Abstract
Purpose
Vasoactive intestinal peptide (VIP) is expressed by corneal endothelial (CE) cells and is present in the aqueous humor, which bathes CE cells in vivo. This study demonstrated the role of CE cell VIP in maintaining the expression level of a CE differentiation marker, N-cadherin, and the hexagonal cell shape.
Methods
To determine the most effective VIP concentration, bovine corneoscleral explants were treated with 0 (control) and 10−12 to 10−6 M VIP. Paired human corneas (nine donors) from an eye bank were used as control; the other corneas were treated with VIP. To silence endogenous VIP, paired fresh human donor corneas (from seven cadavers) were transduced with VIP shRNA or the control lentiviral particles and then bisected/quartered for quantitative analysis by semiquantitative RT-PCR (for mRNA) and Western blot analysis/immunocytochemistry (for protein), whereas alizarin red S staining revealed CE cell shape.
Results
VIP concentration dependently increased bovine CE cell N-cadherin mRNA levels, with the maximal effect observed between 10−10 (1.47 ± 0.06-fold; P = 0.002) and 10−8 M VIP (1.48 ± 0.18-fold; P = 0.012). VIP (10−8 M) treatment increased N-cadherin protein levels in bovine and human CE cells to 1.98 ± 0.28-fold (P = 0.005) and 1.17 ± 0.10 (range, 0.91–187)-fold (P = 0.050) of their respective controls. VIP antagonist (SN)VIPhyb diminished the VIP effect. VIP silencing resulted in deterioration of the hexagonal cell shape and decreased levels of VIP protein and mRNA, N-cadherin (but not connexin-43) mRNA and protein, and the antiapoptotic Bcl-2 protein.
Conclusions
Through its autocrine VIP, CE cells play an active role in maintaining the differentiated state and suppressing apoptosis in the corneal endothelium in situ.
The corneal endothelium, a single-cell layer, functions to maintain the transparency of the cornea by acting as a barrier to the movement of fluid into the cornea and by actively pumping fluid out of the cornea.1 Although their developmental origin is the neural crest,2–5 the corneal endothelial (CE) cells express neuron-specific enolase5,6 and have limited regenerating capacity.7 The mechanisms through which the corneal endothelium maintains its differentiated state throughout life remain unknown. Elucidation of these mechanisms will increase the level of our understanding of the pathogenesis of CE decompensation.
In the corneal endothelium, N-cadherin is a differentiation marker; the expression of N-cadherin coincides with the formation of the CE cell layer during eye development.8,9 The calcium-dependent cell–cell junctional protein N-cadherin mediates cell adhesion through Ca2+-dependent homophilic interaction of the extracellular domain and anchoring its cytoplasmic domain to the actin cytoskeleton.10 As such, N-cadherin plays important roles in shaping cells,11,12 in pattern formation in the developing retina,12 and in the dendritic spine morphogenesis of neurons.13 As an integral component of the cell–cell junctional complex, N-cadherin not only mediates intercellular adhesion strengthening,14,15 it functions as an adhesion-activated receptor capable of initiating distinctive signaling pathways10 leading to cell differentiation and survival. For example, N-cadherin adhesion induces differentiation and lamellipodium extension of myogenic cells,16,17 and stimulation of the N-cadherin signaling pathway increases expression of the antiapoptotic protein Bcl-218 and phosphorylation (inactivation) of the apoptotic protein BAD.19
The mechanisms that regulate N-cadherin gene expression remain largely unknown, though they have been shown to be cAMP dependent.20,21 In bovine and human CE cells, cAMP production is stimulated by vasoactive intestinal peptide (VIP),22 suggesting that VIP may play a role in the modulation of N-cadherin expression in CE cells. VIP is a 28-amino acid neuropeptide that binds to two types of adenylyl cyclase stimulatory heterotrimeric G protein–coupled receptors (VPAC1 and VPAC2) and transduces signal through cAMP-dependent and -independent pathways.23–25 While VIP is widely distributed in the central and peripheral nervous systems, including those in the eye,26,27 and in the immune system,28 we have previously reported that VIP mRNA and protein are also expressed in the CE cells in human and bovine corneas.29 Recently, we have reported that VIP expression in human CE cells in situ is upregulated by the injury factor ciliary neurotrophic factor (CNTF),30 which in turn is an autocrine released by CE cells surviving oxidative stress ex vivo.31 Furthermore, VIP is present and identified as one of the immunosuppressive factors in the aqueous humor,32–34 the fluid that fills the anterior and posterior chambers of the eye and that constantly bathes the CE cells in vivo. Therefore, through modulation of the cAMP level, the endogenous VIP in CE cells may play a role in regulating the N-cadherin level. Thus, in addition to being a trophic factor of CE cells under acute oxidative stress,29 VIP may also play a role as a differentiation-promoting factor by inducing N-cadherin synthesis in CE cells, in agreement with the role of VIP found in neuroendocrine differentiation,35,36 neurite genesis and remodeling,37,38 and melanin genesis in retinal pigment epithelium.39
While it is well known that the corneal endothelium in the adult human is a nonregenerating and differentiated tissue, the distinct possibility exists that the expression of the differentiation marker N-cadherin is maintained at a certain level, perhaps by VIP. In addition to testing the effect of exogenous VIP, the present study investigated effects of silencing endogenous VIP using lentiviral-based short hairpin RNA (shRNA) to knock down VIP mRNA, on the expression of N-cadherin and the normal hexagonal cell shape of CE cells in human corneas ex vivo. The present study is the first in silencing gene expression in CE cells in situ and in silencing VIP gene expression by RNA interference.
Materials and Methods
Media
Medium A
Medium A consisted of Dulbecco modified Eagle medium (DMEM) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin sulfate, and 20 mM HEPES.
Medium B
Medium B consisted of DMEM supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin sulfate, and 0.292 mg/mL l-glutamine. Complete medium B consisted of medium B plus 5% fetal calf serum and fungizone (250 ng/mL amphotericin; Invitrogen; Grand Island, NY).
Bovine Corneoscleral Explants
Bovine eyes obtained from the local abattoir within 6 hours of kill were immersed in a 0.5% iodine solution (1:4 dilution in PBS of povidone iodine preparation solution [Baxter Health Care Co., Deerfield, IL]) for 5 seconds and rinsed in PBS before corneoscleral explants were dissected using a procedure similar to that described previously.40 A scleral incision 1 mm posterior to the limbus was applied, and the explant that was without the iris, ciliary body, or trabecular meshwork was lifted from the eyeball. Explants were rinsed once with 14 mL Dulbecco phosphate-buffered saline (DPBS) in 60 × 15-mm Petri dishes. With its concave side up, the explants were then incubated for 30 minutes at room temperature in 14 mL medium A in 60 × 15-mm Petri dishes and were transferred to 14 mL medium B in 60 × 15-mm Petri dishes and incubated at 37°C in 5% CO2/95% air.
Human Donor Corneas
Viable human corneoscleral explants (human donor corneas) preserved for 5 to 21 days in storage medium (Optisol-GS; Chiron Ophthalmics, Irvine, CA) at 4°C were obtained from the Lions Eye Institute for Transplant & Research, Inc. (Tampa, FL). These corneas were deemed not suitable for transplantation because of the less than optimal CE cell densities and the advanced ages of the donors. Using the same procedure as the eye bank, fresh human donor corneoscleral explants were retrieved from cadavers (within 30 hours after death) in the Anatomy Board of the State of Maryland. The cadavers have been deidentified and were not considered human subjects by the Human Research Protection Office, University of Maryland School of Medicine. Human corneoscleral explants were established in 24-hour organ cultures (with 3.5 mL medium) as the bovine corneoscleral explants.
