Abstract
The 3-triphenylphosphonio-N-(2,6-diisopropylphenyl)-pyrole reacts with two equivalents of methyl lithium to afford a lithium adduct in which a cyclic (amino)[bis(ylide)]carbene, a novel type of NHC, acts as a 1,4-bidentate ligand via the carbene center and the exocyclic ylidic carbon. This species readily undergoes transmetallation reactions, which allows for the synthesis of a variety of transition metal complexes.
N-Heterocyclic carbenes (NHCs) have been extensively studied both as free species1 and as ligands for metal centers.2 Replacement of one of the electronegative amino groups of NHCs A by an alkyl group results in cyclic (alkyl)(amino)carbenes (CAACs) B,3a–c which feature a smaller HOMO-LUMO gap but importantly a HOMO that is higher in energy (stronger σ-donor character) (Fig. 1). The unique ligand properties of CAACs have been demonstrated by the isolation of otherwise unstable low coordinate transition metal species,3d and by the excellent catalytic properties of their palladium,3a gold3e and ruthenium complexes.3f,g It seemed likely that the replacement of the σ-donor alkyl group of B by a carbon based π-donor, such as a phosphorus ylide, as shown in C, would further enhance the nucleophilic character of the carbene center. In fact, metal complexes featuring the benzo-fused version D as ligands have been isolated.4,5 They were first synthesized from isocyanide complexes4 rather than free carbenes, which limited the choice of the substituents as well as of the metals that can be used. However, recently, Kawashima et al.[5] reported the transient formation of carbene D, and the isolation of the ensuing Rh and Pd complexes. Based on the CO stretching frequency of the Rh(carbene)(CO)2Cl complex, they concluded that carbene D was indeed a stronger σ-donor than NHCs A and CAACs B.
Figure 1.
Carbenes A–D, (amino)[bis(ylide)carbene E, and NHC/ylide bidentate ligand F.
Here we report that our search for preparing carbenes of type C has serendipitously led to the discovery of the first stable lithium adduct of a cyclic (amino)[bis(ylide)]carbene E.6 This compound undergoes transmetallation reactions, which allow for the synthesis of a variety of transition metal complexes in which E acts as an LX bidentate ligand. Note that the first complex featuring an NHC/ylide bidentate ligand F was recently reported, and shown to be an effective catalyst in the Tsuji-Trost allylation reaction.7
Phosphonium salt 3 was readily prepared in 48% overall yield by slightly modified reported procedures.8 Addition of 2,5-dimethoxytetrahydrofuran to 2,6-diisopropylaniline gives the N-substituted pyrrole 1.8a Selective bromination leads to compound 2,8b which is converted into the desired phosphonium salt 3 by a nickel-catalyzed coupling with triphenylphosphine.8c Simple anion exchange, using sodium tetraphenyl borate, was done in order to increase the solubility and to facilitate purification of the salt (Scheme 1).
Scheme 1.
Synthesis of phosphonium salt 3.
