Abstract
Purpose
Under certain conditions, the full-field flicker electroretinogram (ERG) of the cone system can show period doubling, such that the shape of the ERG waveform alternates from cycle to cycle. The purpose of this study was to determine the relationship between stimulus temporal frequency and the amplitudes of the spectral components of the ERG that correspond to period doubling.
Method
ERGs were recorded from 10 visually normal subjects in response to full-field sinusoidal flicker presented at frequencies ranging from 12.5 to 100 Hz. Period doubling was apparent over the stimulus frequency range from 25 to 100 Hz and was quantified in terms of the amplitudes of spectral components of the ERG waveform that corresponded to half the stimulus frequency (f/2) and three and five times that frequency (3f/2 and 5f/2).
Results
At stimulus frequencies between 30 and 40 Hz, the amplitude of f/2 was significantly lower than either 3f/2 or 5f/2, which themselves did not differ significantly. At stimulus frequencies between 40 and 60 Hz, all three response components were equivalent in amplitude. At stimulus frequencies above 60 Hz, however, the amplitudes of 3f/2 and 5f/2 were reduced significantly compared to f/2.
Conclusion
There is a frequency-dependent relationship among the spectral components of the flicker ERG that correspond to period doubling. The amplitude of f/2 underestimates the magnitude of period doubling at stimulus frequencies between 30 and 40 Hz, whereas the amplitudes of 3f/2 and 5f/2 underestimate the degree of period doubling at stimulus frequencies above 60 Hz. The explanation for the frequency dependence of these spectral components remains to be resolved.
Keywords: Cone, ERG, Flicker, Temporal modulation
Introduction
The full-field flicker ERG of the cone system is a common clinical method for evaluating retinal dysfunction in human eye diseases. However, a potential complication in the quantitative analysis of the flicker ERG is that the waveform can manifest period doubling, a phenomenon in which the waveform shape repeats itself every two cycles rather than every cycle [1, 2]. Period doubling can be observed as a frank alternation in response amplitude from cycle to cycle, or as a subtle cyclic variation in the shape of the ERG waveform without an amplitude alternation. Period doubling can occur in the ERG response to both pulse trains [1] and sinusoidal stimuli [2]. At a stimulus frequency of f, the presence of period doubling is observable in the amplitude spectrum of the flicker ERG as spectral components that are odd multiples of a frequency that is half the stimulus frequency (f/2) (the even multiples of f/2 are at the same frequencies as f and its harmonic components and therefore cannot be distinguished from those components). It is not necessary that multiple harmonics of f/2 be present in the amplitude spectrum in order to observe an alternation in waveform shape: only one odd multiple of f/2 needs to be present. As discussed previously [3], the specific manifestation of period doubling in the flicker ERG depends on the number of the harmonics of f/2 that are present, as well as on the phase relationships among the various spectral components.
Crevier and Meister [1] noted that the amplitude of the f/2 component of the human flicker ERG was typically relatively small and could be difficult to detect against the noise level. Consequently, the amplitude of the third harmonic of f/2 (3f/2) was employed in that study as a more reliable index of period doubling. A subsequent investigation of period doubling [2] reported a similar reduction of the amplitude of f/2 relative to that of 3f/2. However, in our examination of the flicker ERG waveforms of visually normal subjects at high stimulus frequencies, we observed that there could be a prominent alternation in ERG response amplitude even though the amplitude of the 3f/2 harmonic was relatively small. Thus, it is not clear whether the 3f/2 harmonic is, in fact, the most suitable index of period doubling across the entire frequency range.
The present study explored this issue through a systematic comparison of the amplitudes of the spectral components of the flicker ERG that correspond to f/2 and 3f/2, as well as to 5f/2, which can be an appreciable component of the ERG amplitude spectrum. The amplitudes of these spectral components were compared across stimulus frequency in a group of visually normal subjects. The intent was to provide a more complete characterization of the spectral characteristics of period doubling in the cone flicker ERG.