VIP Treatment of Bovine Corneoscleral Explants and Human Donor Corneas
Bovine Corneoscleral Explants
Twenty-four hours after their establishment in organ cultures, bovine explants were transferred to and incubated in 35-mm culture dishes containing 4 mL fresh medium B plus 5% fetal calf serum and VIP (0, 10−12-10−6 M) for 25 to 48 hours at 37°C in 5% CO2/95% air. To test the effects of blocking VIP binding to its receptor, VIP antagonist (SN)VIPhyb (Bachem, Torrance, CA) was included in the medium during the VIP treatment.
Human Donor Corneas
Paired human donor corneas were removed from the storage medium (Optisol-GS; Bausch & Lomb, Irvine, CA)–containing storage vials and incubated in 2 mL complete medium B and either zero (OD) or 10 −8 M VIP (OS) in 24-well plates for 48 hours at 37°C in 5% CO2/95% air.
Lentiviral Transduction of Paired Fresh Human Donor Corneas
Various lentiviral vectors have been shown, including in human in vitro and in vivo studies,41–44 to efficiently and persistently transduce genes into CE cells of various species. In the present study, conditions of transduction were first characterized closely according to the protocol provided by the manufacturer of the lentiviral particles used (Sigma-Aldrich, St. Louis, MO).
In the initial experiments to determine the efficiency of lentiviral transduction, lentiviral particles that express green fluorescent protein (GFP; Mission TurboGFP control transduction particles SHC003V; Sigma-Aldrich) were added in increasing amounts to 90% confluent SK-N-SH neuroblastoma cells (American Type Culture Collection, Manassas, VA) seeded at 1.6 × 104cells/dish in complete medium B in 35-mm culture dishes. To enhance lentiviral transduction, hexadimethrine bromide was added to the culture medium at a final concentration of 8 μg/mL. After 24 hours of exposure to lentiviral particles, the number of GFP positive SK-N-SH cells was determined. The optimal amount of lentiviral particles (15 μL in 1 mL complete medium B) that resulted in GFP expression in 50% of SK-N-SH cells was determined and used to transduce CE cells in fresh human donor corneas. A representative photomicrograph of a flat-mounted human donor cornea (Supplementary Fig. S1, online at http://www.iovs.org/cgi/content/full/49/8/3491/DC1), showing CE cells transduced with GFP-lentiviral particles (in the presence of 0.5 μg/mL hexadimethrine bromide) for 72 hours, expressed green fluorescent GFP.
The next set of experiments screened five distinctive lentiviral particles produced from five short hairpin RNA (shRNA) lentiviral plasmid vectors. Each could generate intracellularly a distinctive short interfering RNA (siRNA) targeting one of the five human VIP gene sequences (TRCN0000078053-TRCN0000078057; Mission Lentiviral Transduction Particles, SHVRS, Sigma-Aldrich) for the most effective lentiviral particle that caused maximal decrease in VIP mRNA. SK-N-SH cells were exposed to lentiviral particles for 24 hours, as described, the cells were rinsed free of viral particles with DPBS, total RNA was isolated, and semiquantitative RT-PCR for VIP was performed. The lentiviral particle that showed a maximal decrease in VIP mRNA expression was determined to be the shRNA with the following sequence: CCGGCCCGACTTTCCAGAAGAGTTACTCGAGTAACTCTTCTGGAAAGTCGGGTTTTTG (TRCN0000078057), targeting VIP nucleotide sequences 656–676 (NM003381.2) or 653–673 (NM 194435.1).
To study the effect of VIP gene silencing in CE cells, paired fresh human corneas from a 97-year-old female (donor 1), an 84-year-old female (donor 2), a 76-year-old female (donor 3), a 69-year-old female (donor 4), and a 70-year-old female (donor 5) were used in four separate experiments. Within each pair, one of the corneas was transduced with lentiviral particles (TRCN0000078057) determined to be most efficient in silencing VIP gene expression, whereas the paired cornea was transduced with the negative control lentiviral particles produced from the pLKO.1-puro control vector (SHC001V; Sigma-Aldrich). Toward this end, 10-μL stock solutions containing 105 to 106 lentiviral particles in DMEM with 10% heat-inactivated fetal bovine serum and antibiotics was added to the 0.5 mL complete B medium (plus 0.5 g/mL hexadimethrine bromide) incubating the corneas. After 72 hours, corneas of donors 1, 2, and 3 were bisected. Half the cornea was used to isolate CE cell total RNA for semiquantitative RT-PCR, and the other half was further bisected. One quadrant was stained with alizarin red S in calcium and magnesium-free DPBS, followed by washing 2× with DPBS and examination under a microscope, and the other quadrant was fixed with 4% paraformaldehyde in PBS for VIP immunostaining. From each cornea of donors 4 and 5, a quadrant was dissected, stained with alizarin red S, washed 2× with DPBS, and examined under a microscope. From the remaining corneas, CE cells were scraped from the Descemet membrane and extracted for Western blot analysis. A second lentiviral particle that was not as effective as TRCN0000078057 in knocking down VIP mRNA expression in transduced CE cells was used to corroborate the results obtained with TRCN0000078057. This second lentiviral particle (TRCN0000078053) has the shRNA with the sequence CCGGGCTGTGTTAAATAAACCTCAA CTCGAGTTGAGGTTTATTTAACACAGCTTTTTG targeting VIP nucleotide sequences 656–676 (NM003381.2) and/or 1139–1159 (NM 194435.1). Paired corneas from an 88-year-old male (donor 6) and a 38-year-old female (donor 7) were used.
Semiquantitative RT-PCR
Total RNA was isolated from CE cells scraped from bovine and lentiviral particles–stransduced human corneas (RNA-Bee; Tel-Test, Friendswood, TX) and subjected to reverse transcription (RT) using a kit (RETROscript; catalog number 1710; Ambion; Austin, TX). RT products were subjected to PCR using three primer sets corresponding to the gene sequences of (1) human N-cadherin (5′-CACCCAACATGTTTACAATCAACAATGAGAC-3′ [forward] and 5′-CTGCAGCAACAGTAAGGACAAACATCCTATT-3′ [reverse]),45,46 (2) human connexin 43 (5′-CCTTCTTGCTGATCCAGTGGTAC-3′ [forward] and 5′-ACCAAGGACACCACCAGCAT-3′ [reverse]),47 and (3) human VIP (5′-CTTGAGTCTCTTATGGGAAAACGTGT-3′ [forward] and 5′-GTGAAGACTGCATCTGAGTGACG-3′ (reverse).30 For quantifying mRNA levels, semiquantitative PCR was conducted in the presence of the18S primers and the 18S competomers (Universal 18S internal standards; catalog number 1718; Ambion) at a ratio of either 1:9 (N-cadherin and connexin 43) or 0.5:9.5 (VIP). PCR used RT products derived from 0.15 μg bovine RNA (for N-cadherin), 10% of isolated human RNA (for N-cadherin and connexin 43), and 20% of isolated human RNA (for VIP). Between an initial 2-minute (94°C) and a final 5-minute (72°C) treatment, 25 to 38 thermocycles (20 seconds at 94°C, 25 seconds at the annealing temperature, and 40 seconds at 72°C) were conducted. The numbers of cycles and the annealing temperatures (in °C) were 25 and 60 (bovine N-cadherin), 38 and 60 (human N-cadherin), 25 and 54 (human connexin 43), and 35 and 60 (human VIP). RT-PCR products were electrophoresed (2% agarose gel), and the optical densities of bands were measured using a densitometer (NucleoVision; NucleoTech, San Carlos, CA). The identities of specific bands of appropriate sizes revealed after agarose gel electrophoresis were proven by sequencing (Biopolymer Laboratory, University of Maryland) and were demonstrated to be identical with the sequences for bovine N-cadherin (442 bp), human N-cadherin (444 bp), human connexin 43 (154 bp), and human VIP (86 bp).
