5 (24.7) | 87.3 (19.3) | 61.9 (25.8) | 90.6 (15.0) | 83.8 (22.3) | 91.9 (14.0) |
| Role functioning: emotional | 43.8 (41.9) | 65.1 (41.1) | 68.1 (36.2) | 71.2 (37.8) | 50.0 (41.3) | 85.7 (29.6) | 83.7 (33.8) | 85.9 (29.7) |
| Mental health | 62.3 (20.6) | 74.3 (15.4) | 72.2 (18.1) | 73.9 (17.3) | 66.3 (17.9) | 75.4 (14.4) | 77.5 (16.5) | 79.3 (11.8) |
| Functional status and symptoms | ||||||||
| Performance status | 83.3 (9.3) | 87.7 (7.5) | 87.7 (6.8) | 87.7 (7.7) | 86.3 (7.9) | 89.8 (6.0) | 91.3 (6.9) | 91.2 (6.6) |
| Signs/symptoms and toxicities | 0.21 (0.10) | 0.25 (0.12) | 0.25 (0.10) | 0.23 (0.10) | 0.19 (0.10) | 0.22 (0.10) | 0.25 (0.12) | 0.23 (0.12) |
NKCC Natural killer cell cytotoxicity in log-transformed lytic units, Con A Lymphocyte proliferative response to concanavalin A, PHA Lymphocyte proliferative response to phytohemagluttinin
aThere was a statistically significant group difference at baseline, based on one-way analysis of variance. Group comparisons for time 1 through time 3 are summarized in Table 3
Data were examined for the potential influence of nutritional status and anemia. Samples in which albumin was outside of the normal range were not analyzed. Only one patient exhibited signs of anemia (hemoglobin <10 g/dl; [19]) at the baseline assessment, as did one patient during follow-up. Removal of these data yielded identical results to those from the full sample, reported below.
Primary analyses
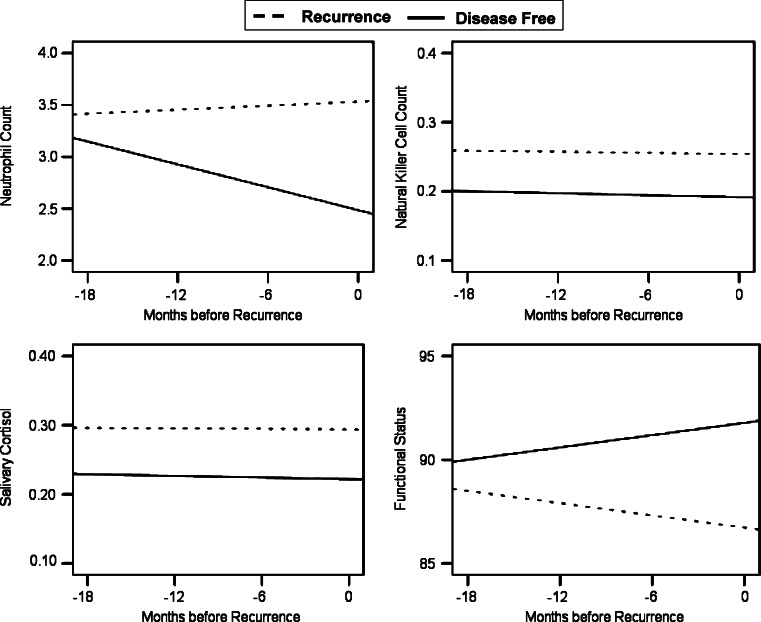
Table 3 summarizes the results. Significant group effects were observed for total WBC, neutrophil counts, lymphocyte counts, and CD56+ (natural killer) lymphocytes, whereby patients in the R group evinced significantly higher cell numbers than those in the DF group. In addition, there was a significant group x time interaction for neutrophil counts. As illustrated in Fig. 1, counts for DF patients were declining over time, while those for R patients remained high. In contrast to cell numbers, indices of cell function (LPR, NKCC) were equivalent between the R and DF groups. These analyses included concurrent cell counts (NK or T4) as a time-variant covariate.
Table 3.