Methods
Subjects
Ten visually normal subjects ranging in age from 23 to 55 years (mean age, 35.4 yrs; standard deviation, 11.5 yrs) participated in the study. All had best-corrected visual acuities that were 20/20 or better in the tested eye as measured with a Lighthouse Distance Visual Acuity Test (Lighthouse International, New York, NY) and normal letter contrast sensitivity as measured with a Pelli-Robson Contrast Sensitivity Chart (Haag-Streit, Koeniz, Switzerland), clear ocular media, and normal-appearing fundi on ophthalmologic examination. Appropriate institutional review board approval was obtained, and the experiments were undertaken with the understanding and written consent of each subject.
Stimuli and Recording System
The test stimulus consisted of sinusoidally modulated full-field flicker that was a sum of middle- and long-wavelength light to minimize chromatic (red/green) adaptation. Stimuli were modulated sinusoidally in luminance rather than presented as pulse trains in order to limit their frequency content. The stimuli were generated by arrays of light-emitting diodes (LEDs) with peak wavelengths of 512 and 632 nm, presented in a Diagnosys ColorDome desktop Ganzfeld (Diagnosys LLC, Littleton, MA). The output of the LEDs was pulse-width-modulated at a frequency of 1 kHz to control the stimulus luminance. Stimulus spectral characteristics were calibrated with a PR-650 SpectraScan colorimeter (Photo Research, Inc., Chatsworth, CA). Luminances were based on the 10-degree luminous efficiency function [V10(λ)], because the non-foveal retina is the major contributor to the full-field ERG [4].
The mean luminance of the flicker was 200 cd/m2, consisting of 100 cd/m2 each of the 512- and 632-nm stimuli (90.4 and 95.4 cd/m2, respectively, based on the 2-degree luminous efficiency function [V(λ)]). The stimulus luminance was maintained at this mean level between presentations of the flicker. The flicker was presented against a steady, rod-saturating adapting field of 12.3 cd/m2 (39.7 scot cd/m2 or 3.3 log scot td, assuming a dilated pupil diameter of 8 mm), generated by an array of LEDs with a peak wavelength of 468 nm. This adapting field was also present during the interstimulus interval. The stimulus contrast was nominally 100%, with contrast C defined according to the Michelson formulation:
| (1) |
where Lmax and Lmin are the maximum and minimum luminances, respectively. Against the short-wavelength adapting field, the effective stimulus contrast was 94.2%.
ERG responses were recorded using a Burian-Allen bipolar contact lens electrode wetted with methylcellulose and inserted under room illumination. An earclip electrode served as ground. Signals were acquired with the Diagnosys Espion electrophysiology console, using an amplifier band-pass setting of 0.3 to 500 Hz and a sampling frequency of 2 kHz. The response sampling was effectively synchronized with the stimulus generation.
Procedure
Prior to the ERG recordings, the pupil of the tested eye was dilated with 2.5% phenylephrine hydrochloride and 1% tropicamide drops, and the cornea was anesthetized with proparacaine drops. Each subject was light-adapted to room illumination prior to testing and was then adapted for 5 min to the stimulus mean luminance in order to achieve a stable level of light adaptation.
ERG responses were recorded to stimuli ranging in frequency from 12.5 to 100 Hz, but the analysis of period doubling was confined to frequencies of 25 Hz and above because that was the frequency range that showed period doubling in this group of subjects, in agreement with previous studies [1-3]. ERG responses from each subject were obtained within a single testing session, and stimuli were presented in order of increasing frequency within the session. The onset of each ERG recording was synchronized with the start of the flicker train in order to maintain the appropriate phase relationship between successive recordings. The recording length for the sweeps was approximately 1 s (the exact length depended on stimulus frequency). At each frequency, ERG responses were recorded to three stimulus presentations.