CE Cell Extract
Bovine CE Cell
At the conclusion of VIP treatment of the corneoscleral explants, corneoscleral explants were removed from the medium, and the corneal endothelium was scraped off the corneas using a razor blade and homogenized in the RIPA buffer (25 mM Tris [pH 7.2], 0.1% SDS, 1% Triton X-100, 1% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA) supplemented with protease inhibitors (one tablet of protease inhibitor cocktail [complete mini; Roche Diagnostics, Mannheim, Germany]; 10 mL). After centrifugation at 12,000g for 10 minutes, the supernatant (cell extract) was analyzed by Western blot analysis.
Human Corneas Previously Stored in Medium
The endothelium and Descemet membrane were hydrodissected from the posterior stroma of human corneoscleral explants using the same method previously described (Rutzen AR, et al. IOVS 2000;41:ARVO Abstract 2376). An injection of DPBS at the posterior limbus was used to create a fluid separation between the endothelium/Descemet membrane and posterior stroma. A small incision was created at the peripheral posterior limbus to release the DPBS from beneath Descemet membrane. A 7.5-mm trephine was used to excise the specimen of endothelium/Descemet membrane, which was then treated as described for the bovine corneal endothelium.
Human Corneas from Cadavers
The corneal endothelium was scraped from each cornea using a razor blade and homogenized in the RIPA buffer, as described.
Western Blot Analysis
Samples of CE cell extracts were electrophoresed under reducing conditions using preformed Tris/Glycine polyacryl amide gradient gels (Nu-Page; Novex, San Diego, CA) and electrophoretically transferred to nitrocellulose membranes for Western blot analysis using the following primary antibodies: affinity-purified polyclonal antibodies against N-cadherin (BTA7 [R&D Systems, Minneapolis, MN] 205606 [Calbiochem, San Diego, CA]), anti–VIP rabbit serum (T-4246 [Peninsula Laboratories, San Carlos, CA]), and mouse monoclonal antibody against Bcl-2 (05–729 [Upstate Biotechnology, Lake Placid, NY]). Except for anti-VIP, immunoreactive molecules were detected using a kit containing the horseradish peroxidase-linked anti–mouse and anti–rabbit IgG secondary antibodies and horseradish peroxidase substrate (ECL kit; Amersham Pharmacia, Piscataway, NJ). For VIP, a secondary antibody-biotinylated goat anti–rabbit IgG in conjunction with extravidin-alkaline phosphatase conjugate (E-2636; Sigma) and a kit (Fast Red TR/Naphthol AS-MX tablet set; Sigma) were used to develop the red reaction product. To reprobe the blots with a monoclonal actin antibody (Ab-1 kit; Oncogene, Cambridge, MA), the blots were stripped with a buffer (Restore Western Blot Stripping buffer; Pierce, Rockford, IL) according to the instructions of the manufacturer. Densities of immunoreactive bands were determined same as in semiquantitative RT-PCR.
VIP Immunostaining
After lentiviral transduction quadrants of human donor corneas were fixed in 4% paraformaldehyde in PBS, embedded in paraffin, and sectioned (6 μm) for immunostaining using an anti–VIP rabbit serum (T-4246, 1:1000 in PBS; Peninsula Laboratories, San Carlos, CA) and a secondary antibody biotinylated goat anti-rabbit IgG. Extravidin-alkaline phosphatase conjugate (E-2636; Sigma) and a kit (Fast Red TR/Naphthol AS-MX tablet set; Sigma) were used to develop the red reaction product in positively stained cells. Rabbit IgG (10 μg/mL) was used as a negative control.
Results
VIP-Concentration Dependency of N-Cadherin mRNA Levels in CE Cells in VIP-Treated Corneoscleral Explants
To determine the maximally effective VIP concentration in increasing the N-cadherin level, bovine corneoscleral explants were used. VIP (0, 10−12-10−6 M) treatment for 25 hours resulted in increased N-cadherin mRNA levels in CE cells in a VIP concentration-dependent manner (Fig. 1). Semiquantitative RT-PCR of N-cadherin and 18S (internal standard) mRNAs showed that the N-cadherin levels in CE cells in 10−12, 10−10, 10−8, and 10−6 M VIP-treated corneoscleral explants increased (in mean ± SEM) to 1.23 0 ± 07-fold (P < 0.01), 1.47 ± 0.14-fold (P < 0.002), 1.48 ± 0.24-fold (P < 0.02), and 1.41 ± 0.14-fold (P < 0.01) of the control corneoscleral explants (significance levels of VIP-treated versus control are shown in parentheses). The difference among various groups was significant at P < 0.05 (ANOVA) (Fig. 1B).
Figure 1.
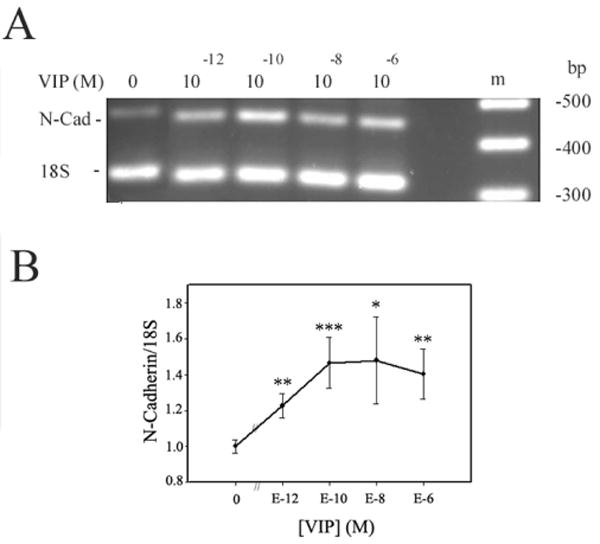
CE cell N-cadherin mRNA level increased in a VIP-concentration dependent manner. (A) N-cadherin and 18S mRNA levels reflected in the electrophoresed (2% agarose) semiquantitative RT-PCR products. (B) The ratio of normalized CE cell N-cadherin cDNA (against the 18S internal standard) levels over that averaged from control corneoscleral explants of the same experiment (y-axis) was shown as a function of VIP concentration. Data were combined from three separate experiments. The difference was significant (P < 0.05, ANOVA) among various groups: corneoscleral explants treated with 0 (N = 6), 10−12 (N = 3), 10−10 (N = 3), 10−8 (N = 3), and 10−6 (N = 5) M VIP. The difference of the control versus VIP-treated corneoscleral explants was significant at the levels of *P < 0.02, **P < 0.01, and ***P < 0.002.
Increased N-Cadherin Protein Level in CE Cells in VIP-Treated Bovine Corneoscleral Explants and Human Donor Corneas
To show the effect of VIP on the protein level of CE cell N-cadherin, bovine corneoscleral explants and paired human donor corneas were treated with VIP at the concentration that produced the maximal effect on increasing the N-cadherin mRNA level (Fig. 1).
