Abstract
Repeated administration of morphine is associated with tolerance to its antinocicpetive properties. Constipation, however, remains the major side-effect of chronic exposure to morphine. In contrast, previous studies suggest that tolerance to opioids develops in the ileum from several species. In this study, we provide evidence that constipation may arise due to lack of tolerance development to morphine in the colon. Mice were implanted with either placebo or 75 mg morphine pellets and examined for morphine tolerance to antinociception, defecation, intestinal and colonic transit after 72 h. Tissues were obtained from the ileum and distal colon and contractile responses measured from longitudinal and circular muscle preparations. In morphine-pelleted mice, a 5.5 fold tolerance developed to antinociception after 72 h and 53.2 fold in mice receiving an additional daily morphine injection. In both models, intestinal transit but not defecation or colonic transit developed tolerance. In isolated longitudinal muscle, electrical field stimulation- induced cholinergic contractions were dose-dependently inhibited by morphine in both ileum and colon of placebo-pelleted with a pD2 or 7.1 ±0.4 and 7.8 ±0.4, respectively. However, the dose-response to morphine inhibition was shifted to the right for the ileum from morphine-pelleted mice (pD2 5.1 ± 0.4) but not the colon (pD2 6.9 ± 0.4). In circular muscle preparations, morphine induced atropine-insensitive contractions in both tissue segments. Tolerance to morphine developed in the ileum but not the colon upon repeated administration of morphine. These findings indicate that lack of tolerance development in the colon is the basis for opioid bowel dysfunction.
Introduction
Morphine is one of the most frequently prescribed drugs for the treatment of moderate to severe pain. Upon repeated administration, tolerance develops to many of its effects including analgesia, nausea, vomiting and respiratory depression (Thompson and Ray, 2003). However, clinical evidence suggests that tolerance does not develop to morphine-induced constipation which limits the chronic use of this excellent pain reliever in man (Ling et al., 1989; Yuan et al., 1998; Thompson and Ray, 2003; Gutstein and Akil, 2006). Indeed, many terminally ill patients choose to discontinue opioid treatment to alleviate the discomfort of chronic opioid-induced bowel dysfunction. Opiate analgesics such as morphine act centrally as well as on peripheral sites within the enteric nervous system. Although gastrointestinal effects may in part be the result of activation of opioid receptors at spinal and supraspinal sites to slow intestinal transit and inhibit secretion, a large body of evidence indicates a direct peripheral activation (Wood and Galligan, 2004). The mechanism of morphine-induced constipation involves inhibition of gastrointestinal peristalsis which occurs as a result of presynaptic inhibition of excitatory cholinergic neurons within the myenteric plexus (Paton, 1957) and may also involve increased tone and non-migrating contractions (Matsumoto et al., 1986; Frantzides et al., 1992). Since the early work of Paton (Paton, 1957), the guinea-pig longitudinal muscle-myenteric plexus (LMPP) preparation has been the tissue preparation of choice to study the in vitro effects of morphine and related opioids in the gastrointestinal tract. Both tolerance and dependence have been demonstrated in this preparation (Rezvani et al., 1983; Leedham et al., 1989; Leedham et al., 1992; David et al., 1993) and in other species (Weisbrodt et al., 1977). This contrasts with the clinical findings of the lack of tolerance to constipation and raises the question of whether similar tolerance occurs within the colon, an important site for the induction of constipation. Remarkably, there are only a few studies addressing this issue although the first indication of the lack of morphine-induced tolerance towards colonic motility was noted in dogs in 1926 (Plant and Miller, 1926). In the rat colon, Burks and colleagues (Williams et al., 1997) demonstrated that continuous infusion of morphine resulted in tolerance in the proximal colon in vivo. In contrast, others have found the lack of tolerance to the anti-transit effects of morphine in mice pretreated twice daily for 10 days (Tan-No et al., 2003) or over 8 h of continuous infusion (Ling et al., 1989), although in these studies the effects of morphine were examined towards inhibition of small intestinal transit. While clinical observations strongly suggest that morphine-induced constipation remains resistant to the development of tolerance (Kreek, 1973), studies defining tolerance in the colon are lacking. The objective of this study was to compare the development of morphine tolerance in the mouse ileum and colon using both in vivo and in vitro methods.
Methods
Animals
Male Swiss Webster mice (Harlan Laboratories, Indianapolis, IN) weighing 25-30 g were housed 6 to a cage in animal care quarters and maintained at 22 ± 2 °C on a 12 h light-dark cycle. Food and water were available ad libitum. The mice were brought to a test room (22 ± 2 °C, 12 h light-dark cycle), marked for identification and allowed 18 h to recover from transport and handling. Protocols and procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Virginia Commonwealth University Medical Center and comply with the recommendations of the IASP (International Association for the Study of Pain).
Surgical implantation of pellets
Mice were anesthetized with 2.5% isoflurane before shaving the hair on the back of the neck. Adequate anesthesia was noted by the absence of the righting-reflex and lack of response to a toe-pinch, according to IACUC guidelines. The skin was cleansed with 10% povidone iodine (General Medical Corp., Prichard, WV) and rinsed with alcohol before making a 1 cm horizontal incision at the base of the skull. The underlying subcutaneous space toward the dorsal flanks was opened using a sterile glass rod. Maintenance of a stringent aseptic surgical field minimized any potential contamination of the pellet, incision and subcutaneous space. A placebo pellet or 75 mg morphine pellet was inserted in the space before closing the site with Clay Adams Brand, MikRon® AutoClip® 9mm Wound Clips (Becton Dickinson and Co., Sparks, MD) and again applying iodine to the surface. The animals were allowed to recover in their home cages where they remained throughout the experiment.
Tail immersion test
The warm-water tail immersion test was performed according to Coderre and Rollman (Coderre and Rollman, 1983) using a water bath with the temperature maintained at 56 ± 0.1 °C. Before injecting the mice, a base-line (control) latency was determined. Only mice with a control reaction time from 2- to 4-sec were used. The average baseline latency for these experiments was 3.0 ± 0.1 sec. The test latency after drug treatment was assessed at the appropriate time, and a 10-sec maximum cut-off time was imposed to prevent tissue damage. Antinociception was quantified according to the method of Harris and Pierson (Harris and Pierson, 1964) as the percentage of maximum possible effect (% MPE) which was calculated as: %MPE = [(test latency – control latency) / (10 – control latency)] X 100. Percent MPE was calculated for each mouse using at least 6 mice per group.
Charcoal meal test for gastrointestinal transit analysis
Forty-eight hours prior to testing, mice were placed in cages with raised mesh wire to suspend them above their bedding and prevent ingestion of feces or bedding. The animals were habituated for 24 h in the presence of food and water and then fasted for 24 h with free access to water (Roy et al., 1998; Raehal et al., 2005). This time frame was chosen to deplete the intestine and colon of any feces. In order to maintain caloric intake and to avoid hypoglycemia, mice had access to a sugar water solution consisting of a final concentration of 5% dextrose for the first 8 h of the fasting period. Mice were treated with either saline (10 μl/g s.c.) or morphine (10 mg/kg s.c.) and 20 min later given an oral gavage consisting of 5% aqueous suspension of charcoal in a 10% gum Arabic solution. At 30 min after the administration of the charcoal meal, the mice were euthanized by cervical dislocation and the small intestine from the jejunum to the caecum was dissected and placed in cold saline to stop peristalsis. The distance traveled by the leading edge of the charcoal meal was measured relative to the total length of the small intestine and the percentage of intestinal transit for each animal was calculated as percentage transit (charcoal distance)/(small intestinal length) × 100. This is referred to as intestinal transit in the text.