Analysis
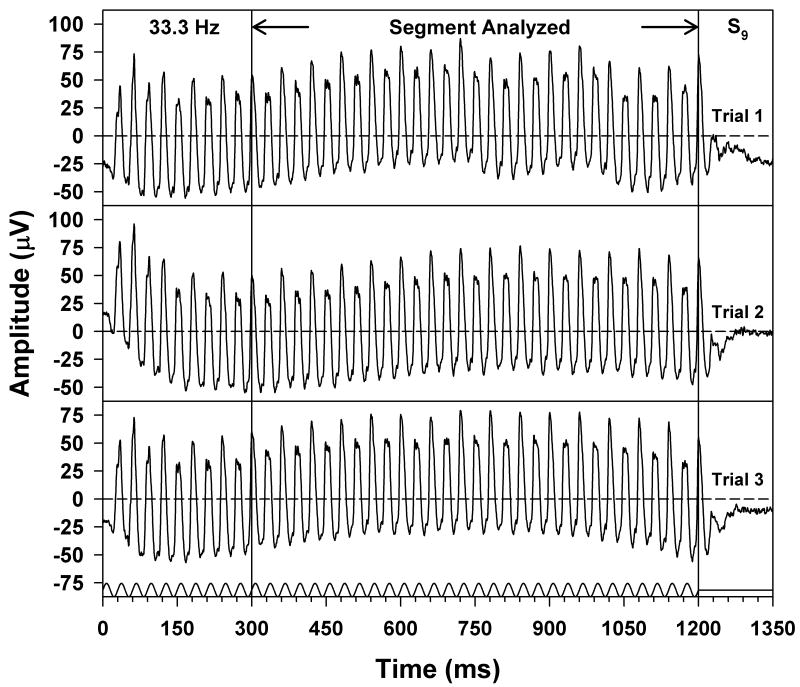
The first 200 to 300 ms of each ERG waveform were omitted from analysis in order to avoid onset transients (the exact length that was omitted was an even number of stimulus cycles). As a result, waveform segments of approximately 800 ms were used for analysis, consisting of an even number of cycles. Figure 1 illustrates this approach applied to the ERGs of subject S9, obtained at a stimulus frequency of 33.3 Hz. Three waveforms are plotted in this figure to illustrate reproducibility, and the segment of the waveforms that was analyzed is indicated by the arrows and vertical lines. The stimulus waveform is illustrated at the bottom. The stimulus was presented in sine phase and contained an integral number of cycles, as was the case at all frequencies.
Figure 1.
Individual ERG responses of S9, obtained at a stimulus frequency of 33.3 Hz, with the stimulus waveform given below the responses and the x-axis labeled in multiples of the stimulus period. Three ERG waveforms are plotted to illustrate reproducibility, and the segment of the waveforms that was used for analysis is indicated by the arrows and vertical lines. Horizontal dashed lines represent the waveform means.
Figure 1 also illustrates the phenomenon of period doubling in the flicker ERG. After an initial onset transient, the ERG waveform began to show an alternation in trough-to-peak amplitude and a change in waveform shape from cycle to cycle. It is apparent from Figure 1 that the onset of the alternation in amplitude occurred on the same response cycle from waveform to waveform. This was also the case at the other stimulus frequencies that were tested, and was true for all subjects. Given this consistency in the onset of period doubling, the data analysis was based on the mean of the three ERG responses obtained at each stimulus frequency for each subject. The amplitude and phase of the ERG response fundamental and of the various harmonics at each stimulus frequency were derived from a discrete Fourier transform (DFT) of each averaged ERG waveform.
To establish that the harmonic components of the flicker ERG were not due to nonlinearities in the stimulus, the waveforms that were generated by the LED arrays were measured with a linear photocell whose output was acquired by the Espion electrophysiology console using amplifier band-pass settings of 0 to 500 Hz and a sampling frequency of 2 kHz. The harmonic components of the stimulus waveforms acquired in this way were analyzed with a DFT. The total harmonic distortion (defined as the ratio of the sum of harmonic power to fundamental power, as per convention) of the stimulus waveforms across frequency was less than 0.5%. Therefore, any spectral components present in the ERG response besides the fundamental must have originated from retinal nonlinearities.
