Abstract
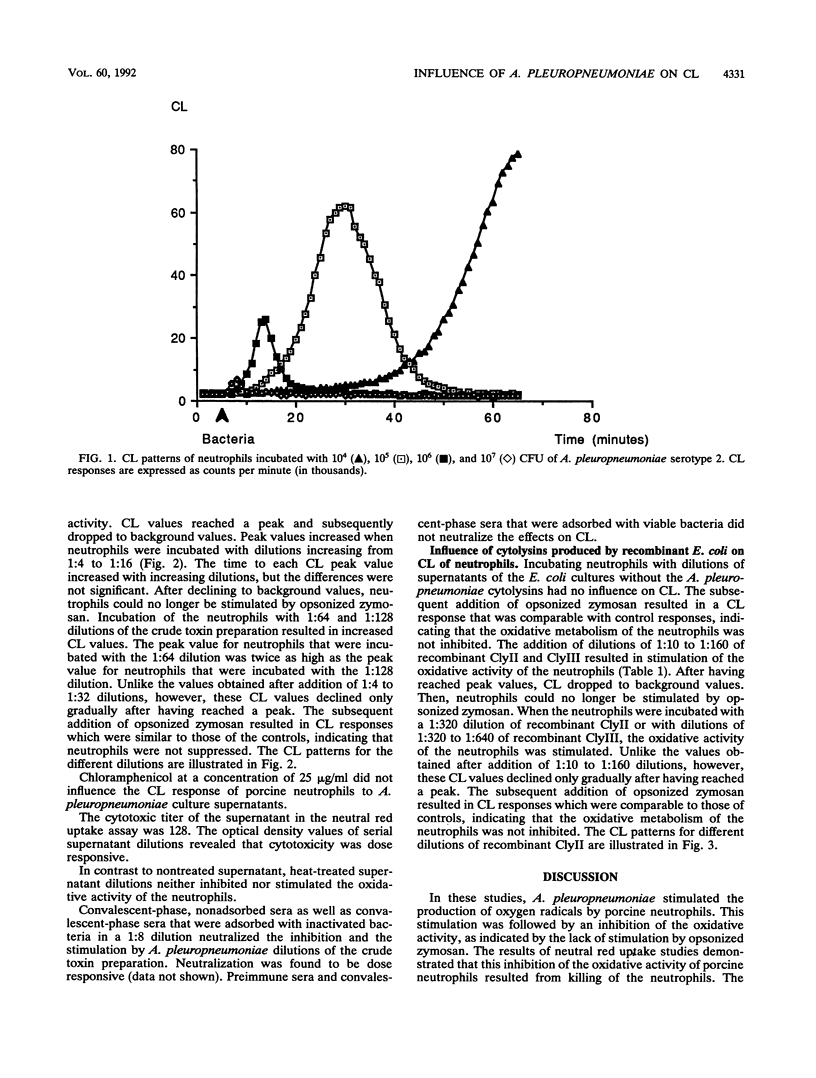
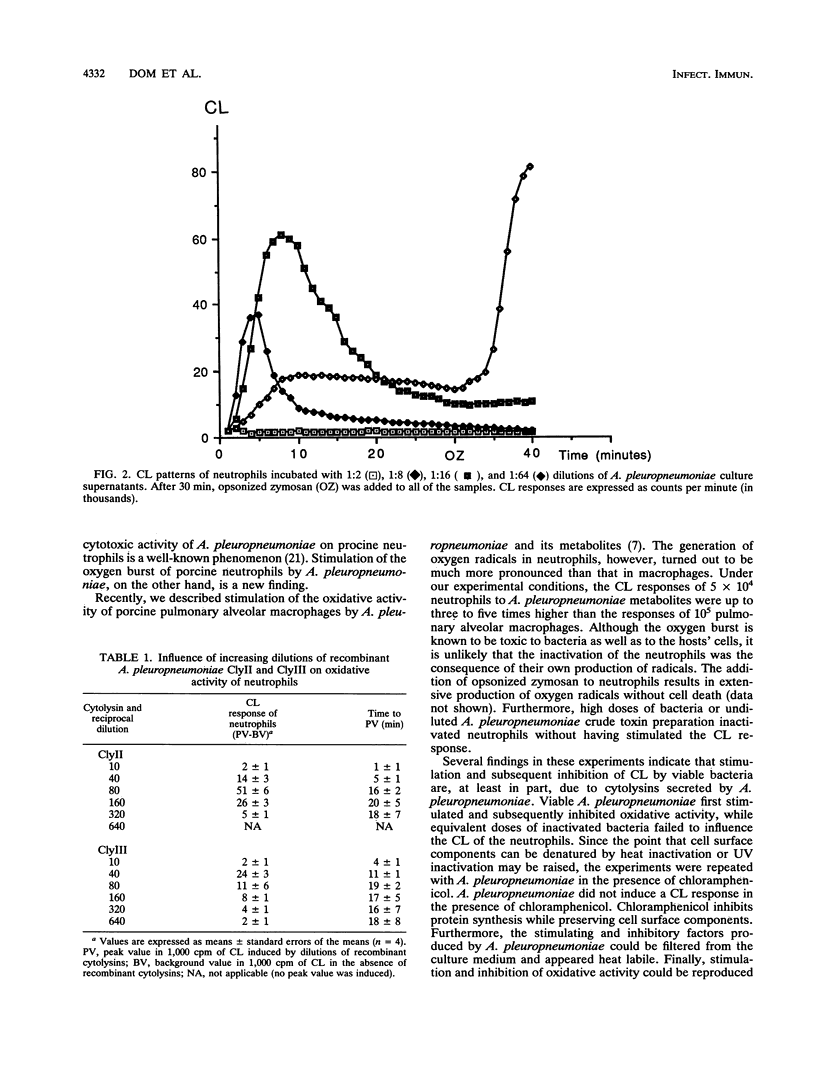
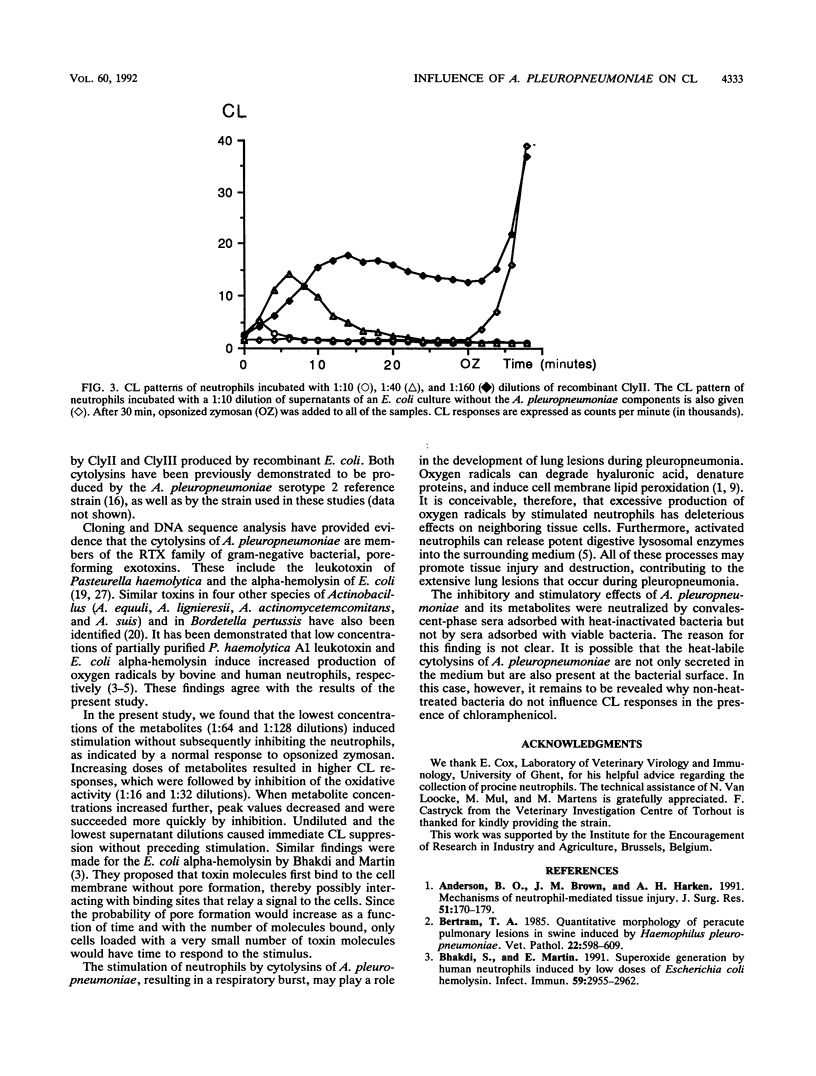
The effects of Actinobacillus pleuropneumoniae serotype 2 and its metabolites on the oxidative activity of porcine neutrophils were studied by using a chemiluminescence technique. Viable A. pleuropneumoniae stimulated the production of oxygen radicals by neutrophils. After having reached a peak value, the oxidative activity decreased until a total inhibition of the oxidative activity of the neutrophils was achieved. All effects were neutralized with homologous convalescent-phase pig sera which had been adsorbed by heat-inactivated A. pleuropneumoniae. Inactivated bacteria and bacteria in the presence of chloramphenicol each had no influence on the oxidative activity of neutrophils. In contrast, a heat-labile factor in A. pleuropneumoniae culture supernatants stimulated and inhibited the oxidative activity of the neutrophils in a dose-dependent manner. Undiluted and low dilutions of culture supernatants were toxic for the phagocytes, while high dilutions stimulated the oxygen radical production of the neutrophils. These effects were neutralized with homologous convalescent-phase pig sera. In order to investigate whether the heat-labile factors in the culture supernatant could be cytolysins, we repeated the experiments with cytolysin II and cytolysin III produced by recombinant Escherichia coli. It was demonstrated that stimulation and inhibition could be reproduced by both cytolysins. In conclusion, the observations made in this study showed that A. pleuropneumoniae secretes heat-labile metabolites that stimulate neutrophil-oxidative metabolism at relatively low concentrations and kill the neutrophils at higher concentrations. Cytolysins may be responsible, at least in part, for these effects.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. O., Brown J. M., Harken A. H. Mechanisms of neutrophil-mediated tissue injury. J Surg Res. 1991 Aug;51(2):170–179. doi: 10.1016/0022-4804(91)90090-9. [DOI] [PubMed] [Google Scholar]
