Abstract
The first hours of a newborn rat’s life entail locating and attaching to the mother’s nipple not only for nutrition but also for protection and warmth. The present study sought to characterize olfactory learning in the rat neonate immediately after birth. Newborn rats were exposed to an odor at various time periods soon after birth and tested for behavioral activation and attachment to a surrogate nipple in the presence of this odor at 4–5 hours postpartum. Regardless of when pups were presented the odor (0, 1, or 2 hours after birth) motor activity was greater among pups previously exposed to the odor than pups with no odor experience. Similarly, latency to attach to the nipple in the presence of the odor was lower among odor-preexposed pups, especially when odor exposure began within an hour of cesarean delivery. Odor exposure immediately after birth for just 15 minutes was sufficient to increase motor activity and to decrease latency to attach to a similarly scented surrogate nipple. These results suggest that olfactory experience very soon after birth can shape subsequent olfactory responses. The relative importance of the dearth of postnatal experience or of elevated neurochemicals immediately after birth and possible associative mechanisms underlying this learning is discussed.
Keywords: surrogate nipple, behavioral activation, olfactory learning, nonassociative learning, newborn, odor, rat
Research has shown that early olfactory learning is ubiquitous in mammals. As early as three hours postpartum, neonatal rats have displayed substantial appetitive (Cheslock, Varlinskaya, Petrov, & Spear, 2000) and aversive olfactory conditioning (Nizhnikov, Petrov, & Spear, 2002), operant conditioning (Bordner, Molina, & Spear, 2007; Johanson & Hall, 1979), and higher order olfactory conditioning (Cheslock, Varlinskaya, High, & Spear, 2003). Olfactory conditioning has been found in various species of mammals, such as newborn mice (Bouslama, Durand, Chauvière, Van den Bergh, & Gallego, 2005), rabbits (Hudson & Distel, 1999) and human neonates (Sullivan et. al., 1991). The widespread presence and robustness of early and diverse olfactory learning in various mammalian species is likely a consequence of its survival value during early postnatal life. Furthermore, since the olfactory system develops early in ontogeny the fetus is also capable of learning about its prenatal environment (Hepper, 1988; Schaal, Hummel, & Soussignan, 2004).
Prenatal olfactory learning is critical for adaptation to the postnatal environment. Research concerning prenatal learning about food preferences in the rat (Hepper, 1988) and rabbit (Hudson & Distel, 1999) illustrates this idea nicely. A primary consequence of prenatal olfactory learning is to maintain the neonate’s physical proximity to the nest. As a neonate, the rat must navigate towards the nipple for its first suckling experience. Perinatal exposure to amniotic fluid in utero and during parturition assists the pup in this endeavor (Teicher & Blass, 1977). If transnatal olfactory continuity (the presence of similar chemosensory cues prenatally and postnatally) is disrupted a decrease in the rabbit (Coureaud, Schaal, Hudson, Orgeur, & Coudert, 2002) or rat (Teicher & Blass, 1977) pup’s ability for a successful first suckling experience may result. It is not surprising then that many similarities exist between prenatal and neonatal olfactory learning. For example, both fetuses and newborns show the ability to learn a conditioned taste aversion (Kehoe & Blass, 1986; Stickrod, Kimble, & Smotherman, 1982). Nevertheless, a provocative question emerges as to what role birth might play in the continuity from fetal to postnatal life?
Postnatal olfactory learning contributes to continued survival throughout early ontogeny. Exposing rat pups to a novel odor from postnatal day one for two to three weeks induces a preference for that odor (Alberts & May, 1984; Coopersmith, Henderson, & Leon, 1986; Sullivan, Wilson, Wong, Correa, & Leon, 1990). While odors associated with maternal care created stronger preferences, odor exposure in the presence of a lactating dam was not necessary and in fact, a warm scented tube or mere exposure was also sufficient (Alberts & May, 1984). Results of these odor exposure studies include preferential orientation, huddling towards the odor as well as odor specific modified olfactory bulb responses. Developing a preference for maternal odors over non-maternal nest odors occurs between postnatal day one and two in the rat (Polan & Hofer, 1998). In human neonates, the mother’s odor can increase mouthing, likely as a consequence of appetitive conditioning during breastfeeding (Sullivan & Toubas, 1998). Appetitive conditioning to a novel odor, with milk as an unconditioned stimulus, has been found in the 3-hour old neonatal rat (Cheslock et al., 2000). Maintained proximity to the nest and mother, promoted by prenatal and postnatal olfactory learning, enhances the pup’s likelihood of staying warm, protected and well fed.
New learning immediately after birth, within minutes or a few hours after parturition, has rarely been a topic of research. Nevertheless, fetal and neonatal learning has important clinical relevance for humans (Schaal et al., 2004). For example, early learning about maternal odors in human neonates prepares them for feeding (Sullivan & Toubas, 1998). So why has research concerning learning in this immediate postpartum time period been largely neglected?
In the past, the behavioral repertoire of the neonatal rat has been considered very limited. Work with anesthetized dams, surrogate nipples, and a simple operant procedure has helped experimenters to operate within the neonatal rat’s motor capabilities (Teicher & Blass, 1977; Petrov, Varlinskaya, Smotherman, 1997; Bordner, Molina, & Spear, 2007; Johanson & Hall, 1979). Nevertheless, even these procedures can sometimes be inappropriate shortly after birth. For example, nipple attachment behavior, seen prenatally but rarely soon after birth, increases over the first five postnatal hours (Smotherman, Goffman, Petrov, & Varlinskaya, 1997). Furthermore, in human research particularly, but also with rodents, there is a desire to leave the mother-infant dyad undisturbed soon after birth. With rats, the neonate cannot see or hear, thus most work has employed gustatory and olfactory stimuli. Nevertheless, immediately after delivery, for a couple of hours the pup has not yet achieved stable breathing patterns (Ronca, Abel, Ronan, Renner, & Alberts, 2006). Presenting an odorant to a newborn rat or fluid into its mouth prior to consistent independent respiration may seem to be problematic due to less sensory perception or possible asphyxiation as a consequence of the erratic breathing patterns. Finally, through processes and stimuli associated with birth (both vaginal and cesarean section), catecholamines, as well as various other neurochemicals in the rat and human neonate, are at levels much greater than those of the adult and also greater than those of the infant just hours later (Ronca, Abel, Ronan, Renner, & Alberts, 2006; Lagercrantz & Herlenius, 2002). Most research on learning in the neonatal rat has delayed conditioning until three hours after cesarean delivery (Cheslock et al., 2000; Nizhnikov, Petrov, & Spear, 2002).
