Abstract
Vasopressin regulates water excretion through effects on the renal collecting duct. Vasopressin signaling in the inner medullary collecting duct (IMCD) is mediated by V2 receptor occupation coupled to the generation of cyclic AMP. Here, we employ a “systems” approach to analysis of vasopressin signaling. The objective is to investigate roles of activation of the Akt and ERK1/2 MAP kinase pathways, as well as Ca2+ mobilization, in IMCD cells isolated from rat kidney. The V2 receptor-selective vasopressin analog dDAVP increased the state of Akt activation (increased phosphorylation at T308 and S473) and decreased the state of ERK1/2 activation (decreased phosphorylation at T202 and Y204). Akt activation was blocked by an inhibitor of PI3K, LY294002. In microdissected IMCD segments, nonperiodic spike-like increases in intracellular Ca2+ (FLUO-4) were accelerated by vasopressin. Chelation of Ca2+ or calmodulin inhibition markedly decreased Akt phosphorylation. Decreased ERK1/2 phosphorylation was associated with a decrease in MEK1/2 phosphorylation and an increase in c-Raf phosphorylation at S259 (an inhibitory site). Based on the current findings integrated with previous findings in the IMCD, we now report a 33-node vasopressin signaling network involved in vasopressin regulation of IMCD function.
Keywords: aquaporin-2, phosphoinositide 3-kinase, cyclic AMP, calmodulin
water transport across the renal collecting duct is controlled by the peptide hormone vasopressin, largely through the long- and short-term regulation of the apical water channel aquaporin-2 (AQP2) (24). Long-term regulation refers to changes in total abundance of AQP2 over a period of days. Short-term regulation refers to changes in AQP2-mediated water transport over periods of minutes through regulation of trafficking of AQP2 vesicles to and from the apical plasma membrane. Beyond vasopressin's role in transport regulation, the growing recognition that vasopressin regulates proliferation of normal inner medullary collecting duct (IMCD) cells (2) as well as of renal cysts derived from collecting duct cells (36) or cysts formed from renal epithelial cells grown in culture (21) brings renewed interest to vasopressin signaling mechanisms in collecting duct cells.
A preliminary vasopressin signaling network in the renal IMCD, based on proteomic analysis and the existing literature, has been published in our previous paper (29) (see discussion for full details). The effects of vasopressin are a consequence of binding of vasopressin to basolateral V2 receptors. Although other processes have been proposed (22), many of the vasopressin effects are believed to be consequent to activation of the heterotrimeric G protein Gαs (GNAS) and to increases in intracellular Ca2+ via calmodulin (8). Oligonucleotide microarrays in IMCDs isolated from rat kidneys revealed a significant level of expression of kinases in the Akt and MAP kinase pathways (37). These kinases and several elements that regulate them are well-studied in many tissues, and their roles in neoplastic diseases have spurred the production of high-quality antibodies for their study. Here, we used these antibodies for immunochemical measurements coupled with fluorometric measurements of intracellular Ca2+ in freshly isolated IMCD cells to expand our understanding of the vasopressin signaling network.
METHODS
Animals and IMCD Preparation
Animal procedures were approved by National Heart, Lung, and Blood Institute Animal Care and Use Committee (no. H-0110). Male Sprague-Dawley rats (200–300 g) were injected intraperitoneally with furosemide (5 mg/rat) 20 min before decapitation and removal of kidneys. Furosemide dissipates the hypertonic medullary osmolality toward plasma osmolality level (∼290 mosmol/kgH2O), thereby preventing osmotic shock to the cells upon isolation of IMCDs (31). IMCDs were isolated from renal medullas as described (6) after digestion (3 mg/ml collagenase B, 2,000 U/ml hyaluronidase, 250 mM sucrose, 10 mM triethanolamine, pH 7.6) at 37°C with continuous stirring for 75–90 min. IMCDs were sedimented by low-speed centrifugation (70 g for 20 s), separating them from the lighter non-IMCD cells. IMCD pellets were washed and sedimented twice in sucrose buffer (250 mM sucrose, 10 mM triethanolamine, pH 7.6), followed by buffer exchange into 290 mosmol/kgH2O bicarbonate buffer (7).
Stimuli and Inhibitors
All experiments were performed in a pH- and temperature-controlled chamber with gentle mixing under an atmosphere of 95% air-5% CO2 at 37°C. IMCD samples were incubated in 290 mosmol/kgH2O bicarbonate buffer in the absence or presence of 0.1 μM EGF, 1 nM dDAVP, 0.1 mM 8-(4-chlorophenylthio)-cAMP (cpt-cAMP) for 1, 2, 5, 10, 15, or 30 min. For inhibitor studies, IMCD samples were incubated in 290 mosmol/kgH2O bicarbonate buffer in the absence or presence of 25 μM LY294002, 10 μM H-89, 50 μM BAPTA-AM, or 25 μM W-7 for 25 min before addition of 1 nM dDAVP or 0.1 mM cpt-cAMP for 5 min.
Antibodies
The antibodies used are summarized in Supplementary Table 1 (the online version of this article contains supplemental data).
Immunoblotting
Immunoblotting was performed as described (11). Briefly, after solubilization in Laemmli buffer, IMCD protein samples (15–20 μg) were resolved by SDS-PAGE gel electrophoresis on 10 or 4–15% polyacrylamide gels (BioRad) and transferred electrophoretically onto nitrocellulose membranes. The membranes were then blocked with Odyssey blocking buffer (Li-Cor, Lincoln, NE), rinsed, and probed with primary antibody overnight at room temperature. After a washing, blots were incubated with species-specific fluorescently labeled secondary antibodies Alexa Fluor 680 (Molecular Probes, Eugene, OR) used at 1:5,000 for detection of all primary antibodies. Fluorescence was imaged and quantified using a Li-Cor Odyssey Imaging System.
Densitometry and Statistical Analysis
A net intensity value of each immunoblot band was quantified using Odyssey application software. Log2 ratio or log2 normalized ratio of experimental band intensity over control band intensity was calculated for each data point. The log2 normalized ratio was performed such that the phosphoprotein values were prenormalized to the total protein values: log2 [(Fep/Fcp)/(Fet/Fct)] where F = band intensity, e = experiment, c = control, p = phosphoprotein, and t = total protein. In time course experiments, we tested for significant differences at each time point by paired t-test. Each experimental value had its own contemporaneous control. For nontime course experiments, paired t-test or one-way ANOVA with Bonferroni contrasts was performed on log2 ratio or log2 normalized ratio as appropriate. P value <0.05 was considered significant. The number of replicates (n) reported in the figures indicates the number of separate IMCD suspension preparations studied.