Bead expulsion test for colonic transit analysis
Mice were habituated and fasted as described above for the small intestine transit analysis. Mice were given an injection of either saline (10 μl/g body weight) or morphine (10 mg/kg s.c.). At 20 min post-injection, animals were anesthetized with isoflurane (1-2 min) in order to insert a single 2 mm glass bead into the distal colon at a distance of 3 cm from the anus. Bead insertion was accomplished using a glass rod with a fire polished end and marked at 3 cm to avoid tissue damage (Raffa et al., 1987; Martinez et al., 2004). After bead insertion, mice were placed in individual cages and the time to bead expulsion was monitored. Animals were monitored for a maximum of 5 h unless the bead expulsion occurred sooner. The quantity of fecal matter expelled was measured and counted as number of fecal boli and the total weight of fecal boli every 24 h over 72 h period.
Evaluation of defecation
Mice were placed in individual cages with raised wire mesh to suspend them above their bedding and prevent ingestion of feces. The amount of food consumed and fecal boli were weighed at 24, 48 and 72 h following pellet implantation. The ratio of the weight of fecal boli to the amount of food consumed was calculated for each group.
Isometric tension recording
One cm segments of ileum and distal colon (about 1 cm proximal to the anus) were dissected, flushed off their contents and trimmed of mesentery. Preparations were suspended in the axis of the circular muscle with a metal triangle or longitudinal muscle tied to a glass hook under 1 g passive tension in 15 ml siliconised organ baths containing Kreb's solution (in mM: NaCl, 118; KCl, 4.6; NaH2PO4, 1.3; MgSO4, 1.2; NaHCO3, 25; glucose, 11 and CaCl2, 2.5) maintained at 37 °C and bubbled with 95% O2 and 5% CO2, tissues were allowed to equilibrate for 60 min prior to start of experiments, with Kreb's solution changed every 15 min. Isometric contractions were recorded by a force transducer (model: #GR –FT03; Radnoti, Monrovia, CA) connected to a personal computer using Acqknowledge 382 software (BIOPAC systems, Santa Barbara, CA). Acetylcholine (3 μM) contractions were measured at the beginning of each experiment as reference control.
Neurogenic contractions by transmural field stimulation
Electrical field stimulation (EFS) (50 V, 7.5 Hz, unless stated otherwise) was applied through concentric electrodes over longitudinal muscle or L-type stimulating electrodes over circular muscle strips to produce neurogenic contractions/relaxations. Single or cumulative doses of morphine or other specific opioid agonists were added over the EFS contractions/relaxations to determine their inhibitory effects on the neurogenic responses.
Drugs and chemicals
The placebo and 75mg morphine pellets were obtained from the National Institute on Drug Abuse (NIDA), Bethesda, MD. Morphine sulfate (Mallinckrodt, St. Louis, MO) was dissolved in pyrogen-free isotonic saline (Baxter Healthcare, Deerfield, IL). Activated charcoal, gum Arabic, atropine sulphate and acetylcholine were purchased from Sigma (St. Louis, MO, USA).
Data analysis
The in vivo data are expressed as mean values ± s.e. Analysis of variance (ANOVA) followed by the post hoc “Student-Newman-Keuls” test were performed to assess significance using the Instat 3.0 software (GraphPad Software, San Diego, CA, U.S.A.). P < 0.05 was considered significant.
Contractile responses after repeated administration of morphine were analyzed by repeated measures – ANOVA followed by Bonferroni post hoc test. Data are presented mean ± s.e. P ≤ 0.05 were considered significant. Effective concentration of agonists to produce half maximal response (reported as negative log (pD2)) was calculated by nonlinear regression and data were analyzed by appropriate statistical tools using GraphPad Pprism software.
Results
Tolerance to morphine antinociception
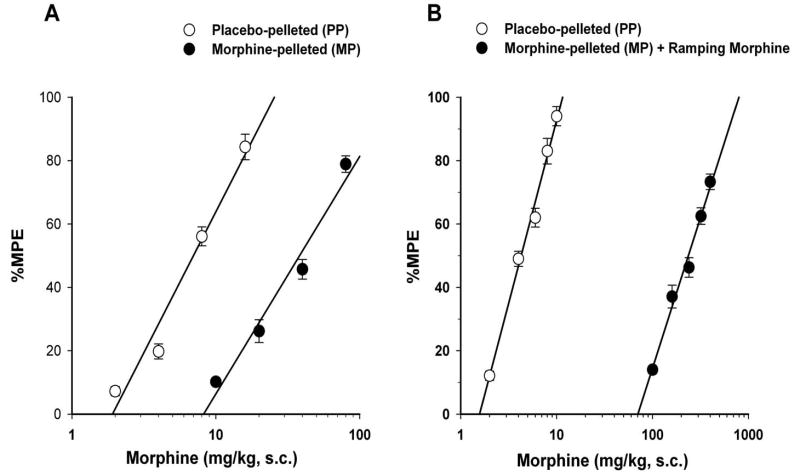
Tolerance developed to morphine antinociception in morphine-pelleted mice as measured in the tail immersion test. The baseline latency for tail-immersion in tolerant mice was similar to that of placebo-pelleted mice at 72-h following pellet implantation (Figures 1-A and 1-B). Morphine administered subcutaneously (s.c.) elicited dose-dependent antinociception in the tail immersion test in both placebo- and morphine-pellet mice at 72-h following pellet implantation. Morphine-pelleted mice (with no ramping morphine injections) showed a 5.5-fold tolerance to morphine antinociception compared to placebo-pelleted mice. The calculated ED50 value for morphine was 38.2 mg/kg (95% C.L. 34.4 to 42.4) in morphine-peletted mice compared to 6.9 mg/kg (95% C.L. 6.3 to 7.6) in placebo-pelleted mice. In a similar but more pronounced fashion, morphine-pelleted mice receiving ramping morphine injections were 53.2-fold tolerant (ED50 = 228.9 mg/kg (95% C.L. 196.8 to 266.2)) to the antinociceptive effect of acute morphine compared to placebo-pelleted mice (ED50 = 4.3 mg/kg (95% C.L. 3.9 to 4.7)).
Figure 1. Antinociceptive effects of morphine in placebo-pelleted (PP) and morphine-pelleted (MP) mice.
Experiments were conducted 72-h following the implantation of the placebo or the 75 mg morphine pellet. Baseline latencies were obtained in the warm-water tail immerstion test and mice were challenged with various doses of morphine s.c. and their tail immersion latencies were re-assessed for construction of dose-response curves. (A) mice implanted with morphine pellet and administered saline (5.3-fold tolerance) and (B) mice implanted with morphine pellet and administered ramping morphine injections (53.2-fold tolerance). Data are expressed as mean % MPE ± S.E.M. Each data point represents 6-10 mice.
Effect of morphine on defecation
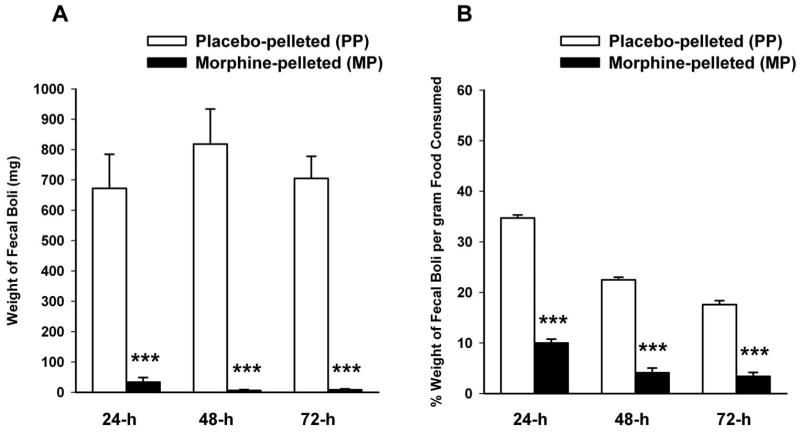
In order to establish whether tolerance develops to constipating effects of morphine as it develops to antinociception, defectation was measured as the weight of fecal boli over the 72-h duration of pellet implantation in the 5.5-fold antinociceptive tolerance model. Figure 2-A shows that defecation was markedly reduced in morphine-pelleted mice compared to placebo-pelleted mice. The average amount of food consumed by placebo -pelleted mice was 672.3 ± 112.1, 818.2 ± 115.2 and 705.1 ± 72.6 mg at 24, 48 and 72 h after pellet implantation respectively, compared to 33.5 ± 5.3, 6.1 ± 1.8 and 8.3 ±2.1 mg in morphine-pelleted mice. Since morphine induced a decrease in food consumption, the ratio of the weight of fecal boli to the amount of food consumed was calculated for each group. Even though morphine-pelleted mice showed a decrease in their food intake, we observed a significant decrease in the ratio of fecal output per gram of food intake in this same group (Figure 2-B). The percent of weight of fecal boli per gram of food consumed in morphine-pelleted mice was 10 ± 0.8, 4.1 ± 0.5 and 3.4 ± 0.6% at 24, 48 and 72h after pellet implantation, respectively compared to 34.7 ± 0.6, 22.5 ± 0.5 and 17.6 ± 0.7% in placebo-pelleted mice. This indicates that constipation is maintained throughout the 72h period while tolerance developed to morphine antinociceptive effects.