Results
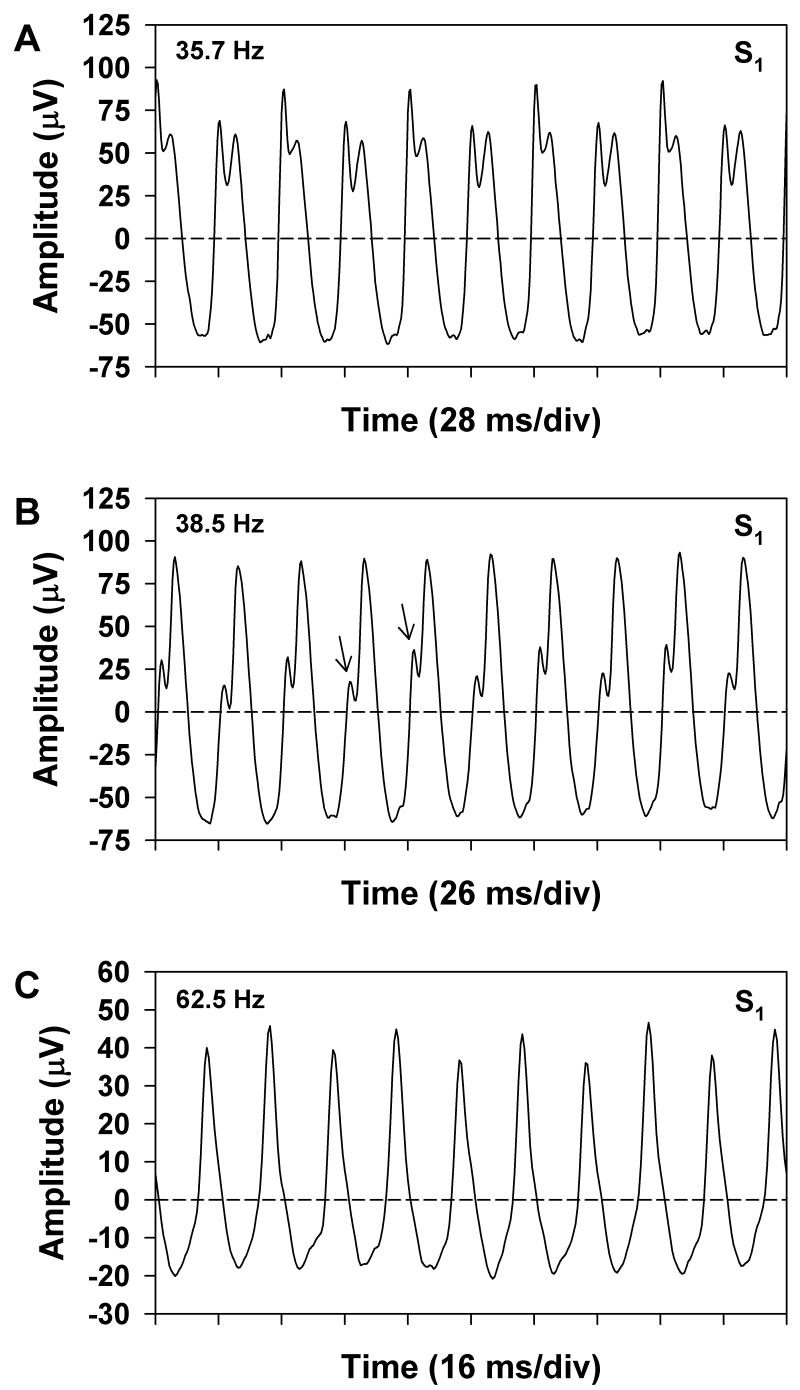
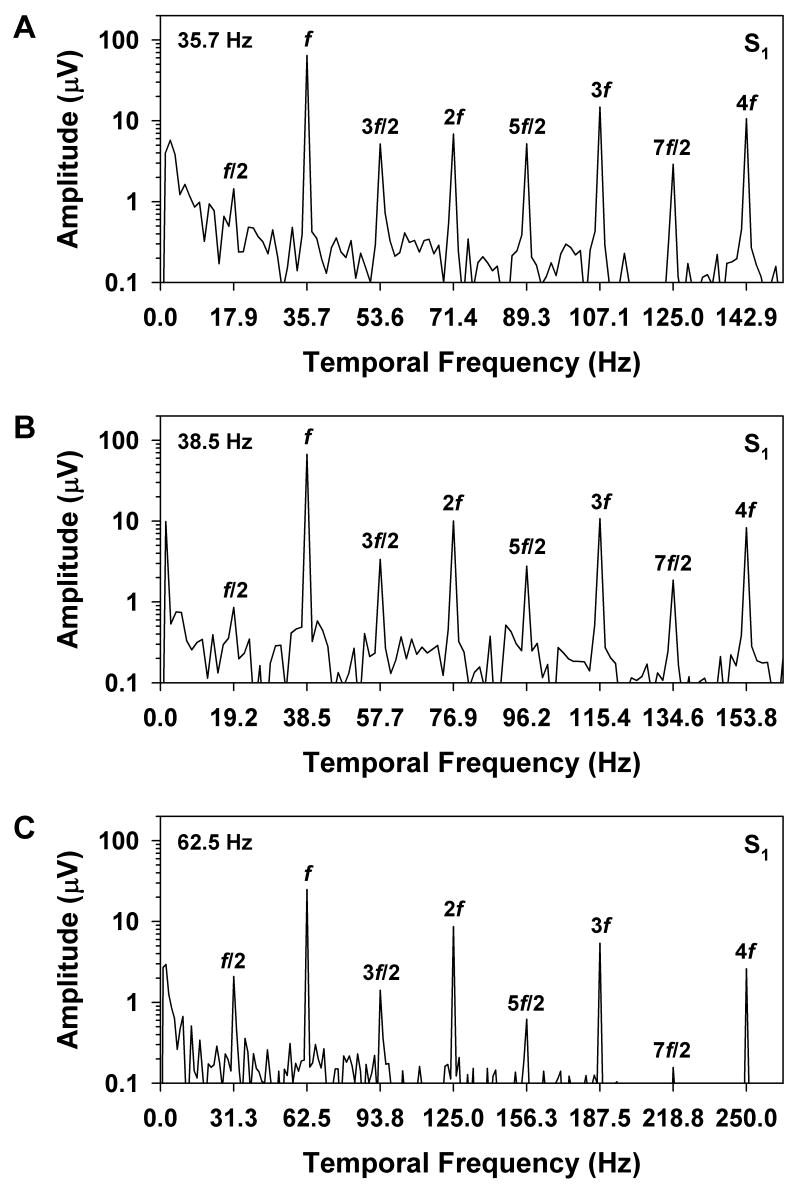
Figure 2 illustrates the various ways in which period doubling can be manifested in the waveform of the flicker ERG. This figure shows the mean ERG responses obtained from S1 at stimulus frequencies of 35.7 (A), 38.5 (B), and 62.5 (C) Hz. Ten response cycles of the analyzed segment of the ERG waveform are plotted at each frequency. For comparison, Figure 3 plots the corresponding amplitude spectra of the entire analyzed waveform segments at these same stimulus frequencies. As per convention, the amplitudes that are plotted in Figure 3 represent half the trough-to-peak amplitude of the harmonic components. At a stimulus frequency of 35.7 Hz (Figure 2A), the waveform alternated in both shape and trough-to-peak amplitude from cycle to cycle. The corresponding amplitude spectrum of the ERG at this stimulus frequency (Figure 3A) showed prominent components at f and its multiples. There were also components at f/2 and its odd multiples, but the amplitude of f/2 was only slightly above the noise level. In comparison, the response components at 3f/2, 5f/2, and 7f/2 were quite robust and were approximately equivalent in amplitude to the component at 2f.
Figure 2.
Three mean ERG waveforms of S1 (10 cycles each) obtained at stimulus frequencies of (A) 35.7 Hz, (B) 38.5 Hz, and (C) 62.5 Hz, with time plotted in multiples of the stimulus period. Horizontal dashed lines represent the waveform means. Arrows indicate the waveform features that alternated from cycle to cycle.
Figure 3.
Amplitude spectra of the mean ERGs of S1 at stimulus frequencies of (A) 35.7 Hz, (B) 38.5 Hz, and (C) 62.5 Hz, with frequency plotted in multiples of the stimulus frequency and amplitude plotted on a logarithmic scale. Labels above the peaks indicate the various multiples of the stimulus frequency.