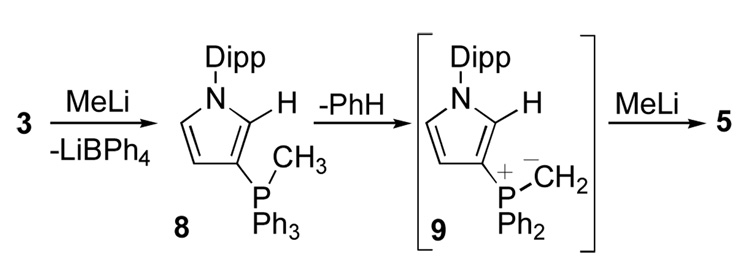
Attempts to deprotonate phosphonium salt 3 with a variety of bases (LDA, TMPLi, t-BuLi, KHMDS) led to non-carbene products or complex mixtures. However, using two equivalents of methyl lithium, a clean reaction took place as evidenced by the presence of a single signal in the 31P NMR spectrum (+20 ppm). The 13C NMR spectrum showed a doublet at 204 ppm (2JCP = 54 Hz), in the range expected for a carbene carbon;1 however, there was a doublet at very high field (−4.4 ppm, 1JCP = 49 Hz), which could not be attributed to 4 (Scheme 2). Interestingly, in the 1H NMR spectrum, a doublet (2JHP = 6.7 Hz; phosphorus coupling was confirmed by decoupling experiments) also appeared at very high field (−0.07 ppm), and integrated for two protons. These data suggested the presence of an ylidic PCH2 moiety. The 1H NMR showed the presence of only 13 aromatic protons in the molecule indicating the loss of one phenyl group from the phosphorus (free benzene can be seen by NMR is the crude reaction mixture). These results as a whole were in favor of a compound featuring both a carbene center and a PCH2 phosphorus ylide fragment, which cannot be rationalized by any reasonable neutral structure. Despite the high solubility of this compound in non polar solvents, including hexanes, it was clear that a salt has been formed. Indeed, 6Li and 7Li NMR demonstrated the presence of Li+ but no Li-C coupling to either the carbenic carbon or the ylidic carbon could be detected, and no correlation was seen in 2D experiments, possibly because of rapid exchange processes. Lastly, the presence of coordinated THF was apparent from 1H and 13C NMR spectroscopy. At that point, it was concluded that the isolated compound was the lithium salt 5,9 which is also in agreement with the stoichiometry of the reaction (2 eqs. of MeLi are necessary to go to completion).
Scheme 2.
Attempted preparation of carbene 4 and synthesis of the lithium adduct 5.
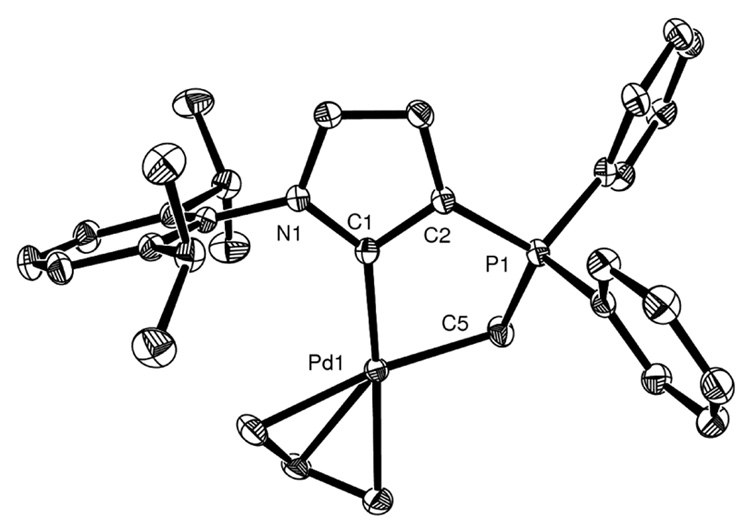
Although 5 is stable under an inert atmosphere, both in the solid state and in solution for several weeks, all attempts to grow single crystals suitable for an X-ray diffraction study failed. Therefore, in order to confirm the structure of 5, we performed transmetallation reactions. The addition of freshly prepared 5 to both [Pd(allyl)Cl]2 and [RhCl(COD)]2 cleanly led to complexes 6 and 7, which were isolated as air stable crystalline solids in 47 and 56% yield (based on the starting phosphonium 3), respectively (Fig. 2 and Fig. 3). The formation of complexes 6 and 7 was clearly evidenced by 13C NMR spectroscopy, the carbene signal shifting from 204 to 180 and 182 ppm, respectively, as is typical for transition metal carbene complexes.2 The bonding of the ylidic carbon to the metal was also suggested by the downfield shift from −4.4 to −1.4 and 13.8 ppm, respectively. In the case of the Rh complex, both the carbenic and ylidic carbon appear as doublet of doublets (Ccarbene JCP = 48 Hz JCRh = 48 Hz, Cylide JCP = 40 Hz JCRh = 25 Hz) further demonstrating the bidentate behavior of ligand E. Single crystals of complex 6 were grown from a concentrated acetonitrile solution, whereas those of complex 7 were obtained from a dichloromethane / ether solution.