Studying the rat pup soon after delivery, however, may be quite advantageous. In view of the dramatic surge of neurochemicals (e.g., neurotransmitters, neurosteroids, neuropeptides, neuromodulators) known to be released within the neonatal rat during the birth process and shortly thereafter, this provides a particularly interesting period of ontogeny in which to study neurobiological correlates of early learning (Lagercrantz & Herlenius, 2002). These neurochemicals are purported to provide a mechanism through which birth-related labor facilitates physiological processes in the rat such as respiration (Ronca & Alberts, 1995) and behavioral processes such as suckling (Abel, Ronca & Alberts, 1998). Some of these same neurochemicals have been shown to affect olfactory conditioning in the rat at various ages (McLean, Darby-King, Sullivan, & King, 1993; Moriceau & Sullivan, 2004; Okutani, Zhang, Yagi, & Kaba, 2002; Robinson & Smotherman, 1995; Rumsey, Darby-King, Harley, & McLean, 2001; Sullivan & Wilson, 1994; Wilson, Fletcher, & Sullivan, 2004; Wilson & Sullivan, 1994). In order to study the function these neurochemicals may have in olfactory learning just after birth, future studies will likely utilize pharmacological agents. As a first step, the following experiments describe olfactory learning soon after birth in the absence of any neurochemical manipulations.
The goal of the current study was to characterize learning in the rat pup within a few minutes or hours after cesarean delivery. The effects of early postnatal odor exposure on later behavioral activation and nipple attachment in the presence of that same odor were tested as a function of how soon after birth the odor was experienced (Experiment 1) and duration of that experience (Experiment 2). Although the newborn rat had seemed unsuitable for associative learning paradigms using the surrogate nipple technique just after birth, until three hours postpartum (Smotherman, Goffman, Petrov, & Varlinskaya, 1997), it remained possible that learning about an odor exposure soon after birth could be expressed on the artificial nipple hours later. A large body of literature demonstrates that younger animals (including humans) forget at a faster rate than older animals (Campbell & Spear, 1972; Rovee-Collier, 1999; Spear & Riccio, 1994). Taking this literature into account, one might predict that odors experienced closer to the test would be recalled most effectively. Nevertheless, we predicted that odor exposure, even if short in duration, occurring immediately after birth would enhance nipple attachment behaviors and alter motor activity to those same odors more than odors experienced later and therefore closer to the test. The nature of our predictions was based on the robust nature of memories found recently in newborn rats (tolerance of trace intervals- Bordner & Spear, 2006a; Cheslock, Varlinskaya, Petrov, & Spear, 2000; resistance to retroactive interference- Cheslock, Sanders, & Spear, 2004).
General Method
Subjects
Subjects were 173 pups cesarean sectioned from 39 Sprague-Dawley females (Taconic, Germantown, NY) bred in wire hanging cages. When a sperm plug was found (embryonic day zero; E0) females were removed from the hanging cages and placed in maternity tubs (45 cm long × 23 cm wide × 20 cm high), partially filled with shavings, with one to two other pregnant rat(s) until E20 when they were separated into individual maternity tubs. The colony room was maintained at 22°C and was on a 14-/10-hour light/dark cycle (lights on at 0700) with ad libitum access to food (Breeders Purina Rat Chow, Lowell, MA) and water. Rats used in these experiments were maintained and treated in accordance with the guidelines for animal care and use established by the National Institutes of Health (1986).
Cesarean Delivery
Near expected term (E21) pups were delivered by cesarean section. Brief ether anesthesia followed by rapid cervical dislocation was administered to the dam. Next, a midline incision was made through the abdominal wall exposing the uterine horns. A small incision into the amniotic sac externalized the pup. Once the umbilical cord was ligated and cut the extraembryonic membranes were removed by a gentle rolling of the neonate on a sanitary paper towel. Pups were placed into a plastic container (12 cm long × 12 cm wide × 6 cm high) lined with moist paper towels. A timer (to mark the birth time of the litter) was started when the median pup was born (e.g., fourth pup out of seven). Any pup not being treated immediately after birth was placed into an incubator maintained at 35°C ± 1°C with 90% humidity.
Materials
Heating Chamber
Odor exposure procedures were at times lengthy and because the neonate is unable to thermoregulate, a heating chamber was constructed to maintain body warmth in the neonates during odor exposure. A 15.24 cm (diameter) × 15.24 cm (height) cylindrical metal duct was connected to a 7.62 cm (width) × 12.7 cm (length) Thermafoil™ heater. Both were encased in aluminum foil insulation. A miniature direct current (24V) controller with a 1A power supply and a 1A fuse kept heat regulated at 35°C ± 1°C as indicated by a resistance temperature detector sensor.
Surrogate nipple
The surrogate nipple was cast from rubber latex (AMACO rubber latex, Indianapolis, IN) and molded into a conical form to measure 12 mm long with a rounded tip measuring 1mm in diameter and the base measuring 2.5 mm in diameter. Polyethylene tubing (Clay Adams, MD) extended throughout the length of the nipple and attached to a syringe filled with water. The small diameter of the tubing, along with the natural viscosity of water, prevented spontaneous effusion from the tip of the nipple. Slight negative pressure, produced by the pups while attached to the nipple, was necessary and sufficient to extract the water from the surrogate nipple (Petrov, Varlinskaya, & Smotherman, 1997). In order to facilitate and standardize the procedure of the nipple presentation, the individual subject was strapped and buckled in semi-supine posture into a ‘vest’ made from ultra-thin, elastic rubber. This light restraint prevented righting attempts but did not otherwise produce discomfort nor hinder the pups’ movements.
Procedure
Odor presentation
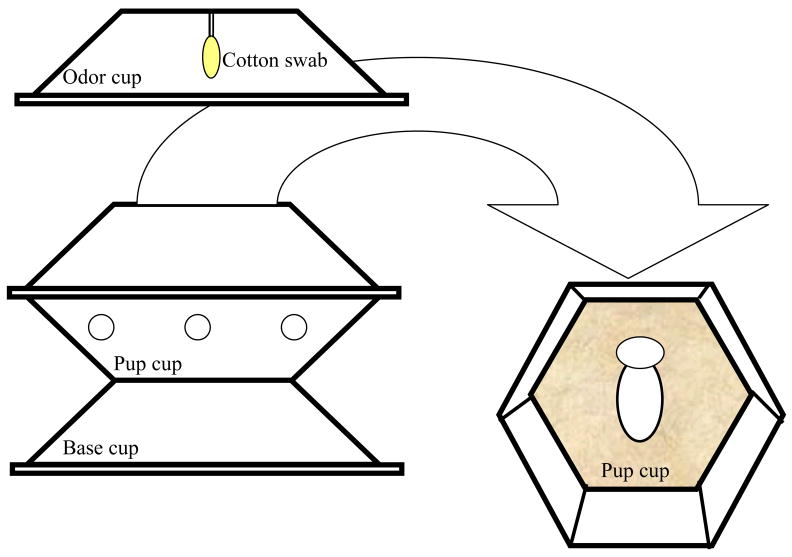
Within the heating chamber, two pups (one male and one female) were placed into a hexagonal shaped shallow cup (8.5 mm wide at the top, 5.5 mm wide at the bottom, 2 mm deep) (see Figure 1). This cup (the pup cup) was set on top of an identical cup placed upside down (the base cup). This base cup added height, which enabled the pups to reach the proper temperature in the heating chamber. A cotton swab was punctured through another identical cup (the odor cup), which was placed over the pup cup to present the odor. This cotton swab was never in contact with the pups. Ventilation was made possible by several holes in the pup cup created with a standard hole-puncher. The pup cup was lined with synthetic fur, which was composed of 100% polyester (Jo-Ann fabric and craft stores™).