Immunofluorescence Confocal Microscopy
Immunofluorescence confocal microscopy was performed as described (42). IMCD samples and kidney slices were fixed with 4% paraformaldehyde overnight and processed using paraffin as the embedding agent. Confocal fluorescence images were taken using a Zeiss LSM 510 microscope (Carl Zeiss MicroImaging, Thornwood, NY).
Imaging of Intracellular Ca2+ in Isolated IMCD Segments
Microdissected IMCDs were transferred to Cell-Tak-coated petri dishes and incubated with 10 μM Fluo-4/AM (Molecular Probes) in HEPES-buffered isolation fluid (in mM: 118 NaCl, 5 HEPES, 5 KCl, 2.5 Na2HPO4, 2 CaCl2, 1.2 MgSO4, 5.5 glucose, 5 Na acetate, pH 7.4 with 0.1% BSA) at room temperature for 60 min. The tubules were washed with the same solution at 37°C for another 30 min to allow deesterification. Fluo-4 fluorescence was monitored dynamically (488-nm excitation; 525-nm emission) using a Zeiss LSM 510 confocal microscope. Fluo-4 exhibits an increase in fluorescence at 525 nm upon binding of Ca2+. Images were sampled at 0.5 Hz and stored digitally. Temporal variations of Fluo-4 emission were monitored in individual IMCD cells during playback of the stored fluorescence image using MetaMorph software.
RESULTS
Positive Controls: Vasopressin and Epidermal Growth Factor Responses
To determine the viability of our IMCD suspension preparation, we tested the ability of vasopressin to alter AQP2 phosphorylation and the ability of epidermal growth factor (EGF) to activate the MAP and Akt kinases.
AQP2 phosphorylation in response to vasopressin in IMCD suspensions.
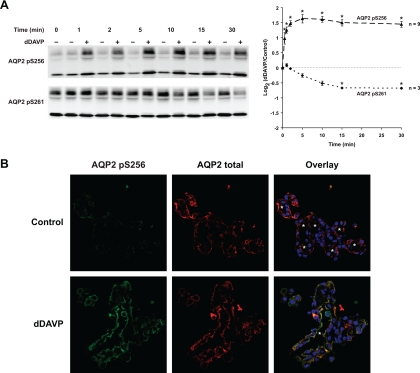
As previously seen (5, 15), the V2 receptor-selective vasopressin analog dDAVP induced a rapid increase in S256 phosphorylation and a slower decrease in S261 phosphorylation (Fig. 1A). Immunofluorescence observations (Fig. 1B) confirmed the increase in phospho-S256 (left) and a shift of the distribution of cellular AQP2 to the apical region of the IMCD cells (middle). Thus, the IMCDs in suspension responded appropriately to vasopressin.
Fig. 1.
Aquaporin-2 (AQP2) phosphorylation in response to vasopressin. A: time course of change in AQP2 phosphorylation at S256 and S261 following 1 nM dDAVP. *Statistically significant. B: immunofluorescence confocal microscopy of inner medullary collecting ducts (IMCDs) double stained with antibodies against AQP2 pS256 (green) and AQP2 total (red). IMCDs were treated with vehicle (top) or 1 nM dDAVP (bottom) for 2 min. White asterisks indicate lumens of the IMCDs.
MAP and Akt kinase activation by EGF in IMCD suspensions.
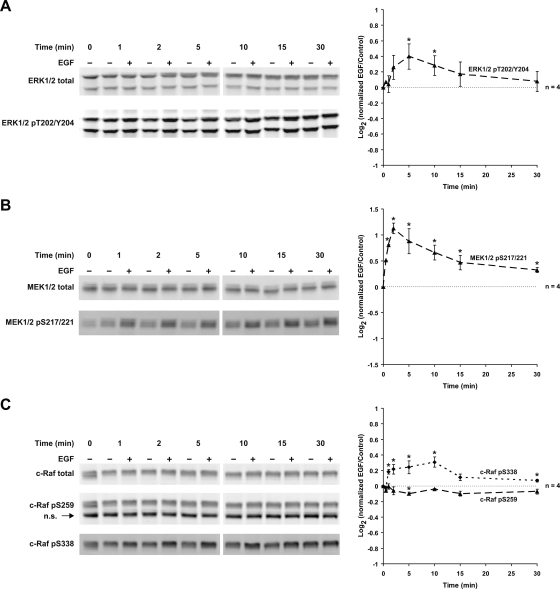
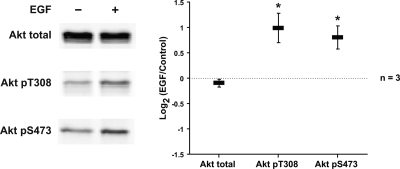
The EGF receptor and other tyrosine kinase receptors are classic activators of MAP kinases as well as Akt kinases (25). Previous studies of microdissected tubules demonstrated that the components of the ERK1/2 MAP kinase pathway are present in IMCD cells and activated by EGF (35). We confirmed the presence of the ERK1/2 MAP kinase pathway components in IMCDs by immunofluorescence staining on tissue sections (Supplementary Fig. 1). In our IMCD suspensions, EGF elicited a biphasic increase in ERK1/2 phosphorylation at T202 and Y204, indicative of activation (Fig. 2A). EGF also induced a biphasic increase in S217/221 phosphorylation of MEK1/2, the kinase responsible for ERK1/2 activation (Fig. 2B). In addition, there were a biphasic increase in activating phosphorylation of c-Raf (the upstream kinase of MEK1/2) at S338 and a small decrease in inhibitory phosphorylation of c-Raf at S259 (Fig. 2C). The feedback mechanisms responsible for the biphasic behavior are unknown. EGF did not measurably affect either p38 MAPK or JNK phosphorylation (Supplementary Fig. 2). The presence of Akt and its upstream activator, phosphoinositide 3-kinase (PI3K) p110γ, in IMCDs was confirmed by immunofluorescence staining on tissue sections (Supplementary Fig. 3). In IMCD suspensions, EGF also increased Akt phosphorylation at T308 and S473, both activating sites (Fig. 3). Thus, EGF strongly activated both the ERK1/2 MAP kinase pathway and Akt in IMCD cell suspensions. We conclude that rat native IMCDs in suspension respond appropriately to both vasopressin and EGF.
Fig. 2.