Figure 2.
Molecular structure of palladium complex 6. Hydrogen atoms omitted for clarity. Thermal ellipsoids at 50% probability. Selected bond lengths [Å] and angles [°]: C1-N1 1.3778(17), C1-C2 1.4013(18), C1-Pd1 2.0302(13), C5-Pd1 2.1268(15), C2-P1 1.7472(14), C5-P1 1.7523(15); N1-C1-C2 104.61(11).
Figure 3.
Molecular structure of rhodium complex 7. Hydrogen atoms omitted for clarity. Thermal ellipsoids at 50% probability. Selected bond lengths [Å] and angles [°]: C1-N1 1.3842(16), C1-C2 1.4045(19), C1-Rh1 2.0698(13), C5-Rh1 2.1250(14), C2-P1 1.7359(13), C5-P1 1.7482(15); N1-C1-C2 103.73(11).
The molecular structure of complexes 6 and 7 determined by X-ray crystallography are shown in Figure 2 and Figure 3.10 A closer look at the geometric parameters of complex 6 shows that the Pd-carbene (2.0302 Å) and Pd-CH2 (2.1268 Å) bond lengths are very similar to those observed for the Pd(Allyl) complex bearing the neutral NHC-ylide bidentate ligand F (2.022-2.014 and 2.148-2.099 Å, respectively).7 In addition, the Rh-carbene bond length (2.0698 Å) in 7 is comparable to that found for other carbene complexes.2
The formation of 5 by addition of two equivalents of MeLi to phosphonium 3 being unexpected, we turned our attention to the mechanism of the reaction. Addition of one equivalent of MeLi to 3 leads to a mixture of unreacted starting material 3 and lithium salt 5. However, when this reaction was monitored by variable temperature multinuclear NMR spectroscopy, signals corresponding to the intermediate 8 were observed (Scheme 3). Indeed, at - 78 °C a new peak at −105.1 ppm appeared in the 31P NMR spectrum, which is in the typical range for a penta-coordinate phosphorus compound.11 In addition, the 1H NMR spectrum shows the presence of a methyl group directly bonded to phosphorus (2.06 ppm, d, 2JHP = 7.56 Hz); this was confirmed by 13C NMR spectroscopy and HSQC experiments. Indeed, these protons correlate with a methyl carbon at 34.8 ppm with the expected large 1JCP coupling constant (65.2 Hz). The formation of penta-coordinate phosphorus compounds by alkylation of phosphonium salts with alkyl lithiums have already been observed.12 Then, upon warming to room temperature, no other intermediate could be detected. Based on literature precedents,13 it is reasonable to postulate, that the next step is the elimination of benzene with concomitant formation of ylide 9. Then, the second equivalent of MeLi can deprotonate the heterocycle affording the observed lithium complex 5.
Scheme 3.
Possible mechanism leading to lithium adduct 5.
In conclusion, a lithium adduct of a new type of bidentate anionic LX ligand in which L is a cyclic (amino)[bis(ylide)]carbene and X is the exocyclic part of the bis(ylide) has been synthesized. This adduct readily undergoes transmetallation reactions, which allows for the synthesis of diverse transition metal complexes. The catalytic activity of the latter is currently under investigation as well as continuing efforts to synthesize a stable cyclic amino ylide carbene of type C.
ACKNOWLEDGMENT
The authors thank the NIH (R01 GM 68825) for financial support of this work.
Footnotes
Supporting Information Available: Crystallographic data including CIF files as well as synthetic and spectroscopic information are available free of charge via the internet at http://pubs.acs.org.