- Bertram T. A. Quantitative morphology of peracute pulmonary lesions in swine induced by Haemophilus pleuropneumoniae. Vet Pathol. 1985 Nov;22(6):598–609. doi: 10.1177/030098588502200615. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Martin E. Superoxide generation by human neutrophils induced by low doses of Escherichia coli hemolysin. Infect Immun. 1991 Sep;59(9):2955–2962. doi: 10.1128/iai.59.9.2955-2962.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri S. J., Snyder I. S. Effect of Escherichia coli alpha-hemolysin on human peripheral leukocyte function in vitro. Infect Immun. 1982 Sep;37(3):966–974. doi: 10.1128/iai.37.3.966-974.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Noel E. J., Ortiz-Carranza O., Srikumaran S. Activation of bovine neutrophils by partially purified Pasteurella haemolytica leukotoxin. Infect Immun. 1991 Sep;59(9):3126–3133. doi: 10.1128/iai.59.9.3126-3133.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenish J., Rosendal S. Identification of the heat-labile hemolysin of Actinobacillus pleuropneumoniae serotype 1. Can J Vet Res. 1989 Apr;53(2):251–254. [PMC free article] [PubMed] [Google Scholar]
- Dom P., Haesebrouck F., De Baetselier P. Stimulation and suppression of the oxygenation activity of porcine pulmonary alveolar macrophages by Actinobacillus pleuropneumoniae and its metabolites. Am J Vet Res. 1992 Jul;53(7):1113–1118. [PubMed] [Google Scholar]
