Abstract
To establish for the first time how mice might differ from rats and humans in terms of copper transport, excretion, and copper binding proteins, plasma and organ cytosols from adult female C57CL6 mice were fractionated and analyzed by directly coupled size exclusion HPLC-ICP-MS, before and after i.p. injection of large doses of 65Cu. Plasma from untreated mice had different proportions of Cu associated with transcuprein/macroglobulin, ceruloplasmin and albumin than in humans and rats, and two previously undetected copper peaks (Mr 700k and 15k) were observed. Cytosols had Cu peaks seen previously in rat liver (Mr >1000k, 45k and 11k) plus one of 110kDa. 65Cu (141 μg) administered over 14h, initially loaded plasma albumin and mainly entered liver and kidney (especially 28kDa and 11kDa components). Components of other organs were less, but still significantly enriched. 63Cu/65Cu ratios returned almost to normal by 14d, indicating a robust system for excreting excess copper. We conclude that there are significant differences but also strong similarities in copper metabolism between mice, rats and humans; that the liver is able to buffer enormous changes in copper status; and that a large number of mammalian copper proteins remain to be identified.
Keywords: copper binding proteins, mouse plasma, cytosol, heavy isotope, excretion
Introduction
Copper is an obligatory trace element in all living organisms and has been identified as important for the functions of a discrete number of redox enzymes involved in varied aspects of mammalian metabolism. These include mitochondrial cytochrome c oxidase - the terminal step of respiration; dopamine-beta-monooxygenase and alpha-amidating enzyme - required for the formation of specific neurotransmitters; lysyl oxidase - needed for the cross-linking maturation of collagen and elastin in connective tissue; tyrosinase – which helps to form the pigment, melanin; and Cu/Zn superoxide dismutase and ceruloplasmin – which protect against reactive oxygen species (Harris 2000; Linder 1991; Linder 2002). More recently, many copper binding proteins necessary for its uptake and transport have been identified, including the membrane transporters, CTR1 (Kuo et al. 2001; Lee et al. 2001) and DMT1 (Fleming et al. 1998; Gunshin et al. 1997); the copper “chaperones” HAH1, CCS and COX17 that shuttle Cu through the cytoplasm to specific sites (Amaravadi et al. 1997; Culotta et al. 1997; Huffman & O’Halloran 2001; Klomp et al. 1997; Linder 2002); and the copper “pumps” ATP7A and B that are defective in Menkes and Wilson diseases, respectively [Camakaris et al. 1999; Mercer 2001; Puig & Thiele 2002). Liver ATP7B is particularly important in helping mammals such as rats and humans efficiently eliminate excess copper (Linder & Roboz 1986; Turnlund et al. 1989; Turnlund et al. 1998) in the bile (Roeser et al. 1980; Schaefer et al. 1999) in a relatively non-re-absorbable form (Linder 1991). In the absence of effective ATP7B, Cu accumulates, particularly in the liver, causing cirrhosis and reduced organ function, leading to an early death in those with this genetic trait (Wilson disease). The identity and role of all the participants needed to transport excess copper to the bile are not yet understood; and other gene products must also be involved, including MURR1/COMMD1 (Burstein et al. 2005; Klomp et al. 2003), a multifunctional protein defective in the Bedlington terrier, a lack of which also results in liver copper accumulation and toxicity. In rats and humans, however, the ability to excrete excess copper via the bile is well developed and largely responsible for maintenance of whole body copper homeostasis (Linder 2001). Thus, there is only short term storage of excess copper (in metallothionein), and excesses are readily excreted, as shown in rats with whole body counting after administration of radioactive 67Cu [32] and in humans with the stable isotopic 65Cu (Turnlund et al. 1989; Turnlund et al. 1998).
It has long been known that ceruloplasmin is the main copper-binding component in the blood plasma and most other body fluids of the mammal. This blue, multi-copper oxidase appears to have pleotropic functions and has been referred to as a prototypic “moonlighting” protein (Bielli & Calabrese 2002), capable of oxidizing a variety of substrates – from amines to Fe(II) (Harris 2000; Linder 1991; Linder 2002); scavenging radicals (Linder 2001; Linder 2002); and delivering copper to tissues (Campbell et al. 1981; Lee et al. 1993), probably via specific receptors (Kataoka & Tavassoli 1985; Linder 2002; Stevens et al. 1984). Its apparent role in Fe transport (attributed to its ferroxidase activity) has been of particular recent interest. Thus, in humans (Harris et al. 1998; Harris et al. 1995; Yoshida et al. 1995) or mice (Harris et al. 1999) lacking ceruloplasmin genetically or through severe Cu deficiency (Linder 1991; Linder 2002; Osaki & Johnson 1969; Ragan et al. 1969) there is a gradual accumulation of excess iron in certain tissues, including the liver and retina, resulting in tissue damage. This has been explained on the basis that ceruloplasmin oxidizes Fe(II) as it leaves cellular storage sites, promoting the binding of the resulting Fe(III) to its blood plasma transporter, transferrin (Frieden 1970; Linder 1991). This concept is supported by experimental evidence that intravenous infusion of ferroxidase-active ceruloplasmin into organs of ceruloplasmin-deficient dogs (Osaki & Johnson 1969) or pigs (Ragan et al. 1969) results in the immediate release of iron into the blood, although it appears that only extremely low levels of active ceruloplasmin (<1% of normal) affect iron transport and accumulation (Roeser et al. 1980).
In human and rat blood, ceruloplasmin accounts for approximately 70% of the total copper in the plasma (Barrow & Tanner 1988; Weiss et al. 1985; Wirth Linder, 1985) rather than the 95% usually quoted. The remainder of the Cu is thought to be associated mainly with two other proteins that may make up most of the exchangeable copper pool in blood fluids. These are albumin, which in most mammals has a single, high affinity copper-binding site at its N-terminus involving a crucial histidine (Linder 1991; Linder 2002); and a macroglobulin, designated alpha-1-inhibitor-3 in rodents and alpha-2-macroglobulin in humans (Liu et al. 2007). The macroglobulins have an even higher affinity for copper than albumin, and rapidly exchange copper with the latter, in vitro (Harris 2000; Linder 1991; Linder 2002; Trang Nguyen, Mizue Moriya, Ann Grana, Mimi Mak and Maria Linder, unpublished results]. Traces of other copper containing proteins are also present in human plasma/serum (Linder 1991), although their contributions to total plasma/serum copper is unclear. These include metallothionein, ferroxidase II, blood clotting factors V and VIII, extracellular SOD and amine oxidase, as well as possibly histidine-rich glycoprotein and unknown small peptides (Linder 1991; Linder 2002).
In the cell cytoplasm, several prominent copper binding proteins have also been separated by size exclusion chromatography (Freedman et al. 1989; Harris 2000; Norton & Heaton 1980; Sharma & McQueen 1981; Terao & Owen 1973). The two most commonly identified are Cu/Zn SOD (about 35,000 Da) and metallothionein (Mr about 15,000), although the previously mentioned (similar-sized) “chaperones” (HAH1, CCS, COX17) for shuttling copper to specific intracellular sites (Amaravadi et al. 1997; Culotta et al. 1997; Huffman & O’Halloran 2001; Klomp et al. 1997), and some other already mentioned soluble copper proteins/enzymes, are also present. A large (> 100 kDa) unknown component has also commonly been observed.
Most of the information about copper in the various proteins described and the kinetics of its transport and excretion has been obtained from trace analysis of the copper in tissues and from by following the distribution of radioactive copper in rats (Linder 1991; Linder 2002; Terao & Owen 1973). In humans, there have also been some studies using the stable isotope, 65Cu Turnlund et al. 1989, 1998). Today, because of its size and ease of genetic manipulation, the mouse is the most widely used organism to model the transport and metabolism of specific nutrients or metabolites in humans; yet the Cu metabolism of mice is not well studied, particularly with regard to copper transport and blood plasma binding components. Indeed, earlier experimental work from our laboratory (Montaser et al. 1992) and that of Prohaska (1983) indicated that copper metabolism in the mouse might differ from that in humans and rats. Thus, the first objective of the studies reported here was to examine this issue. To accomplish this, directly coupled size exclusion high performance liquid chromatography inductively coupled plasma mass spectrometry (HPLC-ICP-MS) was used to compare the copper binding components in blood plasma from mice, humans and rats, as well as in cytosols of a variety of organs. This technique was also used to monitor the biochemical responses and long term fluxes of copper, into and out of organs of the mice, after a large i.p. injection of 65Cu. This stable isotope has been used in low doses in mice too study hepatic Cu turnover (Ting et al. 1990) but has not been used for temporal analyses to study Cu turnover in the blood or other organs (or their individual components). The results reported here are the first showing that the mouse differs considerably from the rat and human with regard to the proportions of copper associated with various plasma proteins; that the major mouse cytoplasmic binding proteins responsible for coping with larger doses of extraneous copper are similar to those previously reported in the rat; and that with high doses of Cu, it is possible to obtain significant, but not substantial, enrichments of copper proteins in organs peripheral to the liver.