Figure 2. Analysis of defecation in morphine antinociceptive tolerance mice.
Mice were implanted with a placebo pellet (PP) or a 75 mg morphine pellet (MP) for 72-h. Equal amounts of water and chow were available for each group and mice placed on grid cages. Total weight of fecal boli (A) and percent of weight of fecal boli per gram of food consumed (B) were calculated at 24, 48, and 72-h. Data are expressed as mean ± S.E.M. (n=6-8). ***significantly different from placebo-pelleted group at P < 0.001.
Gastrointestinal transit
The distance traveled by the leading edge of the charcoal meal was reduced by more than 50% upon acute s.c. administration of morphine (10 mg/kg) in placebo-pelleted mice (Figures 3-A and 3-B). In the 5.5-fold antinociception tolerant group, there was approximately 30% reduction in the baseline distance traveled following saline administration. However acute s.c. administration of this same dose of morphine in this antinociceptive tolerant model did not produce any further reduction in the gastrointestinal transit (Figure 3-A). Similarly, in the 52.3-fold antinociception tolerant group, while basal gastrointestinal transit was reduced in mice injected with saline, acute administration of morphine (10 mg/kg, s.c.) did not further alter the intestinal transit, suggesting that tolerance developed to morphine's effect on intestinal transit (Figure 3-B). An important criteria for tolerance development is that higher concentrations are required to produce a similar effect. Consistent with this fact, morphine at 30 and 50 mg/kg significantly reduced intestinal transit in the 53- fold antinociceptive tolerant mice. At these high doses of morphine, the inhibition of intestinal transit was equal to that achieved with acute atropine (5 mg/kg) over a 30 min period, suggesting that tolerance develops to the opioid but not the cholinergic receptor (Figure 3-B).
Figure 3. Effect of morphine on gastrointestinal transit in moderate (A) and high (B) morphine antinociception tolerance models.
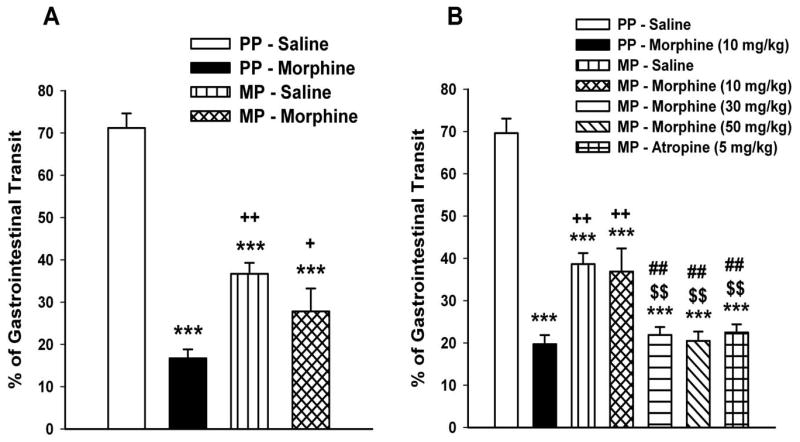
Experiments were conducted 72-h following the implantation of the placebo pellet (PP) or the 75 mg morphine pellet (MP). The low (5.5-fold) tolerant mice were pelleted with a 75 mg morphine pellet, whereas the high (53.2-fold) tolerant mice received ramping morphine injections in addition to the pellet. Mice were fasted for 14-h before testing. A charcoal meal was administered by oral gavage, 20 min following the injection of saline or morphine (10 mg/kg s.c.). Mice were sacrificed 30 min later and the distance traveled by the leading edge of the charcoal was calculated as percentage of the total length from the pylorus to the ileo-caecal junction. Data are expressed as mean ± S.E.M. (n=6-12). *** significantly different from PP-Saline group at P < 0.001; ++significantly different from PP-Morphine group at P < 0.05; +significantly different from PP-Morphine group at P < 0.01; $$significantly different from MP-Saline group at P < 0.05 and ##significantly different from MP-Morphine (10 mg/kg) group at P < 0.05.
Colonic transit
We constructed a dose-response curve for the inhibitory effects of morphine on colonic transit in naïve mice using the bead expulsion test. As shown in Figure 4A, morphine induced a dose dependent increase in bead expulsion time with a maximal effect being achieved at a dose of 10 mg/kg. This dose was equivalent to the maximum analgesia previously identified in tail-immersion assay (data not shown). We next identified whether morphine tolerance develops to colonic transit. In both the 5.5- and 53.2-fold antinociception tolerance models, acute s.c. administration of morphine (10 mg/kg) significantly retarded the expulsion time in morphine-pelleted mice in a similar manner to that in placebo-pelleted mice (Figure 4B and 4C). In the low antinociception tolerance model (5.5 fold), the average bead expulsion time in morphine-pelleted mice was 100.8 ± 11.2 min compared to 127.8 ± 6.5 min in placebo-pelleted mice following morphine administration. In the high antinociception tolerance model (52.3 fold), the morphine-pelleted mice expelled the bead within an average of 76.8 ± 10.4 min compared to 109.8 ± 6.5 min for the placebo-pelleted mice group. This finding suggested that tolerance does not occur to the effects of acute morphine in the colon of morphine-pelleted mice which contrasts with the development of tolerance to morphine antinociception and morphine-induced reduction in gastrointestinal transit.
Figure 4. Effect of morphine on colonic transit.