Summary of mixed-effects models
| Variable | Effect | Estimate | P | Effect size (Cohen’s D) |
|---|---|---|---|---|
| Cell counts | ||||
| White blood cell counta,b,c,d,e | Group | 0.623 | 0.027 | 0.47 |
| Eosinophil countf,g | Group | −0.001 | 0.966 | 0.15 |
| Basophil counta,g | Group | 0.000 | 0.954 | 0.09 |
| Monocyte counth | Group | −0.019 | 0.606 | 0.13 |
| Neutrophil counta,b,c,g | Group | 0.870 | 0.001 | 0.54 |
| Time | −0.024 | 0.037 | ||
| Group × time | 0.035 | 0.022 | ||
| Lymphocyte count a,d,f | Group | 0.200 | 0.008 | 0.57 |
| CD3+ T cell counta,d | Group | 0.120 | 0.084 | 0.37 |
| CD4+ T cell counta,d,h | Group | 0.072 | 0.197 | 0.28 |
| CD8+ T cell countd,f | Group | 0.057 | 0.058 | 0.40 |
| CD56+ NK cell counta,b,e | Group | 0.053 | 0.032 | 0.46 |
| Cell function | ||||
| NKCCf | Group | 0.102 | 0.510 | 0.14 |
| Con Ab,d,f,g | Group | −0.012 | 0.580 | 0.11 |
| PHAg,j | Group | 0.000 | 0.985 | 0.02 |
| Endocrine studies | ||||
| Salivary cortisolc,e | Group | 0.079 | 0.001 | 0.49 |
| ACTH e,f | Group | −2.497 | 0.295 | 0.22 |
| Epinephrinef,g | Group | 0.542 | 0.873 | 0.08 |
| Norepinephrinea,b,g | Group | 56.13 | 0.187 | 0.27 |
| Emotional distress | ||||
| Anxietyb | Group | −0.006 | 0.990 | 0.00 |
| Depressionb,d,g,h | Group | 0.184 | 0.702 | 0.05 |
| Angera,d | Group | 0.738 | 0.118 | 0.21 |
| Vigord,e,f,h | Group | 0.268 | 0.708 | 0.08 |
| Fatiguec,d,e | Group | 1.969 | 0.023 | 0.31 |
| time | −0.099 | 0.029 | ||
| Group × time | 0.086 | 0.145 | ||
| Confusionb,g | Group | −0.044 | 0.924 | 0.02 |
| Quality of life | ||||
| Physical functioningb,e,h | Group | −7.727 | 0.014 | 0.38 |
| Time | 0.209 | 0.118 | ||
| Group ×time | −0.297 | 0.098 | ||
| Role functioning: physical a,e | Group | −12.43 | 0.027 | 0.46 |
| Bodily paina,b,h | Group | −4.032 | 0.241 | 0.24 |
| General health perceptionsa,b,c,h | Group | 1.240 | 0.636 | 0.10 |
| Vitalityd,g,h | Group | −8.898 | 0.039 | 0.32 |
| Time | 0.417 | 0.016 | ||
| Group × time | −0.464 | 0.045 | ||
| Social functioningd | Group | −4.054 | 0.184 | 0.27 |
| Role functioning: emotionalc,f | Group | −13.98 | 0.013 | 0.53 |
| Mental healthd,e | Group | −5.334 | 0.090 | 0.23 |
| Time | 0.223 | 0.108 | ||
| Group × time | −0.332 | 0.075 | ||
| Functional status and symptoms | ||||
| Performance statusb,d,h | Group | −4.513 | 0.003 | 0.45 |
| Time | 00.096 | 0.193 | ||
| Group × time | −0.197 | 0.047 | ||
| Signs/symptoms and toxicitiesa,c,d,e | Group | 0.000 | 0.980 | 0.01 |
aTumor grade (0, well differentiated; 1, moderately or poorly differentiated)
bAge at initial diagnosis
cConcurrent menopausal status (0, pre/peri; 1, post-menopausal)
dConcurrent chemotherapy (0, no; 1, yes)
eConcurrent radiation (0, no; 1, yes)
fConcurrent hormonal therapy (0, no; 1, yes)
gStudy arm (0, assessment-only; 1, psychological intervention with assessment
hBody mass index
iNatural killer cell count
jCD4+ T cell count
All analyses controlled for baseline levels of the outcome. Additional statistical controls were included as indicated
Fig. 1.