At the slightly higher stimulus frequency of 38.5 Hz (Figure 2B), there was no consistent difference in waveform amplitude from cycle to cycle. However, there was a subtle alternation in waveform shape, consisting primarily of a slight difference in the inflection of the rising portion of the response, as indicated by the arrows in the figure. The amplitude spectrum of the ERG at this stimulus frequency (Figure 3B) contained spectral components that were similar to those observed at the lower frequency of 35.7 Hz (Figure 3A), despite the overall differences in waveform shape. As was the case at the lower stimulus frequency, the amplitude of f/2 was only slightly greater than the noise level, whereas the odd harmonics of f/2 were relatively prominent. The difference between the waveform shapes at these two stimulus frequencies was due in large part to the differing phase relationships among the various spectral components. This can be observed qualitatively by the fact that the major peaks of the waveforms in Figures 2A and 2B occurred at different time points in the respective stimulus periods, whereas the more subtle modulations occurred at approximately the same time point for both frequencies.
At the relatively high stimulus frequency of 62.5 Hz (Figure 2C), the overall amplitude of the ERG was less than at the lower frequencies. There was a consistent alternation in response amplitude, but no systematic variation in overall waveform shape. The corresponding amplitude spectrum (Figure 3C) contained a prominent component at f/2, but showed declining amplitudes of the odd harmonics of f/2, in contrast to the results obtained at the lower frequencies. The systematic decline in the amplitudes of the harmonics of f/2 with increasing stimulus frequency paralleled the decrease in the amplitudes of the harmonics of f.
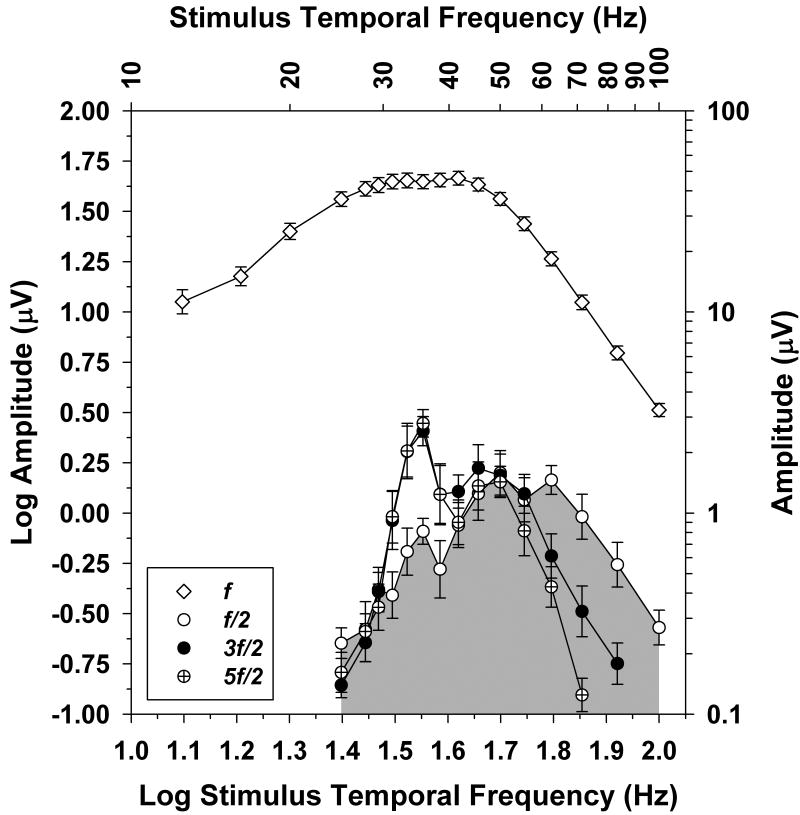
The pattern of spectral component amplitudes shown by S1 was representative of the subjects in general, as is summarized in Figure 4. This figure plots the mean log amplitudes of the ERG spectral components f/2, 3f/2, and 5f/2 for the 10 subjects as a function of log stimulus frequency (for clarity, the 7f/2 component was not plotted). The data points for 3f/2 at 100 Hz and for 5f/2 at 83.3 and 100 Hz were omitted because they were within the noise level, based on the amplitudes of the spectral components at neighboring frequencies. To emphasize the relationships among the spectral components of period doubling, the area under the frequency-response function for f/2 has been shaded.
Figure 4.
Comparison of the mean log amplitudes of f (unfilled diamonds), f/2 (unfilled circles), 3f/2 (filled circles), and 5f/2 (crossed circles) as a function of log stimulus frequency for the 10 subjects. The corresponding stimulus frequencies are given on the top x-axis, and amplitudes are indicated on the right y-axis. Error bars represent standard errors of the means. The region under the f/2 component is shaded to facilitate a comparison of the response functions for the various spectral components.