MATERIALS AND METHODS
Materials
The stable isotope, 65Cu, was obtained as CuO from Oak Ridge National Laboratory (Oak Ridge, TN). Ultrapure nitric acid was from Fisher Scientific (Trace element grade). Heparinized human blood plasma was obtained from healthy, adult volunteers at the university, according to a protocol approved by the California State University, Fullerton, IRB (Approval No. HSR # 05-004). Blood was taken at the Health Center and processed anonymously in the investigators’ research laboratory. Plasma was separated by centrifugation and either used right away (fresh plasma) or stored frozen at −20° or −80° C, in aliquots, until use.
Mice, Rats and treatments
Adult, female C57-BL6 mice, and adult female Sprague Dawley and male Fisher rats were obtained from Simonson Laboratories (Gilroy, CA) and maintained on normal rodent chow (Harlan Teklad rodent chow, Madison, WI). Ceruloplasmin knockout mice were from our own colony, derived from heterozygous breeding pairs obtained from Z. Leah Harris at Johns Hopkins University. Protocols for treatments were approved by the university IACUC (No. 01-R1-05). Some of the C57BL6 mice were injected i.p. with five 25–30 μg doses of 65CuCl2 in 0.9% NaCl (for a total of 141 μg) containing 50 μM nitrilotriacetate (NTA), and they were euthanized in pairs at various times (30 min to 14 days) following the last injection. The NTA was added to assure solubility and bioavailability of the copper injected. Thus, the Cu-NTA complex readily transfers the copper to its plasma binding proteins, as repeatedly demonstrated by comparing additions of 67Cu(II) or 67Cu(II)-NTA to blood plasma (Lee et al. 1993; Weiss et al. 1985), and comparing its tissue and protein distribution after i.v. administration to rats (Vargas et al. 1994; Weiss et al. 1985). Euthanization was by bleeding from the vena cava under pentobarbital anesthesia after treatment with heparin, as previously described (Linder & Roboz 1986; Weiss et al. 1985). Plasma was prepared and stored as described above. Liver, kidney, heart, lungs, spleen and brain were collected and homogenized with trace metal grade 0.15M NH4Cl. Tissue cytoplasm was obtained after centrifugation for 1h at 105,000 x g in a Beckman L8-70M ultracentrifuge, at 4°C. Portions of homogenate and cytoplasm were stored at −80° C until analyzed. Plasma was also obtained from normal adult, female Fisher and male Sprague Dawley rats, euthanized and bled as described for the mice.
Tissue, cytoplasm and plasma copper analysis
For total Cu isotope quantitation, either 100 or 250 μl portions of homogenate or cytoplasm, respectively, were dried to a constant weight at 60° C in acid-washed disposable tubes (Cat. No. 14-958-C; Fisher Scientific), then wet ashed on a hot plate with 250 μl of ultrapure concentrated HNO3 until almost dry. Residues were dissolved in 4.5 ml of 2% ultrapure HNO3 containing 10 ppb of internal standards (Ga, In, Tl and Y). Serum was diluted 100-fold with the same acid-internal standard solution. Individual Cu isotopes were quantified using a Perkin Elmer 6100 DRC ICP-MS from standard curves of Cu generated from dilutions of a multi-element standard with known isotopic ratios of 63Cu:65Cu (SCP Science, Champlain, NY). Analyses of bulk homogenates, serum and cytoplasm samples from control animals showed 63Cu:65Cu ratios consistent with that of the measured natural isotopic abundance of the standards, indicating minimal interference from 63NaAr+ and other polyatomic adducts. Concentrations of 63Cu and 65Cu in the sample were expressed relative to protein content or dry weight. In the injected animals, the content of 65Cu was partitioned into intrinsic 65Cu (pre-existing) and extrinsic 65Cu (experimentally administered). Intrinsic 65Cu was calculated from the measured 63Cu content of the sample using the 63Cu: 65Cu isotope ratio in equivalent control samples. The extrinsic 65Cu was determined by difference (total 65Cu minus the calculated intrinsic 65Cu content).
Protein analysis
Protein content was determined using a Bradford dye binding assay (BioRad, Richmond, CA) and bovine serum albumin as a standard.
Directly coupled size exclusion HPLC-ICP-MS
Chromatographic separations and isotopic determination of samples were performed using a “biocompatible” Beckman 126 programmable solvent delivery module equipped with a Beckman 168 diode array UV/Vis detector that was directly coupled to ICP-MS (Mason & Borja 2002). PEEK@ tubing was used to connect the components in the system. Aspiration was through a quartz Meinhard concentric nebulizer and a cyclonic spray chamber. Standard curves for apparent molecular weight (Mr) were obtained using gel filtration standards that included thyroglobulin (670 kDa), ferritin (480 kDa), bovine gamma-globulin (158 kDa), human ceruloplasmin (132 kDa), chicken ovalbumin (44 kDa), equine myoglobin (16.7 kDa), and vitamin B12 (1.34 kDa). Samples (100 μl) of 5-fold diluted plasma or tissue cytosol were filtered (0.45 μm) and fractionated isocratically (20 mM Tris.HCl, pH 7.4) by size exclusion chromatography, using either a Biosep 2000 or 4000 SEC column (Phenomenex, Torrance, CA). The isotopic ratio of 63Cu: 65Cu of the various peaks was determined at the centroid value of each peak. Analyses of samples from control animals showed protein peaks with 63Cu: 65Cu ratios that were attenuated relative to the measured natural abundance of the Cu standards, indicating some inerference and augmentation of the signal at M/Z 65 from polyatomic adducts, possibly 48Ca16O1H generated from the buffer.
Statistics
Statistical analysis of the data was by one way ANOVA. Probability (p) values of < 0.05 were considered significant.
RESULTS
Comparisons of the copper binding components of mouse plasma in comparison with those of humans and rats
The distribution of copper among binding proteins of the blood plasma of the mouse has not been previously examined. To do this and compare the mouse with the more comprehensively-studied rat and human systems, fresh and previously frozen samples from adult, female C57BL6 mice and from normal adult humans and rats were separated by HPLC size exclusion chromatography and analyzed by UV/Vis spectroscopy prior to coupled ICP-MS elemental analysis. This approach was taken as well to obtain more refined data for copper binding components in all three species than previously available in the literature. Initial fractionation of rat and human plasma using a Biosep 2000 column showed one major copper-containing peak, eluting at 11.3 min, having a narrow shoulder coinciding with the void volume (10.6 min) (Figure 1A). Another (smaller) component eluted at about 14 min. The apparent molecular weights (Mr) of the major (11.3 min) and minor (14 min) components were 124k and 72 k, respectively, which approximate to the molecular weights for ceruloplasmin (132 k) and albumin (69 kDa), respectively. Fractionation of mouse plasma (Figure 1B) showed more copper associated with the presumed albumin peak (around 14 min) and proportionately less with ceruloplasmin, although the latter still predominated. In addition to this difference in Cu distribution, the overall copper content of mouse plasma was considerably lower than that of the human and rat. Concentrations of total copper (ng/ml) in the mouse samples were 400 ± 160 (Mean ± SD; N = 4), whereas those of humans and rats were in the range of 1000–1200 ng/ml [29].
Figure 1.


Size exclusion HPLC separation of copper binding components of human, rat and mouse plasma/serum, on a medium-pore column. Fresh or pre-frozen samples of human (A), rat (A) and mouse (B) blood plasma were fractionated on a Biosep 2000 HPLC column, and the elution of copper-containing components was monitored by coupled ICP-MS. Elution time (min) on the x-axis is plotted against the ion intensity (cps) for 63Cu. The main copper peak (11.3 min) and its shoulders at 10.6 (void vol) and 14 min had Mr values of 124, >200, and 72 k, respectively.