Group differences in predicted trajectories for immune cell counts, salivary cortisol, and functional status in the recurrence and disease-free groups. Differences between the R and DF groups were significant for neutrophils (P = 0.001), natural killer cells (P = 0.032), cortisol (P = 0.001), and functional status (P = 0.003)
For the endocrine data, participants in the R group had significantly higher salivary cortisol levels, as illustrated in Fig. 1. No significant group differences were observed for ACTH, epinephrine, or norepinephrine.
For the behavioral data, the R group reported significantly greater fatigue, and poorer quality of life related to vitality, physical functioning, physical role functioning, and emotional role functioning. Further, a significant group × time interaction for vitality revealed the R group to show no improvement over time, while the DF group showed expected improvements in quality of life (Fig. 1), and there were similar, but non-significant, patterns in fatigue, physical functioning, and mental health. The data are suggestive of poorer energy levels and a range of activity disruption (e.g., difficulty carrying groceries, spending less time on important activities). The R and DF groups were not significantly different on the remaining POMS and SF-36 subscales.
Consistent with the self reports of physical functioning, R patients had significantly lower nurse-rated performance status accompanied by a failure to improve over time. Figure 1 illustrates the group difference in functional status. The groups did not differ in signs, symptoms, or toxicities from cancer treatments.
Follow-up analyses
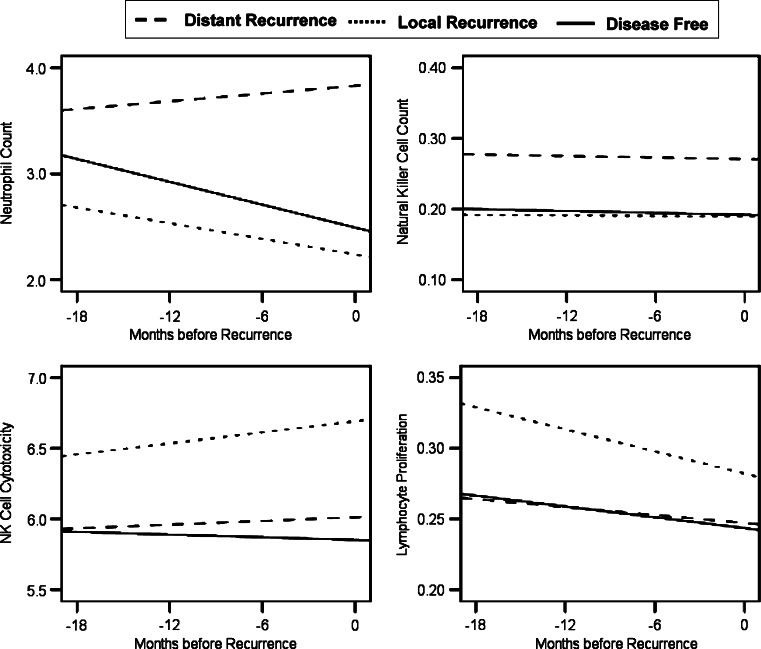
Immune control or response to tumor growth may be different for local and distant disease [25]. Therefore, we further examined the data by comparing loco-regional recurrence (n = 11) with distant recurrence (n = 37). Patients with distant metastases had higher WBC (P = 0.050) and neutrophil counts (P = 0.010) than those with loco-regional recurrence (see Fig. 2). For lymphocyte, T8, and NK cell counts, the pattern of means was identical to those depicted in Fig. 2 with only the distant recurrence patients differing significantly from disease-free; however, the differences between distant and local recurrence groups were non-significant (P = 0.06–0.12). With regard to immune cell function, patients later diagnosed with local recurrence showed higher NKCC (P = 0.035) and LPR to PHA (P = 0.049) than distant recurrence patients (Fig. 2).
Fig. 2.