Figure 1.
Schematic depiction of the odor exposure container.
Nipple attachment test
Surrogate nipple testing occurred in a transparent glove box (63 cm long × 50 cm wide × 25 cm high) kept at 28.0°C ± 0.5°C by a temperature controller (Model 40-90-8B, Frederick Haer, Inc., Brunswick, ME). The surrogate nipple has an alligator clip attached to it such that during nipple presentation the cotton swab, which is attached to the clip on the nipple, is held at approximately 2 cm from the pups’ snout throughout the test. The pup was placed on a mirror maintained at 35.5°C ± 0.5°C. Exposure to the nipple containing water involved the experimenter gently using the nipple to stimulate the pups’ perioral area using a drop of moisture to prevent irritation. Nipple attachment behavior was assessed for five minutes (for a more complete explanation of the surrogate nipple test, see Cheslock, Varlinskaya, Petrov, & Spear, 2000). Measures obtained from the nipple attachment test included: latency to attach (L) to the nipple and total time attached (T). Latency to attach is defined as the time elapsed from the start of nipple presentation to the time of first grasp. Total time attached is the total duration of all grasps summated. However, since latency to attach to the nipple constrains the amount of time a pup has remaining for nipple attachment, total time attached was corrected for by latency in the following manner. Total time attached was divided by the time that remained after the pup had first attached or T/(300 − L), recall that the test is five minutes long, or 300 seconds. Total time attached corrected by latency was expressed as a percentage and so the previous equation was multiplied by 100. Percent total time attached = (T/300 − L) * 100.
Behavioral activation
The first minute of the nipple attachment test was scored (via videotape) for behavioral activation. During the first minute most (85%) of the pups had not yet attached to the nipple. Nevertheless, it should be clear that odor exposure for this test was presented in conjunction with perioral stimulation from the nipple. Subsequent control experiments revealed that perioral stimulation was not necessary for the present effects. At four to five hours after birth the pups were tested for behavioral activation in the presence of a specified odor. The videotape was scored for the frequency of head movements and bursts. Head movements are defined quite simply as any non-twitching movement of the head. Twitches, indicative of active sleep, are rapid, stereotyped, consistently short in duration (< 1 second) and lack obvious muscle tone (Gramsbergen, Schwartze, & Prechtl, 1970). Head movements, however, occur in a variety of directions (e.g., lateral, probing), vary more in duration and demonstrate sustained muscle tone. Bursting behavior is defined as the simultaneous movement of all the pup’s extremities (e.g., head, forelimbs, hindlimbs) in addition to a twisting of the torso. Thus, head movements occur within the burst but a head movement was only classified as such when it occurred in the absence of simultaneous full body movements, which exemplify the burst. The burst tends to be longer in duration than head movements and is characterized by vigorous movement.
Data Analysis
In each of the following experiments no more than one pup per group was represented in each litter. Separate between-subjects univariate analyses of variance were run for each dependent variable (latency, total time attached, head and burst movements) using Statistica version 6.0. Analyses used sex and condition as independent factors. Significance was indicated when p values were less than or equal to .05.
Experiment 1: Effects of odor during postpartum hours 1, 2 or 3
In order to characterize neonatal learning within the first few postnatal hours, an age-appropriate odor exposure procedure was tested. Although the procedure of mere odor exposure was not novel, pups at these ages have not previously been tested with this technique. Experiment 1 was designed to assess the effects of exposure to an artificial odor soon after birth on later behavioral activation and nipple attachment. Exposure to the odor occurred during the first, second or third hour postpartum. Warmth, a conspecific, and a synthetic fur surface were all present during odor exposure in an attempt to resemble characteristics of the nest environment.
Method
Subjects
A total of 98 cesarean delivered neonatal pups from twenty-five dams was used in the current experiment. The number of subjects in each condition is summarized in Table 1.
Table 1.
Number of subjects in Experiment 1
| Condition
|
|||
|---|---|---|---|
| Time of exposure | Sex | Prior odor exposure | No odor exposure |
| 0–60 minutes | Male | 8 | 7 |
| Female | 8 | 8 | |
| 60–120 minutes | Male | 9 | 9 |
| Female | 9 | 9 | |
| 120–180 minutes | Male | 8 | 8 |
| Female | 7 | 8 | |
Procedures
Two orthogonal factors, sex (male, female) and condition (prior odor exposure, no prior odor exposure), combined to total four groups. For each litter, four pups (two males, two females) were delivered by cesarean section and one of each sex (prior odor exposure group) was placed into a heated chamber beginning immediately (0–60 minutes), one hour (60–120 minutes), or two hours (120–180 minutes) after birth. These 3 time periods were run sequentially but in close succession. This sequential method was necessary to control for odor accumulation, from one time period to the next, in the heating chambers and experimental rooms. Accordingly, results within each time period are presented individually, followed by a short paragraph on between-time period comparisons. Control animals (no prior odor exposure group) across experiments did not differ significantly with regard to the dependent variables. Pups in the no prior odor exposure group were placed into an incubator until testing. Inside the heated chamber the two pups provided tactile contact for each other, as did the fur-textured surface, in the presence of 0.1 ml of lemon odor for one hour. After this exposure the pups were numbered by a research assistant—to allow the experimenter to be “blind” at testing—and placed with the other pups in the incubator. All pups remained in the incubator until they were four-hours old. At this time, pups were tested with a surrogate nipple providing water in the presence of 0.1 ml of lemon odor.
Results
Odor exposure 0–60 minutes
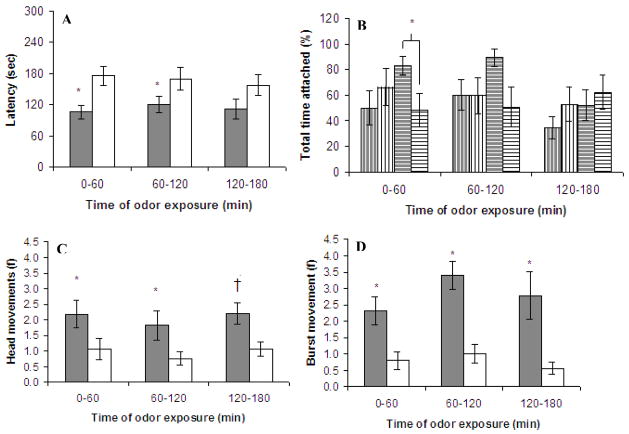
A univariate ANOVA with latency as a dependent measure indicated that pups preexposed to lemon odor attached to the lemon-scented nipple sooner than pups not preexposed to that odor, (Figure 2a, F (1, 27) = 10.46, p <.01). Unexpectedly, females (but not males) with preexposure to lemon odor had longer total attachment (Figure 2b, F (1, 27) = 4.36, p <.05) than non-preexposed females. Analysis of motor activity during the first minute of the surrogate nipple procedure revealed a significant effect of condition on frequency of head movements (Figure 2c, F (1, 27) = 4.25, p <.05) and burst movements, (Figure 2d, F (1, 27) = 7.92, p <.01). For both motor activity measures the lemon-odor-preexposed group was more active in the presence of lemon at test than the non-preexposed group, regardless of sex.