To facilitate further separation of the larger proteins (including the macroglobulins) from ceruloplasmin, the plasma samples were fractionated on a Biosep 4000 column (Figure 2). Typically, five copper peaks could be observed in chromatograms from fresh or frozen (not shown) samples of mouse plasma using this column (Figure 2A). Beginning soon after the void volume, there was a small shoulder of copper (peak 1) followed by a significant minor peak (peak 2) eluting at 15.5 min. The latter accounted for about 8% of the total copper and had an Mr of about 300 k (Figure 2B). This is consistent with the macroglobulin, alpha-1-inhibitor-3 [33], which we have previously identified as the important copper transport protein in rat plasma, transcuprein, of Mr 270 k (Vargas et al. 1994; Weiss et al. 1985). Peak 3 (Fig. 2A) eluted at 16.8 min with a Mr of approximately 190 k, contained about 55% of the plasma copper, and was tentatively identified as ceruloplasmin on the basis that it was markedly diminished in the plasma of knockout mice (data not shown). Peak 4 contained approximately 25% of the total plasma copper, and eluted at 18.8 min (Mr 78 k) just ahead of pure hemoglobin standard (64 kDa) (data not shown), consistent with it being albumin. The final copper peak eluted as a broad shoulder centered around 22.5 min (approximately 15 kDa).
Figure 2.






Size exclusion HPLC separation of copper binding components of fresh mouse plasma, on a large-pore column. A. Samples were fractionated on a Biosep 4000 HPLC column and the copper-containing components monitored by coupled ICP-MS. Data are presented as in Figure 1 for two representative samples. Similar results were obtained with multiple pre-frozen samples. Peaks/shoulders numbered 1–5 were calculated to have Mr values of about 700, 300, 190, 78, and 15 k, respectively. B. Standard curve for the Biosep 4000 column, indicating elution of the following standards, from left to right: thyroglobulin (670 kDa), ferritin (480 kDa), bovine gamma-globulin (158 kDa), human ceruloplasmin (132 kDa), chicken ovalbumin (44 kDa), equine myoglobin (16.7 kDa), and vitamin B12 (1.34 kDa).
Copper profiles of human and rat serum/plasma fractionated on the Biosep 4000 column showed certain differences from the mouse data. Analyses of human plasma obtained from five individuals showed one major and three minor copper peaks (Figure 3A). Peak 1 eluted at 14.1 min (Mr about 680 k) and accounted for 5–10% of the total serum Cu. This Mr is close to that of the major human macroglobulin, alpha-2-macroglobulin (Sottrup-Jensen 1989), which is a tetramer of 180 kDa subunits – in contrast to the monomeric rodent macroglobulin, alpha-1-inhibitor-3 (Liu et al. 2007), eluting at 15.5 min (Figure 2A). Pure alpha-2-macroglobulin, purified from human plasma by size exclusion and Cibacron affinity chromatography (Lonberg-Holm et al. 1987) eluted with the same retention time (data not shown), and we have shown previously that this protein is the human transcuprein that also binds copper tightly (Liu et al. 2007). Peak 2 (Figure 3A) contained approximately 65% of the total serum Cu and eluted at a time corresponding to ceruloplasmin (16.9 min). Peak 3 contained about 15% of the copper, and eluted at 18.7 min as a shoulder on peak 2, with a retention time comparable to albumin. An additional, minor copper-containing component, eluting at about 22 min (about 15 kDa; also seen in mouse plasma) was observed as well.
Figure 3.


Size exclusion HPLC separation of copper binding components of human and rat plasma, on a large-pore column. Results for fresh samples from 5 humans (A) and rats (B) (female Fisher lactating dam and her pups) are shown in the same way as for Figures 1 and 2. Similar results were obtained with multiple pre-frozen samples. For the human plasma, peaks/shoulders 1–4 were calculated to have average Mr values of 676, 187, 82, and 15 k, respectively. Mr values for the rat peaks were about 700, 450, 178, with a broad shoulder ranging from about 80 to 15 kDa, and a small peak at 24 min (about 7 kDa).
Rat plasma had a very similar overall profile to that of the human (Figure 3B), except that the copper eluting with macroglobulin/transcuprein (before the ceruloplasmin peak; peak 3) was approximately 450 rather than 680 kDa, consistent with rodent versus human macroglobulins (Sottrup-Jensen 1989). Collectively, the results confirm that ceruloplasmin is the most dominant copper binding protein in the blood plasma/serum of several mammals, but that it is only one of several proteins accounting for the total copper in this fluid, with other components carrying a substantial portion of the metal, and particularly so in the mouse.
Copper binding components of organ cytoplasms
To detect and compare (with published data for the rat) the major copper binding components of mouse tissue cytoplasms, samples of 100,000 x g supernatants from kidney, liver, heart, spleen, lung and brain homogenates of the same mice used for plasma analysis were fractionated on a Biosep 4000 HPLC column and analyzed by ICP-MS. Comparable copper profiles were observed for all of the tissues (Figure 4). A significant copper binding component (peak 1) eluted close to the void volume (12 min; > 1000 kDa) and, with the exception of the brain, a second peak was observed at approximately 18 min (Mr about 110 k). In all but heart and brain, the dominant copper peak (peak 3) eluted at about 20 min (Mr about 45 k). In the heart (Figure 4B), there was a separate peak at about 23 min (Mr about 11 k) (peak 4), which coincided with shoulders in that region of the elution profiles for other tissues (Figures 4A–C). The brain, which had the lowest amounts of cytosolic copper, had a broad peak encompassing the region of peaks 3–4 in the other tissues (Figure 4C). The major copper peak at 20 min (45 kDa) coincided precisely with the largest peak for Zn in the kidney (Figure 4D) and liver (data not shown) and was tentatively identified as Cu/Zn superoxide dismutase (about 35 kDa). Zn also eluted with peak 2 for copper in the kidney, and in the void. There was no evidence for a discrete peak for metallothionein at 7 kDa (~24 min), although an 11 kDa peak containing Cu with traces of Zn was observed in heart cytosol (Figure 4E), which is consistent with the known retention characteristics of metallothionein in size exclusion chromatography (Kagi & Nordberg 1970).
Figure 4.
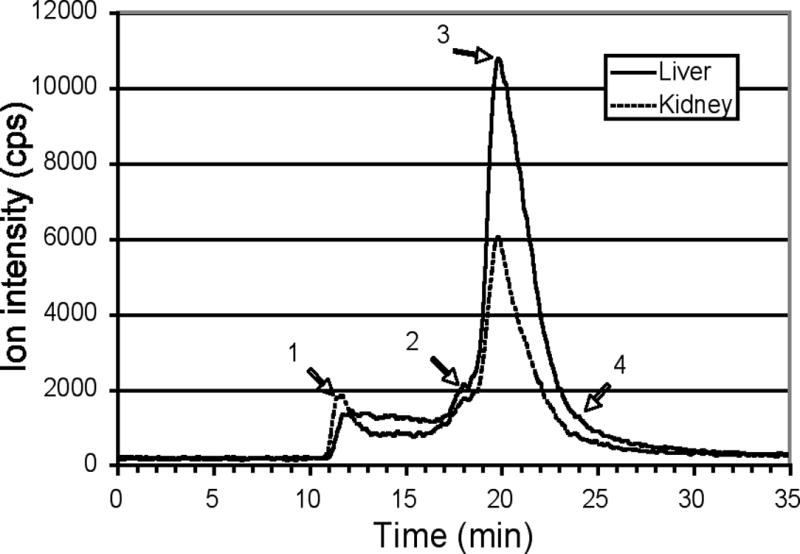




Size exclusion HPLC separation of copper (and zinc) binding components in cytoplasm of tissues of normal adult female mice. Samples of cytoplasm obtained by centrifuging fresh homogenates for 1h at 105,000 x g (see Methods) were applied to the same large-pore size exclusion HPLC column as for plasma (Figures 2–3) and plotted in the same way. Representative 63Cu elution profiles for (A) liver and kidney, (B) heart and spleen, (C) lung and brain have been superimposed. The Mr values for peaks/shoulders 1–4 were calculated to be >1000, 110, 45, and 11 k, respectively. In D and E, the zinc elution profiles for the same samples of kidney (D) and heart (E) cytosol have been superimposed on those for copper.
Effects of injections of excess copper, as 65Cu
To determine how large doses of copper would be accommodated by and eliminated from these various binding proteins and whole organs, mice were injected with a total of 141 μg 65Cu(II)-NTA, given in 5 injections (of 25–30 μg) over 14 h. The total dosages injected were estimated to be 3–4 times the copper already present in a 30 g mouse and were administered to ensure sufficient enrichment of 65Cu in organs and cytosolic components beyond the liver for analytical precision. The excess copper produced no obvious changes in the behavior or activity of the mice, indicating a good toleration.