- Fenwick B. W. Virulence attributes of the liposaccharides of the HAP group organisms. Can J Vet Res. 1990 Apr;54 (Suppl):S28–S32. [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Frey J., Nicolet J. Hemolysin patterns of Actinobacillus pleuropneumoniae. J Clin Microbiol. 1990 Feb;28(2):232–236. doi: 10.1128/jcm.28.2.232-236.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey J., Nicolet J. Regulation of hemolysin expression in Actinobacillus pleuropneumoniae serotype 1 by Ca2+. Infect Immun. 1988 Oct;56(10):2570–2575. doi: 10.1128/iai.56.10.2570-2575.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gygi D., Nicolet J., Frey J., Cross M., Koronakis V., Hughes C. Isolation of the Actinobacillus pleuropneumoniae haemolysin gene and the activation and secretion of the prohaemolysin by the HlyC, HlyB and HlyD proteins of Escherichia coli. Mol Microbiol. 1990 Jan;4(1):123–128. doi: 10.1111/j.1365-2958.1990.tb02021.x. [DOI] [PubMed] [Google Scholar]
- Inzana T. J. Capsules and virulence in the HAP group of bacteria. Can J Vet Res. 1990 Apr;54 (Suppl):S22–S27. [PubMed] [Google Scholar]
- Kamp E. M., Popma J. K., Anakotta J., Smits M. A. Identification of hemolytic and cytotoxic proteins of Actinobacillus pleuropneumoniae by use of monoclonal antibodies. Infect Immun. 1991 Sep;59(9):3079–3085. doi: 10.1128/iai.59.9.3079-3085.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp E. M., van Leengoed L. A. Serotype-related differences in production and type of heat-labile hemolysin and heat-labile cytotoxin of Actinobacillus (Haemophilus) pleuropneumoniae. J Clin Microbiol. 1989 Jun;27(6):1187–1191. doi: 10.1128/jcm.27.6.1187-1191.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggett A. D., Harrison L. R., Farrell R. L. Sequential study of lesion development in experimental haemophilus pleuropneumonia. Res Vet Sci. 1987 Mar;42(2):204–212. [PubMed] [Google Scholar]