After 48-hour treatment of bovine corneoscleral explants with 10−8 M VIP, CE cells were analyzed by Western blot analysis for N-cadherin and actin (as internal standard). As shown in Figure 2A, the N-cadherin level in CE cells from VIP-treated corneoscleral explants was higher than that in the control. Figure 2B showed that VIP treatment increased N-cadherin levels to 1.98 ± 0.28-fold (mean ± SEM; N = 8) of that found in the control corneoscleral explants (N = 7; P = 0.005).
Figure 2.
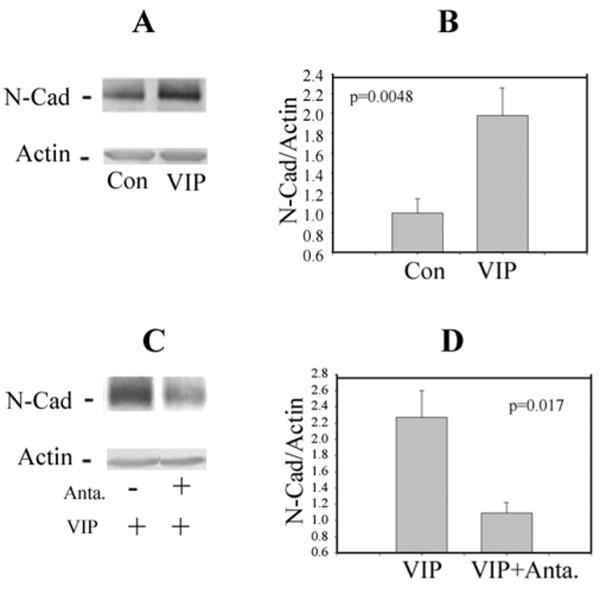
VIP (10−8 M) treatment of bovine corneoscleral explants increased the protein level of N-cadherin in CE cells (A, B), and VIP antagonist (SN)VIPhyb (5 × 10−7M) diminished the VIP effect (C, D). (A) Western blot depicting N-cadherin and actin-immunoreactive bands. Each lane contained 32 μg protein. (B) The ratio of normalized N-cadherin level (against the actin internal standard) over the average of those found in the control corneoscleral explants of the same experiment. Data were combined from five separate experiments showing VIP treatment of corneoscleral explants (N = 8) increased the CE cell N-cadherin level to 1.98 ± 0.28- fold of the control (N = 7) (P = 0.0048). (C) Western blot showing the effect of VIP antagonist on diminishing the CE cell N-cadherin level in VIP-treated corneoscleral explants. (D) Normalized N-cadherin levels (against the actin internal standard) in VIP-treated corneoscleral explants in the absence and presence of the VIP antagonist averaged 2.27 ± 0.33 (N = 4) and 1.09 ± 0.13 (N = 3), respectively (P = 0.017). The data were representative of three separate experiments with similar results.
The effect of VIP (1 × 10−8 M VIP) was diminished by the presence of the VIP antagonist (SN)VIPhyb (5 × 10−7 M) during the VIP treatment of the bovine corneoscleral explants (Figs. 2C, 2D). The level of connexin 43, a protein localized to the gap junction, was not affected by the VIP treatment (data not shown).
Paired human corneas from nine donors were used as control versus VIP (10−8 M)-treated. Western blot analysis of CE cells showed that VIP was effective in increasing the N-cadherin level (Fig. 3A) in six of nine human donor cornea pairs. In all nine pairs, VIP-treated corneas demonstrated N-cadherin levels that were 0.91- to 1.87-fold of their respective controls (1.173 ± 0.099; P = 0.050; Fig. 3B). There was no correlation between VIP responsiveness and either donor age or cause of death (data not shown).
Figure 3.
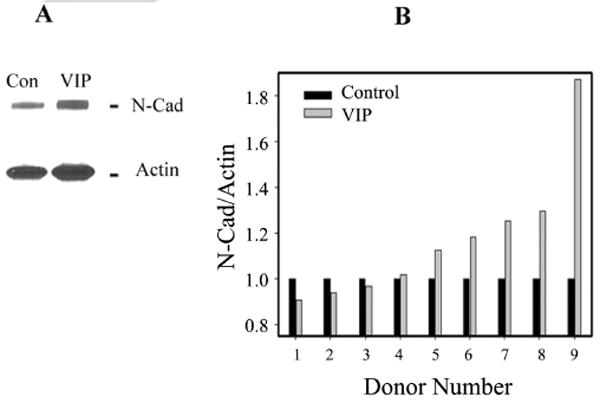
VIP-increased N-cadherin levels demonstrated in paired donor human corneas, as control versus VIP (10−8 M) treated. (A) Western blot analysis of N-cadherin and actin (as internal standard) in CE cells of control (Con) and VIP-treated (VIP) paired corneas. Lanes contained extract of CE cells in sheets of corneal endothelium (7.5 mm in diameter) trephined from the centers of the corneas. (B) Normalized N-cadherin level (against the actin internal control) of nine pairs of human corneas, ranked by the effectiveness of VIP, from the lowest (donor 1) to the highest (donor 9). VIP treatment increased N-cadherin level to (mean ± SEM) 1.173 ± 0.10-fold of the control (P = 0.050). Ages of donors 1 to 9 were, respectively, 75, 58, 72, 64, 66, 73, 61, 71, and 67 years.
Knockdown of Endogenous VIP Expression in Human CE Cells by Lentiviral Based shRNA Leading to Deterioration of the Hexagonal Cell Shape and Decreased N-Cadherin Level
Given that VIP protein and mRNA are expressed by CE cells,29 knocking down the endogenous VIP expression would lead to disturbances of the VIP signaling pathways and would help identify the normal functions of endogenous VIP. Toward this end, three pairs of fresh human donor corneas were used. In each pair, one cornea was transduced with shRNA lentiviral particles targeted at knocking down VIP gene expression, whereas the paired cornea was transduced with the negative control lentiviral particles. VIP gene knockdown dramatically affect the morphology of the CE mosaic (Figs. 4A, 4B). Alizarin red S staining of the CE cell–cell borders in the corneal quadrant from all three pairs of corneas (see Materials and Methods) demonstrated that VIP gene knockdown resulted in deterioration of the hexagonal cell shape and appearance of irregularly shaped CE cells. Each individual CE cell in VIP knockdown corneas covered a larger area than that in the control corneas (Figs. 4A, 4B). Immunocytochemical studies of the VIP protein expression (Figs. 4C, 4D), semiquantitative RT-PCR of VIP mRNA (Figs. 4E, 4F), and Western blot analysis (Figs. 4G, 4H) confirmed the effectiveness of the VIP shRNA lentiviral particles in knocking down endogenous VIP expression. The normalized VIP (against 18S) mRNA level in the control CE cells of two human corneas (donors 1 and 2) averaged 0.49, whereas that in the VIP knockdown CE cells in the paired human corneas was 0.28, a 43% reduction. Previously, a specific VIP-immunoreactive molecule with the same molecular size as the prepro-VIP (20 kDa) has been demonstrated in fresh human CE cells.29 In two pairs of human corneas (donors 4 and 5), VIP gene knockdown in CE cells reduced the level of the 20-kDa VIP-immunoreactive molecule (Fig. 4G) to 18% of that found in control corneas (Fig. 4H).
Figure 4.