Epidermal growth factor (EGF) increases MAP kinase activation; 0.1 μM EGF significantly activated ERK1/2 phosphorylation at T202 and Y204 (5 and 10 min; A), markedly activated MEK1/2 phosphorylation at S217/221 (1 to 30 min; B), and significantly activated c-Raf phosphorylation at S338 (1 to 10 and 30 min) and slightly inhibited c-Raf phosphorylation at S259 (5 min; C). *Statistically significant. n.s., Nonspecific bands.
Fig. 3.
EGF increases Akt activation; 0.1 μM EGF significantly activated Akt phosphorylation at T308 and S473 after 5-min exposure. *Statistically significant.
Vasopressin Increases Akt Activation
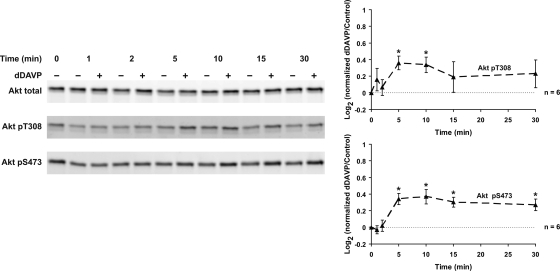
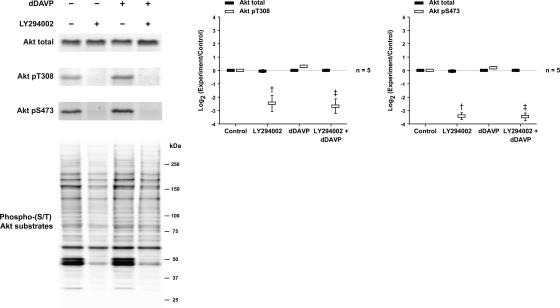
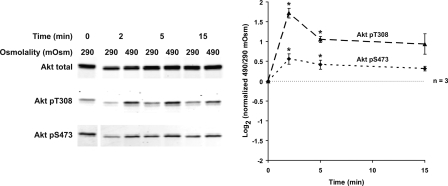
The vasopressin analog dDAVP increased Akt activation in IMCD suspensions as evidenced by a biphasic increase in Akt phosphorylation at T308 and S473 (Fig. 4). Maximal activation was seen 5–10 min after dDAVP addition. Akt activation is generally dependent on localized production of phosphatidylinositol (3,4,5)-trisphosphate (PIP3) through PI3K. When IMCD suspensions were pretreated with an inhibitor of PI3K, LY294002, Akt phosphorylation at both sites was markedly diminished and the dDAVP-induced increase was eliminated (Fig. 5). In addition, experiments were performed to assess the phosphorylation of Akt's downstream substrates, detected by a phospho-(S/T) Akt substrate (RXRXXpS/pT motif) antibody (Fig. 5). A marked inhibition of Akt substrate phosphorylation by LY294002 was observed. There appeared to be a small increase in Akt substrate phosphorylation in response to dDAVP. However, when we immunoblotted for known Akt substrates (GSK-3α/β, Bad, TSC2, MDM2, and FoxO1), no change in their phosphorylation was detected in response to dDAVP addition (Supplementary Fig. 4).
Fig. 4.
Vasopressin increases Akt activation; 1 nM dDAVP significantly increased Akt phosphorylation at T308 (5 and 10 min) and S473 (5 to 30 min). *Statistically significant.
Fig. 5.
Effects of phosphoinositide 3-kinase (PI3K) inhibition on Akt and Akt substrate phosphorylation. IMCD suspensions were preincubated for 25 min in the absence or presence of 25 μM LY294002 and then incubated with vehicle or 1 nM dDAVP for 5 min. †Statistically significant vs. control. ‡Statistically significant vs. dDAVP alone.
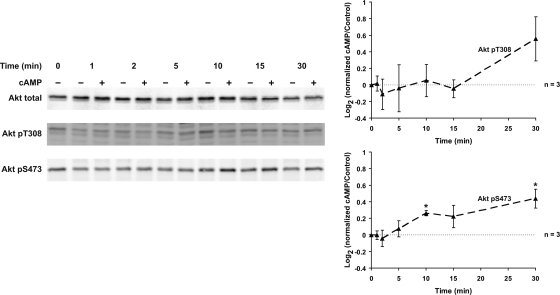
cAMP Increases Akt Activation
The cAMP analog cpt-cAMP mimicked dDAVP with regard to phosphorylation at S473, but did not significantly increase phosphorylation at T308 (Fig. 6).
Fig. 6.
cAMP increases Akt activation; 0.1 mM 8-(4-chlorophenylthio)-cAMP (cpt-cAMP) significantly increased Akt phosphorylation only at S473 (10 and 30 min). *Statistically significant.
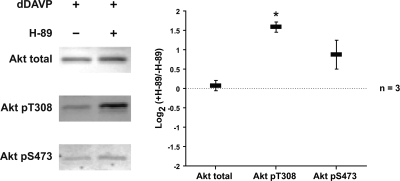
H-89 Increases Akt Activation
We tested whether the PKA inhibitor H-89 inhibits dDAVP-mediated activation of Akt. As shown in Fig. 7, H-89 did not inhibit the effect, but instead strongly increased Akt phosphorylation at T308. This suggests that PKA is not responsible for increased Akt phosphorylation in the presence of vasopressin or that H-89 has nonspecific effects that obscure its effect on PKA.
Fig. 7.
H-89 increases Akt activation. IMCD suspensions were preincubated for 25 min in the absence or presence of 10 μM H-89 and then incubated with 1 nM dDAVP for 5 min. *Statistically significant.
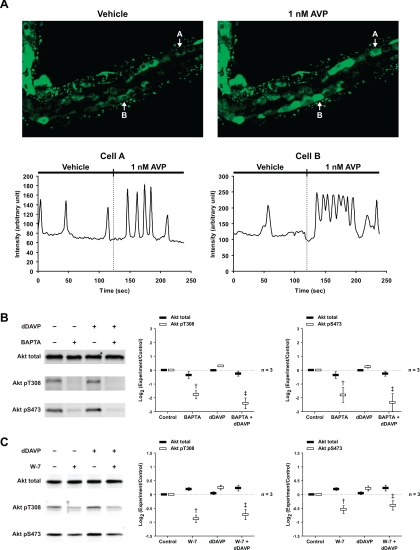
Vasopressin Causes Spike-Like Increases in Intracellular Ca2+; BAPTA and W-7 Block Akt Activation
Dynamic measurements in microdissected rat IMCDs using the calcium-sensitive fluorophore Fluo-4 revealed aperiodic spike-like increases in intracellular Ca2+ that were accelerated by vasopressin, confirming findings of Yip (41) [Fig. 8A and Supplementary Video (http://dir.nhlbi.nih.gov/papers/lkem/ca_spikes_video/)]. Pretreatment with the intracellular Ca2+ chelator BAPTA or the calmodulin inhibitor W-7 inhibited dDAVP-induced increases in Akt phosphorylation at both sites (Fig. 8, B and C).