- Lo R. Y. Molecular characterization of cytotoxins produced by Haemophilus, Actinobacillus, Pasteurella. Can J Vet Res. 1990 Apr;54 (Suppl):S33–S35. [PubMed] [Google Scholar]
- Rosendal S., Devenish J., MacInnes J. I., Lumsden J. H., Watson S., Xun H. Evaluation of heat-sensitive, neutrophil-toxic, and hemolytic activity of Haemophilus (Actinobacillus) pleuropneumoniae. Am J Vet Res. 1988 Jul;49(7):1053–1058. [PubMed] [Google Scholar]
- Rycroft A. N., Williams D., Cullen J. M., Macdonald J. The cytotoxin of Actinobacillus pleuropneumoniae (pleurotoxin) is distinct from the haemolysin and is associated with a 120 kDa polypeptide. J Gen Microbiol. 1991 Mar;137(3):561–568. doi: 10.1099/00221287-137-3-561. [DOI] [PubMed] [Google Scholar]
- Sebunya T. N., Saunders J. R. Haemophilus pleuropneumoniae infection in swine: a review. J Am Vet Med Assoc. 1983 Jun 15;182(12):1331–1337. [PubMed] [Google Scholar]
- Serebrin S., Rosendal S., Valdivieso-Garcia A., Little P. B. Endothelial cytotoxicity of Actinobacillus pleuropneumoniae. Res Vet Sci. 1991 Jan;50(1):18–22. doi: 10.1016/0034-5288(91)90047-r. [DOI] [PubMed] [Google Scholar]
- Smits M. A., Briaire J., Jansen R., Smith H. E., Kamp E. M., Gielkens A. L. Cytolysins of Actinobacillus pleuropneumoniae serotype 9. Infect Immun. 1991 Dec;59(12):4497–4504. doi: 10.1128/iai.59.12.4497-4504.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee C. A., Lo R. Y. Extensive homology between the leukotoxin of Pasteurella haemolytica A1 and the alpha-hemolysin of Escherichia coli. Infect Immun. 1987 Dec;55(12):3233–3236. doi: 10.1128/iai.55.12.3233-3236.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utrera V., Pijoan C. Fimbriae in A pleuropneumoniae strains isolated from pig respiratory tracts. Vet Rec. 1991 Apr 13;128(15):357–358. doi: 10.1136/vr.128.15.357. [DOI] [PubMed] [Google Scholar]