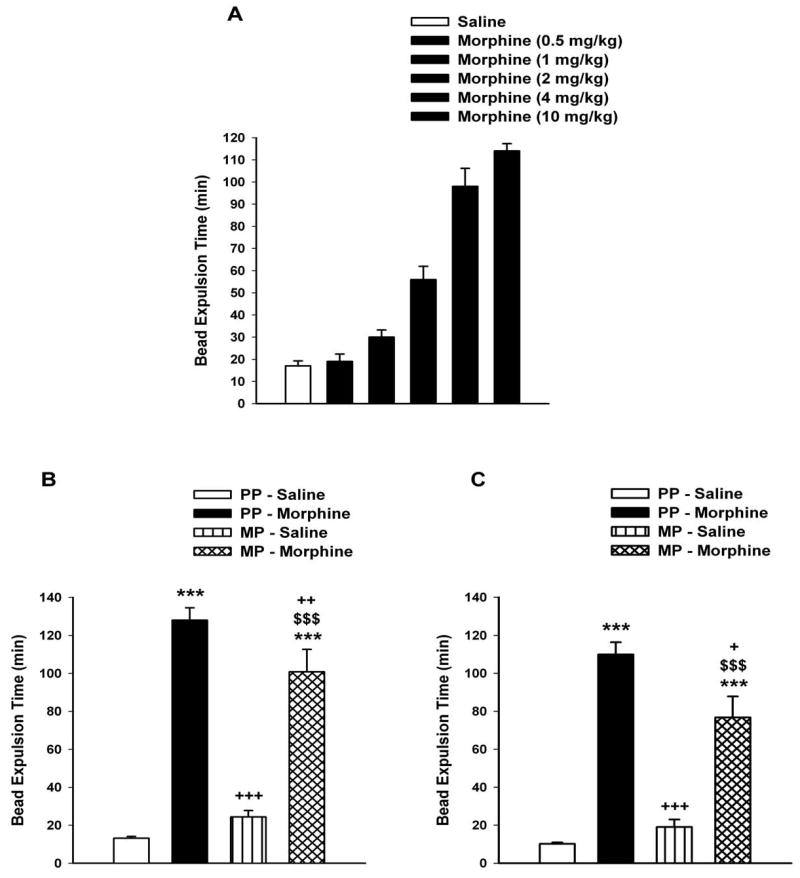
A) Dose-response to the inhibitory effects of morphine on colonic transit in drug naïve mice. Mice (n=6) were injected with either saline or morphine (0.5 -10 mg/kg s.c.). At 20 mins post-injection, a single 2 mm glass bead was inserted into the distal colon and the time for bead expulsion was determine. B) Effect of morphine on colonic transit in low- and high (C) morphineantinociception tolerance models. Experiments were conducted 72-h following the implantation of the placebo pellet (PP) or the 75 mg morphine pellet (MP). The low (5.5-fold) tolerant mice were pelleted with a 75 mg morphine pellet, whereas the high (53.2-fold) tolerant mice received ramping morphine injections in addition to the pellet. Mice were fasted for 14-h before testing. Saline or morphine (10 mg/kg) was administered s.c. and 30 min later, a 2 mm glass bead was inserted into the distal colon at a distance of 3 cm from the anus. The time for expulsion of the bead was determined as an indication of colonic transit. Data are expressed as mean ± S.E.M. (n=6-12). ***significantly different from PP-Saline group at P < 0.001; +++significantly different from PP-Morphine group at P < 0.001; ++significantly different from PP-Morphine group at P < 0.01; +significantly different from PP-Morphine group at P < 0.05 and $$$significantly different from MP-Saline group at P < 0.001.
These in vivo data show that while 72 h exposure to morphine induced tolerance to antinociception and intestinal transit, colonic transit remained responsive to morphine inhibition thus resulting in prolonged constipation.
2. Morphine tolerance in vitro
Constipation can generally be ascribed to both a decrease in the propulsive activity and increased non-propulsive contractions. While propulsive activity is largely neurally mediated, non-propulsive contractions are likely to include a myogenic component. To determine the effects of morphine on each of these components in both ileum and colon, we utilized an in vitro whole tissue approach. Longitudinal muscle preparations were used to study the effect of morphine on neurally mediated cholinergic contractions, while circular muscle preparations were used to test the effect of morphine on muscle contraction.
2.1 Longitudinal muscle-myenteric plexus preparations
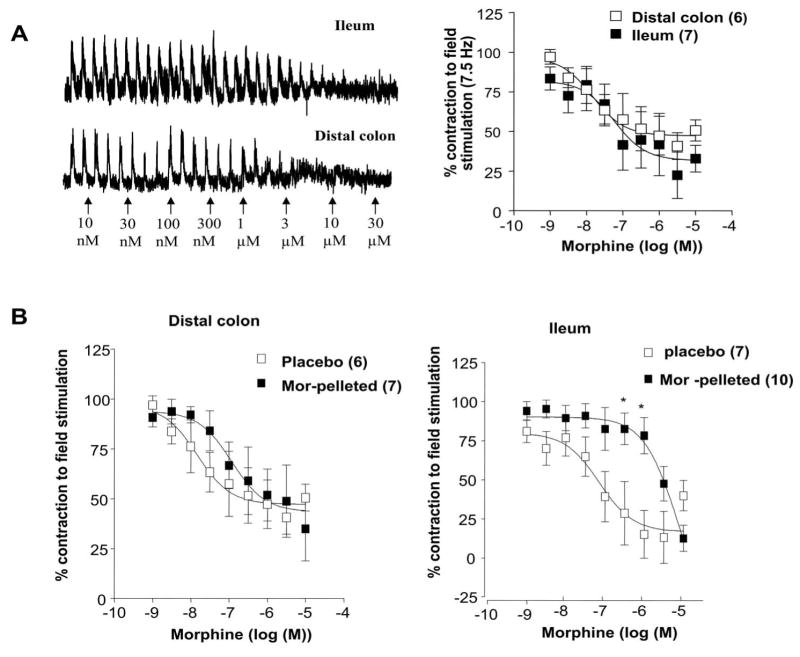
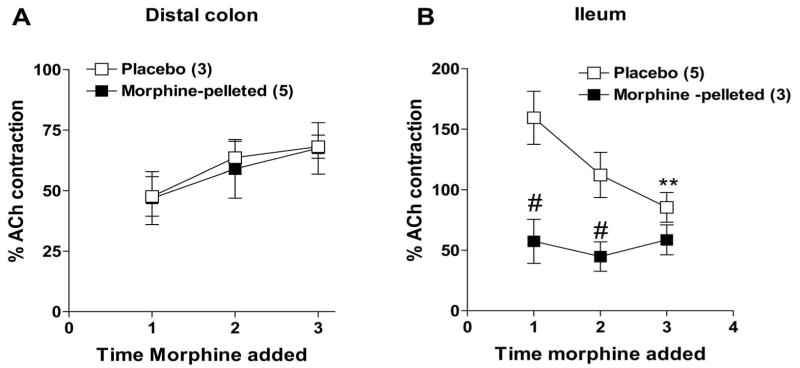
In the first set of experiments, we examined the effect of morphine on field-stimulated (FS) cholinergic contractions in the longitudinal muscle preparations from the ileum and colon. The FS contractions were abolished by TTX (100 nM) and atropine (100 nM) (not shown). Morphine dose-dependently inhibited FS contractions in both ileum and colon (Figure 5a), consistent with it's widely known effects on inhibition of acetylcholine release from enteric neurons. The pD2 values for morphine induced inhibition were 7.8 ± 0.4 in the colon and 7.1 ± 0.4 in the ileum. The inhibition of FS contractions in the colon from the 5.5 fold antinociceptive tolerant mice was similar between the placebo and morphine-pelleted mice (pD2 in placebo 7.8 + 0.4 and 6.9 + 0.4 morphine-pelleted). In contrast, there was a significant rightward shift in morphine-sensitivity in the ileum from the morphine-tolerant mice with the pD2 value being 7.1 + 0.4 in placebo and 5.1 + 0.4 in morphine-pelleted (Figure 5b). This suggested that tolerance to morphine developed in the ileum but not the colon to the inhibition of cholinergic-mediated contractions and is consistent with the above in vivo data on intestinal and colonic transit. The pD2 values for morphine were similar in the ileum (7.1 ± 0.4) and colon (7.8 ± 0.4) from the placebo-pelleted mice indicative that there is no difference in the morphine receptor sensitivity towards inhibition of cholinergic neurons in these two tissue segments.
Figure 5. Inhibition of field-stimulated contractions by morphine in the colon and ileum longitudinal muscle –myenteric plexus preparations.
(a) Left Panel shows raw traces of field stimulated contractions and the inhibitory effect of morphine in both ileum and colon. Right panel is the graph depicting concentration-dependent inhibition response of morphine in both ileum and colon. (b) Morphine-induced concentration-dependent inhibition of field-stimulated (7.5Hz) contractions in the longitudinal-myenteric plexus preparations from mice colon and ileum from placebo- and morphine-pelleted mice. Note the concentration-response curve is shifted to the right demonstrating tolerance development only in the ileum but not in the colon from morphine-pelleted mice. Data points are mean responses ± s.e.mean; n=values in parentheses. *P ≤0.05 vs corresponding concentration response.