Figure 2.
Experiment 1. These graphs illustrate the mean (± SE) of a) latency to attach to and b) percent total time attached to a scented surrogate nipple as a function of time of odor exposure. Also depicted are the mean (± SE) values of c) head movements and d) burst movements during the first minute of presentation of the scented surrogate nipple. For all graphs: gray bars = pups with prior odor exposure; white bars = pups with no prior odor exposure. For graph b) percent total time attached: vertical stripes = male rats, horizontal stripes = female rats. †Figure 2C (120–180) significant effects were found only for males * indicates p <.05
Odor exposure 60–120 minutes
Pups receiving odor exposure from 60–120 minutes after cesarean section had a shorter latency to attach to a nipple in the presence of lemon odor (Figure 2a, F (1, 32) = 7.27, p =.01). There was no significant effect of sex, condition nor an interaction thereof on total time attached. The apparent interaction seen in Figure 2b is nonsignificant (F (1, 32) = 2.35, p >.10) but it is interesting to note that it is in the same direction as the interaction seen during odor exposure from 0–60 minutes postnatally. Pups preexposed to lemon odor were more active in the presence of lemon odor than pups without this preexposure (head movements, Figure 2c, F (1, 32) = 4.30, p <.05; and burst movements, Figure 2d, F (1, 32) = 21.25, p <.0001).
Odor exposure 120–180 minutes
Lemon odor exposure from 120–180 minutes after cesarean section did not significantly affect nipple attachment behaviors (i.e., latency and time attached, see Figures 2a and 2b). Nevertheless, lemon preexposure increased level of head movements upon reexposure at test (Figure 2c, F (1, 27) = 9.35, p <.01) and bursting (Figure 2d, F (1, 27) = 11.53, p <.01). With regard to head movements, however, an interaction showed that this was only true for male pups (F (1, 27) = 10.62, p <.01).
Cross-experimental comparisons
Including time of exposure (0–60, 60–120, 120–180 minutes) as an independent variable along with sex and condition in a univariate ANOVA did not change the basic conclusions. When comparing across experiments, latency was decreased in the presence of a preexposed odor regardless of when the odor was experienced during the first three hours after delivery (F (1, 85) = 13.36, p <.001). Nevertheless, it will be recalled that odor exposure from 120–180 minutes postnatal did not result in a decreased latency to attach to a scented nipple with the separate experimental analyses listed above. Since the experiments were run sequentially, these cross-experimental comparisons remain tentative until future experiments can test these three time periods within the scope of one study. In terms of total time attached, analyses revealed a significant interaction of sex and condition, F (1, 85) = 5.82, p <.05. There were no main effects or interactions with time of exposure. Tukey’s honestly significant difference post hoc tests clarified that female pups preexposed to lemon odor attached to the surrogate nipple for longer than male pups with the same preexposure. Like latency, behavioral activation in the presence of lemon odor was increased regardless of sex or when the odor was experienced during the first three hours after delivery (head F (1, 85) = 14.76, p <.001 and burst movements, F (1, 85) = 36.46, p <.000001). The same caution used in interpretation of the latency results applies to the behavioral activation results. Although time of odor exposure was not a factor in the cross-experimental analyses, the separate analyses revealed that head movements subsequent to later odor exposures (120–180 minutes) were only detected in male pups.
In summary, latency to attach to a lemon scented nipple was decreased whereas motor activity was increased in pups exposed to 60 minutes of lemon odor soon after birth. Other measures of nipple attachment (e.g., total time attached), however, were less clearly affected by odor preexposure. Thus, it seems that latency to attach to a scented nipple or simple motor activation are the more sensitive indices of prior odor exposure effects.
Experiment 2: Effect of duration of odor exposure beginning immediately after birth
Experiment 2 varied duration of odor exposure during the first postnatal hour and analyzed its effect on behavioral activation and nipple attachment behaviors. Immediate odor exposure had the most profound and consistent effects on behavioral activation and nipple attachment measures in Experiment 1. In view of its ecological relevance, onset of odor exposure in the current experiment always began immediately following cesarean delivery.
Methods
Subjects
A total of 106 cesarean-delivered newborn pups from twenty-two different dams were used in Experiment 2. Male and female pups were exposed to lemon odor for 0, 15, 45, or 60 minutes, beginning immediately after birth. Analysis included 31 pups from Experiment 1 (in the present experiment these are the 0 and 60 minute groups). Comparisons between these 31 pups and pups in the 0 and 60 minute groups tested at the same time as all other pups in Experiment 2 revealed no significant differences. There were 10–11 pups per group, except that this number was doubled in the 0 minute exposure group due to the convergence of experimental data sets (see Table 2).
Table 2.
Number of subjects in Experiment 2
| Duration of exposure | Sex | Litter 1–8 | Litter 9–16 | Litter 17–22 | Total |
|---|---|---|---|---|---|
| 0 minutes | Male | 7 | 8 | 6 | 21 |
| Female | 8 | 8 | 6 | 22 | |
| 15 minutes | Male | - | 8 | 3 | 11 |
| Female | - | 8 | 3 | 11 | |
| 45 minutes | Male | - | 8 | 3 | 11 |
| Female | - | 8 | 2 | 10 | |
| 60 minutes | Male | 8 | - | 2 | 10 |
| Female | 8 | - | 2 | 10 |
Note. Litter 1–8 were from Experiment 1 (0–60 minutes), then Litter 9–16 were run, and in an attempt to merge these data sets 6 litters were run with all groups represented in each litter.
Procedures
Pups were placed into an odorized (lemon), heated chamber (2 pups per cup) immediately after birth and taken out 15, 45 or 60 minutes later. Pups in the 0 minute group were placed immediately into an incubator without odor exposure. At four hours postpartum, all pups were tested on a nipple providing water in the presence of 0.1 ml lemon odor.