Fig. 8.
Vasopressin causes spike-like increases in intracellular Ca2+; BAPTA and W-7 block Akt activation. A: 1 nM AVP strikingly induced aperiodic spike-like increases in intracellular Ca2+ in microdissected IMCD cells, measured by Fluo-4 fluorescence [see also Supplementary Video (http://dir.nhlbi.nih.gov/papers/lkem/ca_spikes_video/)]. These increases occurred independently in each cell in an unsynchronized fashion throughout the IMCD. The signal intensities of selected cells (A and B) as a function of time (s) are represented below the images. Injection of vehicle did not alter frequency of Ca2+ spikes. B and C: IMCD suspensions were preincubated for 25 min in the absence or presence of 50 μM BAPTA (B) or 25 μM W-7 (C) and then incubated with vehicle or 1 nM dDAVP for 5 min. †Statistically significant vs. control. ‡Statistically significant vs. dDAVP alone.
Elevated Osmolality Increases Akt Activation
An increase in incubation medium osmolality from 290 to 490 mosmol/kgH2O (addition of NaCl) increased Akt activation, with an increase in Akt phosphorylation at T308 and S473 (Fig. 9). T308 phosphorylation increased more than S473 phosphorylation, in contrast to the dDAVP and cAMP effects on Akt activation (S473 ≥ T308).
Fig. 9.
Elevated osmolality increases Akt activation. An increase in incubation medium osmolality from 290 to 490 mosmol/kgH2O (by addition of NaCl) significantly increased Akt phosphorylation at T308 and S473 (2 and 5 min). *Statistically significant.
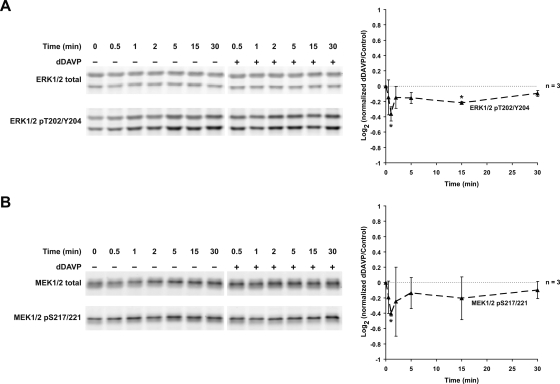
Vasopressin Decreases ERK1/2 and MEK1/2 Activation
The vasopressin analog dDAVP reduced ERK1/2 phosphorylation at T202 and Y204 (Fig. 10A). This response was associated with a fall in MEK1/2 phosphorylation at S217/221 (Fig. 10B). Preincubation with the PI3K inhibitor LY294002 did not inhibit dDAVP-mediated inactivation of ERK1/2 and MEK1/2 phosphorylation, and in fact independently decreased ERK1/2 and MEK1/2 phosphorylation (Supplementary Fig. 5).
Fig. 10.
Vasopressin decreases ERK1/2 and MEK1/2 activation; 1 nM dDAVP significantly decreased ERK1/2 phosphorylation at T202 and Y204 (1 and 15 min; A) and MEK1/2 phosphorylation at S217/221 (1 min; B). *Statistically significant.
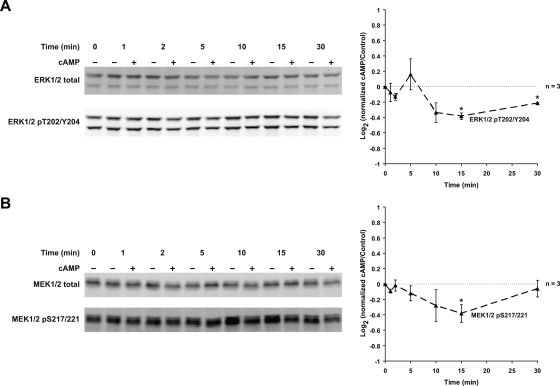
cAMP Decreases ERK1/2 and MEK1/2 Activation
Addition of cpt-cAMP reproduced the effects seen with dDAVP (Fig. 11), suggesting that the inhibition of the MAP kinase pathway by vasopressin is mediated by cAMP.
Fig. 11.
cAMP decreases ERK1/2 and MEK1/2 activation; 0.1 mM cpt-cAMP significantly decreased ERK1/2 phosphorylation at T202 and Y204 (15 and 30 min; A) and MEK1/2 phosphorylation at S217/221 (15 min; B). *Statistically significant.
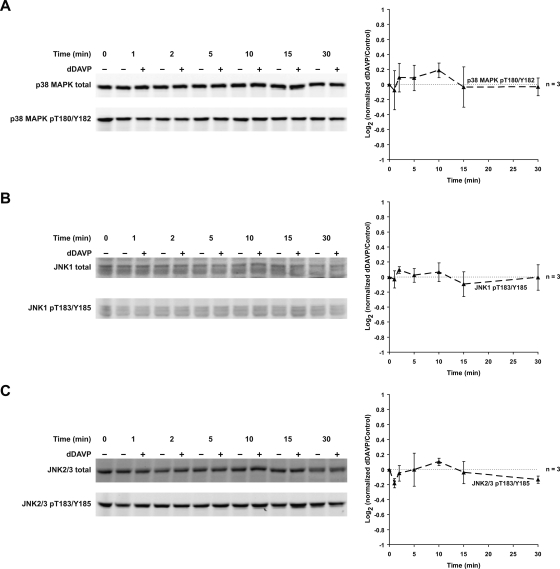
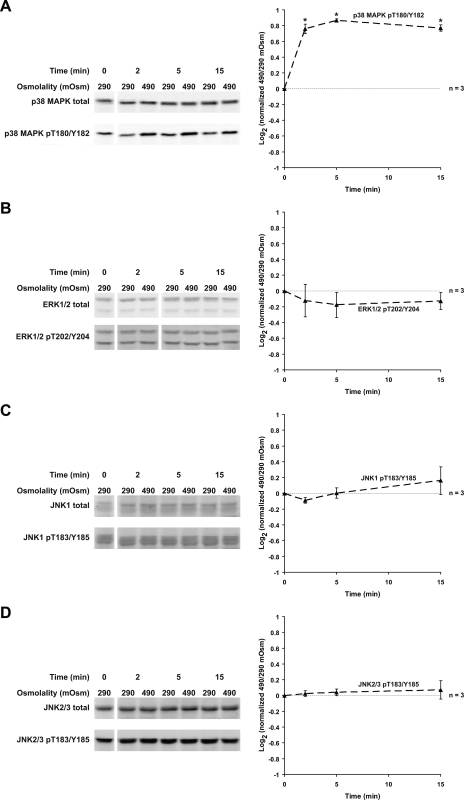
Vasopressin Does Not Affect p38 MAPK or JNK Phosphorylation
dDAVP did not alter p38 MAPK and JNK phosphorylation (Fig. 12). p38 MAPK phosphorylation has been reported to be increased by high osmolality (17, 28). To provide a positive control for p38 MAPK activation, we switched IMCD suspensions from a solution with osmolality 290 to 490 mosmol/kgH2O. High osmolality increased p38 MAPK phosphorylation at T180 and Y182 with no effect on ERK1/2 or JNK phosphorylation (Fig. 13).