There was no difference in the sensitivity to exogenous acetylcholine between the placebo and morphine tolerant mice (ED50 of 11.4 nM (placebo) and 10.9 nM.(morphine-pelleted)) (Figure 6a) or to neurally mediated frequency dependent contractions (Figure 6b). Furthermore, acetylcholine-induced contractions in the presence of morphine (1 uM) were also not affected (Figure 6c). Morphine-dependent inhibition of FS contractions were reversed by naloxone (Figure 6d).
Figure 6.
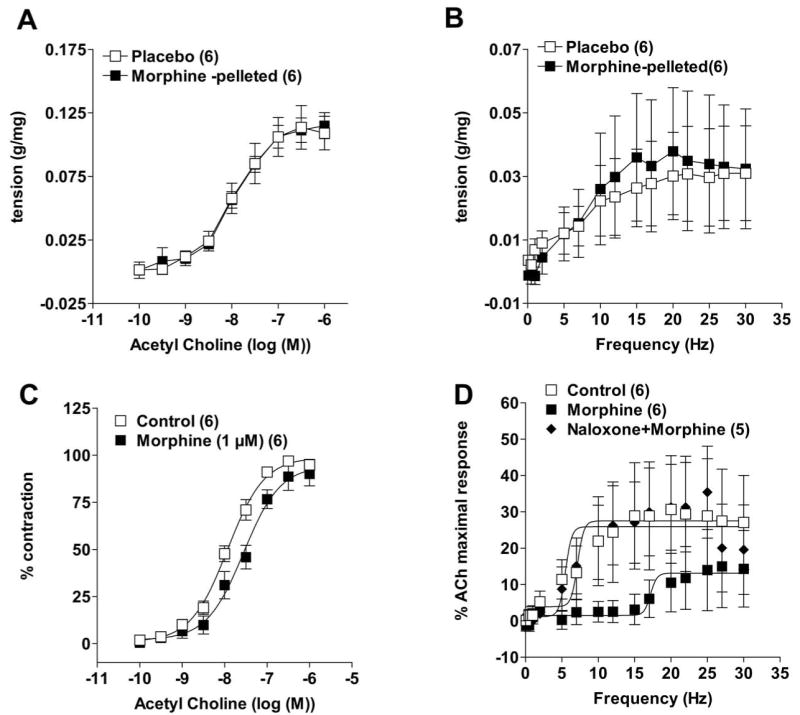
Contractility of the muscle strips were unaffected by acute or chronic morphine (pellets) treatment. (a) Dose response curve of acetylcholine-induced contractions and (b) frequency (electrical field)- dependent contractions curves were not different between placebo- and morphine-pelleted mice. (c) dose-response to acetylcholine in the absence and presence of 1μM morphine for 10 min. Acute administration of morphine did not affect the acetylcholine-induced contractions and (d) reversal of morphine-induced inhibition by naloxone of field-stimulated contractions in distal colon longitudinal muscle preparations. Data points are mean responses ± s.e.mean; n = values in parentheses.
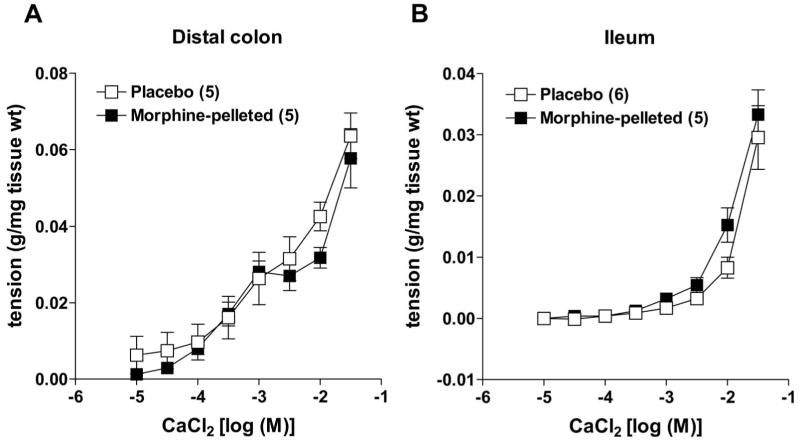
To further verify that morphine pellet implantation for three days did not alter smooth muscle contractility, we measured longitudinal muscle contractions to calcium in depolarizing K+ solution. Figure 7 shows that in either colon or ileum, calcium-dependent contractions are similar in placebo- and morphine-pelleted mice.
Figure 7.
Calcium-induced contractions of muscle strip preparations from (a) colon and (b) ileum in a depolarizing (80 mM K+ physiological saline) solution in both placebo- and morphine-pelleted mice. There was no difference in the contractile ability of either muscle strip between placebo- and morphine-pelleted groups. Data points are mean ± s.e.mean; n = values in parentheses.
In order to test whether acute tolerance occurred upon repeated administration, FS stimulated contractions were measured in drug naïve mice i.e. tissues isolated from non-pelleted mice. As shown in Figure 8, repeated administration of morphine continues to inhibit FS contractions in the colon, whereas in the ileum FS contractions become resistant to morphine by the 3rd application.
Figure 8.
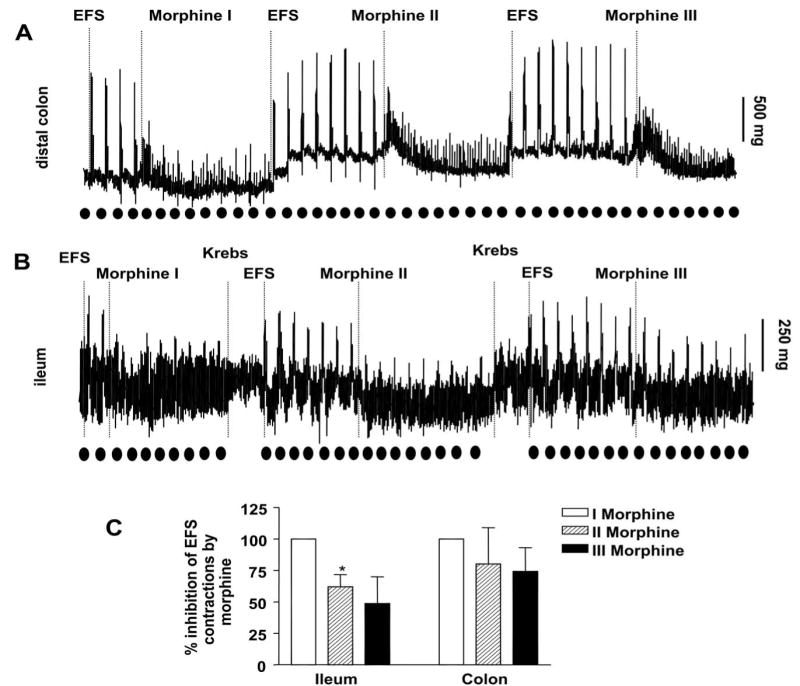
Effect of repeated administration of morphine (300 nM) on FS contractions of longitudinal muscle preparations from (a) colon and (b) lleum of drug naïve mice. The repeated administration of morphine inhibits FS contractions in the colon. In the ileum, morphine was less effective at inhibition on repeated administration. The time point of EFS (Electrical field stimulation) is represented by dark circles. (c) Graphical representation of data (mean ± s.e.mean) of morphine induced –inhibition of FS contractions. *P ≤ 0.05 is considered significantly different compared to respective initial morphine response (Morphine I); n = 3 (paired ‘t’ test).