References
- 1.For reviews on metal free NHCs, see: Enders D, Niemeier O, Henseler A. Chem. Rev. 2007;107:5606–5655. doi: 10.1021/cr068372z.Marion N, Diez-Gonzalez S, Nolan SP. Angew. Chem., Int. Ed. 2007;46:2988–3000. doi: 10.1002/anie.200603380.Canac Y, Soleilhavoup M, Conejero S, Bertrand G. J. Organomet. Chem. 2004;689:3857–3865.Bourissou D, Guerret O, Gabbaï FP, Bertrand G. Chem. Rev. 2000;100:39–92. doi: 10.1021/cr940472u.Arduengo AJ., III Acc. Chem. Res. 1999;32:913–921.
- 2.For recent reviews on carbenes as ligands, see: Kantchev E, O'Brien C, Organ M. Angew. Chem. Int. Ed. 2007;46:2768–2813. doi: 10.1002/anie.200601663.Lee HM, Lee CC, Cheng PY. Curr. Org. Chem. 2007;11:1491–1524.Liddle ST, Edworthy IS, Arnold PL. Chem. Soc. Rev. 2007;36:1732–1744. doi: 10.1039/b611548a.Diez-Gonzalez S, Nolan SP. Synlett. 2007:2158–2167.Pugh D, Danopoulos AA. Coord. Chem. Rev. 2007;251:610–641.Lin IJB, Vasam CS. Coord. Chem. Rev. 2007;251:642–670.Douthwaite RE. Coord. Chem. Rev. 2007;251:702–717.Gade LH, Bellemin-Laponnaz S. Coord. Chem. Rev. 2007;251:718–725.Colacino E, Martinez J, Lamaty F. Coord. Chem. Rev. 2007;251:726–764.Dragutan V, Dragutan I, Delaude L. Coord. Chem. Rev. 2007;251:765–794.Sommer WJ, Weck M. Coord. Chem. Rev. 2007;251:860–873.Diez-Gonzalez S, Nolan SP. Coord. Chem. Rev. 2007;251:874–883.Hahn FE. Angew. Chem. Int. Ed. 2006;45:1348–1352. doi: 10.1002/anie.200503858.Nolan SP. N-Heterocyclic Carbenes in Synthesis. Wiley-VCH: 2006. Glorius F. N-Heterocyclic Carbenes in Transition Metal Catalysis (Topics in Organometallic Chemistry) Springer Verlag: 2006.
- 3.a) Lavallo V, Canac Y, Prasang C, Donnadieu B, Bertrand G. Angew. Chem. Int. Ed. 2005;44:5705–5709. doi: 10.1002/anie.200501841. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Jazzar R, Dewhurst RD, Bourg J-B, Donnadieu B, Canac Y, Bertrand G. Angew. Chem. Int. Ed. 2007;46:2899–2902. doi: 10.1002/anie.200605083. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Jazzar R, Bourg J-B, Dewhurst RD, Donnadieu B, Canac Y, Bertrand G. J. Org. Chem. 2007;72:3492–3499. doi: 10.1021/jo0703909. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Lavallo V, Canac Y, DeHope A, Donnadieu B, Bertrand G. Angew. Chem., Int. Ed. 2005;44:7236–7239. doi: 10.1002/anie.200502566. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Lavallo V, Frey GD, Kousar S, Donnadieu B, Bertrand G. Proc. Natl. Acad. Sci. USA. 2007;104:13569–13573. doi: 10.1073/pnas.0705809104. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Anderson DR, Lavallo V, O'Leary DJ, Bertrand G, Grubbs RH. Angew. Chem. Int. Ed. 2007;46:7262–7265. doi: 10.1002/anie.200702085. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Anderson DR, Ung T, Mkrtumyan G, Bertrand G, Grubbs RH, Schrodi Y. Organometallics. 2008;27:563–566. doi: 10.1021/om7008028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Facchin G, Campostrini R, Michelin RA. J. Organomet. Chem. 1985;294:C21–C25. [Google Scholar]; b) Michelin RA, Facchin G, Braga D, Sabatino P. Organometallics. 1986;5:2265–2274. [Google Scholar]; c) Michelin RA, Mozzon M, Facchin G, Braga D, Sabatino P. J. Chem. Soc. Dalton Trans. 1988:1803–1811. [Google Scholar]; d) Facchin G, Mozzon M, Michelin RA, Robeiro MTA, Pombeiro AJL. J. Chem. Soc. Dalton Trans. 1992:2827–2835. [Google Scholar]; e) Tamm M, Hahn FE. Coord. Chem. Rev. 1999;182:175–209. [Google Scholar]; f) Pombeiro AJL. J. Organomet. Chem. 2005;690:6021–6040. [Google Scholar]
- 5.Nakafuji S, Kobayashi J, Kawashima T. Angew. Chem. Int. Ed. 2008;47:1141–1144. doi: 10.1002/anie.200704746. [DOI] [PubMed] [Google Scholar]
- 6.For a review on phosphorus diylides [R2P+(CH2−)2]: Taillefer M, Cristau HJ. Top. Curr. Chem. 2003;229:41–73.