Fig. 12.
Vasopressin does not affect p38 MAPK or JNK phosphorylation; 1 nM dDAVP did not alter p38 MAPK phosphorylation at T180 and Y182 (A), JNK1 phosphorylation at T183 and Y185 (B), or JNK2/3 phosphorylation at T183 and Y185 (C).
Fig. 13.
Effects of high osmolality on MAP kinase activation. An increase in incubation medium osmolality from 290 to 490 mosmol/kgH2O (by addition of NaCl) significantly increased p38 MAPK phosphorylation at T180 and Y182 (2 to 15 min; A) with no effect on ERK1/2 phosphorylation (B), JNK1 phosphorylation (C), or JNK2/3 phosphorylation (D). *Statistically significant.
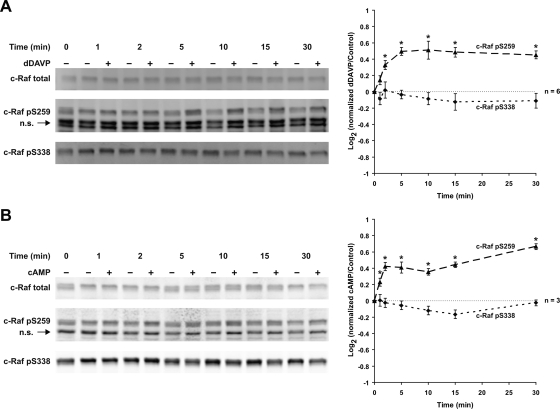
Vasopressin and cAMP Increase c-Raf Phosphorylation at Serine-259
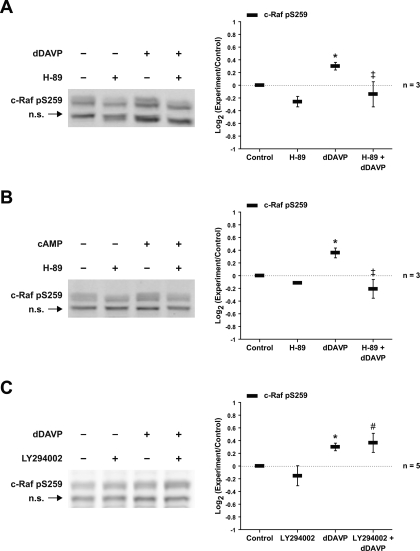
The kinase c-Raf is a mediator of MEK1/2 phosphorylation in many cell types and is strongly expressed in the renal IMCD (37). c-Raf has multiple activating and inhibitory sites (4). Figure 14A shows the effects of dDAVP on phosphorylation of S338 (an activating site) and S259 (an inhibitory site). dDAVP did not affect the level of phosphorylation at S338, but increased phosphorylation at S259. cpt-cAMP produced similar responses (Fig. 14B). The dDAVP- and cpt-cAMP-induced phosphorylation at S259 was blocked by the PKA inhibitor H89 (Fig. 15, A and B). However, dDAVP-induced phosphorylation at S259 was not blocked by the PI3K inhibitor LY294002 (Fig. 15C). These findings point to a role for PKA but not Akt in c-Raf inactivation.
Fig. 14.
Vasopressin and cAMP increase c-Raf phosphorylation at serine-259; 1 nM dDAVP (A) and 0.1 mM cpt-cAMP (B) significantly activated c-Raf phosphorylation at S259 (1 to 30 min) with no effect on c-Raf phosphorylation at S338. *Statistically significant.
Fig. 15.
PKA inhibition but not PI3K inhibition decreases c-Raf phosphorylation at serine-259. IMCD suspensions were preincubated for 25 min in the absence or presence of 10 μM H-89 (A and B) or 25 μM LY294002 (C) and then incubated with vehicle or 1 nM dDAVP (A and C) or 0.1 mM cpt-cAMP (B) for 5 min. *Statistically significant vs. control. ‡Statistically significant vs. dDAVP alone. #Statistically significant vs. LY294002 alone.
DISCUSSION
Here, we demonstrated that the V2 receptor-selective vasopressin analog dDAVP affects states of activation of both the Akt and ERK1/2 MAP kinase pathways in native rat renal IMCD cells. Akt activation is increased, whereas ERK1/2 activation is decreased under the conditions of this study.