2.2 Circular muscle preparations
Unlike the longitudinal muscle, circular smooth muscle cells are known to express opiate receptors (Bitar and Makhlouf, 1982; Bitar and Makhlouf, 1985). To test the effect of morphine on the circular muscle, tissues were set up as ring preparations in the organ bath. Following 1 h of equilibration, morphine- (applied in a cumulative fashion) induced contractions in both distal colon and in the ileum from placebo-pelleted mice. As shown in Figure 9, the response to morphine markedly differed between the colon and ileum. While morphine induced a sustained increase in the phasic and tonic contractions in the colon, those in the ileum consisted of an initial increase in tone and phasic contraction amplitudes which gradually diminished towards baseline values within 30 min. The pD2 values for morphine-induced phasic contractions were 6.9 + 0.3 and 7.2 + 0.1 n=9) in the colon and ileum, respectively. We have found that exposure of mouse colon to morphine causes an increase in spontaneous contractions for at least two hours whereas in the ileum the increase in contractions lasts for only 10-15 min. These findings suggested that changes in receptor desensitization may be more prominent in the ileum than the colon.
Figure 9.
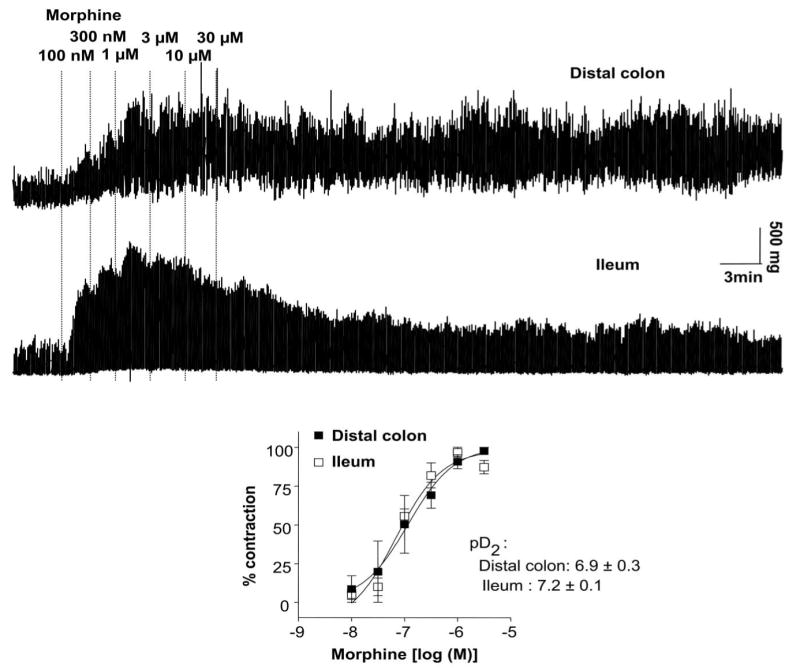
Circular muscle contractions to increasing concentrations of morphine in the colon (top tracing) and ileum (bottom tracing) from the same animal. Isometric tension recordings were obtained from placebo-pelleted mice. Bottom panel shows dose-response relationship for the peak contractions (phasic) to morphine in distal colon and ileum. There was no significant difference in the potency of morphine to cause contraction responses between colon and ileum. Qualitatively similar data (expressed as mean response ± s.e.mean in the graph) were obtained in all placebo-pelleted mice examined (n = 9)
In order to determine whether colonic contractions were resistant to morphine-induced tolerance and compare it to tolerance in the ileum, repeated administration of morphine (a submaximal dose of 3 μM) were applied every 20 min with in-between washes in drug naïve mice. Morphine induced contractions of equal or greater magnitude to all repeated administration (3 μM) in the colon but showed desensitization in the ileum (Figure 10). By the fourth administration the total contractions in the colon appeared to be enhanced while those in the ileum were reduced by almost 40%.
Figure 10.
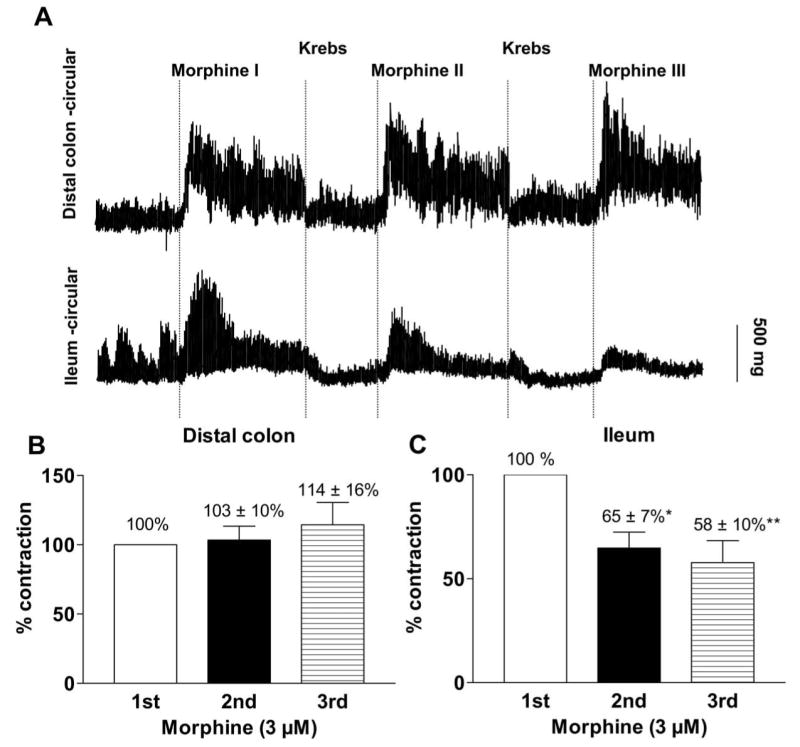
(a) Representative isometric tension recording traces from colon (top trace) and ileum (bottom) circular muscle tissue ring preparations showing effects of repeated administration of morphine; Paired experiments in placebo-pelleted mice. Bar graphs depict peak contractions (mean ± s.e.mean) of circular muscle strips from (b) colon and (c) ileum induced by repeated administration of same concentration of morphine (3 μM) at an interval of 20-30 min, keeping the initial response as 100%. The repeated administration of morphine was less effective (to cause contractions) compared to the first administration of morphine in the ileum while colon exhibited similar responsiveness to all the repeated administrations of morphine, demonstrating lack of tolerance development to morphine in the colon. * P≤ 0.05 is considered significant compared to the corresponding initial response, n = 3 (repeated measures ANOVA).
In order to test whether tolerance was evident in the gastrointestinal tract in morphine-pelleted mice, colon and ileum preparations were subjected to repeated administration to morphine (300 nM) as above. The amplitude of peak contraction, measured as percentage of acetylcholine-induced contraction, was enhanced by the 3rd application in the colon in both the placebo- and morphine-pelleted mice. In the ileum, the contractions in the placebo declined by the 3rd application, but remained constant in the tolerant mice (Figure 11).
Figure 11.
Peak amplitude of morphine-induced contractions in distal colon and ileum upon repeated administration of same concentration of morphine (3 μM) in placebo and morphine-pelleted mice. (a) Contractions were slightly augmented (no tolerance development) in the colon after each morphine administration in both placebo and morphine-pelleted. In contrast, (b) the morphine-induced contractions of ileum preparations in the placebo group declined substantially declined but remained constant in morphine-pelleted mice. Data are expressed as respective mean contraction ± s.e.m; n = values in parentheses. ** P <0.01 vs initial contraction (repeated measures ANOVA, Bonferroni posttest); #P <0.05 vs corresponding morphine administration in the placebo group (unpaired ‘t’ test).
Discussion
In this work, we have systematically compared the development of tolerance to morphine in the mouse small intestine and colon. While both the ileum and colon from several species have been studied with respect to the acute effects of morphine, studies on the development of tolerance have largely been limited to the upper gastrointestinal tract, particularly the small intestine (Rezvani et al., 1983; Leedham et al., 1989; Ling et al., 1989). There are only a few studies that have looked at the chronic effect of morphine in the colon (Williams et al., 1997). The lack of tolerance to morphine towards its constipating effects in man is widely accepted (Gutstein and Akil, 2006). To the best of our knowledge the effects of chronic opioids on the ileum and colon has not been comprehensively evaluated combining an in vivo and in vitro approach in any species.