Pairs of mice were euthanized at various times after the last 65Cu injection to analyze the distribution of 65Cu. Figure 5 shows the calculated values for the distribution of injected (extrinsic) 65Cu in the various mouse organs, as well as for preexisting, intrinsic 63Cu and 65Cu, 2 h (Figure 5A) and 24 h (Figure 5B) after the last 65Cu injection. Values were calculated from the measured 65Cu and 63Cu concentrations and the natural 63Cu/65Cu ratio (see Methods). Analysis of the partitioning of the extrinsic 65Cu in the various tissues 2 h after the last injection showed that the liver had by far the highest concentration, followed by the kidney, with minimal accumulation in heart and brain. Extrinsic 65Cu in the plasma was also greatly elevated, being 3x the intrinsic content 2 h post injection, as would be expected, since the copper was distributed to the tissues through the blood. Indeed, analyses of plasma samples 30 min after the last 65Cu injection showed levels of extrinsic 65Cu in plasma 5x higher than at 2 h (data not shown). This implied rapid loss of the copper from the circulation. Levels of extrinsic 65Cu in plasma fell to about twice normal levels by 24 h (Figure 5B), while minimal losses were observed from the liver. The total concentrations of copper in heart, spleen and brain increased significantly between 2 and 24 h post injection. These increases were caused by an influx of intrinsic copper, suggesting that the injected 65Cu had induced displacement and transfer of preexisting metal from other sites in the animal.
Figure 5.


Copper contents of mouse tissues 2 and 24 h after injections of large doses of 65Cu. Concentrations of intrinsic 63Cu (black bars), intrinsic 65Cu (clear/white bars), and extrinsic (excess) 65Cu (grey bars), in nmol/mg tissue protein, were determined 2h (A) and 24 h (B) after the last i.p. injection of a 25–30 μg doses of 65Cu. Error bars are average deviations of values for pairs of mice.
Figure 6 shows the temporal changes in extrinsic 65Cu in the tissues and plasma extrinsic 65Cu, from 30 min after the last injection until 24 h (A) or two weeks (B) later, expressed as a percentage of the administered dose. (Values for plasma and brain in the figure have been multiplied for visual clarity.) Again, the overwhelming importance of liver deposition of the injected copper is apparent, with the kidney being of secondary importance, and relatively little deposition occurring in any of the other organs analyzed. Data from liver taken 30 min after the last (of 5) injection of 65Cu showed that this organ contained approximately 30% of the total administered dose. By 6–24 h post-injection, over 50% of the dose was retained in the liver, after which time rapid loss was observed. Thus, from day 1 to day 7, the liver eliminated 40% of the injected copper, a further 17% being lost by the end of the second week. This indicates a highly efficient excretory mechanism, presumably involving the bile (Burstein et al. 2005; Harada et al. 2005; Linder 1991; Linder 2002; Linder & Roboz 1986; Turnlund et al. 1989 and 1998). Indeed, mass balance calculations of the total extrinsic 65Cu content of the analyzed tissues at each time period showed incomplete recovery of the administered dose, with no more than 60% being accounted for at any time tested. This is consistent with either a very high initial rate of excretion (during the period of isotope administration prior to the last injection) and/or partitioning into tissues such as the bone, skin, viscera, etc. that were not analyzed.
Figure 6.


Time course of distribution of extrinsic (excess) 65Cu to tissues and plasma at various times after the last injection of the stable isotope (as % of dose). Error bars are average deviations of values for pairs of mice. Data for plasma and brain have been multiplied 10x and 100x, respectively, to make the changes more visible. (A) Results for the first 24h; (B) results for the full time course of 14 days (after the last injection).
The changes in the proportion of the total extrinsic 65Cu accounted for by the cytoplasm of each organ, and temporal changes in these proportions, were also calculated (Table 1). In the case of the liver, most of the extrinsic 65Cu was cytoplasmic after 24 h, and this proportion remained essentially constant for the duration of the experiment (data not shown). In the case of the kidney, just over half was initially in the cytoplasm, and this declined slowly over the following 7–14 days. In this respect, it is of interest to note that the kidney remained enriched with 65Cu longer than the liver (Figure 6B), and that most of the extrinsic 65Cu remaining at 7 and 14 days was not in the cytoplasm (Table 1). Similarly, the spleen showed a marked reduction in the proportion of extrinsic 65Cu retained in the cytoplasm. In contrast, the cytoplasmic content of extrinsic 65Cu in the brain and lung increased from initial values of about 65% to account for more than 89% of the retained dose by 7–14 days. However in heart cytoplasm, the proportion stayed relatively low and constant.
Table 1.
Distribution of extrinsic 65Cu among tissues and the proportion in their cytoplasms
| Tissue | Total Extrinsic 65Cu (nmol/mg protein) | Extrinsic 65Cu in Cytoplasm (nmol/mg protein) (percent) |
|---|---|---|
| Liver (up to 24h) | 1.15 ± 0.38 (8) | 0.95 ± 0.35 (8) 83% |
| Kidney (up to 6h) | 0.19 ± 0.06 (6) | 0.11 ± 0.04 (6) 55% |
| (7–14d) | 0.039 ± 0.023 (4) | 0.015 ± 0.009 (4) 38% |
| Heart (up to 7d) | 0.092 ± 0.026 (10) | 0.023 ± 0.007 (10) 25% |
| Lung (up to 7d) | 0.084 ± 0.013 (8) | 0.058 ± 0.009 (9) 69% |
| (14d) | 0.043, 0.035 (2) | 0.036, 0.033 (2) 89% |
| Spleen (up to 24h) | 0.058 ± 0.015 (8) | 0.040 ± 0.030 (4) 69% |
| (7–14d) | 0.016 ± 0.010 (4) | 0.005 ± 0.003 (4) 31% |
| Brain (up to 24h) | 0.0085 ± 0.0027 (8) | 0.0056 ± 0.0019 (7) 65% |
| (7–14d) | 0.0072 ± 0.0019 (4) | 0.0069 ± 0.0023 (4) 96% |
Values are Means ± SD (N) for samples taken at different times after the last injection of 65Cu
To detect and compare the changes in enrichment of 65Cu in the cytoplasmic copper binding proteins, portions from the liver (Figure 7A), kidney (Figure 7B) and brain (Figure 7C) 2 h, 6 h and 14 days after the last 65Cu injection were fractionated on the Biosep 4000 column. The relative degree of Cu enrichment of each protein was determined by comparing the increases in the absolute intensities of the 65Cu signal and the 63Cu/65Cu ratios at the peak centroid. In the untreated mice, the isotopic ratios of Cu 63Cu/65Cu were invariably lower than the expected natural geological abundance, presumably due to interferences caused by the buffer, averaging 1.70 ± 0.13 (Mean ± SD; N=9). Nevertheless, following injection of 65Cu, a pronounced decrease in the 63Cu/65Cu ratios was noted in all the liver and kidney components (Figures 7A and 7B). In contrast, the 63Cu/65Cu ratios in the brain showed little change over the treatment period (Figure 7C) indicating minimal accumulation of 65Cu in the protein components of this organ.
Figure 7.


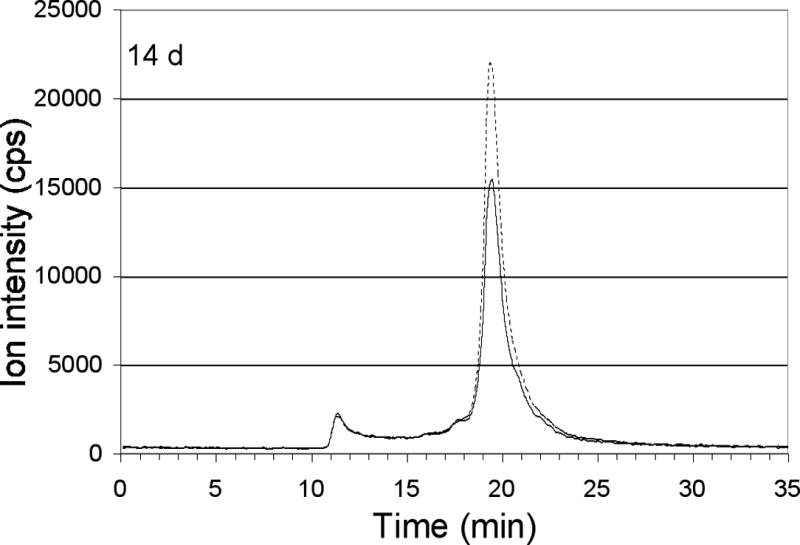









Distribution of excess copper among components of tissue cytoplasm at various times after the final injection of 65Cu. Profiles of total 63Cu (dashed line) and total 65Cu (solid line) associated with copper binding components of the cytoplasm, 2h, 6h and 14 days after the last 65Cu injection, separated by size exclusion HPLC on the large-pore Biosep 4000 column. Elution time (min) on the x-axis is plotted against the ion intensity, which indicates copper quantitation. Also shown for each tissue (at bottom) is the ratio of 63Cu/65Cu for individual peaks, at the times indicated (control, 0-time ratios being from un-injected mice). Error bars represent average deviations for values from pairs of mice. Results are for liver (A), kidney (B) and brain (C). Copper peaks/shoulders 1–5 were calculated to have Mr values of >1000, 110, 45, 28 and 11 k, respectively.