It is apparent that the relationship among the log amplitudes of f/2, 3f/2, and 5f/2 varied with stimulus frequency. There was a prominent peak in the amplitude of 3f/2 (and also 5f/2) at a stimulus frequency of 35.7 Hz, but there was a relative attenuation of f/2 compared to 3f/2 and 5f/2 across the frequency range from approximately 30 to 40 Hz. At stimulus frequencies above 55.5 Hz, the opposite pattern was observed; there was a relative attenuation of 3f/2 and 5f/2 compared to f/2. The log amplitudes of all three spectral components were similar at stimulus frequencies near 50 Hz.
The log amplitudes of f/2, 3f/2, and 5f/2 were compared statistically using a repeated-measures analysis of variance and post-hoc t-tests with a Bonferroni correction for multiple comparisons. At stimulus frequencies of 31.2, 33.3, 35.7, and 38.5 Hz, the log amplitude of f/2 was significantly lower than the log amplitudes of either 3f/2 or 5f/2 (all P < 0.001), but the log amplitudes of 3f/2 and 5f/2 did not differ significantly. At stimulus frequencies of 41.7, 45.4, 50.0, and 55.5 Hz, there were no significant differences among the log amplitudes of the three harmonic components. At stimulus frequencies of 62.5 Hz and higher, however, the log amplitude of f/2 was significantly greater than the log amplitudes of either 3f/2 or 5f/2 (all P < 0.001). At a stimulus frequency of 71.4 Hz, the log amplitudes of 3f/2 and 5f/2 also differed significantly (P < 0.001).
Shown for comparison in Figure 4 are the mean log amplitudes of the response fundamental f as a function of log stimulus frequency. Consistent with previous studies (e.g., ref. [5] and ref. [6]), the frequency-response function for f showed a band-pass shape, with a broad region of high amplitudes between approximately 30 and 40 Hz and an amplitude attenuation at lower and higher frequencies. There was also a small inflection in the amplitude function for f in the region of the peak amplitudes of 3f/2 and 5f/2. At stimulus frequencies above 60 Hz, the amplitudes of the f/2 and 3f/2 components decreased with increasing stimulus frequency in parallel with the reduction in the amplitude of f. This indicates that the amplitudes of the f/2 and 3f/2 spectral components were proportional to fundamental response amplitude over this frequency region.
Discussion
The aim of this study was to characterize the frequency characteristics of period doubling in the human cone flicker ERG, with an emphasis on the properties of the spectral components as derived from Fourier analysis. The results demonstrate that the degree of correspondence among the various spectral components that represent period doubling varies with stimulus frequency, as shown in Figure 4. At stimulus frequencies between approximately 30 and 40 Hz, there was a relative attenuation of f/2 compared to both 3f/2 and 5f/2, so that either 3f/2 or 5f/2 would be a more appropriate index of period doubling than f/2. At stimulus frequencies above 60 Hz, however, the amplitude of f/2 was significantly larger than that of either 3f/2 or 5f/2, indicating that over this frequency region, the amplitude of f/2 is a more appropriate measure of period doubling. Between approximately 40 and 60 Hz, all three spectral components had statistically equivalent amplitudes.
Our results emphasize the point that, although period doubling can be quantified in terms of an alternation in trough-to-peak amplitude, a more appropriate method is to derive the amplitudes of the spectral components of the ERG response that correspond to period doubling. For example, the trough-to-peak amplitude may not vary even though period doubling is present, as is illustrated in Figure 2B. Further, the spectral components are less susceptible to random eye movements and to the noise intrinsic to the ERG that may interfere with trough-to-peak measurements.
As discussed by Crevier and Meister [1], period doubling is a phenomenon that is frequently encountered in nonlinear dynamical systems and may indicate that the system is approaching a chaotic state. Based on electrophysiological recordings from salamander retina, these investigators proposed a quantitative model of period doubling that involves a nonlinear feedback signal with an exponential decay that alters response gain, in combination with electrical coupling between neural elements that leads to synchronous responses. According to the Crevier and Meister model [1], the f/2 spectral component of the flicker ERG is generated by this feedback mechanism. The harmonics of f/2 are then presumably the result of retinal nonlinearities that also generate the harmonics of f.