Group differences in predicted trajectories for immune cell counts and function and salivary cortisol in the local recurrence, distant recurrence, and disease-free groups. Differences between the local and distant recurrence groups were significant for neutrophil counts (P = 0.010), natural killer cell cytotoxicity (P = 0.035), and lymphocyte proliferative response to phytohemaggluttinin (P = 0.049). The difference in natural killer cell count between local and distant recurrence groups did not reach significance (P = 0.060)
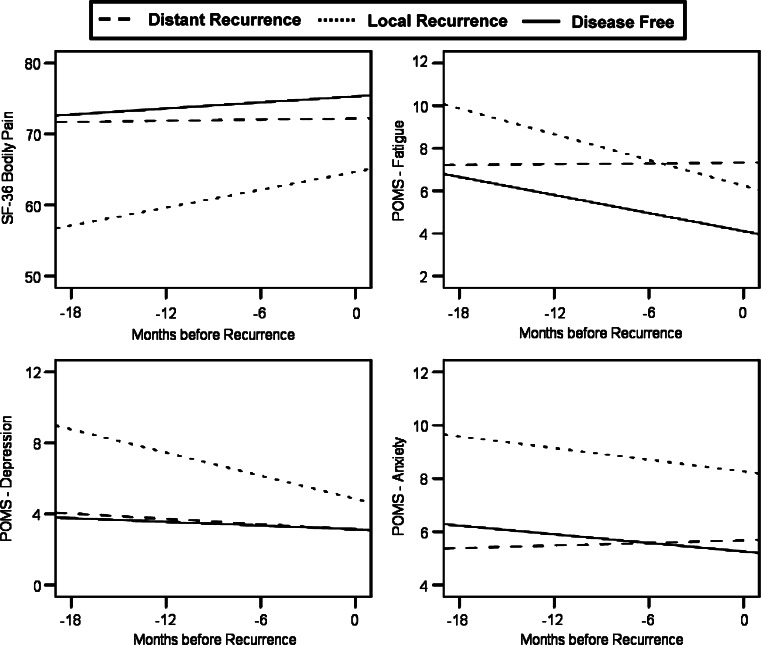
Figure 3 illustrates subgroup differences in the behavioral data. Data showed the patients with local recurrence to have higher fatigue (P = 0.041), depression (P < 0.001), and anxiety (P < 0.001), as well as poorer quality of life related to mental health (P = 0.026) and pain (P = 0.043), relative to patients with distant metastases. There was also a trend for patients with local recurrence to have lower vigor (P = 0.063) than those with distant disease.
Fig. 3.
Group differences in predicted trajectories for pain, fatigue, and depressed and anxious mood in the local recurrence, distant recurrence, and disease-free groups. Higher scores indicate less interference of pain in quality of life and more feelings of fatigue, anxiety and depression. Differences between the local and distant recurrence groups were significant for quality of life related to pain (P = 0.043), fatigue (P = 0.041), depressed mood (P < 0.001), and anxiety (P < 0.001)
Discussion
This exploratory study offers a unique look at the hematologic and behavioral context preceding a breast cancer recurrence diagnosis. Two groups were selected, similar in prognostic characteristics and received treatments, which differed in the event of recurrence. Reliable group differences in immune cell counts, cortisol, and behavioral factors were observed. Analyses control for important potential correlates of recurrence: age, concurrent treatments, and other prognostic characteristics. To make the test more stringent, baseline values of the variables of interest are controlled in all analyses. Thus, the results are reflective of group differences which emerge between the initial and recurrence diagnoses, rather than group differences which may have been present at initial diagnosis. The present study hypothesizes that differences such as these between disease-free and recurrence patients, unexplained by known correlates such as treatment, could be related to disease processes.
Researchers have suggested that tumor cells can evoke a specific immune reaction via their altered antigenicity, a non-specific inflammatory reaction, or a reaction to cytokines produced by the tumor or its microenvironment [25]. Our data show patients with distant recurrence to have elevated neutrophil counts, which could have resulted from granulocyte colony stimulating factor (G-CSF) released by the tumor [14, 16], albeit of a lesser severity than reported case studies [31]. Other research has reported neutrophilia to be accompanied by lymphopenia [33], but this was not the case for our patients. Notably, the increase in lymphocyte numbers in peripheral blood was not accompanied by enhancement of NK or T cell activity (i.e., NKCC, LPR). It is plausible that elevated cortisol, an immunosuppressive hormone, may have been responsible for this pattern [29].