In general, the elution profiles and patterns of turnover of 65Cu in the fractionated cytoplasmic proteins of liver and kidney (Figure 7A and B) were very similar. In addition to having elevated 65Cu in the void volume and 110 kDa components (peaks 1 and 2), and in the dominant 45 kDa peak (No. 3) seen in untreated mouse cytoplasm (Figure 4A), most of the administered extrinsic 65Cu 2 h post injection was bound by peaks 4 and 5 (Mr about 28 and 11 k). Neither peak 4 nor 5 bound significant amounts of Cu in control animals (Figure 4A; and 7A, B, dashed line). After the 65Cu treatment, the general background signal (between 12 and 17 min) of 65Cu in the chromatogram, increased markedly as well. In the liver (Figure 7A), there was considerably less copper at 6 h than 2 h, with 65Cu being eliminated most rapidly from the largest and the 28 kDa component and least rapidly from the 11 kDa moiety. Similar patterns of 65Cu depletion were observed in the cytoplasmic components of the kidney (Figure 7B), although significantly less 65Cu was associated and consequently lost from the void volume peak. Turnover of the metal in peak 3 was significantly more rapid in the liver than the kidney. There was almost complete turnover of metal (or specific loss of the injected 65Cu) in the former (but not the latter) by day 14. By 14 days post injection, much of the injected copper had been eliminated from the liver and kidney cytosol, although the isotopic ratios clearly indicated long term retention of a proportion of the administered 65Cu. At this time, the profiles of 63Cu and 65Cu on the various peaks were closely aligned, implying exchange and equilibration of the intrinsic and extrinsic Cu between the cytoplasmic proteins in these organs (Figures 7A and B).
Unlike the liver and kidney, the ratios of 63Cu/65Cu in the cytosolic components in the brain did not change significantly from that of the untreated mice, even 30 min (data not shown) and 2 h post injection. The pool of copper in this organ was thus essentially pharmacokinetically isolated from the administered injection of 63Cu.
The response of the blood plasma proteins to the excess copper was also examined. Figures 8A–E show that 30 min to 6 h post injection, most of the extrinsic 65Cu was associated with albumin (peak 3, at 18.7 min). In comparison, ceruloplasmin and macroglobulin (peaks 1 and 2, at 15.5 and 16.8 min) bound much less 65Cu, although their isotopic ratios still indicated a substantial enrichment with 65Cu. Plasma concentrations of 65Cu diminished rapidly, as shown by the increase in the 63Cu/65Cu peak ratios from 30 min to 2 h after the last isotope injection (Figure 8A and B), and subsequently between 6 h and 14 d after injection (Figure 8C and D). It should be noted that except right after injection, when albumin was primarily responsible for binding the excess copper, enrichment of the three main copper components (macroglobulin, ceruloplasmin and albumin) appeared to be about the same, consistent with the rapid entry, turnover and equilibration of the copper in these components.
Figure 8.



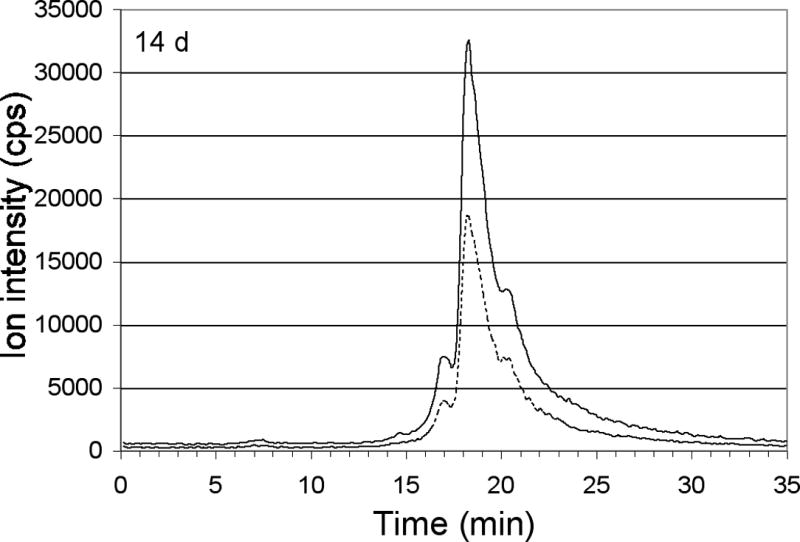

Distribution of excess copper to components of blood plasma at various times after the final injection of 65Cu. Profiles of total 63Cu (dashed line) and total 65Cu (solid line) associated with copper binding components 30 min, 2h, 6h, and 14 days after the last 65Cu injection (A–D), as separated by size exclusion HPLC on the large-pore Biosep 4000 column (as in Figure 7). (E) Ratios of 63Cu/65Cu for the peaks over time, the error bars indicating average deviations for values from pairs of mice. Copper peaks/shoulders 1–4 were calculated to have Mr values of about 450, 190, 78 and 45 k, respectively. [The numbering of peaks is not identical to that in the untreated mice (Figure 2), where an additional component (peak 1) was detectable. Here, peak 2 (rather than 3) is ceruloplasmin.]
DISCUSSION
Our analyses of copper in the plasma/serum and tissues indicates that its distribution in the mouse is similar to the human and rat but also differs significantly in some aspects. The most striking difference is that mice have less than half as much copper in the circulating blood plasma as humans and rats. This is consistent with our earlier findings (Montaser et al. 1992) and is also in accord with the observation that mice also have less copper in their livers and kidneys. Thus, Meyer et al. (2001) reported liver concentrations of 2.6 ± 0.2 μg/g for 10–12 week old mice of the same strain used here, which is significantly lower than the values of 4.6 ± 1.1 and 6.2 ± 0.8 reported for rats and humans, respectively (Means ± SD, N=9–23; Linder 1991. Similarly, mouse kidney values of 3.8 ± 0.1 (Meyer et al. 2001) are significantly less than the values of 7.9 and 12 for rats and humans, respectively (Linder 1991). Heart and brain did not show marked interspecies differences.
As concerns the copper binding components of plasma and serum, we report here for the first time the distribution of copper in the circulation of the mouse together with a refined picture for the human and rat. The current analyses confirm our previous studies and those of others that ceruloplasmin contains approximately two-thirds of the total copper in human and rat blood plasma (Barrow & Tanner 1988; Gless et al. 1992; Weiss et al. 1985; Wirth & Linder 1985). This is contrary to the 95% still often quoted from the old and outdated literature (see Weiss et al. 1985). Approximately 15% of the plasma copper from humans and rats eluted in the position of albumin, which is known to bind one copper ion very tightly at the N-terminus (Linder 1991; Masuoka et al. 1993); and 5–10% eluted with components of Mr consistent with the transcupreins alpha-2-macroglobulin (in humans) or alpha-1-inhibitor-3 (in rats) which bind copper even more tightly than albumin (Linder 1991; Linder 2002; Liu et al. 2007; Masuoka et al. 1993). Our values for unclotted human blood plasma differ from those of Gless et al. (1992) for serum who reported 2.3, 84 and 14% of total copper co-eluting with alpha-2-macroglobulin, ceruloplasmin and albumin, respectively. This difference may relate to the observation that a substantial proportion of the large (750 kDa) alpha-2-macroglobulin can become trapped in the clots removed when serum is harvested from whole blood (Salvatore Pizzo, personal communication). The current studies indicate the mouse has a significantly smaller proportion of its plasma copper (about half) associated with the ceruloplasmin peak than humans and rats, and more associated with the macroglobulin/transcuprein and albumin peaks (about 10 and 25%, respectively).