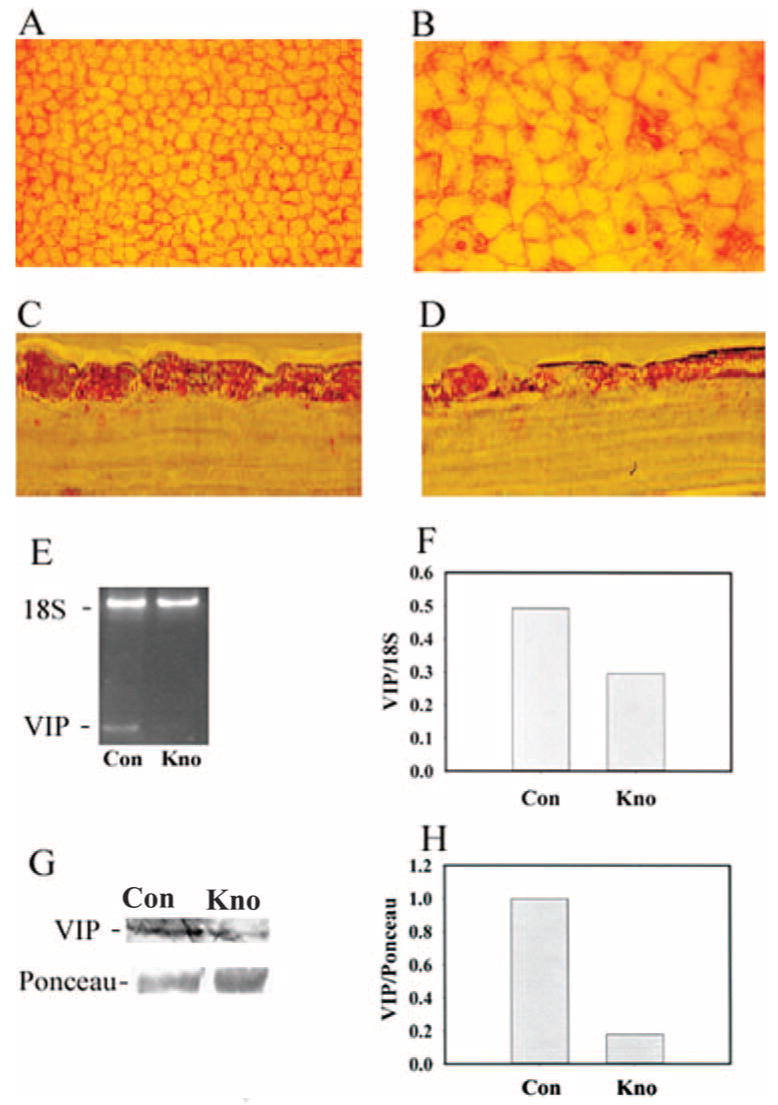
Lentiviral-mediated VIP shRNA in human CE cells altered cell shape and decreased VIP expression. Five pairs of human donor corneas were used. Within each pair, one cornea was transduced with control lentiviral particles (Con) and the other cornea was transduced with those with the VIP mRNA-interfering nucleotide sequence (Kno). Alizarin red S staining of the cell- cell junctions revealed in flat-mounted corneas the normal hexagonal CE cell shape in the control (A) and deterioration of the hexagonal cell shape after VIP knockdown (B). Downregulation of CE cell VIP expression was demonstrated by VIP immunostaining of 6-μm corneal sections (C, D), semiquantitative RT-PCR followed by agarose gel electrophoresis of RT-PCR products (E), densitometry of the VIP and 18S (internal standard) bands in the gel (F), and Western blot analysis (G) followed by densitometry (H) of the VIP-immunoreactive molecule and the Ponceau S-stained 65-kDa molecule, which was the most abundant protein in CE cell extract. Identical results were obtained from all five pairs (A, B) and both donor pairs (donor 1 and donor 2) (C-F) examined. (F) Data were averaged from the two pairs; the third pair did not yield a sufficient number of CE cells for RT-PCR. Two additional pairs of corneas (from donors 4 and 5) were used in Western blot analysis for the CE cell VIP immunoreactive molecule (G, H). Original magnifications: ×200 (A, B); ×1000 (C, D).
Deterioration of the hexagonal CE cell shape was accompanied by a decrease in N-cadherin expression in VIP knockdown CE cells. As shown in Figure 5A, semiquantitative RT-PCR of N-cadherin mRNA demonstrated that VIP knockdown in CE cells of human corneas decreased the N-cadherin mRNA level. The normalized N-cadherin (against 18S) mRNA levels in the control CE cells of two human corneas (from two donors) and those in the VIP knockdown CE cells of the paired corneas averaged 10.2 and 2.3, respectively (Fig. 5B). In the meantime, VIP gene expression knockdown did not affect the expression of connexin 43, a gap junctional protein (Figs. 5C, 5D).
Figure 5.
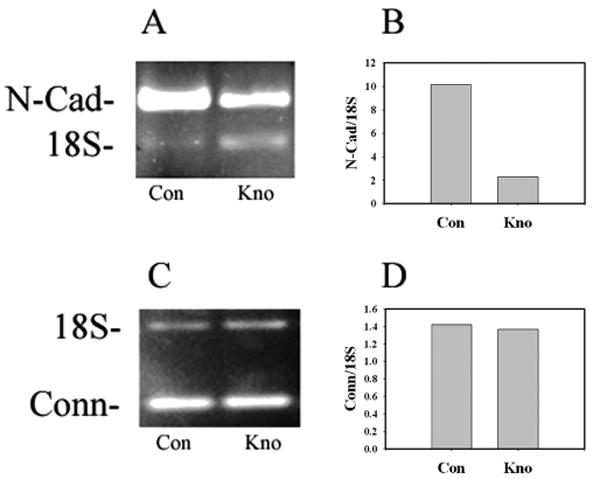
Semiquantitative RT-PCR demonstrating VIP knockdown in CE cells in fresh human donor corneas resulted in decreased N-cadherin (A, B) but showed no effect on connexin 43 (C, D) mRNA levels. CE cells in paired human donor corneas were transduced with control lentiviral particles (Con) or those with VIP mRNA-interfering nucleotide sequences (Kno). (A, C) Agarose gel electrophoresis of RT-PCR products. (B, D) Densitometry of the N-cadherin, connexin, and 18S (internal standard) bands in the gels obtained using two pairs of human donor corneas, similar to those described in Figures 4C–4F.
To show the effect of VIP gene knockdown on the protein level of CE cell N-cadherin, Western blot analysis was performed using four pairs of human corneas. Figure 6A demonstrates results from two pairs of corneas (donors 4 and 5). In VIP gene knockdown CE cells transduced by TRCN0000078057 particles, the N-cadherin protein level was drastically reduced; the protein levels of actin and Bcl-2 were also reduced, but to lesser extents. In VIP gene knockdown CE cells, the protein levels of N-cadherin, actin, and Bcl-2 were 1% (Fig. 6B), 31% (Fig. 6C), and 54% (Fig. 6D) of their respective controls. Results were corroborated with those from two additional pairs of corneas (donors 6 and 7), in which CE cells were transduced with the second lentiviral particle (TRCN0000078053), which was not as effective as the TRCN0000078057 particle in knocking down VIP gene expression (see Materials and Methods), resulting in decreases in the protein levels of N-cadherin, actin, and Bcl-2 to 11% (Fig. 6B), 67% (Fig. 6C), and 90% (Fig. 6D) of their respective controls.
Figure 6.