For patients with local disease, high NK cell cytotoxicity and T cell proliferation were accompanied by patient reports of pain, fatigue, and emotional distress including depression and anxiety. While the immune and behavioral effects may be independent, they may also arise from a common mechanism. It has been hypothesized that fatigue among cancer patients can result from a ‘cytokine cascade’, triggered by post-treatment elevation of the pro-inflammatory cytokines tumor necrosis factor (TNF-α), interleukin-1β (IL-1β), and IL-6 (see [24] for a discussion). IL-2, also a pro-inflammatory cytokine, is known to produce fatigue, depressed mood, and other ‘sickness behaviors’ in addition to the enhanced immune function (particularly NK cytotoxicity) [6, 31].
Altered immune or endocrine regulation, such as an increase of pro-inflammatory cytokines, could arise either as a cause or a consequence of recurrent disease; that is, it could be an immune reaction to the presence of disease or a fertile environment within which recurrent disease can develop [7, 10]. We note that the analytic strategy we selected implies (but, of course, does not demonstrate) a direction of effect. We are testing the hypothesis that recurrence processes yield differential signs/symptoms rather than the converse—signs or symptoms affecting an individual’s risk for recurrence. For this reason, mixed model analyses were used rather than an analysis in which recurrence status was an outcome, such as logistic regression. Separate from our assumptions, however, there is evidence to suggest that the latter scenario—that the observed effects are a reaction to the presence of disease is a plausible interpretation. Namely, because the analyses controlled for baseline values, these results reveal patterns which developed only after the initial diagnosis. Future research testing indicators of inflammation (e.g., C-reactive protein, IL-6) could test these hypotheses.
The results of the present study suggest avenues for research. For earlier recurrence detection, for example, it may be fruitful to test cytokines which relate to the elevated cell counts and behavioral data observed here. Tumor cells have been observed to secrete GCS-F [14, 16] and, considering the present data, this increase may reach levels which are detectable in the peripheral blood. IL-2, stimulating a Th2-mediated immune response, may also be differentially elevated in patients who are about to recur. An exploration of these variables in archival data could test these hypotheses.
A second line of potential investigation concerns the possible causal relationship between our outcomes and recurrence. Neutrophils and lymphocytes have been shown to be cytotoxic against tumor cells [13, 17], yet a recurrence of cancer was observed despite their high numbers. Future research could test whether, in such cases, insufficient cells infiltrated the tumor [25], or whether the cells exhibited poor cytotoxicity.
Use of clinical trial data for this analysis was advantageous. Periodic evaluation of the sample provided data prior to the recurrence diagnosis and measures assessing multiple systems. An added benefit of the large trial was the ability to identify a comparison sample whose initial disease was similar to the recurrence patients. Further, follow-up of the disease-free patients continues, and they are known to remain disease-free for at least a year (mean = 84, range: 13–135 months) after the collection of the data presented here.
Significance levels were not adjusted for multiple statistical tests. However, the number of significant statistical tests [11 of 33 (33%)] was higher than chance (i.e., 5%) and produced a conceptually consistent pattern. Data were also limited by a small sample size, and results will need to be replicated in a larger study. Groups were equivalent on established prognostic variables. HER-2 data were not available, but the literature does not suggest that HER-2/neu status is correlated with any of the outcomes reported here.
Specific clinical recommendations cannot be made from the present data. However, earlier detection, prediction, and even prevention of recurrence require an understanding of disease activity before its clinical appearance. At present, these results invite the possibility of identifying metastatic disease months or even years sooner than is currently possible.
Acknowledgments
We thank the participants and the professional and research staff of the Stress and Immunity Cancer Projects. Special thanks to Dr. Hae-Chung Yang for assistance with data analysis. This research was funded by the American Cancer Society (PBR-89, RSGPB-03-248-01-PBP), Longaberger Company-American Cancer Society Grant for Breast Cancer Research (PBR-89A), U.S. Army Medical Research Acquisition Activity Grants (DAMD17-94-J-4165; DAMD17-96-1-6294; DAMD17-97-1-7062), National Institutes of Mental Health (1 R01 MH51487), the National Cancer Institute (K05 CA098133; R01 CA92704), the General Clinical Research Center (M01-RR0034), and The Ohio State University Comprehensive Cancer Center (P30 CA16058).