Using size-exclusion HPLC-ICP-MS, we detected additional copper binding components in plasma, not previously reported. The first (Mr 15k) was present in humans and mice and might be metallothionein, which is known to occur in serum (Garvey & Chang 1981; Mehra & Bremner 1983) and to elute as a seemingly larger protein (Kagi & Nordberg 1979). A second in mice and humans was larger than transcuprein, eluting close to the void volume. A third (~45kDa) was evident in plasma from all three species. (It is noteworthy that no very low molecular weight copper components were detected.) Thus, ceruloplasmin is clearly only one of a number of proteins in the blood fluid that bind quantitatively significant amounts of copper.
Although albumin normally accounts for only 15–25% of the copper in human, rat and mouse blood plasma, it has the potential for binding a great deal more. Indeed, calculations indicate that the albumin in 1 ml of plasma can theoretically bind more than 600 μg of copper with high affinity. This is many orders of magnitude more than that normally bound [about 100 ng/ml in mouse and 120–200 ng/ml in human plasma (Barrow & Tanner 1988; Linder 1991)]. This available binding capacity is clearly illustrated in the current study where approximately 28 μg 65Cu was sequestered by the circulating albumin of one ml of mouse plasma within 30 min of the last injection (one ml being about equivalent to the total plasma volume of the mouse). Thus, albumin may be viewed as an important component of the buffering system of most mammals that acts in concert with other transfer and transport proteins to protect against acute copper toxicity that could otherwise be induced by free copper ions (Linder 2002; Rae et al. 1999).
The cytoplasmic distribution of copper in all of the major organs examined in the mouse showed a consistent association of with 2 or 3 major components that were separable by size exclusion HPLC. This finding is comparable to earlier reports for Cu distribution in a more limited number of rat organs. Thus, rat liver, kidney (and intestinal) cytosols fractionated on Sephadex G75 or 100 showed three consistent components, one eluting in the void volume, one at about 35 kDa and another at about 15 kDa (Linder 1991; Norton & Heaton 1980; Sharma & McQueen 1981; Terao & Owen 1973). It has generally been accepted that the second and third peaks contain Cu/Zn superoxide dismutase and metallothionein (MT), respectively (Linder 1991). However, these reports preceded awareness of several other proteins, including the copper “chaperones”, ATOX1 and COX 17, presumed to be present in most cells, and which transport copper to the TransGolgi network and mitochondria, respectively. Since these are very similar to MT in apparent size (Linder 2002), they will contribute to copper in the 15 kDa peak range. These proteins and MT did not separate very well from Cu/Zn SOD on the larger pore columns used in this study, generally eluting together in a broad (and large) peak. SOD and the others were best differentiated by comparing the heart (which has mainly the reputed MT/ATOX1/COX17 peak) with the kidney (which has mainly the SOD peak). The leading edge of the latter also co-eluted with a major Zn peak, consistent with Cu/Zn SOD. However, the SOD chaperone, CCS, is about the same size as SOD (Huffman & O’Halloran 2001) and would also elute there. A monomeric (48 kDa) form of S-adenosyl homocysteine hydrolase [4], which also binds copper tightly and accounts for a significant portion of liver copper in the cytoplasm, should elute just ahead of SOD and would contribute to this peak as well. A dimer of the hydrolase might also account for the 110 kDa component in the various cytosols, since it binds copper as well, at least in liver, kidney and brain (to decreasing degree). Aggregates of the 23 kDa COMMD proteins [which include MURR1/COMMD1 implicated in biliary excretion and defective in the Bedlington terrier; Burstein et al. 2005)] also probably contribute to the copper components eluting in the 20–50 kDa range, as should the dimeric “S100b” protein (Nishikawa et al. 1997) particularly prevalent in brain. Thus, the large and broad copper peak in the 20–50 kDa region is presumably comprised not just of SOD and MT but several other proteins. Clearly, further studies involving more refined and powerful separatory techniques, such as multidimensional HPLC together with confirmatory molecular identification by techniques such as MALDI-TOF are clearly necessary to resolve the specific subcellular moieties involved.
The identity of the largest copper binding component is still a complete mystery. Although a number of new, larger copper binding proteins have recently been identified in mammalian cells, most of these are bound to intracellular membranes or the plasma membrane (ATP7A and B; hephaestin; DMT1; CTR1) (Arredondo & Nunez 2005; Linder 2002; Puig & Thiele 2002). Prior studies had indicated it was larger than 100 kDa. Our current work using a larger pore column shows it to be a great deal larger (>1000 kDa). The absence of very small copper components may again be noted. Although evidence from cell culture has shown that copper can bind the tripeptide, glutathione, when given in high doses and preserved under anaerobic conditions, it is questionable whether this happens in vivo, since others have shown that cytoplasmic proteins can successfully out-compete GSH for Cu (Ohta et al. 2001).
Our observations that mice were able to tolerate the potentially toxic effects of large doses of copper and were able to rapidly eliminate the metal is consistent with previous observations made in humans and rats (Linder & Roboz 1986; Turnlund 1989). The level of tolerance exhibited by the mice in the current study was particularly impressive. Based on the copper concentrations of various organs and tissues in mice and rats, we estimate that an adult mouse of 25–35g contains a total of 35–50 μg copper. In this study we injected of 25–30 μg copper 5x over 14 hours, for a total of about 141 μg, a dosage three times greater than the amounts of copper already present. This was not only tolerated (the mice did not alter their behavior after an injection) but our analyses of the amounts of copper remaining in the animals at various times after the last injection indicated that elimination was very rapid. Consistent with the well-established observation that primarily the liver (and kidney) take up most of the new copper entering the circulation (Linder 1991), these organs were the only ones examined that responded to the copper treatment with large increases in tissue copper accumulation. Thirty min after the last injection, liver concentrations of copper (most of which was extrinsic 65Cu) were 8x higher than normal, while those in the kidney were 2x the normal. The liver accounted for 30–55% of the total 65Cu injected in the first hours after the last injection, the kidney only about 5%. Mass balance calculations indicated only 65% of the injected dose could be accounted for in the organs examined. This implied that either a considerable portion of the dose may have been already excreted during the 14 h injection period, or that the 65Cu was sequestered in tissues not analyzed, such as the skin, viscera and skeleton. In humans, the bones are known to account for 40% of the total body copper (Linder 1991), and the release of newly absorbed 65Cu from these compartments back into the blood would explain its continuing buildup of 65Cu noted in the liver up to 6 h after the last injection. This buildup could not be reconciled with the fall in blood plasma 65Cu alone, which could account for only 10–20% of that copper.
Over the following two weeks, the liver copper content showed an exponential decline, with a half-life of about 4 days. Except for the kidney, which remained enriched longer due to 65Cu retained in non-cytoplasmic components (Table 1), similar rates of decline were observed in other organs, with only about 20% of the injected 65Cu dose remaining after 7 days and approximately 5% after 14 days. This is similar to our previous findings for whole body copper in rats where we followed the loss of ng quantities of injected 67Cu for two weeks (Linder & Roboz 1986). Most of the radioisotope turned over with a half-life of 2.5–3 days, with component having a much longer half-life (8–10 days). Prior injection of 25 μg of copper (about 10% of the estimated total in a 150g rat) increased liver copper concentrations about 50% but did not alter the turnover rate. The same and a previous study (Owen et al. 1975) indicated that 10–14% of the injected copper was lost in the bile and feces (and about 2% in the urine) over the first 24 h. All of this is consistent with the established concepts that (a) copper homeostasis is mainly controlled at the level of excretion, and mainly by the liver, which eliminates most of the excess through the bile (Linder 1991); (b) with the exception of dogs and sheep and individuals with specific genetic defects, such as in ATP7B in Wilson disease, most mammals are capable of dealing with unexpectedly large intakes of copper.
Finally, the current studies have shown the utility of stable isotopic Cu to study the long term metabolism of the metal over physiologically relevant time scales beyond those feasible with radioisotopic forms of the element. Relatively small doses of the stable copper isotope, 65Cu, have been used successfully in humans to study whole body intestinal absorption, transport and excretion kinetics, with the finding that long term retention and excretion of copper are inversely related to copper status and intake, those with low intakes retaining more and excreting less and vice versa (Turnlund et al. 1989, 1998). Studies in humans are obviously more limiting, however, and do not allow us to follow the turnover of copper in specific organs and their proteins, which is the advantage of using experimental animals. The current feasibility studies have shown that stable isotopic copper 65Cu administered i.p. to mice results in the detectable enrichment of specific copper proteins in all organs but the brain and particularly so in the liver and kidney. Although large doses of 65Cu were administered, the data indicate that physiologically relevant doses of stable isotopic copper could be used in conjunction with HPLC-quadrupole ICP-MS or, more preferably, multicollector magnetic sector instruments to successfully model the long-term homeostatic capabilities and turnover of copper in the individual binding components of the tissues more actively involved in the metabolism and processing of this essential, but potentially toxic, trace element.