Results
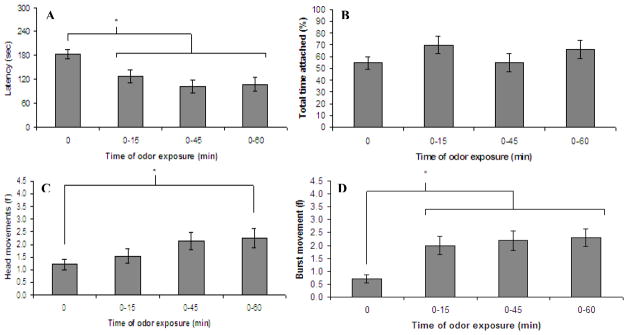
Exposure to lemon odor immediately after birth decreased latency to attach to a nipple in the presence of lemon odor four hours postpartum (F (3, 98) = 8.35, p <.0001). As Figure 3a illustrates, this was true whether pups were exposed to lemon odor for 15, 45 or 60 minutes (p <.05,.0005, and.01 respectively). Total time attached was not significantly affected by sex, condition or an interaction thereof (see Figure 3b). The reason for failure to replicate the sex by condition interaction seen in Exp 1 (0–60 minute condition, F (1, 27) = 4.36, p =.046) is unclear but is likely due to type I error in Exp 1.
Figure 3.
Experiment 2. These graphs illustrate the mean (± SE) of a) latency to attach to and b) percent total time attached to a scented surrogate nipple as a function of duration of prior odor exposure. Also depicted are the mean (± SE) values of c) head movements and d) burst movements during the first minute of presentation of the scented surrogate nipple. * indicates p <.05
Pups exposed to lemon odor for 60 minutes had more head movements than nonexposed animals when later reexposed to lemon odor (Figure 3c, F (3, 98) = 3.39, p <.05). Although not quite significantly different from nonexposed animals (p =.08), pups exposed to lemon odor for 45 minutes were intermediate to and not statistically different from pups exposed for a full hour (p >.5) or from pups exposed for 15 minutes (p >.5). Pups exposed to lemon odor immediately after birth displayed significantly more bursting behaviors to a lemon odor than did nonexposed animals (Figure 3d, F (3, 98) = 9.15, p <.0001) regardless of the duration of exposure (p <.01 for all three exposure durations).
In summary, latency to attach to a lemon scented nipple was decreased whereas burst movements were increased in pups exposed to lemon odor immediately after birth regardless of whether this odor exposure lasted 15, 45, or 60 minutes. Total time attached to the scented surrogate nipple was not affected by preexposure to that scent. Finally, head movements were only increased in response to a preexposed odor when this exposure period was an entire hour long. Thus, it seems that latency to attach to a scented nipple and bursting movements are sensitive indices of brief prior odor exposure effects.
General Discussion
Our predictions, that odor exposure occurring immediately after birth would enhance nipple attachment behaviors and increase motor activity to the same odor more than odor exposure experienced later, were partially supported. Although increased burst movements to a preexposed odor occurred regardless of when odor exposure took place, faster attachment to the scented surrogate nipple was most robust when odor preexposure took place soon after birth. Our prediction that short odor durations would be sufficient to see enhanced nipple attachment behaviors and increased motor activity was generally supported. Burst movements and latency to attach to a scented nipple were altered by immediate odor pre-exposure lasting as little as 15 minutes. Head movements, however, were only affected by an entire hour of odor pre-exposure, whereas total time attached was not affected whatsoever. Earlier it was discussed that this immediate postnatal time period may be problematic for olfactory study due to the erratic breathing patterns soon after birth. These results indicate that, despite unstable breathing patterns, the neonatal rat can detect olfactory stimuli within minutes after cesarean delivery.
Total time attached and head movements in Exp 1 both demonstrated effects dependent on the sex of the animal. Although not predicted, these sex effects may be influenced by the presence of a testosterone surge, only in male rats, with peak levels occurring at around one to two hours postpartum (Corbier, Edwards, & Roffi, 1992; Corbier, Kerdelhue, Picon, & Roffi, 1978; Lee, DeKretser, Hudson, & Wang, 1974; Weisz & Ward, 1980). Previous research in our lab has found that levels of testosterone in the male pup were higher than females beginning between 30 and 60 minutes postnatally and continuing until four hours postnatally (Miller & Spear, 2006). Although the effects of testosterone manipulations on rats’ olfactory learning have not yet been studied during this period, there has been some research on the effects of testosterone on learning in other species, ages, and preparations. For example, preexposure to a colored bead (to peck) in two-day-old domestic chicks weakened later avoidance training to that same color bead only when testosterone was injected just before or after pre-training (Andrew, Clifton, & Gibbs, 1981). Although the perinatal testosterone surge may play a role in sex effects found in learning soon after birth, this possibility must be qualified by the fact that sex effects found in Exp 1 were not replicated in Exp 2.
Behavioral changes elicited by reexposure to an odor occurring soon after birth could be indicative of facilitated perception or alterations in affect towards this odor (these are not mutually exclusive). In order to test these hypotheses future experiments may conduct threshold detection experiments. Transfer learning experiments may also be useful. For example, after odor preexposure a newborn rat might be conditioned by pairing that same odor with either an appetitive or aversive stimulus. If, for example, the odor preexposure procedure potentiates learning that the preexposed odor predicts an appetitive stimulus but attenuates learning about an aversive stimulus, we could conclude that something appetitive was learned during odor preexposure. Chotro and colleagues (1991) used these procedures and found that acute exposure to ethanol odor just prior to cesarean section yielded an appetitive olfactory conditioning. If further learning about the preexposed odor is facilitated regardless of the affective nature of the unconditioned stimulus, this would seem to support the view of facilitated perception after familiarization.
An appetitive-consummatory dissociation, like that used in ethological analysis of behavioral systems (Timberlake & Silva, 1973), may be useful for understanding why the measures of latency and motor activity were more sensitive to odor preexposure than total time attached. In this view, general and focal search comprise appetitive-seeking behavior such as locomotion and orientation (i.e., behavioral activation for an altricial pup) and obtainment (i.e., latency to attach) of a desired stimulus, such as food, whereas consummatory phases involve ingestion activities (i.e., total time attached). It makes sense that early olfactory experiences would primarily promote later appetitive (versus consummatory) behaviors in the presence of this odor because olfactory cues help orient the pup toward its food source. Increased motor activation, however, was not likely a direct cause of the decreased latency, because activity tends to decrease just before attachment to a nipple (Bacher, Robertson, & Smotherman, 2000). In contrast with the present findings, odors familiar to the infant rat such as amniotic fluid or maternally derived odors have been found to reduce motor activity upon reexposure (Dominguez, Lopez, & Molina, 1998; Schapiro & Salas, 1970). Consistent with our data, however, amniotic fluid decreases latency to attach to an artificial nipple in newborn rats (Koffman, Petrov, Varlinskaya, & Smotherman, 1998).