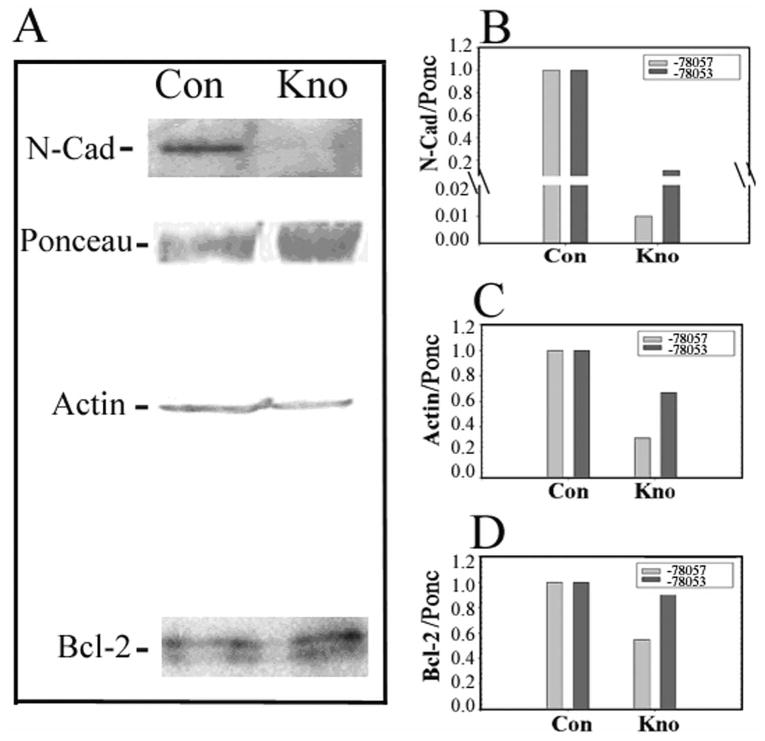
Western blot analysis demonstrating VIP knockdown in CE cells in fresh human donor corneas resulted in decreased protein levels of N-cadherin, actin, and Bcl-2. (A) Western blot analysis of CE cell extracts obtained from paired corneas transduced with control lentiviral particles (Con) or those with VIP mRNA-interfering nucleotide sequences (TRCN0000078057; Kno). Results were obtained from two pairs of corneas from donors 4 and 5 and were corroborated by those obtained from two additional pairs of corneas from donors 6 and 7 using TRCN0000078053 lentiviral particles to knock down VIP gene expression. (B) Densitometry of the N-cadherin, actin, Bcl-2 immunoreactive bands, and a Ponceau S–stained 65-kDa molecule, which was the most abundant protein in CE cell extract and was used as the internal standard to normalize the intensities of the N-cadherin, actin, and Bcl-2 bands in the blots.
Discussion
The present study demonstrated that the CE cell autocrine VIP increased N-cadherin expression and maintained the normal hexagonal cell shape in the corneal endothelium, whereas our previous reports demonstrate that VIP protects the corneal endothelium against the acute killing effect of oxidative stress,29 that endogenous VIP is upregulated by CNTF,30 and that CNTF in turn is released by CE cells surviving oxidative stress.31 We therefore hypothesize that the CE cell autocrine factors CNTF and VIP work in concert to maintain the differentiated state and to promote the survival of the corneal endothelium.
Although a few growth factors such as the hepatocyte growth factor and epidermal growth factor have been shown to upregulate N-cadherin expression,10 how N-cadherin gene expression is regulated is unknown. The proximal promoter sequence of the N-cadherin gene has been characterized.48 It has high GC content and contains an Sp1/Sp3-binding site, which functions as the basal regulatory element of the promoter. Whereas Sp1 binding to the promoter is increased by cAMP,49 activated cAMP signaling pathways increase the activity of the Sp1-binding site-containing promoter of various genes.50,51 In particular, the Sp1-binding site is required for cAMP-dependent, follicle-stimulating hormone (FSH)-stimulated insulin-like growth factor binding protein gene expression.51 Although FSH-stimulated N-cadherin gene expression in Sertoli cells is mediated by cAMP,20 the role of the Sp1-binding site in cAMP-stimulated N-cadherin gene expression has not been demonstrated. We have previously demonstrated VIP-stimulated cAMP production in CE cells in human and bovine corneas.22 The observation in the present study—supported by our preliminary study showing that actinomycin dose dependently diminished the effect of VIP on increasing N-cadherin level—of VIP-increased levels of N-cadherin was likely the result of VIP upregulation of N-cadherin gene expression.
Various lentiviral vectors efficiently and persistently transduce genes into CE cells of several species, including humans, as observed in in vitro and in vivo studies, resulting in no detrimental effect on the corneal endothelium morphology.41–44 What is the sequence of events leading to the deterioration of the normal hexagonal geometry of the apical surface of the CE cells after VIP gene knockdown (Figs. 4A, 4B)? VIP knockdown decreased only N-cadherin, but not connexin 43, expression (Fig. 5), and exogenous VIP increased N-cadherin level in CE cells (Figs. 1–3) but had no effect on that of connexin 43 (data not shown). Furthermore, loss of N-cadherin, but not of E-cadherin, alters cone cell shape in Drosophila eyes.11 We tentatively concluded that, in the VIP knockown CE cells, decreased VIP expression (Figs. 4C–H) led to decreased levels of N-cadherin (Figs. 5A, 5B, 6A, 6B), cell–cell adhesion, actin (Figs. 6A, 6C), and stability of the actin cytoskeleton to which the cytoplasmic domain of N-cadherin normally anchors,10 and to the deterioration of the normal hexagonal cell shape. Future studies will investigate the possibility that exogenous VIP may rescue the VIP knockdown CE cell and restore its hexagonal shape. Future studies of N-cadherin gene knockdown will ascertain the cause-and-effect relationship between the loss of N-cadherin expression and hexagonal cell shape.
After VIP knockdown, the area covered by individual CE cells increased (Figs. 4A, 4B), indicative of cell spreading, which is a well-recognized repair mechanism in this nonregenerating tissue. Consistent with this finding was that more denuded areas devoid of CE cells were present in the VIP knockdown corneas (Figs. 4A, 4B). Given that VIP is a trophic factor capable of protecting CE cells against oxidative stress,29 reduced endogenous VIP levels might have increased the vulnerability of CE cells to stress induced by lentiviral transduction.52 In addition, the signaling pathway that is initiated by N-cadherin in the junctional complex and that leads to increased levels of antiapoptotic protein Bcl-218 and phosphorylation (inactivation) of the proapoptotic protein BAD19 might have been affected as a result of VIP gene knockdown.
Indeed, decreased levels of the antiapoptotic protein Bcl-2 (Figs. 6A, 6D) were observed in CE cells after the VIP gene was knocked down by either the most effective lentiviral particles (TRCN0000078057) or the less effective (see Materials and Methods) ones (TRCN0000078053). Although the extent of Bcl-2 reduction correlated well with that of N-cadherin such that the more N-cadherin was downregulated the more Bcl-2 level was reduced (Figs. 6A, 6B, 6D), N-cadherin expression was more sensitive to VIP gene knockdown than Bcl-2, suggesting the involvement of other signaling pathways in the regulation of Bcl-2 expression. In the present study, phosphorylated BAD was not detected in the Western blot analysis of CE cells either in the control corneas or in those with VIP gene knockdown. It remains to be studied whether the level of BAD was higher in VIP knockdown CE cells; these results nonetheless predicted increased apoptotic CE cell death after VIP gene knockdown. Furthermore, because N-cadherin mediates intercellular adhesion strengthening,14,15 CE cells with decreased N-cadherin levels resulting from VIP knockdown may detach from the corneal endothelium sheet.
In conclusion, the present study demonstrated for the first time a definite role of an autocrine in maintaining the differentiated state of the corneal endothelium and the feasibility of using RNA interference for studying the functions of specific genes in corneal endothelium in situ.
