Abstract
Histopathological analysis is a basic methodology for assessing testicular injury after exposure to candidate therapeutics or toxicants. One possible injury response in rat testis is the failure of step 19 spermatids to spermiate. Such spermatids are transported towards the basement membrane where they are retained for degradation by Sertoli cells. In control rats, these retained spermatids heads (RSH) were observed at stages IX-XII. Exposure to the Sertoli cell toxicant, 2,5-hexanedione (HD), for 18d at 0.08% − 1.0% in drinking water resulted in a dose-dependent increase in the number of RSH at stages IX-XII (no observed effect level [NOEL], 0.14%). To explore the dynamics of spermatid head retention, rats were treated with 0.33% or 1% HD for various durations and RSH were assessed across all stages. After 0.33% HD exposure for 18d, there were more RSH present in stage IX-XII tubules compared to control. Numbers of RSH dropped back to control levels after 4wk of recovery post-18d exposure. Exposure of rats to 1% HD for 18d resulted in markedly elevated numbers of RSH at stages IXII/III. There was no evidence of other histopathological alterations. These data identify RSH as a sensitive histopathological marker of testicular toxicity for subacute HD exposure.
Keywords: Spermatid Head Retention; Delayed Spermiation; Spermatid Heads; Rat; 2,5-Hexanedione; Testis; Toxicology; Histopathology
INTRODUCTION
There are numerous, well-characterized histopathological endpoints for assessing testicular toxicity, including germ cell death, sloughing, multi-nucleated germ cells, Sertoli cell vacuoles, altered seminiferous tubule diameter, and atrophy (Moffit et al. 2007). Even testis weight is a sensitive indicator of toxicity (Creasy 2002). Toxicants with different cellular targets and mechanisms of action exhibit different endpoint responses (Moffit et al. 2007). In adult rats, testis weight and sloughing were the most sensitive endpoints 24h after carbendazim (CBZ) exposure, germ cell death was the most sensitive endpoint 12h after mono-2-ethylhexyl phthalate (MEHP) exposure, and the presence of elongated spermatid heads in the basal half of the seminiferous tubule epithelium at stages IX-XI was the most sensitive endpoint of toxicity after exposure to 2,5-hexanedione (HD) in drinking water for 18d. This study characterizes the dose-dependence, onset, and clearance of retained spermatid heads (RSH) in the basal compartment of the seminiferous tubule after exposure to the Sertoli cell toxicant, HD.
It is important to be familiar with the spermatogenic cycle as certain histopathological events occur at specific time points in spermatogenesis. The spermatogenic cycle is a coordinated development of spermatogonia, spermatocytes and spermatids. A comprehensive review is available in Russell et al. 1990, and will be described in brief here. This cycle has been divided into 14 stages in the rat based on histological characteristics of the germ cell components. The entire cycle takes approximately 14 days, and each stage lasts a fixed amount of time. At the beginning of the spermatogenic cycle (Stage I), elongated spermatids are near the lumen, and then move basally through stage V. These cohorts of spermatids are in crypts surrounded by ectoplasmic specializations, Sertoli cell structures consisting of adhesion proteins with interacting microtubules (MT) that assist in moving the spermatids luminally at stage VI (Russell 1993). Spermatids reach the lumen at stage VII and the ectoplasmic specializations dissociate while the spermatids remain adherent to the Sertoli cell by the tubulobulbar complex, which is primarily made up of actin filaments. At the end of stage VIII, spermiation occurs as the tubulobulbar complex dissociates in a testosterone-dependent manner, releasing the step 19 spermatids into the lumen (Saito et al. 2000). It is generally accepted that step 19 spermatids that fail to undergo spermiation are engulfed by Sertoli cells and transported to the basal compartment for degradation (Russell 1991) where they are observed as RSH from stages IX-XII. The basal movement of unreleased spermatids immediately after spermiation will be referred to as spermatid head retention. At stage IX, the oldest population of spermatids commences morphological changes into elongated spermatids that will be released in the next cycle of spermatogenesis.
Retained spermatid heads have been observed after suppression of FSH or testosterone and after exposure to ethane dimethanesulfonate (EDS), boric acid, dibromoacetic acid (DBA) and sodium dichloroacetic acid (NaDCA) (Bartlett et al. 1986; Toth et al. 1992; Saito et al. 2000; Linder et al. 1997; Linder et al. 1994; Treinen and Chapin 1991). Selective in vivo suppression of FSH, testosterone, or both for 1 week caused 11, 14, and 50% failure in spermiation, respectively (Saito et al. 2000). Toxicants such as EDS destroy Leydig cells, the testosterone producing cells in the testis, thereby lowering testicular testosterone (Bartlett et al. 1986). Three days after an injection of EDS, testosterone levels in an adult rat were undetectable and RSH were seen at stages X and XI 4d later. Similarly, exposure to boric acid decreased levels of basal testosterone after 4d of dosing and inhibited spermiation after 7d of dosing (Treinen and Chapin 1991). Exposure to the drinking water disinfection by-product, DBA, but not NaDCA, decreased serum testosterone in rats (Linder et al. 1994, Toth et al. 1992). The presence of RSH was noted after exposure to either toxicant.