As mentioned previously, the rapid forgetting characteristic of infancy would predict stronger effects of prior odor exposure with shorter retention intervals. Nevertheless, latency to attach to a surrogate nipple was decreased in the presence of an odor primarily when preexposure to that odor occurred shortly after birth and not closer to test. Furthermore, retention interval did not exert an effect in terms of motor activity measures. There are several possible explanations for such effects. We list a few hypotheses, which are not necessarily mutually exclusive, and discuss each briefly. First, cognitive primacy, associated with the first exemplar experienced among a larger set, is an attribute of stimuli encountered soon after birth, although the precise effect of this is unknown (Cheslock, Sanders, & Spear, 2004). Better recall for the first salient event experienced in a given context may explain why earlier memories would be longer lasting than later ones. This notion was given some support by recent research in our lab (submitted for publication), which presented 2 odors sequentially with an hour interstimulus interval. Odors were presented either immediately after birth or two hours later so the odor exposure, which occurred 120–180 minutes postnatally, was the first odor exposure for some pups and the second odor exposure for others. Order of odor exposure (whether an odor was presented first or second) affected responsiveness to that odor at test more than the time at which the odor exposure occurred.
Second, potential reinforcers present during odor exposure could have had more rewarding properties sooner after birth. Despite the absence of an explicit reinforcer (e.g., milk) in the current experiments, the ambient warmth, synthetic fur, or conspecific during odor exposure may have been a sufficient unconditioned stimulus. This seems unlikely given the similar ambient temperatures and presence of conspecifics that preceded and followed odor exposure in the incubator. Odor exposures occurring soon after birth may have been paired with stimulation from cesarean section procedures or with lingering amniotic fluid on the neonate. So it remains unclear whether the present results reflect the consequences of nonassociative or associative conditioning. Third, depending on how long consolidation takes at these ages longer retention intervals may have provided more time for consolidation. Similarly, sensitization, or increased responding after repeated exposures, may have been expressed at test and is induced preferentially after spaced versus massed exposures.
Mentioned briefly in the introduction, higher levels of neurochemicals such as norepinephrine (NE) immediately after birth may support better odor learning at that time. Odor exposure immediately after birth likely occurred under a different internal neurochemical milieu for the pup than odors experienced later (Ronca, Abel, Ronan, Renner, & Alberts, 2006). An apparently sensitive period during the first postnatal week of the rat, marked by exaggerated NE release from the locus ceruleus during olfactory conditioning, is characterized by rapid and robust early olfactory conditioning (Sullivan & Wilson, 1994; Wilson & Sullivan, 1994). Rewarding stimuli are often the source of NE release. The neonatal surge of NE, however, may induce an olfactory imprinting-like occurrence in mammals wherein an explicit reinforcer might not be necessary to form a lasting odor preference (Sullivan, McGaugh, & Leon, 1991). In fact, artificially stimulating some of these surging neurochemicals later on in infancy has been shown to support odor preference learning in the absence of a reinforcer (Bordner & Spear, 2006b; Sullivan & Wilson, 1994; Wilson & Sullivan, 1994).
Other studies using non-reinforced odor presentations have shown strong long-lasting odor preferences due to this early experience (Caza & Spear, 1984; Hudson, 1993). However, some studies have been unable to see effects of non-reinforced odor exposure. For instance, Terry and Johanson (1996) found increased activity, mouthing, and preference to an odor only when it had been paired with maternal behavior. Familiarity, in this case, did not seem sufficient to produce effects. These experiments, however, had tested older rats, 3–12 days old, and perhaps more importantly, had exposed the pups to the odor for 24 hours a day from birth until the day of testing, with no retention interval. The present experiments, which used newborn rats, an hour of odor exposure and at least an hour retention interval, found that long durations of odor exposure may not be necessary to see behavioral effects. Perhaps the pups without reinforced odor exposure in Terry and Johanson’s study were still habituated to the odor at test. Future studies may reveal in greater detail precisely how these variables (e.g., age, odor exposure duration, retention interval) affect responding to an odor experienced soon after birth.
The current experiments developed a procedure that can be used to study very early postnatal learning. Just fifteen minutes of odor exposure immediately after cesarean delivery decreased latency to attach to a similarly scented artificial nipple as well as increased motor activity to this same odor at four hours postpartum. Nevertheless, some questions remain. Does prior exposure to an odor increase behavioral activation to only that odor or to any similar or equally salient odor upon reexposure hours later? Also, what are the effects of prior odor exposure on responding to stimulation of an unscented nipple? Regarding the first point, other experiments in our laboratory, using the same procedure, have indicated that odor exposure immediately after birth for an hour is odor-specific (Miller & Spear, 2007). This study found that neither ethanol odor exposure nor similar handling in a no odor exposure group increased activity to lemon odor at test. The second question has yet to be answered.
The immediate postpartum period used in the current experiments was chosen due to the naturally elevated neurochemical and hormonal levels at this time. Not only is this time-dependent change in neurochemical levels convenient for sampling different internal milieus based on time since birth, but raised neurochemical levels may exist in part due to the special ability they afford the pup to learn about its mother soon after birth, a feature of value for survival. Future studies can explicitly test the neurochemical hypothesis outlined above by manipulating NE pharmacologically. Additionally, natural manipulations can be utilized. For example, prenatal ethanol can decrease the testosterone surge seen in males soon after birth (McGivern, Handa, Redei, 1993; Ward, Ward, Denning, French, & Hendricks, 2002). Furthermore, evidence suggests that NE levels (and possibly other neurochemicals) may differ between cesarean and vaginally delivered infants. In one study, a positive correlation existed between duration of labor and umbilical NE levels of human neonates (Varendi, Porter, & Winberg, 2002). A study charting NE soon after birth explicitly compared cesarean- and vaginally-delivered rat pups and found that immediately after birth vaginally delivered pups had higher levels of NE (Ronca, Abel, Renner, Ronan, & Alberts, 2006). Nevertheless, NE levels in the cesarean delivered pups still showed the same general pattern of highest NE level immediately after birth with a significant decline by 120 minutes after cesarean delivery.
Acknowledgments
The research presented in this article was supported by grants from the National Institute of Mental Health (MH035219) and the National Institute of Alcohol Abuse and Alcoholism (AA011960, AA013098, and AA015992) to Norman E. Spear. We express our appreciation to Teri Tanenhaus for assistance with the manuscript and to Paul Zatley for equipment design and manufacture.