Acknowledgments
This work was supported in part by USPHS Grant RO1 HD46949 and NSF REU grant CHE 0354159 to M. C. Linder; and NSF grants DBI-9978806 and OCE-9977564 to A. Z. Mason. We are grateful to Dr. Z. Leah Harris for the breeding pairs for our colony of ceruloplasmin knockout mice.
References
- Amaravadi R, Glerum DM, Tzagoloff A. Isolation of a cDNA encoding the human homolog of COX17, a yeast gene essential for mitochondrial copper recruitment. Hum Genet. 1997;99(3):329–333. doi: 10.1007/s004390050367. [DOI] [PubMed] [Google Scholar]
- Arredondo M, Nunez MT. Iron and copper metabolism. Mol Aspects Med. 2005;26(4–5):313–327. doi: 10.1016/j.mam.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Barrow L, Tanner MS. Copper distribution among serum proteins in paediatric liver disorders and malignancies. Eur J Clin Invest. 1988;18(6):555–560. doi: 10.1111/j.1365-2362.1988.tb01267.x. [DOI] [PubMed] [Google Scholar]
- Bethin KE, Cimato TR, Ettinger MJ. Copper binding to mouse liver S-adenosylhomocysteine hydrolase and the effects of copper on its levels. J Biol Chem. 1995;270(35):20702–20711. doi: 10.1074/jbc.270.35.20703. [DOI] [PubMed] [Google Scholar]
- Bielli P, Calabrese L. Structure to function relationships in ceruloplasmin: a ‘moonlighting’ protein. Cell Mol Life Sci. 2002;59(9):381–387. doi: 10.1007/s00018-002-8519-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein E, Hoberg JE, Wilkinson AS, Rumble JM, Csomos RA, Komarck CM, Maine GN, Wilkinson JC, Mayo MW, Duckett CS. COMMD proteins, a novel family of structural and functional homologs of MURR1. J Biol Chem. 2005;280(23):22222–22232. doi: 10.1074/jbc.M501928200. [DOI] [PubMed] [Google Scholar]
- Camakaris J, Voskoboinik I, Mercer JFB. Molecular mechanisms of copper homeostasis. Biochem Biophys Res Comm. 1999;261(2):225–232. doi: 10.1006/bbrc.1999.1073. [DOI] [PubMed] [Google Scholar]
- Campbell CH, Brown R, Linder MC. Circulating ceruloplasmin is an important source of copper for normal and malignant cells. Biochim Biophys Acta. 1981;678 (1):27–38. doi: 10.1016/0304-4165(81)90044-1. [DOI] [PubMed] [Google Scholar]
- Culotta VC, Klomp LW, Strain J, Casareno RL, Krems B, Gitlin JD. The copper chaperone for superoxide dismutase. J Biol Chem. 1997;272(38):23469–23472. doi: 10.1074/jbc.272.38.23469. [DOI] [PubMed] [Google Scholar]
- Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci USA. 1998;95(3):1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman JH, Ciriolo MR, Peisach J. The role of glutathione in copper metabolism and toxicity. J Biol Chem. 1989;264(10):5598–5606. [PubMed] [Google Scholar]
- Frieden E. Ceruloplasmin, a link between copper and iron metabolism. Nutr Rev. 1970;28(1):87–91. [PubMed] [Google Scholar]
- Garvey JS, Chang CC. Detection of circulating metallothionein in rats injected with zinc or cadmium. Science. 1981;214(1):805–807. doi: 10.1126/science.7292012. [DOI] [PubMed] [Google Scholar]
- Gless U, Schmitt Y, Ziegler S, Kruse-Jarres JD. chromatographic separation of serum proteins and estimation of their zinc and copper content. J Trace Elem Electrolytes Health Dis. 1992;6(4):245–50. [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388(6641):482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Harada M, Kawaguchi T, Kumemura H, Terada K, Ninomiya H, Taniguchi E, Hanada S, Baba S, Maeyama M, Koga H, Ueno T, Furuta K, Suganuma T, Sugiyama T, Sata M. The Wilson disease protein ATP7B resides in the late endosomes with Rab7 and the Niemann-Pick C1 protein. Am J Path. 2005;166(2):499–510. doi: 10.1016/S0002-9440(10)62272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris ED. Cellular copper transport and metabolism. Annu Rev Nutri. 2000;20:291–310. doi: 10.1146/annurev.nutr.20.1.291. [DOI] [PubMed] [Google Scholar]
- Harris ZL, Durley AP, Man TM, Gitlin JD. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc Natl Acad Sci USA. 1999;96(19):10812–10817. doi: 10.1073/pnas.96.19.10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris ZL, Klomp LW, Gitlin JD. Aceruloplasminemia: an inherited neurodegenerative disease with impaired iron homeostasis. Am J Clin Nutr (5 Suppl) 1998;67:972S–977S. doi: 10.1093/ajcn/67.5.972S. [DOI] [PubMed] [Google Scholar]
- Harris ZL, Takahashi Y, Miyajima H, Serizawa M, MacGillivray RTA, Gitlin JD. Aceruloplasminemia: molecular characterization of this disorder of iron metabolism. Proc Natl Acad Sci USA. 1995;92(7):2539–2543. doi: 10.1073/pnas.92.7.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman DL, O’Halloran TV. Function, structure and mechanism of intracellular copper trafficking proteins. Annu Rev Biochem. 2001;70:677–701. doi: 10.1146/annurev.biochem.70.1.677. [DOI] [PubMed] [Google Scholar]
- Kagi KHR, Nordberg M, editors. Metallothionein. Birkhauser Verlag; Basel: 1979. [Google Scholar]
- Kataoka M, Tavassoli M. Identification of ceruloplasmin receptors on the surface of human blood monocytes, granulocytes, and lymphocytes. Exp Hematol. 1985;13(8):806–810. [PubMed] [Google Scholar]
- Klomp AE, van der Sluis B, Klomp LWJ, Wijmenga C. The ubiquitously expressed MURR1 protein is absent in canine copper toxicosis. J Hepatol. 2003;39(5):703–709. doi: 10.1016/s0168-8278(03)00380-5. [DOI] [PubMed] [Google Scholar]
- Klomp LW, Lin SJ, Yuan DS, Klausner RD, Culotta VC, Gitlin JD. Identification and functional expression of HAH1: a novel human gene involved in copper homeostasis. J Biol Chem. 1997;272(14):9221–9226. doi: 10.1074/jbc.272.14.9221. [DOI] [PubMed] [Google Scholar]
- Kuo YM, Zhou B, Cosco D, Gitschier J. The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc Natl Acad Sci USA. 2001;98(12):6836–6841. doi: 10.1073/pnas.111057298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Prohaska JR, Thiele DJ. Essential role for mammalian copper transproter Ctr1 in copper homeostasis and embryonic development. Proc Natl Acad Sci USA. 2001;98(12):6842–6847. doi: 10.1073/pnas.111058698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lancey R, Montaser A, Madani N, Linder MC. Transfer of copper from mother to fetus during the latter part of gestation in the rat. Proc Soc Exp Biol Med. 1993;203(6):428–439. doi: 10.3181/00379727-203-43619. [DOI] [PubMed] [Google Scholar]
- Linder MC. Biochemistry of Copper. Plenum Press; New York: 1991. [Google Scholar]
- Linder MC. Copper and genomic stability in mammals. Mutation Res. 2001;475(1–2):141–152. doi: 10.1016/s0027-5107(01)00076-8. [DOI] [PubMed] [Google Scholar]
- Linder MC. Biochemistry and molecular biology of copper in mammals. In: Massoro EJ, editor. Handbook of Copper Pharmacology and Toxicology. Humana Press; Totowa NJ: 2002. [Google Scholar]
- Linder MC, Roboz M. Turnover and excretion of copper in rats as measured with 67Cu. Am J Physiol. 1986;251(11):E551–E555. doi: 10.1152/ajpendo.1986.251.5.E551. [DOI] [PubMed] [Google Scholar]