2,5-Hexanedione (HD), a metabolite of n-hexane, is a testicular toxicant that targets the Sertoli cell, the support cell within the seminiferous tubule. Manifestations of in vivo HD toxicity take some time to appear as HD must form adducts with proteins, including tubulin, and alter MT dynamics, an important structural element in Sertoli cells. Tubulin purified from rats exposed to 1% HD for 2 wk exhibited decreased nucleation time and more rapid assembly into microtubules (Boekelheide 1988b). Extensive and irreversible atrophy of the seminiferous tubule occurred in adult rats after exposure to 1% HD in drinking water for 5 weeks (Hall et al. 1991). However, 18d of exposure to 1% HD in drinking water resulted in few histopathological changes, with the exception of an accumulation of step 19 spermatid heads in the basal compartment of stage IX-XI tubules (Moffit et al. 2007). Testosterone levels do not change after exposure to 1% HD (unpublished data), so the retention of spermatid heads is likely caused by another mechanism, potentially the disruption of microtubules.
None of the studies that reported the observation of RSH in the basal compartment of the seminiferous tubule in adult rats investigated the dynamics of this phenomenon, nor characterized its utility and sensitivity as an endpoint for assessing toxicity. In this report, we investigate the dynamics of the retention and degradation of spermatid heads and characterize spermatid head retention as a sensitive marker for subacute HD toxicity.
MATERIALS AND METHODS
Animal care
Adult Fischer 344 rats weighing 200−225g (approximately 2 months of age) were ordered from Charles River Laboratory and housed at 21±1°C on a 12h light/dark schedule with access to water and Purina Rodent Chow 5001 ad libitum. After acclimation for 1wk, rats in HD dose groups were given a percentage of HD (98+% pure) dissolved in water ad libitum. The HD solution was mixed a day before dosing began, and more solution was prepared as needed during the time course of exposure. Rats were euthanized by CO2 asphyxiation. Average body weights and testis weights at time of necropsy are reported in Supplemental Table 1. All procedures involving animals were performed in accordance with the guidelines of Brown University's Institutional Animal Care and Use Committee, in compliance with National Institute of Health guidelines.
Experiments
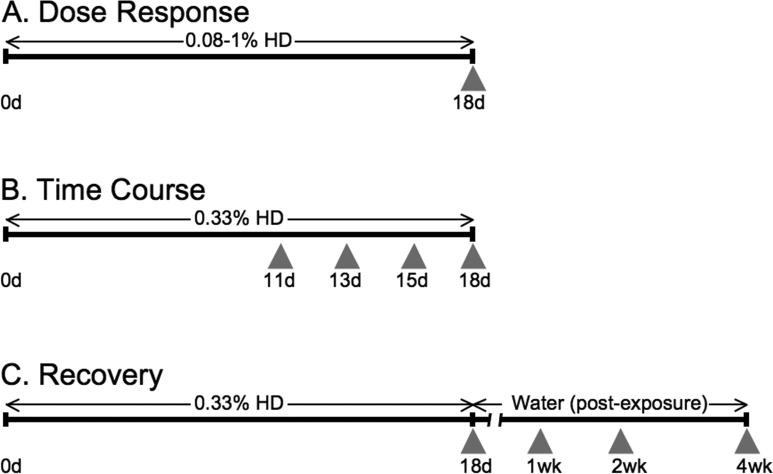
Three experiments were conducted to assess the endpoint of spermatid head retention: a Dose Response experiment, a Time Course experiment, and a Recovery experiment. Figure 1 shows a schematic of the dosing regiment for each experiment. When possible, data were pooled across the experiments (control, and 0.33 and 1% HD for 18d).
Figure 1.
Timeline and experimental design. For Dose Response experiments (A), animals were treated with 0.08, 0.14, 0.21, 0.33, 0.625, or 1.0% HD for 18d. For Time Course experiments (B), animals were exposed to 0.33% HD and sacrificed at 11, 13, 15, and 18d. For Recovery experiments (C), animals were given 0.33% HD for 18d and then switched to water and sacrificed 1, 2, and 4 weeks post-exposure. Gray arrowheads indicate sacrificing timepoints. The duration of HD or water administration is indicated by horizontal arrows above the timeline.
Dose Response
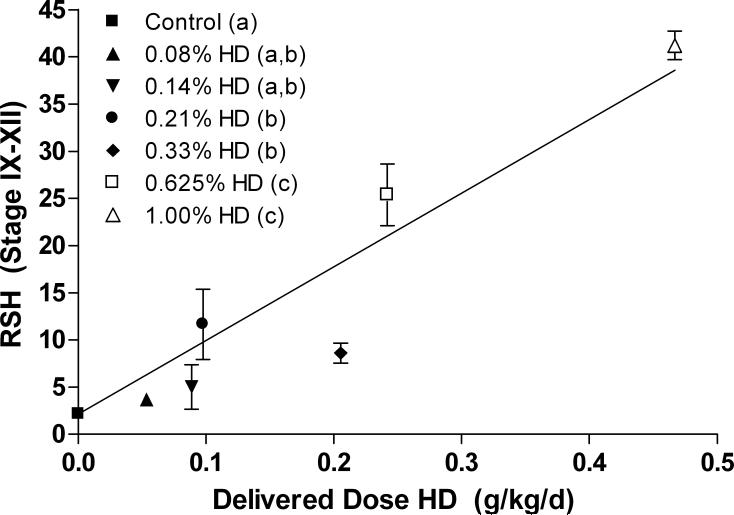
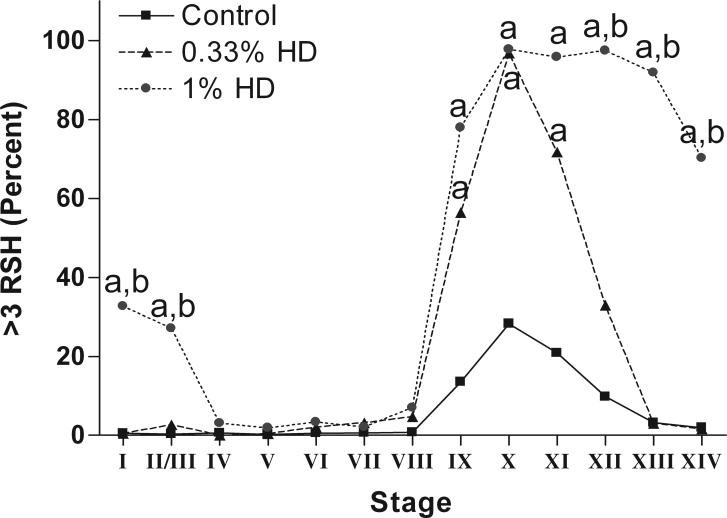
The aim of this experiment was to establish a dose response by quantifying the total number of RSH after exposure to different doses of HD. All rats were subjected to the same dosing regiment at the following doses: 0.08, 0.14, 0.21, 0.33, 0.625, or 1% HD in drinking water for 18d (control, n=9; 0.33 and 1.0%, n=6, n=3 for all other doses). The data is reported in two ways. Figure 6 shows the average number of RSH per seminiferous tubule at stages IX-XII plotted against the average delivered dose. The delivered dose was calculated by determining the g of HD administered per rat over the 18d dosing period based on total water consumption and rat weight at 18d. Rats that received the higher doses of HD drank less water (Moffit et al. 2007) and gained less weight through the time course (Supplemental Table 1). Rats that received 1% HD lost weight. Figure 7 quantified the total number of RSH across all stages for dose groups 0.33 and 1.0% only.