- 7.Canac Y, Duhayon C, Chauvin R. Angew. Chem. Int. Ed. 2007;46:6313–6315. doi: 10.1002/anie.200701490. [DOI] [PubMed] [Google Scholar]
- 8.a) Fang Y, Leysen D, Ottenheijm HCJ. Synt. Comm. 1995;25:1857–1861. [Google Scholar]; b) Dvornikova E, Kamienska-Trela K. Synlett. 2002:1152–1153. [Google Scholar]; c) Manabe K. Tet. Lett. 1998:5807–5810. [Google Scholar]
- 9.Several examples of carbene-Li+ adducts have already been reported: Alder RW, Blake ME, Bortolotti C, Bufali S, Butts CP, Linehan E, Oliva JM, Orpen AG, Quayle MJ. Chem. Commun. 1999:241–242.Arduengo AJ, III, Tamm M, Calabrese JC, Davidson F, Marshall W. J. Chem. Lett. 1999;10:1021–1022.Lavallo V, Ishida Y, Donnadieu B, Bertrand G. Angew. Chem. Int. Ed. 2006;45:6652–6655. doi: 10.1002/anie.200602701.
- 10.Structural data have been deposited in the Cambridge Crystallographic Data Center under CCDC 676576 (6), and 676577 (7) and can be obtained free of charge at www.ccdc.cam.ac.uk/conts/retrieving.html.
- 11.a) Dennis LW, Bartuska VJ, Maciel GE. J. Am. Chem. Soc. 1982;104:230–235. [Google Scholar]; b) Schmidbaur H, Holl PZ. Anorg. Allg. Chem. 1979;458:249–256. [Google Scholar]
- 12.a) Monkowius U, Mitzel NW, Schier A, Schmidbaur H. J. Am. Chem. Soc. 2002;124:6126–6132. doi: 10.1021/ja012041g. [DOI] [PubMed] [Google Scholar]; b) Hellwinkel D. Top Curr Chem. 1983;109:1–63. [Google Scholar]; b) Hellwinkel D, Lindner W. Chem. Ber. 1976;109:1497–1505. [Google Scholar]; c) Hellwinkel D, Lindner W, Wilfinger H-J. Chem. Ber. 1974;107:1428–1443. [Google Scholar]; d) Turnblom EW, Katz TJ. J. Am. Chem. Soc. 1973;95:4292–4311. [Google Scholar]; e) Richards EM, Tebby JC. J. Chem. Soc. C. 1970:1425–1428. [Google Scholar]
- 13.a) Seyferth D, Hughes WB, Heeren JK. J. Am. Chem. Soc. 1965;87:2847–2854. [Google Scholar]; b) Seyferth D, Hughes WB, Heeren JK. J. Am. Chem. Soc. 1965;87:3467–3474. [Google Scholar]