It was suggested previously [2] that the relative attenuation of f/2 compared to 3f/2 at stimulus frequencies in the region between 30 and 40 Hz might be due to the operation of a band-pass filter that was located at or following a nonlinearity that generated the harmonics of f/2. This band-pass filter was presumed to act on the actual harmonic frequencies (not the stimulus frequencies) and thus reduced the response amplitude of f/2 in a manner similar to the way in which the amplitude of f is attenuated at stimulus frequencies below 30 Hz (see Figure 4). However, the robustness of the 5f/2 (and 7f/2) spectral components across the stimulus frequency range of 30 to 40 Hz (Figures 2 and 4) argues against such a band-pass filter. A further consideration is that, at stimulus frequencies above 60 Hz, the attenuation of the harmonics of f/2 across stimulus frequency was similar to the attenuation of the harmonics of f (e.g., Figure 3C). This similarity implies that both sets of harmonics were subject to a form of low-pass filtering over this frequency range. Thus, the nature of the filtering that governs the response properties of f/2 and its harmonics across frequency remains to be resolved.
In conclusion, period doubling is a replicable characteristic of the flicker ERG of the cone system, and it may prove useful in probing the nature of feedback mechanisms within the outer retina (e.g., ref [3]). As depicted in Figure 2, the phenomenon can be observed in the ERG waveform as a cyclic variation in trough-to-peak amplitude, but also as a change in waveform shape from cycle to cycle without a frank alternation in amplitude. The particular manifestation of period doubling depends on the relationship between the amplitudes and phases of the various spectral components of the flicker ERG response. The present results show that the appropriate ERG spectral component to use in quantifying period doubling varies with stimulus frequency. Either the 3f/2 or the 5f/2 harmonic is preferable at stimulus frequencies below 40 Hz, whereas the f/2 harmonic is a more representative measure at frequencies higher than 60 Hz. Alternatively, the mean amplitude of the f/2, 3f/2, and 5f/2 harmonics might serve as a useful measure of period doubling. The explanation for the frequency-dependent behavior of the spectral components of the flicker ERG response that represent period doubling remains to be determined. However, this frequency dependence should be considered in any quantitative analysis of period doubling in the human cone flicker ERG.
Acknowledgments
This research was supported by NIH Research Grant EY08301 (KRA), NIH Core Grant EY01792, and an unrestricted departmental grant from Research to Prevent Blindness. KRA is a Research to Prevent Blindness Senior Scientific Investigator.
References
- 1.Crevier DW, Meister M. Synchronous period-doubling in flicker vision of salamander and man. J Neurophysiol. 1998;79:1869–1878. doi: 10.1152/jn.1998.79.4.1869. [DOI] [PubMed] [Google Scholar]
- 2.Alexander KR, Levine MW, Super BJ. Characteristics of period doubling in the human cone flicker electroretinogram. Vis Neurosci. 2005;22:817–824. doi: 10.1017/S0952523805226111. [DOI] [PubMed] [Google Scholar]
- 3.Alexander KR, Raghuram A. Effect of contrast on the frequency response of synchronous period doubling. Vision Res. 2007;47:555–563. doi: 10.1016/j.visres.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birch DG. The focal and multifocal electroretinogram. In: Fishman GA, Birch DG, Holder GE, Brigell MG, editors. Electrophysiologic testing in disorders of the retina, optic nerve, and visual pathway. 2nd. The Foundation of the American Academy of Ophthalmology; San Francisco: 2001. pp. 177–195. [Google Scholar]
- 5.Burns SA, Elsner AE, Kreitz MR. Analysis of nonlinearities in the flicker ERG. Optom Vision Sci. 1992;69:95–105. doi: 10.1097/00006324-199202000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Alexander KR, Barnes CS, Fishman GA. ON-pathway dysfunction and timing properties of the flicker ERG in carriers of X-linked retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2003;44:4017–4025. doi: 10.1167/iovs.02-0989. [DOI] [PubMed] [Google Scholar]