References
- Abel RA, Ronca AE, Alberts JR. Perinatal stimulation facilitates suckling onset in newborn rats. Developmental Psychobiology. 1998;32:91–99. doi: 10.1002/(sici)1098-2302(199803)32:2<91::aid-dev2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Alberts JR, May B. Nonnutritive, thermotactile induction of filial huddling in rat pups. Developmental Psychobiology. 1984;17:161–181. doi: 10.1002/dev.420170207. [DOI] [PubMed] [Google Scholar]
- Andrew RJ, Clifton PG, Gibbs ME. Enhancement of effectiveness of learning by testosterone in domestic chicks. Journal of Comparative Physiology and Psychology. 1981;95:406–417. doi: 10.1037/h0077786. [DOI] [PubMed] [Google Scholar]
- Bacher LF, Robertson SS, Smotherman WP. An intrinsic source of behavioral regulation that influences discrete responses to cues important for the initiation of suckling. Behavioral Neuroscience. 2000;114:594–601. [PubMed] [Google Scholar]
- Bordner KA, Molina JC, Spear NE. 2007. Operant conditioning supported by ethanol reinforcement in the newborn rat. Manuscript submitted for publication. [Google Scholar]
- Bordner KA, Spear NE. Trace conditioning in 1-day-old rats. Developmental Psychobiology. 2006a;48:58–70. doi: 10.1002/dev.20108. [DOI] [PubMed] [Google Scholar]
- Bordner KA, Spear NE. Olfactory learning in the one-day old rat: reinforcing effects of isoproterenol. Neurobiology of Learning and Memory. 2006b;86:19–27. doi: 10.1016/j.nlm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Bouslama M, Durand E, Chauvière L, Van den Bergh O, Gallego J. Olfactory classical conditioning in newborn mice. Behavioral Brain Research. 2005;161:102–106. doi: 10.1016/j.bbr.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Spear NE. Ontogeny of memory. Psychological Review. 1972;79:215–236. doi: 10.1037/h0032690. [DOI] [PubMed] [Google Scholar]
- Caza PA, Spear NE. Short-term exposure to an odor increases its subsequent preference in preweanlings rats: a descriptive profile of the phenomenon. Developmental Psychobiology. 1984;17:407–422. doi: 10.1002/dev.420170407. [DOI] [PubMed] [Google Scholar]
- Cheslock SJ, Varlinskaya EI, High JM, Spear NE. Higher order conditioning in the newborn rat: effects of temporal disparity imply infantile encoding of simultaneous events. Infancy. 2003;4:157–176. [Google Scholar]
- Cheslock SJ, Varlinskaya EI, Petrov ES, Spear NE. Rapid and robust olfactory conditioning with milk before suckling experience: promotion of nipple attachment in the newborn rat. Behavioral Neuroscience. 2000;114:484–495. [PubMed] [Google Scholar]
- Cheslock SJF, Sanders SK, Spear NE. Learning during the newborn’s first meal: special resistance to retroactive interference. Developmental Science. 2004;7:581–598. doi: 10.1111/j.1467-7687.2004.00382.x. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Cordoba NE, Molina JC. Acute prenatal experience with alcohol in the amniotic fluid: interactions with aversive and appetitive alcohol orosensory learning in the rat pup. Developmental Psychobiology. 1991;24:431–451. doi: 10.1002/dev.420240605. [DOI] [PubMed] [Google Scholar]
- Coopersmith R, Henderson SR, Leon M. Odor specificity of the enhanced neural response following early odor experience in rats. Brain Research. 1986;392:191–197. doi: 10.1016/0165-3806(86)90245-2. [DOI] [PubMed] [Google Scholar]
- Corbier P, Edwards DA, Roffi J. The neonatal testosterone surge: a comparative study. Archives Internationales de Physiologie, de Biochimie et de Biophysique. 1992;100:127–131. doi: 10.3109/13813459209035274. [DOI] [PubMed] [Google Scholar]
- Corbier P, Kerdelhue B, Picon R, Roffi J. Changes in testicular weight and serum gonadotropin and testosterone levels before, during, and after birth in the perinatal rat. Endocrinology. 1978;103(6):1985–1991. doi: 10.1210/endo-103-6-1985. [DOI] [PubMed] [Google Scholar]
- Coureaud G, Schaal B, Hudson R, Orgeur P, Coudert P. Transnatal olfactory continuity in the rabbit: behavioral evidence and short-term consequence of its disruption. Developmental Psychobiology. 2002;40:372–390. doi: 10.1002/dev.10038. [DOI] [PubMed] [Google Scholar]
- Dominguez HD, Lopez MF, Molina JC. Neonatal responsiveness to alcohol odor and infant alcohol intake as a function of alcohol experience during late gestation. Alcohol. 1998;16:109–117. doi: 10.1016/s0741-8329(97)00169-9. [DOI] [PubMed] [Google Scholar]
- Gramsbergen A, Schwartze P, Prechtl HFR. The postnatal development of behavioral states in the rat. Developmental Psychobiology. 1970;3:267–280. doi: 10.1002/dev.420030407. [DOI] [PubMed] [Google Scholar]
- Hepper PG. Adaptive fetal learning: prenatal exposure to garlic affects postnatal preferences. Animal Behaviour. 1988;36:935–936. [Google Scholar]
- Hudson R. Olfactory imprinting. Current Opinion in Neurobiology. 1993;3:548–552. doi: 10.1016/0959-4388(93)90054-3. [DOI] [PubMed] [Google Scholar]
- Hudson R, Distel H. The flavor of life: perinatal development of odor and taste preferences. Schweizerische Medizinische Wochenschrift. 1999;129:176–181. [PubMed] [Google Scholar]
- Johanson IB, Hall WG. Appetitive learning in 1-day-old rat pups. Science. 1979;205:419–421. doi: 10.1126/science.451612. [DOI] [PubMed] [Google Scholar]
- Kehoe P, Blass EM. Conditioned aversions and their memories in 5-day old rats during suckling. Journal of Experimental Psychology: Animal Behavior Processes. 1986;12:40–47. [PubMed] [Google Scholar]
- Koffman DJ, Petrov ES, Varlinskaya EI, Smotherman WP. Thermal, olfactory, and tactile stimuli increase oral grasping of an artificial nipple by the newborn rat. Developmental Psychobiology. 1998;33:317–326. [PubMed] [Google Scholar]
- Lagercrantz H, Herlenius E. Neurotransmitters and neuromodulators. In: Lagercrantz H, Hanson M, Evrard P, Rodeck CH, editors. The Newborn Brain: Neuroscience and Clinical Applications. New York: Cambridge University Press; 2002. pp. 139–163. [Google Scholar]
- Lee VW, de Kretser DM, Hudson B, Wang C. Proceedings: FSH, LH and testosterone levels in the rat from birth to sexual maturity. Journal of Reproduction and Fertility. 1974;36:479–480. doi: 10.1530/jrf.0.0360479. [DOI] [PubMed] [Google Scholar]
- McGivern RF, Handa RJ, Redei E. Decreased postnatal testosterone surge in male rats exposed to ethanol during the last week of gestation. Alcoholism: Clinical and Experimental Research. 1993;17:1215–1222. doi: 10.1111/j.1530-0277.1993.tb05232.x. [DOI] [PubMed] [Google Scholar]
- McLean JH, Darby-King A, Sullivan RM, King SR. Serotonergicinfluence on olfactory learning in the neonate rat. Behavioral and Neural Biology. 1993;60:152–162. doi: 10.1016/0163-1047(93)90257-i. [DOI] [PubMed] [Google Scholar]