Acknowledgments
The authors thank the Lions Eye Institute for Transplant & Research, Inc. (Tampa, FL) and the Anatomy Board of the State of Maryland (Baltimore, MD) for the human donor corneas, and Dante Gloria and Jason M. Cheng for excellent technical support.
Supported in part by National Institutes of Health Grant RO1EY-11607 (S-WMK) and by Research to Prevent Blindness, Inc.
Footnotes
Disclosure: S.-W.M. Koh, None; K. Chandrasekara, None; C.J. Abbondandolo, None; T.J. Coll, None; A.R. Rutzen, None
References
- 1.Waring GO, Bourne WM, Edelhauser HF, Kenyon KR. The corneal endothelium: normal and pathologic structure and function. Ophthalmology. 1982;89:531–590. [PubMed] [Google Scholar]
- 2.Johnston MC, Noden DM, Hazelton RD, Coulombre JL, Coulombre AJ. Origins of avian ocular and periocular tissues. Exp Eye Res. 1979;29:27–43. doi: 10.1016/0014-4835(79)90164-7. [DOI] [PubMed] [Google Scholar]
- 3.LeLievre CS, LeDouarin NM. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryo Exp Morphol. 1975;34:125–154. [PubMed] [Google Scholar]
- 4.Noden DM. The control of avian cephalic neural crest cytodifferentiation, I: skeletal and connective tissues. Dev Biol. 1978;67:296–312. doi: 10.1016/0012-1606(78)90201-4. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi K, Sueishi K, Tanaka K, et al. Immunohistochemical evidence of the origin of human corneal endothelial cells and keratocytes. Graefes Arch Clin Exp Ophthalmol. 1986;224:452–456. doi: 10.1007/BF02173362. [DOI] [PubMed] [Google Scholar]
- 6.Adamis AP, Molnar ML, Tripathi BJ, Emmerson MS, Stefansson K, Tripathi RC. Neuron-specific enolase in human corneal endothelial cells and posterior keratocytes. Exp Eye Res. 1985;41:665–668. doi: 10.1016/0014-4835(85)90039-9. [DOI] [PubMed] [Google Scholar]
- 7.Joyce NC. Proliferative capacity of the corneal endothelium. Pro Retin Eye Res. 2003;22:359–389. doi: 10.1016/s1350-9462(02)00065-4. [DOI] [PubMed] [Google Scholar]
- 8.Beebe DC, Coats JM. The lens organizes the anterior segment: specification of neural crest cell differentiation in the avian eye. Dev Biol. 2000;220:424–431. doi: 10.1006/dbio.2000.9638. [DOI] [PubMed] [Google Scholar]
- 9.Reneker LW, Silversides DW, Xu L, Overbeek PA. Formation of corneal endothelium is essential for anterior segment development- a transgenic mouse model of anterior segment dysgenesis. Development. 2000;127:533–542. doi: 10.1242/dev.127.3.533. [DOI] [PubMed] [Google Scholar]
- 10.Derycke LDM, Bracke ME. N-cadherin in the spotlight of cell-cell adhesion, differentiation, embryogenesis, invasion and signaling. Int J Dev Biol. 2004;48:463–476. doi: 10.1387/ijdb.041793ld. [DOI] [PubMed] [Google Scholar]
- 11.Carthew RW. Adhesion proteins and the control of cell shape. Curr Opin Genet Dev. 2005;15:358–363. doi: 10.1016/j.gde.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi T, Carthew RW. Surface mechanics mediate pattern formation in the developing retina. Nature. 2004;431:647–652. doi: 10.1038/nature02952. [DOI] [PubMed] [Google Scholar]
- 13.Togashi H, Abe K, Mizoguchi A, Takaoka K, Chisaka O, Takeichi M. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- 14.El Sayegh TY, Arora PD, Laschinger CA, et al. Cortactin associates with N-cadherin adhesions and mediates intercellular adhesion strengthening in fibroblasts. J Cell Sci. 2004;117:5117–5131. doi: 10.1242/jcs.01385. [DOI] [PubMed] [Google Scholar]
- 15.Chan MWC, El Sayegh TY, Arora PD, et al. Regulation of intercellular adhesion strength in fibroblasts. J Biol Chem. 2004;279:41047–41057. doi: 10.1074/jbc.M406631200. [DOI] [PubMed] [Google Scholar]
- 16.Gavard J, Marthiens V, Monnet C, Lambert M, Mege RM. N-cadherin activation substitutes for the cell contact control in cell cycle arrest and myogenic differentiation: involvement of p120 and β-catenin. J Cell Sci. 2004;117:257–270. doi: 10.1074/jbc.M401705200. [DOI] [PubMed] [Google Scholar]
- 17.Gavard J, Lambert M, Grosheva I, et al. Lamellipodium extension and cadherin adhesion: two cell responses to cadherin activation relying on distinct signalling pathways. J Biol Chem. 2004;279:36795–36802. doi: 10.1242/jcs.00857. [DOI] [PubMed] [Google Scholar]
- 18.Tran NL, Adams DG, Vaillancourt RR, Heimark RL. Signal transduction from N-cadherin increases Bcl-2: regulation of the phosphatidylinositol 3-kinase/Akt pathway by homophilic adhesion and actin cytoskeletal organization. J Biol Chem. 2002;277:32905–32914. doi: 10.1074/jbc.M200300200. [DOI] [PubMed] [Google Scholar]
- 19.Koutsouki E, Beeching CA, Slater SC, Blaschuk OW, Sala-Newby GB, George SJ. N-cadherin dependent cell-cell contacts promote human saphenous vein smooth muscle cell survival. Arterioscler Thromb Vasc Biol. 2005;25:982–988. doi: 10.1161/01.ATV.0000163183.27658.4b. [DOI] [PubMed] [Google Scholar]
- 20.MacCalman CD, Getsios S, Farookhi R, Blaschuk OW. Estrogen potentiates the stimulatory effects of follicle-stimulating hormone on N-cadherin messenger ribonucleic acid levels in cultured mouse Sertoli cells. Endocrinology. 1997;138:41–48. doi: 10.1210/endo.138.1.4831. [DOI] [PubMed] [Google Scholar]
- 21.Bozdagi O, Shan W, Tanaka H, Benson DL, Huntley GW. Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites, and required for potentiation. Neuron. 2000;1:245–259. doi: 10.1016/s0896-6273(00)00100-8. [DOI] [PubMed] [Google Scholar]
- 22.Koh SWM, Yue BYJT. VIP stimulation of cAMP production in cultures of rabbit corneal endothelial cells. Cornea. 2002;21:270–274. doi: 10.1097/00003226-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Ishihara TR, Shigemoto K, Mori K, Takahashi, Nagata S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron. 1992;8:811–819. doi: 10.1016/0896-6273(92)90101-i. [DOI] [PubMed] [Google Scholar]
- 24.Lutz EM, Sheward WJ, West KM, Morrow JA, Frank G, Harmar AJ. The VIP2 receptors: molecular characterization of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett. 1993;334:3–8. doi: 10.1016/0014-5793(93)81668-p. [DOI] [PubMed] [Google Scholar]
- 25.Langer I, Robberecht P. Mutations in the carboxy-terminus of the third intracellular loop of the human recombinant VPAC1 receptor impair VIP-stimulated [Ca2+]i increase but not adenylate cyclase stimulation. Cell Signal. 2005;17:17–24. doi: 10.1016/j.cellsig.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Brecha NC, Eldred W, Kuljis RO, Karten HJ. Identification and localization of biologically active peptide in the vertebrate retina. In: Chader G, Osborne N, editors. Progress in Retinal Research. 1984. pp. 185–226. [Google Scholar]