It is well known that acute and chronic morphine results in constipation. We now show that constipation also exists in morphine antinociceptive tolerant mice. Additionally we found that tolerance exists to the effects of chronic morphine to intestinal transit but not to bead expulsion in the colon. In this study, we measured intestinal transit using charcoal meal as a marker. Acute morphine-induced inhibition of small intestinal transit has also been observed with radioactive chromium (Weisbrodt et al., 1980) and by fluorescence markers (Schmidt et al., 2008). We found that while basal intestinal transit was significantly reduced in the morphine-pelleted compared to the placebo-pelleted mice, the administration of morphine did not further decrease intestinal transit. This indicated that tolerance to an additional bolus of morphine occurs towards intestinal transit. In contrast to the effects in the ileum, tolerance did not occur in the colon. Colonic transit time was significantly retarded by morphine in placebo- as well as in morphine-pelleted mice. It is however noteworthy that basal transit time was not significantly retarded in the morphine-pelleted mice. The reason for this is not obvious as further administration of morphine to these mice resulted in inhibition of colonic transit time, negating the possibility of tolerance. It is possible, that the colon may have become partially tolerant to low concentrations of morphine as may occur at 72 h after morphine pellet implantation, and that a 10 mg/kg dose is sufficient to further retard colonic transit. Nevertheless, this development differs from that observed in the ileum in vivo and the difference was further confirmed in isolated tissue segments.
Two preparations were used to study the effects of chronic morphine in vitro. In the longitudinal muscle preparation, morphine inhibits field-stimulated release of acetylcholine from excitatory enteric nerves (Paton, 1957), whereas in the circular muscle preparations morphine induces contractions (Grider and Makhlouf, 1987; Iwata et al., 2007). In both types of preparations, there was no difference in the sensitivity to morphine's effects between the ileum and colon in the naïve mice. However, repeated administration of morphine to drug naïve mice, or those with placebo pellet implantations resulted in tolerance development in the ileum. This was observed as a rightward shift of the concentration –dependent inhibition of field-stimulated contractions by morphine in morphine-pelleted mice, and in decreased ability to inhibit the neurogenic contractions upon repeated administration in ileum from drug naïve mice. Similarly, in circular muscle, contractions to morphine were reduced by repeated administration in drug naïve mice, and remained suppressed in the ileum but not the colon from morphine-pelleted mice. Our findings in the mouse ileum using chronic morphine treatment and repeated administration in the organ bath confirm other studies that have demonstrated tolerance to morphine to neurogenic contractions in the longitudinal muscle preparations, especially in the guinea-pig ileum (Collier et al., 1981; Rezvani et al., 1983). These studies show for the first time that tolerance also occurs to circular muscle contractions in the ileum but not the colon.
The decrease in neuronal excitability primarily results in the suppression of peristaltic propulsion. Morphine also increases phasic contractions and resting contractile tone of circular muscle in both the ileum and colon. In the rat colon, Grider and Makhlouf (Grider and Makhlouf, 1987) demonstrated that phasic contractions of circular muscle strips were induced by tetrodotoxin and vasoactive intestinal polypeptide (VIP) antiserum suggesting that inhibition of an inhibitory VIPergic component by morphine leads to the unmasking of a myogenic phasic activity of the circular muscle. However, a TTX-insensitive component was also identified by these authors consistent with a possible direct smooth muscle stimulation by morphine. Early work by Bitar and Maklhouf (Bitar and Makhlouf, 1982; Bitar and Makhlouf, 1985) showed opiate mediated contractions of isolated smooth muscle cells from the guinea-pig and human stomach, intestine and gall-bladder and evidence for specific opiate receptors on circular smooth muscle was further identified by receptor protection assays (Grider and Makhlouf, 1991). Thus morphine-induced contractile responses in the circular muscle may include both a direct smooth muscle component as well as inhibition of an inhibitory tone. Non-propulsive segmental contractions in addition to inhibition of peristalsis contribute towards constipation (Pasricha, 2006). In addition to morphine's effect on inhibition of acetylcholine release, enhanced contractions are likely to exacerbate constipation. In several species, including humans, morphine increases non-propulsive activity (Plant and Miller, 1926; Frantzides et al., 1990; Frantzides et al., 1992).
The present studies were done in mice, and further studies are necessary to establish that there is differential ileal vs. colonic morphine tolerance across species. In humans, colonic propulsion and peristalsis are reduced and tone increases that may lead to spasms. This may occur as a result of dis-inhibition of inhibitory neurons (Burleigh and Trout, 1986), an effect that may be reflected as an increase in contractions of isolated circular muscle. Furthermore, differences in the absorptive/secretory functions between the ileum and colon as well the intrinsic and extrinsic neuronal input may account for the differential tolerance. Future studies addressing these issues will be required to delineate the differential basis for tolerance development.
The rewarding and analgesic effects of morphine occur through the activation of μ opioid receptors. Inhibition of gastrointestinal transit by morphine is also a μ opioid receptor function. In μ receptor knock-out mice, inhibition of intestinal transit by morphine is absent (Roy et al., 1998). In these studies, subcutaneous administration of morphine significantly inhibited gastrointestinal transit in wild-type and heterozygous mice, but not in homozygous mice. Furthermore, δ and κ agonists given either intracerebroventricular (i.c.v.) or subcutaneously did not produce significant gastrointestinal transit effect in μ receptor knock-out mice. Although gastrointestinal effects may be the result of activation of opioid receptors at spinal and supraspinal sites to slow intestinal transit and inhibit secretion, a large body of evidence indicates a direct peripheral activation (Wood and Galligan, 2004). Our findings that in vivo tolerance correlates with those from isolated tissue segments further confirms a peripheral mechanism for the morphine tolerance.
The mechanisms by which tolerance to antinociception or any other effect occurs are complex and still not well understood (Ueda et al., 2003). In the intact animal, morphine tolerance may include adaptive changes of the opioid receptor and/or changes in gene expression (Williams et al., 2001). There is still controversy concerning the role of receptor desensitization in tolerance to morphine in vivo (Finn and Whistler, 2001). This may be further complicated as morphine tolerance can develop to different effects at different rates depending upon the dose and frequency of dose administration (Smith et al., 1999; Smith et al., 2003). The finding that the colon does not develop tolerance is consistent with the clinical impression of the lack of tolerance towards constipation (Gutstein and Akil, 2006). The present studies highlight the need for further defining the cellular basis for the differential expression of morphine tolerance in the ileum and colon.
Acknowledgments
We thank Dr. Krsita L. Scoggins, David L. Stevens, Joshua A. Seager and Sanaa M. Akbarali for their input in the in vivo studies.
This work was supported in part by the National Institutes of Health grants DK-46367, DA020836 and K05DA000480.