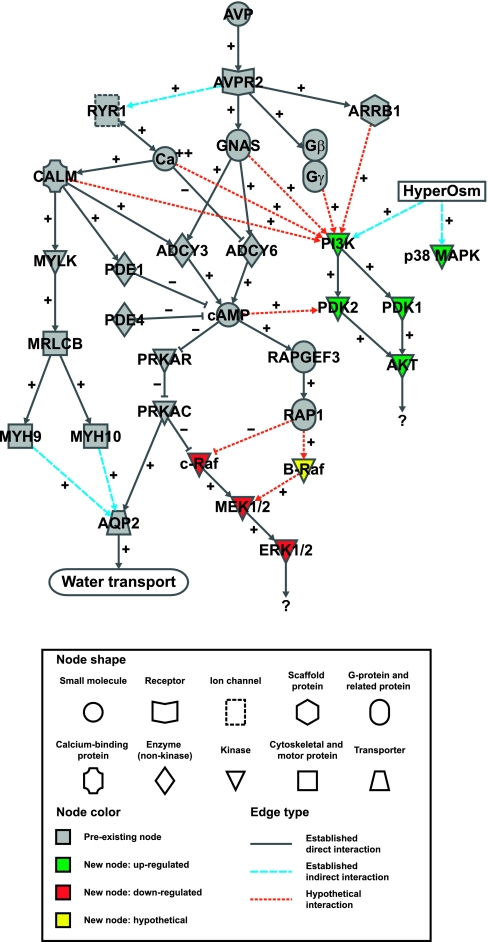
Figure 16 is a 33-node signaling network diagram showing preexisting knowledge (in gray) about vasopressin signaling in native IMCD cells coupled to the new findings in this paper in green (activated by vasopressin) or red (inhibited by vasopressin). The nodes are either proteins (indicated by gene names), small molecules/ions, or conditions/processes. Each protein has been detected in native IMCD cells by protein mass spectrometry, transcriptional profiling by Affymetrix arrays, or both (http://dir.nhlbi.nih.gov/labs/lkem/rm/proteomics_db.asp). Existing knowledge connects vasopressin (top) with AQP2 (bottom) via the V2 receptor (AVPR2). The heterotrimeric G protein α subunit GNAS and the corresponding Gβ/Gγ complex dissociate and become activated. GNAS in its GTP-bound form activates at least two adenylyl cyclases in IMCD, ADCY3 (14) and ADCY6 (3). The former is activated by Ca2+ calmodulin while the latter is inhibited by Ca2+. These enzymes mediate production of cAMP, increasing cAMP concentration either locally or globally within the cell. cAMP is degraded by cyclic nucleotide phosphodiesterases PDE1 and PDE4 in IMCD cells (37). cAMP binds to the regulatory subunit of PKA (PRKAR). Several PKA regulatory subunits are expressed in the IMCD, although PRKAR1A is the most abundant (37). cAMP binding to the regulatory subunit removes its inhibition of the catalytic subunit (presumably PRKACA), thereby activating kinase activity. Although there are presumably many PKA targets, a well-documented one is AQP2 at serine-256 (13). cAMP can also bind to and activate RapGEF3 (Epac1), which is strongly expressed in the IMCD in contrast to very low expression of RapGEF4 (Epac2) (37). Another important vasopressin-dependent signaling process is intracellular Ca2+ mobilization, which is also downstream from the V2 receptor AVPR2. This process is mediated by releasing of Ca2+ from the endoplasmic reticulum via the ryanodine-sensitive Ca2+ release channel RYR1 (8). The increase in intracellular Ca2+ is manifest as a series of aperiodic Ca2+ spikes (41) [confirmed in this paper (Fig. 8A)]. The rise in intracellular Ca2+ increases activation of calmodulin (CALM1, CALM2, and CALM3 have the same amino acid sequence) and activates various calmodulin-regulated proteins including myosin light chain kinase (MYLK), ADCY3, and phosphodiesterase-1 (PDE1). MYLK phosphorylates the B isoform of the regulatory light chain of nonmuscle myosin (MRLCB) (5). MRLC isoforms regulate conventional nonmuscle myosin II including myosin II-A (MYH9) and myosin II-B (MYH10), both expressed in the IMCD (5). Myosin II isoforms are actin-dependent motors that are involved in organization of the actin cytoskeleton and are believed to be involved with AQP2 trafficking. In this paper, we add further information implicating both the PI3K/Akt pathway and the ERK1/2 MAP kinase pathway in vasopressin signaling in the IMCD. The PI3K/Akt pathway is activated by vasopressin (green nodes), while the ERK1/2 MAP kinase pathway is inhibited by vasopressin (red nodes). Changes in both pathways were relatively small as assessed by whole cell immunoblotting, but were extraordinarily consistent in the many experiments carried out in this study. Small but consistent changes are compatible with important physiological functions, considering the likelihood that these pathways can be activated in very localized subregions of the cell where changes may be much larger than seen in whole cell measurements.
Fig. 16.
Network diagram of vasopressin signaling in the IMCD. See discussion and Supplementary Table 2 for details.
Several prior studies investigated the role of vasopressin in regulation of the ERK1/2 MAP kinase pathway. Depending on the cell preparation and conditions, vasopressin has been found either to increase (38), to decrease (39), or to have no effect (20) on ERK1/2 activation in cultured kidney cells. In the present paper, we show in freshly isolated IMCDs that vasopressin normally inhibits ERK1/2 activation. One factor that could play a role in vasopressin-mediated inhibition of ERK1/2 and MEK1/2 phosphorylation is PKA-mediated inhibition of c-Raf (10) (Fig. 16). We demonstrated in this study that dDAVP enhances the phosphorylation of c-Raf at S259, an inhibitory event. This event was reproduced by cAMP (Fig. 11), suggesting that the inhibition of c-Raf is downstream from vasopressin-induced cAMP production. H-89 (a PKA inhibitor) decreased vasopressin- and cAMP-triggered phosphorylation of c-Raf at S259 (Fig. 15, A and B). Inhibition of c-Raf activity by phosphorylation at S259 can also be mediated by Akt (43). In the present study, Akt was activated by vasopressin in a PI3K-dependent manner. However, vasopressin-induced phosphorylation of c-Raf at S259 was not blocked by the PI3K inhibitor LY294002 (Fig. 15C). In addition, PI3K inhibition did not increase ERK1/2 and MEK1/2 activation or block the decreases mediated by vasopressin (Supplementary Fig. 5). Another possible mechanism by which cAMP might contribute to regulation of ERK1/2 and MEK1/2 is a downstream effect of cAMP-mediated Epac1 activation of Rap1 (Fig. 16). Rap1 inhibits c-Raf by competing with Ras (27). However, Rap1 can also activate B-Raf (26), resulting in MEK1/2 activation. The effects of Epac1 and Rap1 activation on the MAP kinase pathway in IMCD cells require further study.