Figure 6.
The average number of total RSH per seminiferous tubules at stages IX-XII in control and HD treated rats is plotted against the average total delivered dose of HD over 18d to each dose group (g/kg/day). There is a significant trend in the presence of RSH with dose; a linear regression fit to the data with the y-intercept set to the control value gives r=0.9135. The legend is expressed as the corresponding percent of HD administered in drinking water for each dose group. Significance between dose groups is indicated by the letters in parentheses in the legend; different letters indicated statistically different average numbers of RSH. ANOVA analysis revealed a NOEL and LOEL at 0.14 and 0.21% HD, respectively. Error bars indicate SEM.
Figure 7.
The percentage of seminiferous tubules at each stage with greater than three total RSH in control and HD-treated testes. Rats treated with 0.33% HD for 18d are significantly different from control at stages IX-XII; rats treated with 1% HD for 18d are significantly different from controls at stages IX-II/III, and different from 0.33% HD treated rat at stages XII-II/III. Significant difference from control is indicated by an “a” and significant differences between 0.33% and 1% HD is indicated by a “b”. See Supplemental Table 2 for SEM values at each point.
Time Course
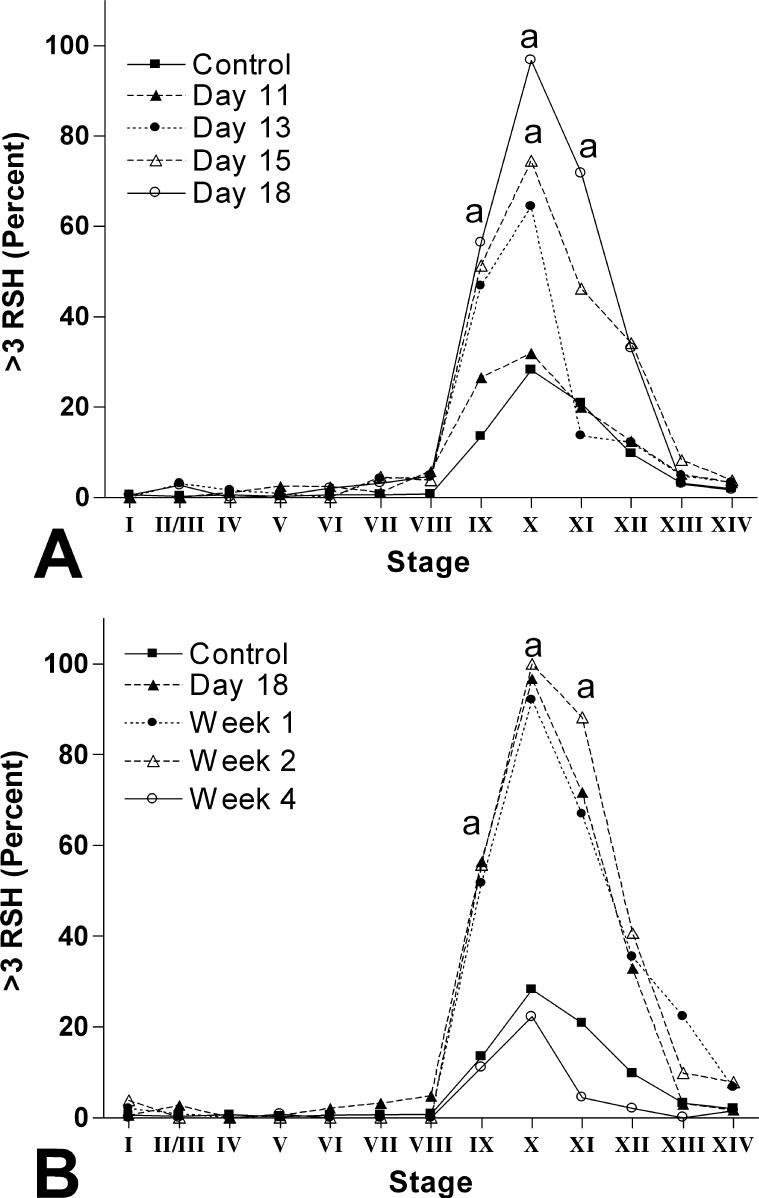
The aim of this experiment was to examine the time-dependent onset of RSH. The LOEL from Moffit et al. 2007 (0.33% HD) was chosen as the dose for this extensive evaluation. Rats were given 0.33% HD and sacrificed 11, 13, 15, or 18d later (n=3 per dose group except 18d, where n=6). The total number of RSH in a testis cross section was recorded in every seminiferous tubule cross section with a major:minor diameter less than 1.5:1. The percentage of seminiferous tubules at each stage with greater than three RSH is reported in Figure 8A.