- Liu NM, Lo LSL, Askary SH, Goforth J, Vivas EM, Tsai M, Linder MC. Transcuprein is a macroglobulin regulated by copper and iron availability. J Nutr Biochem. 2007 doi: 10.1016/j.jnutbio.2006.11.005. Epub, March 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K, Reed DL, Roberts RC, Damato-McCabe D. Three high molecular weight protease inhibitors of rat plasma. Reactions with trypsin. J Biol Chem. 1987;262(10):4844–4853. [PubMed] [Google Scholar]
- Mason AZ, Borja MR. A study of Cu turnover in proteins of the visceral complex of Littoria littorea by stable isotopic analysis using coupled HPLC-ICP-MS. Mar Environ Res. 2002;54(3–5):351–355. doi: 10.1016/s0141-1136(02)00171-x. [DOI] [PubMed] [Google Scholar]
- Masuoka J, Hegenauer J, Van Dyke BR, Saltman P. Intrinsic stoichiometric equilibrium constants for the binding of zinc(II) and copper (II) to the high affinity site of serum albumin. J Biol Chem. 1993;268(29):21533–21537. [PubMed] [Google Scholar]
- Mehra RK, Bremner I. Development of a radioimmunoassay for rat liver metallothionein-1 and its application to the analysis of rat plasma and kidneys. Biochem J. 1983;213(2):459–465. doi: 10.1042/bj2130459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer JF. The molecular basis of copper-transport diseases. Trends Mol Med. 2001;7(2):64–69. doi: 10.1016/s1471-4914(01)01920-7. [DOI] [PubMed] [Google Scholar]
- Meyer LA, Durley AP, Prohaska JR, Harris ZL. Copper transport and metabolism are normal in aceruloplasminemic mice. J Biol Chem. 2001;276(39):36857–36861. doi: 10.1074/jbc.M105361200. [DOI] [PubMed] [Google Scholar]
- Montaser A, Tetreault C, Linder MC. comparison of copper binding proteins in dog serum with those in other species. Proc Soc Ex Biol Med. 1992;200(4):321–329. doi: 10.3181/00379727-200-43437. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Lee ISM, Shiraishi N, Ishikawa T, Ohta Y, Mishikimi M. Identification of S100b protein as copper-binding protein and its suppression of copper-induced cell damage. J Biol Chem. 1997;272(37):23037–23041. doi: 10.1074/jbc.272.37.23037. [DOI] [PubMed] [Google Scholar]
- Norton DS, Heaton FW. Distribution of copper and zinc among protein fractions in the cytoplasm or rat tissues. J Inorg Biochem. 1980;13(1):1–9. doi: 10.1016/s0162-0134(00)80208-1. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Sharaishi N, Inai Y, Lee IS, Ishihashi H, Nishikimi M. Ascorbate-induced high-affinity binding of copper to cytosolic proteins. Biochem Biophys Res Comm. 2001;287(4):888–894. doi: 10.1006/bbrc.2001.5679. [DOI] [PubMed] [Google Scholar]
- Osaki S, Johnson DA. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J Biol Chem. 1969;244(20):5757–5761. [Google Scholar]
- Owen CA, Jr, Randall RV, Goldstein NP. Effect of dietary D-penicillamine on metabolism of copper in rats. Am J Physiol. 1975;228(1):88–91. doi: 10.1152/ajplegacy.1975.228.1.88. [DOI] [PubMed] [Google Scholar]
- Prohaska JR. Changes in tissue growth, concentrations of copper, iron, cytochrome oxidase and superoxide dismutase subsequent to dietary or genetic copper deficiency in mice. J Nutr. 1983;113(8):2148–2158. doi: 10.1093/jn/113.10.2048. [DOI] [PubMed] [Google Scholar]
- Puig S, Thiele DJ. Molecular mechanisms of copper uptake and distribution. Current Opin Chem Biol. 2002;6(2):171–180. doi: 10.1016/s1367-5931(02)00298-3. [DOI] [PubMed] [Google Scholar]
- Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O’Halloran TV. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science. 1999;284(5415):805–808. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- Ragan HA, Nacht S, Lee GR, Bishop CR, Cartwright GE. Effect of ceruloplasmin on plasma iron in copper deficiency swine. Am J Physiol. 1969;217(5):1320–1323. doi: 10.1152/ajplegacy.1969.217.5.1320. [DOI] [PubMed] [Google Scholar]
- Roelofsen J, Wolters H, Van Luyn JA, Miura N, Kuipers F, Vonk RJ. Copper-induced apical trafficking of ATP7B in polarized hepatoma cells provides a mechanism for biliary copper excretion. Gastroenterol. 2000;119(3):782–793. doi: 10.1053/gast.2000.17834. [DOI] [PubMed] [Google Scholar]
- Roeser HP, Lee GR, Nacht S, Cartwright GE. The role of ceruloplasmin in iron metabolism. J Clin Invest. 1980;49(12):2408–2417. doi: 10.1172/JCI106460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M, Roelofsen J, Wolters H, Hofmann WJ, Muller M, Kuipers F, Stremmel W, Vonk RJ. Localization of the Wilson’s disease protein in human liver. Gastroenterol. 1999;117(6):1380–1385. doi: 10.1016/s0016-5085(99)70288-x. [DOI] [PubMed] [Google Scholar]
- Sharma RP, McQueen EG. Effects of gold sodium thiomalate on cytosolic copper and zinc in the rat kidney and liver tissues. Clin Exp Pharmacol Physiol. 1981;8(6):591–599. doi: 10.1111/j.1440-1681.1981.tb00771.x. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L. Alpha-macroglobulins: structure, shape, and mechanisms of proteinase complex formation. J Biol Chem. 1989;264(20):11539–11542. [PubMed] [Google Scholar]
- Stevens MD, DiSilvestro RA, Harris ED. Specific receptor for ceruloplasmin in membrane fragmentsfrom aortic and heart tissues. Biochem. 1984;23(2):261–266. doi: 10.1021/bi00297a014. [DOI] [PubMed] [Google Scholar]
- Stevenson FT, Greene S, Kaysen GA. Serum a2-macroglobulin and a1-inhibitor 3 concentrations are increased in hypoalbuminemia by post-transcriptional mechanisms. Kidney Internat. 1998;53(7):67–75. doi: 10.1046/j.1523-1755.1998.00734.x. [DOI] [PubMed] [Google Scholar]
- Terao T, Owen CA., Jr Nature of copper compounds in liver supernate and bile of rats: studies with 67Cu. Am J Physiol. 1973;224(3):682–686. doi: 10.1152/ajplegacy.1973.224.3.682. [DOI] [PubMed] [Google Scholar]
- Ting BTG, Lee CC, Janghobani M, Prohaska JR. Development of the stable isotope tracer approach for studies of copper turnover in the rat and mouse. J Nutr Biochem. 1990;1(5):249–255. doi: 10.1016/0955-2863(90)90074-u. [DOI] [PubMed] [Google Scholar]
- Turnlund JR, Keyes WR, Anderson HL, Acord LL. Copper absorption and retention in young men at three levels of dietary copper using the stable isotope, 65Cu. Am J Clin Nutr. 1989;49(5):870–878. doi: 10.1093/ajcn/49.5.870. [DOI] [PubMed] [Google Scholar]
- Turnlund JR, Keyes WR, Peiffer GL, Scott KC. Copper absorption, excretion, and retention by young men consuming low dietary copper determined by using the stable isotope 65Cu. Am J Clin Nutr (Suppl) 1998;67(6):1219–1225. doi: 10.1093/ajcn/67.6.1219. [DOI] [PubMed] [Google Scholar]
- Vargas EJ, Shoho AR, Linder MC. Copper transport in the Nagase analbuminemic rat. Am J Physiol. 1994;267(2):G259–G269. doi: 10.1152/ajpgi.1994.267.2.G259. [DOI] [PubMed] [Google Scholar]
- Weiss KC, Linder MC. Copper transport in rats involving a new plasma protein. Am J Physiol. 1985;249(1):E77–E88. doi: 10.1152/ajpendo.1985.249.1.E77. [DOI] [PubMed] [Google Scholar]
- Wirth PL, Linder MC. Distribution of copper among multiple components of human serum. J Natl Cancer Inst. 1985;75(2):277–284. [PubMed] [Google Scholar]
- Yoshida K, Furihata K, Takeda S, Nakamura A, Yamamoto K, Hiyamuta S, Ikeda S, Shimizu N, Yanagisawa N. A mutation in the ceruloplasmin gene is associated with systemic hemosiderosis in humans. Nature Genet. 1995;9(3):267–272. doi: 10.1038/ng0395-267. [DOI] [PubMed] [Google Scholar]