Vasopressin increased Akt phosphorylation at two sites (T308 and S473), both associated with activation of kinase activity. The network shown in Fig. 16 summarizes hypothetical mechanisms that could be responsible for Akt activation based on studies in other cell types and the present study (indicated by dashed edges). Conceivably, the coupling could be mediated through several different downstream effects of vasopressin: 1) Ca2+ oscillations in the cell (33); 2) calmodulin activation (9); 3) release of activated α and β/γ subunit complexes in association with G protein activation by the V2 receptor (1, 18, 32); 4) local or global increases in cAMP (12, 19); or 5) activation of the GPCR interactor β-arrestin (30). Chelation of intracellular Ca2+ with BAPTA or inhibition of calmodulin with W-7 markedly diminished Akt phosphorylation at both of the key activating sites (Fig. 8, B and C). This finding is consistent with previous studies that established a role for Ca2+ in regulation of Akt via effects on PI3K activation (33). Ca2+/calmodulin-regulated Akt activation has also been reported to be mediated through mechanisms other than those involving calmodulin-activated kinases or PI3K (9). In contrast, Akt phosphorylation was not inhibited by the PKA inhibitor H-89 in the present study (Fig. 7), suggesting that the activation of Akt is not mediated by PKA. In agreement with earlier studies on hyperosmolality-induced Akt activation in rat inner medulla (34), an elevation in medium osmolality from 290 to 490 mosmol/kgH2O increased Akt phosphorylation at T308 and S473 (Fig. 9). Interestingly, T308 phosphorylation increased more than S473 phosphorylation, in contrast to the dDAVP and cAMP effects on Akt activation (S473 ≥ T308). This observation leads to speculation that vasopressin activates Akt through a different signaling pathway from hyperosmolality. The downstream substrates of vasopressin-induced Akt activation were investigated by immunoblotting for known Akt substrates (GSK-3α/β, Bad, TSC2, MDM2, and FoxO1). However, no change was found after dDAVP addition (Supplementary Fig. 4). Further studies are required to identify the downstream targets of PI3K/Akt pathway activation in response to vasopressin in IMCDs. Interestingly, it has been recently shown that phosphorylation of Akt (at both T308 and S473) and its substrate GSK-3β (at S9) were increased in IMCD suspensions of rats with lithium-induced nephrogenic diabetes insipidus (23). This change in Akt and GSK-3β phosphorylation, however, might not be directly related to vasopressin effects due to the fact that lithium treatment leads to various other changes in the kidney, including change in local osmolality in the renal medulla.
The present study was not designed to address signaling mechanisms in autosomal dominant polycystic kidney disease (ADPKD). However, the findings regarding the role of intracellular Ca2+ in vasopressin signaling may be relevant to the pathogenesis of cyst formation and growth and are therefore discussed here. It has been hypothesized that a proximate event in the disordered signaling responsible for cyst growth in ADPKD is a reduction in the intracellular Ca2+ level due to loss of polycystin-mediated Ca2+ entry (40). This reduction in Ca2+ is proposed to decrease Akt activation, as was seen in the present study when intracellular free Ca2+ concentration was reduced by BAPTA-mediated chelation. This model of ADPKD signaling assumes that intracellular Ca2+ is relatively invariant in a minute-by-minute time scale. However, in the present study we confirmed the observation of Yip (41) that the cells of the native IMCD normally undergo rapid spike-like increases in Ca2+ concentration (Fig. 8A and Supplementary Video). These Ca2+ spikes are due to Ca2+ release by RYR1, which exhibits Ca2+-induced Ca2+ release. That is, the spikes result when intracellular Ca2+ rises above some threshold that triggers the release. Vasopressin increases the frequency of the Ca2+ spikes, possibly owing to a cAMP-dependent lowering of the threshold (16). In the context of the model of ADPKD signaling, a decrease in intracellular Ca2+ due to loss of polycystin-mediated Ca2+ entry would make it harder for Ca2+ to rise to the Ca2+ release threshold and would decrease the spike frequency, a prediction that has not yet been tested.
Ca2+ oscillations in the cells raise technical issues that are difficult to address experimentally. Ca2+-dependent enzymes such as myosin light chain kinase are rapidly activated and deactivated in response to the spikes. Thus, it is difficult to assess the net effect of this very dynamic pattern of Ca2+ release using steady-state measurements on mixtures of cells that are experiencing changes in intracellular Ca2+ at different times. In the same way, it is possible that vasopressin-induced changes in Akt activity (or possibly ERK1/2 activity) may occur rapidly, in step with the Ca2+ oscillations. Thus, full assessment of collecting duct signaling, both in the context of normal function and ADPKD, may require methods capable of resolving rapid changes in enzyme activity in individual cells, presumably via appropriate imaging techniques.
GRANTS
This work was supported by the intramural budget of the NHLBI (Z01-HL-01285-KE).
Supplementary Material
Acknowledgments
We thank C. Combs and D. Malide of the National Heart, Lung, and Blood Institute (NHLBI) Light Microscopy Core Facility for assistance in confocal microscopy and G. Ma for creating the LKEM Ca2+ spikes web site.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Brock C, Schaefer M, Reusch HP, Czupalla C, Michalke M, Spicher K, Schultz G, Nurnberg B. Roles of Gβγ in membrane recruitment and activation of p110γ/p101 phosphoinositide 3-kinase γ. J Cell Biol 160: 89–99, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai Q, McReynolds MR, Keck M, Greer KA, Hoying JB, Brooks HL. Vasopressin receptor subtype 2 (V2R) activation increases cell proliferation in the renal medulla of AQP1 null mice. Am J Physiol Renal Physiol 293: F1858–F1864, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Chabardes D, Firsov D, Aarab L, Clabecq A, Bellanger AC, Siaume-Perez S, Elalouf JM. Localization of mRNAs encoding Ca2+-inhibitable adenylyl cyclases along the renal tubule. Functional consequences for regulation of the cAMP content. J Biol Chem 271: 19264–19271, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Chong H, Vikis HG, Guan KL. Mechanisms of regulating the Raf kinase family. Cell Signal 15: 463–469, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Chou CL, Christensen BM, Frische S, Vorum H, Desai RA, Hoffert JD, de Lanerolle P, Nielsen S, Knepper MA. Nonmuscle myosin II and myosin light chain kinase are downstream targets for vasopressin signaling in the renal collecting duct. J Biol Chem 279: 49026–49035, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Chou CL, DiGiovanni SR, Luther A, Lolait SJ, Knepper MA. Oxytocin as an antidiuretic hormone. II. Role of V2 vasopressin receptor. Am J Physiol Renal Fluid Electrolyte Physiol 269: F78–F85, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Chou CL, Rapko SI, Knepper MA. Phosphoinositide signaling in rat inner medullary collecting duct. Am J Physiol Renal Physiol 274: F564–F572, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Chou CL, Yip KP, Michea L, Kador K, Ferraris J, Wade JB, Knepper MA. Regulation