Figure 8.
The percentage of seminiferous tubules at each stage with greater than three total RSH in control and 0.33% HD treated testes. (A) Exposure to 0.33% HD for 11, 13, 15, and 18d. Treated testes first show a increase in the percentage of seminiferous tubules with greater than three RSH at stage X after 15d of exposure, which further increases to include stages IX-XI after 18d of exposure. Points significantly different from control are indicated with an “a”. (B) Exposure to 0.33% HD for 18d followed by a post-exposure recovery period of 1, 2, or 4 wk. After 1 and 2 wk of recovery, the percentage of seminiferous tubules with greater than three RSH is statistically identical to 18d of exposure (indicated with “a”). After 4 wk of recovery, the percentage of seminiferous tubules with greater than three RSH returns to the levels seen in control. See Supplemental Table 2 for SEM values at each point.
Recovery
The aim of this experiment was to determine the time dependence for the resolution of RSH after stopping HD exposure. Rats were given 0.33% HD for 18d and were then switched to water alone and euthanized 1, 2, or 4wk later (n=3 for each recovery time point, except 18d, where n=6). The total number of RSH in a testis cross section was recorded in every seminiferous tubule cross section with a major:minor diameter of less than 1.5:1. The percentage of seminiferous tubules at each stage with greater than three RSH is reported in Figure 8B.
Light microscopy
All reagents were from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. The left testes were fixed in 10% neutral buffered formalin and kept at 4°C for at least 3d before processing. Cross-sections from the middle of each testis were embedded in 2-hydroxyethyl methacrylate (Technovit 7100, Kulzer, Germany) and 3μm sections were stained with periodic acid Schiff's reagent and hematoxylin (PASH). Testis cross-sections were viewed on a Zeiss (New York, NY) standard light microscope or Aperio Scan Scope (Aperio Technologies, Vista, CA). The stage of each seminiferous tubule cross section with a major:minor axis less than 1.5:1 was determined in one testis cross-section per rat and the number of RSH in the bottom half of each seminiferous tubule (basal compartment) was recorded. The average and range of seminiferous tubule cross-sections counted per rat is reported in Supplemental Table 1. Scoring for intact and degraded RSH was performed on the same cross-section on a light microscope to allow for fine focusing. An intact RSH was defined as closely resembling normal step 19 spermatid heads in density, thickness, and contour. A degraded RSH was defined as having less dense nuclear staining than a normal spermatid or an intact RSH and a thinner profile with jagged contours and abrupt, rather than gradual, bends. Figures 2B and 5 show the average number of intact and degraded RSH per seminiferous tubules at each stage in control and 1% HD treated rats, respectively. High magnification images of RSH were taken on a Zeiss Axiovert 35 microscope using a Spot RT camera (Diagnostic Instruments Inc., Sterling Heights, MI). Images were re-sized proportionally and arrows and letters were added in Adobe Photoshop 6.0 (Adobe Systems Incorporated, San Jose, CA).
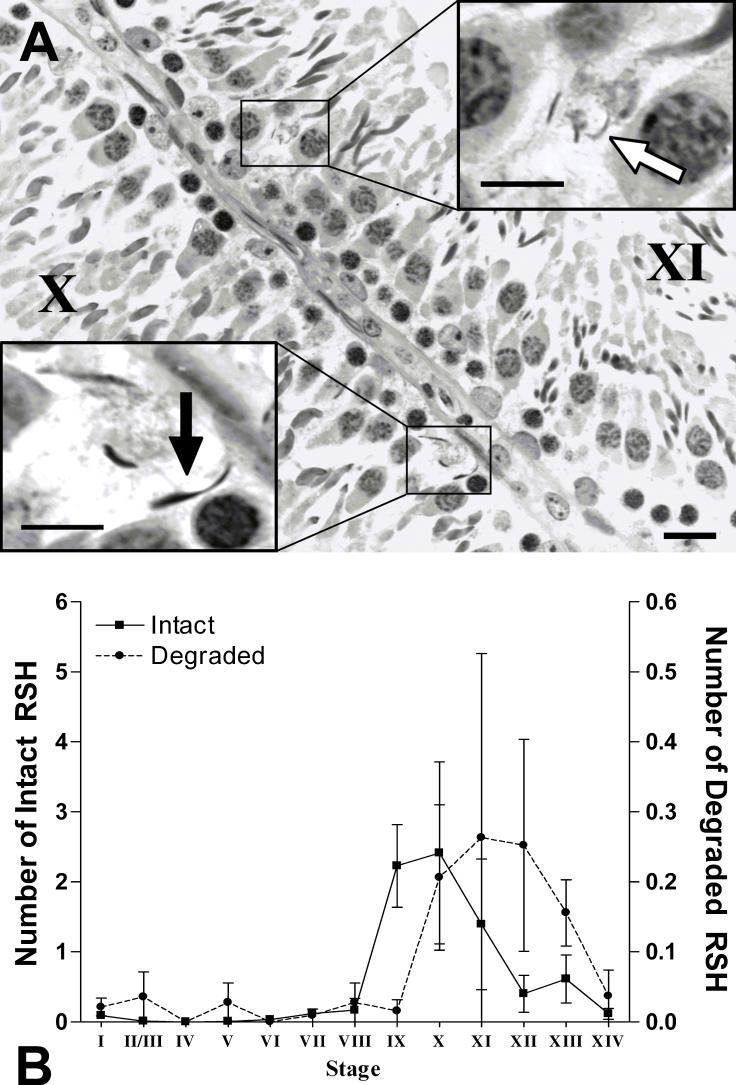
Figure 2.