References
- 1.SPSS 14.0 for Windows. Release 14.0.0. SPSS, Inc, Chicago
- 2.American Joint Committee on Cancer . AJCC cancer staging manual. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 3.American Joint Committee on Cancer (2002) AJCC cancer staging manual. In: Greene FL (ed). Springer, New York
- 4.Andersen BL, Farrar WB, Golden-Kreutz DM, et al. Distress reduction from a psychological intervention contributes to improved health for cancer patients. Brain Behav Immun. 2007;7:953–961. doi: 10.1016/j.bbi.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen BL, Farrar WB, Golden-Kreutz DM, et al. Psychological, behavioral, and immune changes following a psychosocial intervention: a clinical trial. J Clin Oncol. 2004;17(1):3570–3580. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anisman H, Hayley S, Turrin N, et al. Cytokines as a stressor: implications for depressive illness. Int J Neuropsychopharmacol. 2002;5(4):357–373. doi: 10.1017/S1461145702003097. [DOI] [PubMed] [Google Scholar]
- 7.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 8.Bryant J, Day R, Whiteside TL, et al. Calculation of lytic units for the expression of cell-mediated cytotoxicity. J Immunol Methods. 1992;146(1):91–103. doi: 10.1016/0022-1759(92)90052-U. [DOI] [PubMed] [Google Scholar]
- 9.Colozza M, Azambuja E, Cardoso F, et al. Proliferative markers as prognostic and predictive tools in early breast cancer: where are we now? Ann Oncol. 2005;16(11):1723–1739. doi: 10.1093/annonc/mdi352. [DOI] [PubMed] [Google Scholar]
- 10.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demicheli R, Retsky MW, Swartzendruber DE, et al. Proposal for a new model of breast cancer metastatic development. Ann Oncol. 1997;8(11):1075–1080. doi: 10.1023/A:1008263116022. [DOI] [PubMed] [Google Scholar]
- 12.Demicheli R, Terenziani M, Bonadonna G. Estimate of tumor growth time for breast cancer local recurrences: rapid growth after wake-up? Breast Cancer Res Treat. 1998;51(2):133–137. doi: 10.1023/A:1005887422022. [DOI] [PubMed] [Google Scholar]
- 13.di Carlo E, Iezzi M, Pannellini T. Neutrophils in anti-cancer immunological strategies: old players in new games. J Hematother Stem Cell Res. 2001;10(6):739–748. doi: 10.1089/152581601317210836. [DOI] [PubMed] [Google Scholar]
- 14.Furihata M, Sonobe H, Ohtsuki Y, et al. An immunohistochemical study on a case of granulocyte-colony stimulating factor-producing gall-bladder carcinoma. Pathol Int. 1999;49(11):1010–1013. doi: 10.1046/j.1440-1827.1999.00970.x. [DOI] [PubMed] [Google Scholar]
- 15.Imai K, Matsuyama S, Miyake S, et al. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356(Nov 25):1795–1799. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 16.Inoue M, Minami M, Fujii Y, et al. Granulocyte colony-stimulating factor and interleukin-6-producing lung cancer cell line, LCAM. J Surg Oncol. 1997;64(4):347–350. doi: 10.1002/(SICI)1096-9098(199704)64:4<347::AID-JSO18>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Jakobisiak M, Lasek W, Golab J. Natural mechanisms protecting against cancer. Immunol Lett. 2003;90(2–3):103–122. doi: 10.1016/j.imlet.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Karnofsky . The clinical evaluation of chemotherapeutic agents in cancer. In: Macleod CM, editor. Evaluation of chemotherapeutic agents. New York: Columbia University Press; 1949. pp. 199–205. [Google Scholar]
- 19.Kee JL. Laboratory and diagnostic tests. Upper Saddle River: Pearson Education; 2005. [Google Scholar]
- 20.Levy SM, Herberman RB, Lippman M, et al. Immunological and psychosocial predictors of disease recurrence in patients with early-stage breast cancer. Behav Med. 1991;17:67–75. doi: 10.1080/08964289.1991.9935161. [DOI] [PubMed] [Google Scholar]
- 21.Levy SM, Wise BD. Psychosocial risk factors, natural immunity, and cancer progression: Implications for intervention. Health Psychol. 1987;6(3):229–43. [Google Scholar]