Abbreviations
- ANOVA
Analysis of variance
- EFS
electrical field stimulation
References
- Bitar KN, Makhlouf GM. Specific opiate receptors on isolated mammalian gastric smooth muscle cells. Nature. 1982;297:72–74. doi: 10.1038/297072a0. [DOI] [PubMed] [Google Scholar]
- Bitar KN, Makhlouf GM. Selective presence of opiate receptors on intestinal circular muscle cells. Life Sci. 1985;37:1545–1550. doi: 10.1016/0024-3205(85)90187-0. [DOI] [PubMed] [Google Scholar]
- Burleigh DE, Trout SJ. Morphine attenuates cholinergic nerve activity in human isolated colonic muscle. Br J Pharmacol. 1986;88:307–313. doi: 10.1111/j.1476-5381.1986.tb10206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coderre TJ, Rollman GB. Naloxone hyperalgesia and stress-induced analgesia in rats. Life Sci. 1983;32:2139–2146. doi: 10.1016/0024-3205(83)90103-0. [DOI] [PubMed] [Google Scholar]
- Collier HO, Cuthbert NJ, Francis DL. Model of opiate dependence in the guinea-pig isolated ileum. Br J Pharmacol. 1981;73:921–932. doi: 10.1111/j.1476-5381.1981.tb08747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David C, Davis N, Mason R, Wilson VG. Evidence for functional dissociation of dependence and tolerance in guinea-pig isolated ileal segments following 20 hour exposure to morphine in vitro. Br J Pharmacol. 1993;110:1522–1526. doi: 10.1111/j.1476-5381.1993.tb13995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn AK, Whistler JL. Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron. 2001;32:829–839. doi: 10.1016/s0896-6273(01)00517-7. [DOI] [PubMed] [Google Scholar]
- Frantzides CT, Condon RE, Schulte WJ, Cowles V. Effects of morphine on colonic myoelectric and motor activity in subhuman primates. Am J Physiol. 1990;258:G247–252. doi: 10.1152/ajpgi.1990.258.2.G247. [DOI] [PubMed] [Google Scholar]
- Frantzides CT, Cowles V, Salaymeh B, Tekin E, Condon RE. Morphine effects on human colonic myoelectric activity in the postoperative period. Am J Surg. 1992;163:144–148. doi: 10.1016/0002-9610(92)90267-u. discussion 148-149. [DOI] [PubMed] [Google Scholar]
- Grider JR, Makhlouf GM. Suppression of inhibitory neural input to colonic circular muscle by opioid peptides. J Pharmacol Exp Ther. 1987;243:205–210. [PubMed] [Google Scholar]
- Grider JR, Makhlouf GM. Identification of opioid receptors on gastric muscle cells by selective receptor protection. Am J Physiol. 1991;260:G103–107. doi: 10.1152/ajpgi.1991.260.1.G103. [DOI] [PubMed] [Google Scholar]
- Gutstein HB, Akil H, editors. Opioid Analgesics: Goodman & Gilman's The Pharmacological Basis of Therapeutics. 11th. McGraw-Hill; 2006. [Google Scholar]
- Harris LS, Pierson AK. Some Narcotic Antagonists in the Benzomorphan Series. J Pharmacol Exp Ther. 1964;143:141–148. [PubMed] [Google Scholar]
- Iwata H, Tsuchiya S, Nakamura T, Yano S. Morphine leads to contraction of the ileal circular muscle via inhibition of the nitrergic pathway in mice. Eur J Pharmacol. 2007;574:66–70. doi: 10.1016/j.ejphar.2007.06.029. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Medical safety and side effects of methadone in tolerant individuals. Jama. 1973;223:665–668. [PubMed] [Google Scholar]
- Leedham JA, Doak N, Taylor DA, Fleming WW. The development and reversal of the tolerance to morphine in the longitudinal smooth muscle-myenteric plexus preparation of the guinea pig. Life Sci. 1989;45:1483–1489. doi: 10.1016/0024-3205(89)90039-8. [DOI] [PubMed] [Google Scholar]
- Leedham JA, Kong JQ, Taylor DA, Johnson SM, Fleming WW. Membrane potential in myenteric neurons associated with tolerance and dependence to morphine. J Pharmacol Exp Ther. 1992;263:15–19. [PubMed] [Google Scholar]
- Ling GS, Paul D, Simantov R, Pasternak GW. Differential development of acute tolerance to analgesia, respiratory depression, gastrointestinal transit and hormone release in a morphine infusion model. Life Sci. 1989;45:1627–1636. doi: 10.1016/0024-3205(89)90272-5. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Sarna SK, Condon RE, Cowles VE, Frantzides C. Differential sensitivities of morphine and motilin to initiate migrating motor complex in isolated intestinal segments. Regeneration of intrinsic nerves. Gastroenterology. 1986;90:61–67. doi: 10.1016/0016-5085(86)90075-2. [DOI] [PubMed] [Google Scholar]
- Pasricha PJ. Treatment of disorders of bowel motility and water flux: Goodman & Gilman's The Pharmacological Basis of Therapeutics: 11th edition, 2006. mcGraw-Hill; 2006. [Google Scholar]
- Paton WD. The action of morphine and related substances on contraction and on acetylcholine output of coaxially stimulated guinea-pig ileum. Br J Pharmacol Chemother. 1957;12:119–127. doi: 10.1111/j.1476-5381.1957.tb01373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant OH, Miller GH. Effects of Morphine and some other opium alkaloids on the muscular activity of the alimentary canal 1. Action on the small intestine in unanesthetized dogs and man. Journal of Pharmacology and Experimental Therapeutics. 1926;27:361–383. [Google Scholar]
- Rezvani A, Huidobro-Toro JP, Hu J, Way EL. A rapid and simple method for the quantitative determination of tolerance development to opiates in the guinea-pig ileum in vitro. J Pharmacol Exp Ther. 1983;225:251–255. [PubMed] [Google Scholar]
- Roy S, Liu HC, Loh HH. mu-Opioid receptor-knockout mice: the role of mu-opioid receptor in gastrointestinal transit. Brain Res Mol Brain Res. 1998;56:281–283. doi: 10.1016/s0169-328x(98)00051-5. [DOI] [PubMed] [Google Scholar]
- Schmidt J, Stoffels B, Nazir A, Dehaven-Hudkins DL, Bauer AJ. Alvimopan and COX-2 inhibition reverse opioid and inflammatory components of postoperative ileus. Neurogastroenterol Motil. 2008;20:689–699. doi: 10.1111/j.1365-2982.2007.01078.x. [DOI] [PubMed] [Google Scholar]
- Smith FL, Javed RR, Elzey MJ, Dewey WL. The expression of a high level of morphine antinociceptive tolerance in mice involves both PKC and PKA. Brain Res. 2003;985:78–88. doi: 10.1016/s0006-8993(03)03170-6. [DOI] [PubMed] [Google Scholar]
- Smith FL, Lohmann AB, Dewey WL. Involvement of phospholipid signal transduction pathways in morphine tolerance in mice. Br J Pharmacol. 1999;128:220–226. doi: 10.1038/sj.bjp.0702771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan-No K, Niijima F, Nakagawasai O, Sato T, Satoh S, Tadano T. Development of tolerance to the inhibitory effect of loperamide on gastrointestinal transit in mice. Eur J Pharm Sci. 2003;20:357–363. doi: 10.1016/j.ejps.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Thompson AR, Ray JB. The importance of opioid tolerance: a therapeutic paradox. J Am Coll Surg. 2003;196:321–324. doi: 10.1016/S1072-7515(02)01800-8. [DOI] [PubMed] [Google Scholar]
- Ueda H, Inoue M, Mizuno K. New approaches to study the development of morphine tolerance and dependence. Life Sci. 2003;74:313–320. doi: 10.1016/j.lfs.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Weisbrodt NW, Badial-Aceves F, Dudrick SJ, Burks TF, Castro GA. Tolerance to the effect of morphine on intestinal transit. Proc Soc Exp Biol Med. 1977;154:587–590. doi: 10.3181/00379727-154-39724. [DOI] [PubMed] [Google Scholar]
- Weisbrodt NW, Sussman SS, Stewart JJ, Burks TF. Effect of morphine sulfate on intestinal transit and myoelectric activity of the small intestine of the rat. J Pharmacol Exp Ther. 1980;214:333–338. [PubMed] [Google Scholar]
- Williams CL, Bihm CC, Rosenfeld GC, Burks TF. Morphine tolerance and dependence in the rat intestine in vivo. J Pharmacol Exp Ther. 1997;280:656–663. [PubMed] [Google Scholar]
- Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- Wood JD, Galligan JJ. Function of opioids in the enteric nervous system. Neurogastroenterol Motil. 2004;16 2:17–28. doi: 10.1111/j.1743-3150.2004.00554.x. [DOI] [PubMed] [Google Scholar]
- Yuan CS, Foss JF, O'Connor M, Moss J, Roizen MF. Gut motility and transit changes in patients receiving long-term methadone maintenance. J Clin Pharmacol. 1998;38:931–935. doi: 10.1002/j.1552-4604.1998.tb04389.x. [DOI] [PubMed] [Google Scholar]