(A) Histopathology of a seminiferous tubule cross-section at stage X and XI from a control rat. Densely stained intact RSH (black arrow) and thinner and fainter degraded RSH (white arrow) are present near the basement membrane of the seminiferous tubule. PASH stained 3μm plastic section; bar = 20μm. (B) Average number of intact RSH (left y-axis) or degraded RSH (right y-axis) per seminiferous tubule at each stage in control testes (n=3). The average number of intact RSH increases just after spermiation, while the average number of degraded RSH increases one stage later (∼7h). The degraded RSH persist for longer than the intact RSH. There is a 10-fold difference between the scale of intact and degraded RSH. Error bars indicate SEM.
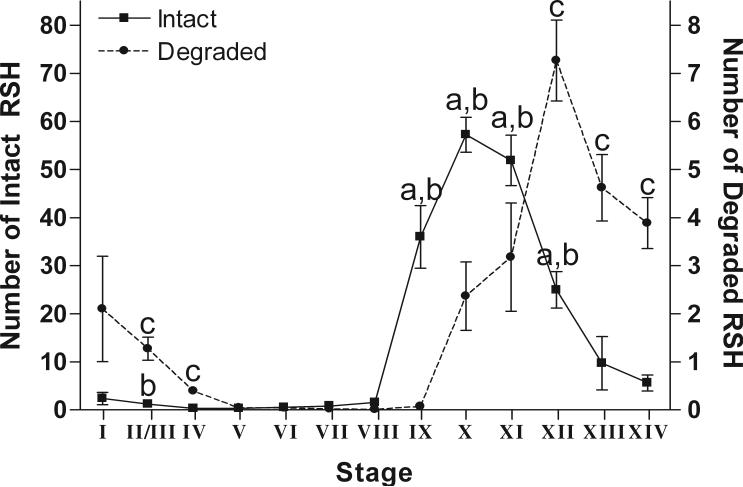
Figure 5.
The average number of intact RSH (left y-axis) or degraded RSH (right y-axis) per seminiferous tubule at each stage from rats treated with 1% HD for 18d. The average number of intact RSH increases soon after spermiation and the average number of degraded RSH increases one stage later. Intact RSH are significantly different from degraded RSH in stages IX-XII (indicated by “a”). There are significantly more intact RSH at stages IX-XII and stage II/III in treated testes compared to control, (indicated by “b”). There is a significantly higher average of degraded RSH in treated versus control testes at stages XII to IV, excluding stage I (indicated by “c”). There is a 10-fold difference between the scale of intact and degraded RSH. Error bars indicate SEM.
Electron Microscopy
Electron microscopy images of intact and degraded RSH were obtained to show the different ultrastructures as RSH were degraded. Rats were from a different experiment, but were of a similar age and subjected to the same dosing regiment. The right testes from 2 control rats and 3 rats treated with 1% HD for 18d were perfused with 5% w/v glutaraldehyde in 0.1 mol/L phosphate buffer (pH 7.4) via the testicular artery using a 30-gauge needle. After perfusion, the testes were cut into small pieces and immersed in 2.5% w/v glutaraldehyde in 0.1 mol/L phosphate buffer (pH 7.4) for 1h, then post-fixed with 1.0% w/v osmium tetroxide in 0.1 mol/L sodium cacodylate buffer (pH 7.4), dehydrated in a series of graded ethanol solutions, and embedded in Spurr epoxy resin (Electron Microscopy Sciences, Hatfield PA). Sections were stained with toluidine blue, and the spermatogenic stages (stage IX and XII) were identified using light microscopy. Stage IX was chosen to demonstrate an intact RSH because none of the RSH appeared degraded at that stage (by light microscopy), and stage XII was chosen to demonstrate a degraded RSH because that is the stages where they are the most prevalent. Thin sections (85nm) were stained with uranyl acetate and lead citrate, and examined with a transmission electron microscope (Philips 410, Philips Electronics Instruments, Mahwah, NJ).
Data analysis
Unless specifically designated as intact or degraded, RSH in the text refers to the total number of spermatid heads (intact plus degraded) retained in the basal compartment. The percent of seminiferous tubules at stages IX-XI with greater than three RSH proved to be the most sensitive endpoint in a previous publication reporting the dose response to HD exposure (Moffit et al. 2007). This endpoint of greater than three RSH compensated for the low level of RSH in control rats, wherein the vast majority of all seminiferous tubules lacked RSH and less than 4% had greater than three RSH. For all data, significance was assessed by one way ANOVA with p<0.05, unless otherwise noted.
RESULTS
In control rats, RSH were observed in the basal compartment of seminiferous tubules throughout the spermatogenic cycle with 4% of the seminiferous tubules containing greater than three RSH. RSH most often appeared dense, much like normal step 19 spermatid heads and were designated as intact (Figure 2A). Some RSH at stages X-XIII stained less densely with hematoxylin, appearing PAS positive and were designated as degraded (see Materials and Methods for a full description of intact and degraded RSH). Quantification of the average number of intact RSH and degraded RSH per seminiferous tubule showed that the intact RSH first appeared in stage IX, right after spermiation, while degraded RSH first appeared at stage X. (Figure 2B). The average number of intact RSH peaked at stage X and was 10 fold higher than the peak number of degraded RSH, maximal at stage XI. There was no statistical difference between the average number of intact or degraded RSH in control testes at each stage.