- 22.McNair DM, Lorr M, Droppleman LF. EITS manual for the profile of mood states. San Diego: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 23.Moinpour CM, Feigl P, Metch B, et al. Quality of life end points in cancer clinical trials: Review and recommendations. J Natl Cancer Inst. 1989;81(7):485–495. doi: 10.1093/jnci/81.7.485. [DOI] [PubMed] [Google Scholar]
- 24.Morrow GR, Andrews PL, Hickok JT, et al. Fatigue associated with cancer and its treatment. Support Care Cancer. 2002;10:389–398. doi: 10.1007/s005200100293. [DOI] [PubMed] [Google Scholar]
- 25.Nagtegaal ID, Marijnen CA, Kranenbarg EK, et al. Local and distant recurrences in rectal cancer patients are predicted by the nonspecific immune response; specific immune response has only a systemic effect—a histopathological and immunohistochemical study. BMC Cancer. 2001;1:7. doi: 10.1186/1471-2407-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norton L. A Gompertzian model of human breast cancer growth. Cancer Res. 1988;49(22):6443–6444. [PubMed] [Google Scholar]
- 27.Ownby HE, Roi LD, Isenberg RR, et al. Peripheral lymphocyte and eosinophil counts as indicators of prognosis in primary breast cancer. Cancer. 1983;52(1):126–130. doi: 10.1002/1097-0142(19830701)52:1<126::AID-CNCR2820520123>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 28.Papatestas AE, Lesnick GJ, Genkins G, et al. The prognostic significance of peripheral lymphocyte counts in patients with breast carcinoma. Cancer. 1976;37(1):164–168. doi: 10.1002/1097-0142(197601)37:1<164::AID-CNCR2820370123>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 29.Rook GA. Glucocorticoids and immune function. Baillieres Clin Endocrinol Metab. 1999;13(4):567–581. doi: 10.1053/beem.1999.0044. [DOI] [PubMed] [Google Scholar]
- 30.Rotstein S, Blomgren H, Petrini B, et al. Blood lymphocyte counts with subset analysis in operable breast cancer. Relation to the extent of tumor disease and prognosis. Cancer. 1985;56(6):1413–1419. doi: 10.1002/1097-0142(19850915)56:6<1413::AID-CNCR2820560632>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 31.Ruka W, Rutkowski P, Kaminska J, et al. Alterations of routine blood tests in adult patients with soft tissue sarcomas: relationships to cytokine serum levels and prognostic significance. Ann Oncol. 2001;12(10):1423–1432. doi: 10.1023/A:1012527006566. [DOI] [PubMed] [Google Scholar]
- 32.Thornton LM, Andersen BL, Crespin TR, et al. Individual trajectories in stress covary with immunity during recovery from cancer diagnosis and treatments. Brain Behav Immun. 2007;21(2):185–194. doi: 10.1016/j.bbi.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh SR, Cook EJ, Goulder F, et al. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91(3):181–184. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 34.Ware JE, Snow KK, Kosinski M. SF-36 health survey: manual and interpretation guide. Lincoln: QualityMetric Incorporated; 2000. [Google Scholar]
- 35.Whitehead RH, Thatcher J, Teasdale C, et al. T and B lymphocytes in breast cancer stage relationship and abrogation of T-lymphocyte depression by enzyme treatment in vitro. Lancet. 1976;14(1):330–33. doi: 10.1016/S0140-6736(76)90085-4. [DOI] [PubMed] [Google Scholar]
- 36.Wiltschke C, Krainer M, Budinsky AC, et al. Reduced mitogenic stimulation of peripheral blood mononuclear cells as a prognostic parameter for the course of breast cancer: a prospective longitudinal study. Br J Cancer. 1995;71:1292–1296. doi: 10.1038/bjc.1995.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamasaki S, Kan N, Harada T, et al. Relationship between immunological parameters and survival of patients with liver metastases from breast cancer given immuno-chemotherapy. Breast Cancer Res Treat. 1993;26(1):55–65. doi: 10.1007/BF00682700. [DOI] [PubMed] [Google Scholar]