- Miller SS, Spear NE. Unpublished master’s thesis. State University of New York; Binghamton: 2006. Nonassociative and associative learning in the neonatal rat and parallel changes in neurohormone and brain monoamine levels. [Google Scholar]
- Moriceau S, Sullivan RM. Corticosterone influences on mammalian neonatal sensitive-period learning. Behavioral Neuroscience. 2004;118:274–281. doi: 10.1037/0735-7044.118.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. Guide for the care and use of laboratory animals (DHEW Publication No. 86–23) Washington, DC: U.S. Government Printing Office; 1986. [Google Scholar]
- Nizhnikov ME, Petrov ES, Spear NE. Olfactory aversive conditioning in the newborn (3-Hr-Old) rat impairs later suckling for water and milk. Journal of Experimental Psychology: Animal Behavioral Processes. 2002;28:277–283. [PubMed] [Google Scholar]
- Okutani F, Zhang J-J, Yagi F, Kaba H. Non-specific olfactory aversion induced by intrabulbar infusion of the GABAA receptor antagonist bicuculline in young rats. Neuroscience. 2002;112:901–906. doi: 10.1016/s0306-4522(02)00117-3. [DOI] [PubMed] [Google Scholar]
- Petrov ES, Varlinskaya EI, Smotherman WP. The newborn rat ingests fluids through a surrogate nipple: a new technique for the study of early suckling behavior. Physiology & Behavior. 1997;62:1155–1158. doi: 10.1016/s0031-9384(97)00310-7. [DOI] [PubMed] [Google Scholar]
- Polan HJ, Hofer MA. Olfactory preference for mother over home nest shavings by newborn rats. Developmental Psychobiology. 1998;33:5–20. [PubMed] [Google Scholar]
- Polan HJ, Hofer MA. Maternally directed orienting behaviors of newborn rats. Developmental Psychobiology. 1999;34:269–279. doi: 10.1002/(sici)1098-2302(199905)34:2<269::aid-dev3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Smotherman WP. Habituation and classical conditioning in the rat fetus: opioid involvements. In: Lecanuet J–P, Fifer WP, Krasnegor NA, Smotherman WP, editors. Fetal Development: A Psychobiological Perspective. Hillsdale, NJ: Lawrence Erlbaum; 1995. pp. 295–314. [Google Scholar]
- Ronca AE, Abel RA, Ronan PJ, Renner KJ, Alberts JR. Effects of labor contractions on catecholamine release and breathing frequency in newborn rats. Behavioral Neuroscience. 2006;120:1308–1314. doi: 10.1037/0735-7044.120.6.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronca AE, Alberts JR. Simulated uterine contractions facilitate respiratory behavior in fetal and newborn rats. Physiology & Behavior. 1995;58:1035–1041. doi: 10.1016/0031-9384(95)00155-c. [DOI] [PubMed] [Google Scholar]
- Rovee-Collier C. The development of infant memory. Psychological Science. 1999;8:80–85. [Google Scholar]
- Rumsey JD, Darby-King A, Harley CW, McLean JH. Infusion of the metabotropic receptor agonist, DCG-IV, into the main olfactory bulb induces olfactory preference learning in rat pups. Developmental Brain Research. 2001;128:177–179. doi: 10.1016/s0165-3806(01)00156-0. [DOI] [PubMed] [Google Scholar]
- Schaal B, Hummel T, Soussignan R. Olfaction in the fetal and premature infant: functional status and clinical implications. Clinics in Perinatology. 2004;31:261–285. doi: 10.1016/j.clp.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Schapiro S, Salas M. Behavioral response of infant rats to maternal odor. Phisiology and Behavior. 1970;5:815–817. doi: 10.1016/0031-9384(70)90285-4. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Goffman D, Petrov ES, Varlinskaya EI. Oral grasping of a surrogate nipple by the newborn rat. Developmental Psychobiology. 1997;31:3–17. doi: 10.1002/(sici)1098-2302(199707)31:1<3::aid-dev2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Stickrod G, Kimble DP, Smotherman WP. In utero taste/odor aversion conditioning in the rat. Physiology & Behavior. 1982;28:5–7. doi: 10.1016/0031-9384(82)90093-2. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, McGaugh JL, Leon M. Norepinephrine-induced plasticity and one-trial olfactory learning in neonatal rats. Brain Research: Developmental Brain Research. 1991;60:219–228. doi: 10.1016/0165-3806(91)90050-s. [DOI] [PubMed] [Google Scholar]
- Spear NE, Riccio DC. Memory: phenomena and principles. Boston, MA: Allyn and Bacon; 1994. Developmental change in the processing of memories; pp. 177–211. [Google Scholar]
- Sullivan RM, Taborsky-Barba S, Mendoza R, Itano A, Leon M, Cotman CW, et al. Olfactory classical conditioning in neonates. Pediatrics. 1991;87:511–518. [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Toubas P. Clinical usefulness of maternal odor in newborns: soothing and feeding preparatory responses. Biology of the Neonate. 1998;74:402–408. doi: 10.1159/000014061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. The locus coeruleus, norepinephrine, and memory in newborns. Brain Research Bulletin. 1994;35:467–472. doi: 10.1016/0361-9230(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Wong R, Correa A, Leon M. Modified behavioral and olfactory bulb responses to maternal odors in preweanling rats. Brain Research. Developmental Brain Research. 1990;53:243–247. doi: 10.1016/0165-3806(90)90013-o. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Blass EM. First suckling response of the newborn albino rat: the roles of olfaction and amniotic fluid. Science. 1977;198:635–636. doi: 10.1126/science.918660. [DOI] [PubMed] [Google Scholar]
- Terry LM, Johanson IB. Effects of altered olfactory experiences on the development of infant rats’ responses to odors. Developmental Psychobiology. 1996;29:353–377. doi: 10.1002/(SICI)1098-2302(199605)29:4<353::AID-DEV4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Timberlake W, Silva KM. Appetitive behavior in ethology, psychology, and behavior systems. In: Thompson NS, editor. Perspectives in Ethology. Vol. 11. New York: Plenum Press; 1973. pp. 211–247. [Google Scholar]
- Varendi H, Porter RH, Winberg J. The effect of labor on olfactory exposure learning within the first postnatal hour. Behavioral Neuroscience. 2002;116:206–211. doi: 10.1037//0735-7044.116.2.206. [DOI] [PubMed] [Google Scholar]
- Ward OB, Ward IL, Denning JH, French JA, Hendricks SE. Postparturitional testosterone surge in male offspring of rats stressed and/or fed ethanol during late pregnancy. Hormones and Behavior. 2002;41:229–235. doi: 10.1006/hbeh.2001.1746. [DOI] [PubMed] [Google Scholar]
- Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106:306–316. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Fletcher ML, Sullivan RM. Acetylcholine and olfactory perceptual learning. Learning & Memory. 2004;11:28–34. doi: 10.1101/lm.66404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Neurobiology of associative learning in the neonate: early olfactory learning. Behavioral and Neural Biology. 1994;61:1–18. doi: 10.1016/s0163-1047(05)80039-1. [DOI] [PubMed] [Google Scholar]