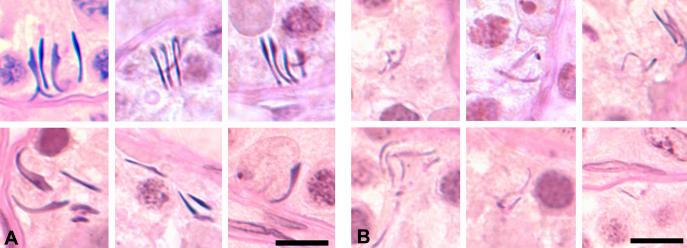
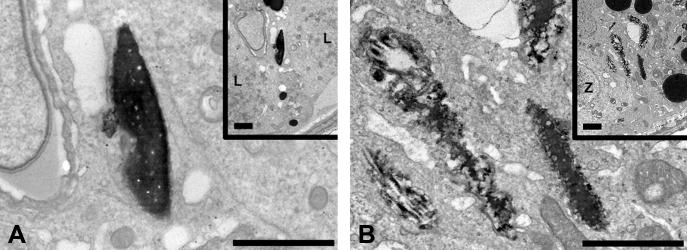
The distinction between intact and degraded RSH was more prominent after exposure to 1% HD for 18 days. Numerous intact and degraded RSH were present in clusters in the basal compartment (Figure 3). To better visualize the distinction between intact and degraded RSH, electron microscopy was performed on testes from rats treated with 1% HD for 18d (Figure 4). The density and contour of RSH at stage IX (Figure 4A) was clearly distinct from RSH at stage XII (Figure 4B).
Figure 3.
Histopathological appearance of intact (A) and degraded (B) RSH from a rat treated with 1% HD for 18d. Intact RSH appear similar to step 19 spermatids with smooth curved edges and dense hematoxylin staining. Degraded RSH appear thinner with jagged edges, are sometimes bent at more acute angles, and stain less intensely with hematoxylin, often appearing PAS positive. PASH stained 3μm plastic sections; bars = 10μm.
Figure 4.
Transmission electron microscopy of intact RSH in stage IX (A) and degraded RSH in stage XII (B) from a rat treated with 1% HD for 18d. The degraded RSH at stage XII show rougher surfaces compared to the intact RSH at stage IX. Each inset is at a lower magnification and shows the basement membrane. L, leptotene spermatocyte; Z, zygotene spermatocyte. Uranyl acetate and lead citrate stained 85nm sections; bars = 2μm.
In rats treated with 1% HD for 18d, quantification of the average number of intact and degraded RSH showed a one-stage delay in the appearance of degraded RSH and an 8-fold difference between the peak average number of intact RSH (stage X) and degraded RSH (stage XII) (Figure 5). Statistically, there were more intact RSH than degraded RSH at stages IX-XII. Additionally, in the 1% HD treated samples, the average number of RSH extended into later stages with intact RSH present until stage II/III and degraded RSH extending to stage IV.
To better define the dose response and the dynamics of the onset and resolution of RSH after HD exposure, a series of Dose Response, Time Course, and Recovery experiments were performed (see Figure 1 for an overview of these experiments).
Dose Response
Exposure to HD greatly increased the occurrence of spermatid head retention, predominately at stages IX-XII. Rats dosed with 0.08% to 1% HD in drinking water for 18d (delivered doses of 0.05 − 0.47 g/kg/day per 18d) showed a dose dependent increase in the average number of RSH in seminiferous tubules at stages IX-XII, with a lowest observed effect level (LOEL) of 0.21% HD, with 1.72- fold as many RSH as control (Figure 6). The NOEL was 0.14% HD (delivered dose of 0.09 g/kg/day over 18d). A linear regression fit to the dose response with the y-intercept set to the control value gave r = 0.9135.
Exposure to 0.33% HD for 18d resulted in a significant increase in the percent of seminiferous tubules with greater than three RSH at stages IX-XI, peaking at 96.8% at stage X (Figure 7). Exposure to 1% HD showed a significant increase from stages IX-II/III that plateaued at stages X-XII, with over 95% of the seminiferous tubules containing more than three RSH (Figure 7).
Time Course
Time course experiments were conducted with 0.33% HD in drinking water for various durations to investigate the onset of increased spermatid head retention. Exposure to 0.33% HD for 11−18d showed a steady increase in the percent of seminiferous tubules at stages IX-XII with greater than three RSH in the basal compartment (Figure 8A). After 13d of exposure, there was a non-significant increase at stages IX and X (47% and 64% compared to control, respectively). After 15d of exposure, stages IX-XII had a higher percent of seminiferous tubules with greater than three RSH, but only stage X showed a significant increase at 74%. After 18d of exposure, the percentage of seminiferous tubules with greater than three RSH peaked at stage X (96.8%) and was significantly elevated across stages IX-XI.
Recovery
To assess the ability to recover from RSH, rats were treated with 0.33% HD for 18d and then switched to water and evaluated 1, 2, and 4 wk post-exposure (Figure 8B). The percentage of tubules with greater than three RSH remained high 1 and 2 wk post-exposure (92% and 100% at stage X, respectfully). After 4 wk of recovery, however, RSH returned to the level observed in control rats (23% of the stage X seminiferous tubules containing greater than three RSH). There were no other histopathological changes apparent at any of these time points.