- 27.Stone RA. Vasoactive intestinal polypeptide and ocular innervation. Invest Ophthalmol Vis Sci. 1984;27:951–957. [PubMed] [Google Scholar]
- 28.Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev. 2004;56:249–290. doi: 10.1124/pr.56.2.7. [DOI] [PubMed] [Google Scholar]
- 29.Koh SWM, Waschek JA. Corneal endothelial cell survival in organ cultures under acute oxidative stress: effect of VIP. Invest Ophthalmol Vis Sci. 2000;41:4085–4092. [PubMed] [Google Scholar]
- 30.Koh SW, Guo Y, Bernstein SL, Waschek JA, Liu X, Symes AJ. Vasoactive intestinal peptide induction by ciliary neurotrophic factor in donor human corneal endothelium in situ. Neurosci Lett. 2007;16:89–94. doi: 10.1016/j.neulet.2007.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koh SW. Ciliary neurotrophic factor released by corneal endothelium surviving oxidative stress ex vivo. Invest Ophthalmol Vis Sci. 2002;43:2887–2896. [PubMed] [Google Scholar]
- 32.Taylor AW, Streilein JW, Cousins SW. Immunoreactive vasoactive intestinal peptide contributes to the immunosuppressive activity of normal aqueous humor. J Immunol. 1994;153:1080–1086. [PubMed] [Google Scholar]
- 33.Troger J, Kieselbach G, Gottinger W, Metzler R, Kahler C, Saria A. Different concentrations of vasoactive intestinal peptide in aqueous humor of patients with proliferative vitreoretinopathy and cataract patients. Ger J Ophthalmol. 1994;3:245–247. [PubMed] [Google Scholar]
- 34.Koh SWM, Rutzen AR, Coll TJ, Hemady RK, Higginbotham EJ. VIP-immunoreactivity in human aqueous humor. Curr Eye Res. 2005;30:189–194. doi: 10.1080/02713680490908715. [DOI] [PubMed] [Google Scholar]
- 35.Collado B, Gutierrez-Canas I, Rodriguez-Henche N, Prieto JC, Carmena MJ. Vasoactive intestinal peptide increases vascular endothelial growth factor expression and neuroendocrine differentiation in human prostate cancer LNCaP cells. Regul Pept. 2004;119:69–75. doi: 10.1016/j.regpep.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 36.Gutierrez-Canas I, Juarranz MG, Collado B, et al. Vasoactive intestinal peptide induces neuroendocrine differentiation in the LNCaP prostate cancer cell line through PKA, ERK, and PI3K. Prostate. 2005;63:44–55. doi: 10.1002/pros.20173. [DOI] [PubMed] [Google Scholar]
- 37.Heraud C, Hilairet S, Muller JM, Leterrier JF, Chadeneau C. Neuritogenesis induced by vasoactive intestinal peptide, pituitary adenylate cyclase-activating polypeptide, and peptide histidine methionine in SH-SY5y cells is associated with regulated expression of cytoskeleton mRNAs and proteins. J Neurosci Res. 2004;75:320–329. doi: 10.1002/jnr.10866. [DOI] [PubMed] [Google Scholar]
- 38.Alleaume C, Eychene A, Harnois T, et al. Vasoactive intestinal peptide-induced neurite remodeling in human neuroblastoma SH-SY5Y cells implicates the Cdc42 GTPase and is independent of Ras-ERK pathway. Exp Cell Res. 2004;299:511–524. doi: 10.1016/j.yexcr.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 39.Koh SWM, Kane GJ. VIP stimulates proliferation and differentiation of the cultured retinal pigment epithelium with disparate potencies. Cell Biol Int Rep. 1992;16:175–183. doi: 10.1016/s0309-1651(06)80111-6. [DOI] [PubMed] [Google Scholar]
- 40.Koh SWM, Yeh TH, Morris SM, et al. Vasoactive intestinal peptide stimulation of human trabecular meshwork cell growth. Invest Ophthalmol Vis Sci. 1997;38:2781–2789. [PubMed] [Google Scholar]
- 41.Wang X, Appukuttan B, Ott S, et al. Efficient and sustained transgene expression in human corneal cells mediated by a lentiviral vector. Gene Ther. 2000;7:196–200. doi: 10.1038/sj.gt.3301075. [DOI] [PubMed] [Google Scholar]
- 42.Bainbridge JW, Stephens C, Parsley K, et al. In vivo gene transfer to the mouse eye using an HIV-based lentiviral vector: efficient long-term transduction of corneal endothelium and retinal pigment epithelium. Gene Ther. 2001;8:1665–1668. doi: 10.1038/sj.gt.3301574. [DOI] [PubMed] [Google Scholar]
- 43.Beutelspacher SC, Ardjomand N, Tan PH, et al. Comparison of HIV-1 and EIAV-based lentiviral vectors in corneal transduction. Exp Eye Res. 2005;80:787–794. doi: 10.1016/j.exer.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Parker DG, Kaufmann C, Brereton HM, et al. Lentivirus-mediated gene transfer to the rat, ovine and human cornea. Gene Ther. 2007;14:760–767. doi: 10.1038/sj.gt.3302921. [DOI] [PubMed] [Google Scholar]
- 45.Chen Q, Chen TJ, Letourneau PC, Costa Lda F, Schubert D. Modifier of cell adhesion regulates N-cadherin-mediated cell-cell adhesion and neurite outgrowth. J Neurosci. 2005;25:281–290. doi: 10.1523/JNEUROSCI.3692-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee HS, Tomarev SI. Optimedin induces expression of N-cadherin and stimulates aggregation of NGF-stimulated PC12 cells. Exp Cell Res. 2007;313:98–108. doi: 10.1016/j.yexcr.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li DQ. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355–366. doi: 10.1634/stemcells.22-3-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le Mee S, Fromigue O, Marie PJ. Sp1/Sp3 and the myeloid zinc finger gene MZF1 regulate the human N-cadherin promoter in osteoblasts. Exp Cell Res. 2005;302:129–142. doi: 10.1016/j.yexcr.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Wang S, Wesley RA, Danner RL. Adjacent sequence controls the response polarity of nitric oxide-sensitive Sp factor binding sites. J Biol Chem. 2003;278:29192–291200. doi: 10.1074/jbc.M213043200. [DOI] [PubMed] [Google Scholar]
- 50.Zheng XL, Matsubara S, Diao C, Hollenberg MD, Wong NC. Activation of apolipoprotein AI gene expression by protein kinase A and kinase C through transcription factor, Sp1. J Biol Chem. 2000;275:31747–3154. doi: 10.1074/jbc.M000621200. [DOI] [PubMed] [Google Scholar]
- 51.Ongeri EM, Verderame MF, Hammond JM. Follicle stimulating hormone induction of ovarian IGF binding protein-3 transcription requires a TATA-box binding protein, and the PKA and PI-3 kinase pathways. Mol Endocrinol. 2005;19:1837–1848. doi: 10.1210/me.2004-0487. [DOI] [PubMed] [Google Scholar]
- 52.An DS, Qin FX, Auyeung VC, et al. Optimization and functional effects of stable short hairpin RNA expression in primary human lymphocytes via lentiviral vectors. Mol Ther. 2006;14:494–504. doi: 10.1016/j.ymthe.2006.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