DISCUSSION
The events that lead to spermatid head retention include spermiation failure, movement of the unreleased spermatid heads to the basal compartment, and the degradation of phagocytosed spermatid heads. Spermiation failure and the presence of RSH in stages IX to XII have previously been observed after exposure to various toxicants, including those that decrease serum testosterone, such as EDS, as well at those that do not, such as the haloacetic acids; however, the dynamics of induction and removal of RSH have not been explored in detail (Bartlett et al. 1986; Linder et al. 1994; Toth et al. 1992). This study describes the dose-dependent onset and resolution of RSH in a well-established model of testicular injury, exposure to HD in drinking water.
Spermiation failure is known to occur spontaneously. Spermatids that fail spermiation are phagocytosed and moved to basal compartments for degradation (Russell and Clermont 1977), resulting in basal levels of RSH at stages IX-XII. Very little is known about the transport of RSH to the basal compartment. It has been suggested that Sertoli cells engulf the spermatid heads and transport the heads within vesicles along MT to the basal compartment (Russell 1991).
Exposure to HD exaggerated this phenomenon, as illustrated by the dose trend of the average number of RSH at stage IX-XII. The underlying mechanism for HD-induced spermatid head retention is unknown, but may be related to the well-described effects of HD as a MT stabilizer (Graham et al. 1985; Boekelheide 1988b; Boekelheide 1988a; Boekelheide 1987b; Boekelheide 1987a). This MT stabilizing effect of HD exposure may have impaired ectoplasmic specialization function, resulting in failed dissociation of step 19 heads from the seminiferous epithelium. The RSH often appeared in clusters in 1% HD treated rats, suggesting that large groups of spermatid heads failed to spermiate and were retained at the same time.
The distribution of RSH across stages and the quantification of intact and degraded RSH have provided insight into the kinetics of degradation. The delayed onset of the appearance of degraded RSH versus intact RSH in control animals indicated that at least 7h must pass for this partial degradation to be observed. As RSH are only detected during stages IX-XII in controls, they are fully degraded within this period, a span of 3d. The restriction of RSH to stages IX-XII in 0.33% HD treated rats indicated that the degradation function of Sertoli cells was not impaired at this dose. On the other hand, the presence of RSH in stages XIII-II/III after 18d of 1% HD treatment suggested that Sertoli cells were more severely damaged compared to 0.33% HD exposure. In fact, there were significantly elevated levels of degraded RSH over control from stages XII-IV at the 1% HD dose. Sertoli cells exposed to 1% HD for 18d were less efficient at removing the RSH, either because the volume of RSH overwhelmed the capacity of Sertoli cells to degrade, or the degradation machinery was compromised in some way.
The Time Course experiment suggests that 0.33% HD caused a biologically significant increase in RSH by 13d, which progressed to statistical significant by 15d of exposure at stage X. Based on the timing of spermatogenesis (Russell et al. 1990), those RSH seen at stage X at 13d failed to spermiate approximately half a day earlier, after 12.5d of exposure to HD. The RSH present at stage II/III after 18d of exposure to 1% HD must have failed to spermiate 5−6 days earlier, based on the known duration of each stage, which is consistent with onset at 12−13d of exposure.
In the Recovery experiment, the elevated levels of RSH at 1 and 2wk post-exposure suggested continued spermiation failure. This is not surprising given the known dynamics of clearance of HD-induced MT-adducts with approximately 2 weeks required for the HD-induced alterations in MT function to resolve (Boekelheide 1988a; Boekelheide 1988b). The eventual decline of RSH by 4wk showed that the seminiferous epithelium was able to recover from this injury.
Previous studies of Sertoli cell toxicants from this laboratory showed that RSH at stages IX-XI was not a sensitive endpoint for short-term exposures on the order of a day or less. Two other Sertoli cell toxicants were tested: a 24h exposure to carbendazim, which depolymerizes MT, and a 12h exposure to mono-2-ethyhyl phthalate, a non-MT dependent toxicant (Moffit et al. 2007). Only the highest dose of carbendazim tested (200mg/kg) showed a significant increase in the percentage of seminiferous tubules with greater than three RSH 24h after exposure. This suggests that the endpoint of RSH is more useful for longer exposures that allow time for the progression of the cycle of spermatogenesis and accumulation of RSH. Additionally, different toxicants may have various periods of latency after exposure before an effect is observed.
This study has shown that elevated numbers of RSH are a sensitive histopathological marker of the extent of injury following HD exposure. Further experiments are required to define the underlying mechanism for this histopathological marker, but it may involve, at least in part, altered MT at the ectoplasmic specialization. Comparing the frequency of spermatid head retention after subacute exposure to toxicants that work through different mechanisms may help elucidate the processes that contribute to spermiation failure and retention. This laboratory is currently investigating longer-term exposures of other Sertoli cell toxicants to determine if spermatid head retention is a general marker for subacute Sertoli cell toxicity. The presence of RSH at stages IX-XII may be the only histopathological marker with low-dose exposures, and may indicate a decrease in testosterone, disrupted MT dynamics, or a more generalized alteration of Sertoli cell function. We suggest that the accumulation of RSH at stages IX to XII be qualitatively or quantitatively assessed as an indicator of Sertoli cell disruption in subacute and longer-term toxicity studies.
ACKNOWLEDGEMENTS
Authors would like to thank Paula Weston for her help with electron microscopy. The project described was supported by grant number 5 P42 ES013660-02 from the National Institute of Environmental Health Sciences (NIEHS), NIH.
Abbreviations
- HD
2,5-hexanedione
- RSH
Retained spermatid heads
- MT
Microtubules
- CBZ
Carbendazim
- MEHP
Mono-2-ethylhexyl phthalate
- FSH
Follicular stimulating hormone
- LH
Luteinizing hormone
- DBA
Dibromoacetic acid
- NaDCA
Sodium dichloroacetic acid
- EDS
Ethane dimethanesulfonate
Footnotes
Publisher's Disclaimer: This PDF receipt will only be used as the basis for generating PubMed Central (PMC) documents. PMC documents will be made available for review after conversion (approx. 2−3 weeks time). Any corrections that need to be made will be done at that time. No materials will be released to PMC without the approval of an author. Only the PMC documents will appear on PubMed Central -- this PDF Receipt will not appear on PubMed Central.
Supplementary Material
REFERENCES
- Bartlett JM, Kerr JB, Sharpe RM. The effect of selective destruction and regeneration of rat Leydig cells on the intratesticular distribution of testosterone and morphology of the seminiferous epithelium. Journal of Andrology. 1986;7:240–53. doi: 10.1002/j.1939-4640.1986.tb00924.x. [DOI] [PubMed] [Google Scholar]
- Boekelheide K. 2,5-Hexanedione alters microtubule assembly. I. Testicular atrophy, not nervous system toxicity, correlates with enhanced tubulin polymerization. Toxicol Appl Pharmacol. 1987a;88:370–82. doi: 10.1016/0041-008x(87)90212-2. [DOI] [PubMed] [Google Scholar]
- Boekelheide K. 2,5-Hexanedione alters microtubule assembly. II. Enhanced polymerization of crosslinked tubulin. Toxicol Appl Pharmacol. 1987b;88:383–96. doi: 10.1016/0041-008x(87)90213-4. [DOI] [PubMed] [Google Scholar]
- Boekelheide K. Rat testis during 2,5-hexanedione intoxication and recovery. I. Dose response and the reversibility of germ cell loss. Toxicol Appl Pharmacol. 1988a;92:18–27. doi: 10.1016/0041-008x(88)90223-2. [DOI] [PubMed] [Google Scholar]
- Boekelheide K. Rat testis during 2,5-hexanedione intoxication and recovery. II. Dynamics of pyrrole reactivity, tubulin content, and microtubule assembly. Toxicol Appl Pharmacol. 1988b;92:28–33. doi: 10.1016/0041-008x(88)90224-4. [DOI] [PubMed] [Google Scholar]
- Creasy DM. In: Histopathology of the male reproductive system II - evaluation. Chapin RE, Boekelheide K, editors. John Wiley and Sons; Inc, New York: 2002. [Google Scholar]
- Graham DG, Anthony DC, Szakál-Quin G, Gottfried MR, Boekelheide K. Covalent crosslinking of neurofilaments in the pathogenesis of n-hexane neuropathy. Neurotoxicology. 1985;6:55–63. [PubMed] [Google Scholar]
- Hall ES, Hall SJ, Boekelheide K. 2,5-Hexanedione exposure alters microtubule motor distribution in adult rat testis. Fundam Appl Toxicol. 1991;24:173–82. doi: 10.1006/faat.1995.1021. [DOI] [PubMed] [Google Scholar]
- Linder RE, Klinefelter GR, Strader LF, Suarez JD, Dyer CJ. Acute spermatogenic effects of bromoacetic acids. Fundam Appl Toxicol. 1994;22:422–30. doi: 10.1006/faat.1994.1048. [DOI] [PubMed] [Google Scholar]
- Linder RE, Klinefelter GR, Strader LF, Suarez JD, Roberts NL. Spermatotoxicity of dichloroacetic acid. Reproductive toxicology (Elmsford, N.Y) 1997;11:681–8. doi: 10.1016/s0890-6238(97)00031-2. [DOI] [PubMed] [Google Scholar]
- Moffit JS, Bryant BH, Hall SJ, Boekelheide K. Dose-Dependent Effects of Sertoli Cell Toxicants 2,5-Hexanedione, Carbendazim, and Mono-(2-ethylhexyl) phthalate in Adult Rat Testis. Toxicol Pathol. 2007;35:719–27. doi: 10.1080/01926230701481931. [DOI] [PubMed] [Google Scholar]
- Russell LD. The perils of sperm release-- 'let my children go'. International Journal of Andrology. 1991;14:307–11. doi: 10.1111/j.1365-2605.1991.tb01097.x. [DOI] [PubMed] [Google Scholar]
- Russell LD. Role in Spermiation. In: Russell LD, M.D. G, editors. The Sertoli Cell. Cache River Press; Clearwater, FL: 1993. [Google Scholar]
- Russell LD, Clermont Y. Degeneration of germ cells in normal, hypophysectomized and hormone treated hypophysectomized rats. The Anatomical record. 1977;187:347–66. doi: 10.1002/ar.1091870307. [DOI] [PubMed] [Google Scholar]
- Russell LD, R.A. E, A.P. SH, E.D. C. Histological and Histopathological Evaluation of the Testis. 1990 [Google Scholar]
- Saito K, O'Donnell L, McLachlan RI, Robertson DM. Spermiation failure is a major contributor to early spermatogenic suppression caused by hormone withdrawal in adult rats. Endocrinology. 2000;141:2779–85. doi: 10.1210/endo.141.8.7628. [DOI] [PubMed] [Google Scholar]
- Toth GP, Kelty KC, George EL, Read EJ, Smith MK. Adverse male reproductive effects following subchronic exposure of rats to sodium dichloroacetate. Fundam Appl Toxicol. 1992;19:57–63. doi: 10.1016/0272-0590(92)90028-g. [DOI] [PubMed] [Google Scholar]
- Treinen KA, Chapin RE. Development of testicular lesions in F344 rats after treatment with boric acid. Toxicol Appl Pharmacol. 1991;107(2):325–35. doi: 10.1016/0041-008x(91)90212-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.