of aquaporin-2 trafficking by vasopressin in renal collecting duct: roles of ryanodine-sensitive Ca2+ stores and calmodulin. J Biol Chem 275: 36839–36846, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Deb TB, Coticchia CM, Dickson RB. Calmodulin-mediated activation of Akt regulates survival of c-Myc-overexpressing mouse mammary carcinoma cells. J Biol Chem 279: 38903–38911, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Dhillon AS, Pollock C, Steen H, Shaw PE, Mischak H, Kolch W. Cyclic AMP-dependent kinase regulates Raf-1 kinase mainly by phosphorylation of serine 259. Mol Cell Biol 22: 3237–3246, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiGiovanni SR, Nielsen S, Christensen EI, Knepper MA. Regulation of collecting duct water channel expression by vasopressin in Brattleboro rat. Proc Natl Acad Sci USA 91: 8984–8988, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filippa N, Sable CL, Filloux C, Hemmings B, Van OE. Mechanism of protein kinase B activation by cyclic AMP-dependent protein kinase. Mol Cell Biol 19: 4989–5000, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fushimi K, Sasaki S, Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem 272: 14800–14804, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Hoffert JD, Chou CL, Fenton RA, Knepper MA. Calmodulin is required for vasopressin-stimulated increase in cyclic AMP production in inner medullary collecting duct. J Biol Chem 280: 13624–13630, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffert JD, Nielsen J, Yu MJ, Pisitkun T, Schleicher SM, Nielsen S, Knepper MA. Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct. Am J Physiol Renal Physiol 292: F691–F700, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Islam MS, Leibiger I, Leibiger B, Rossi D, Sorrentino V, Ekstrom TJ, Westerblad H, Andrade FH, Berggren PO. In situ activation of the type-2 ryanodine receptor in pancreatic beta cells requires cAMP-dependent phosphorylation. Proc Natl Acad Sci USA 95: 6145–6150, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kultz D, Garcia-Perez A, Ferraris JD, Burg MB. Distinct regulation of osmoprotective genes in yeast and mammals. Aldose reductase osmotic response element is induced independent of p38 and stress-activated protein kinase/Jun N-terminal kinase in rabbit kidney cells. J Biol Chem 272: 13165–13170, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Leopoldt D, Hanck T, Exner T, Maier U, Wetzker R, Nurnberg B. Gβγ stimulates phosphoinositide 3-kinase-γ by direct interaction with two domains of the catalytic p110 subunit. J Biol Chem 273: 7024–7029, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Mei FC, Qiao J, Tsygankova OM, Meinkoth JL, Quilliam LA, Cheng X. Differential signaling of cyclic AMP: opposing effects of exchange protein directly activated by cyclic AMP and cAMP-dependent protein kinase on protein kinase B activation. J Biol Chem 277: 11497–11504, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Michlig S, Mercier A, Doucet A, Schild L, Horisberger JD, Rossier BC, Firsov D. ERK1/2 controls Na,K-ATPase activity and transepithelial sodium transport in the principal cell of the cortical collecting duct of the mouse kidney. J Biol Chem 279: 51002–51012, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Neufeld TK, Douglass D, Grant M, Ye M, Silva F, Nadasdy T, Grantham JJ. In vitro formation and expansion of cysts derived from human renal cortex epithelial cells. Kidney Int 41: 1222–1236, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Nickols HH, Shah VN, Chazin WJ, Limbird LE. Calmodulin interacts with the V2 vasopressin receptor: elimination of binding to the C terminus also eliminates arginine vasopressin-stimulated elevation of intracellular calcium. J Biol Chem 279: 46969–46980, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen J, Hoffert JD, Knepper MA, Agre P, Nielsen S, Fenton RA. Proteomic analysis of lithium-induced nephrogenic diabetes insipidus: mechanisms for aquaporin 2 downregulation and cellular proliferation. Proc Natl Acad Sci USA 105: 3634–3639, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen S, Frokiær J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82: 205–244, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Oda K, Matsuoka Y, Funahashi A, Kitano H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol Syst Biol 1: 1–17, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohtsuka T, Shimizu K, Yamamori B, Kuroda S, Takai Y. Activation of brain B-Raf protein kinase by Rap1B small GTP-binding protein. J Biol Chem 271: 1258–1261, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Okada T, Hu CD, Jin TG, Kariya K, Yamawaki-Kataoka Y, Kataoka T. The strength of interaction at the Raf cysteine-rich domain is a critical determinant of response of Raf to Ras family small GTPases. Mol Cell Biol 19: 6057–6064, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Padda R, Wamsley-Davis A, Gustin MC, Ross R, Yu C, Sheikh-Hamad D. MEKK3-mediated signaling to p38 kinase and TonE in hypertonically stressed kidney cells. Am J Physiol Renal Physiol 291: F874–F881, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Pisitkun T, Bieniek J, Tchapyjnikov D, Wang G, Wu WW, Shen RF, Knepper MA. High-throughput identification of IMCD proteins using LC-MS/MS. Physiol Genomics 25: 263–276, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Povsic TJ, Kohout TA, Lefkowitz RJ. β-Arrestin1 mediates insulin-like growth factor 1 (IGF-1) activation of phosphatidylinositol 3-kinase (PI3K) and anti-apoptosis. J Biol Chem 278: 51334–51339, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Sands JM, Knepper MA. Urea permeability of mammalian inner medullary collecting duct system and papillary surface epithelium. J Clin Invest 79: 138–147, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stoyanov B, Volinia S, Hanck T, Rubio I, Loubtchenkov M, Malek D, Stoyanova S, Vanhaesebroeck B, Dhand R, Nurnberg B, Gierschik P, Seedorf K, Hsuan JJ, Waterfield MD, Wetzker R. Cloning and characterization of a G protein-activated human phosphoinositide-3 kinase. Science 269: 690–693, 1995. [DOI] [PubMed] [Google Scholar]
- 33.Tang X, Batty IH, Downes CP. Muscarinic receptors mediate phospholipase C-dependent activation of protein kinase B via Ca2+, ErbB3, and phosphoinositide 3-kinase in 1321N1 astrocytoma cells. J Biol Chem 277: 338–344, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Terada Y, Inoshita S, Hanada S, Shimamura H, Kuwahara M, Ogawa W, Kasuga M, Sasaki S, Marumo F. Hyperosmolality activates Akt and regulates apoptosis in renal tubular cells. Kidney Int 60: 553–567, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Terada Y, Yamada T, Takayama M, Nonoguchi H, Sasaki S, Tomita K, Marumo F. Presence and regulation of Raf-1-K (Kinase), MAPK-K, MAP-K, and S6-K in rat nephron segments. J Am Soc Nephrol 6: 1565–1577, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Torres VE, Harris PC. Mechanisms of disease: autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol 2: 40–55, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Uawithya P, Pisitkun T, Ruttenberg BE, Knepper MA. Transcriptional profiling of native inner medullary collecting duct cells from rat kidney. Physiol Genomics 32: 229–253, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Umenishi F, Narikiyo T, Vandewalle A, Schrier RW. cAMP regulates vasopressin-induced AQP2 expression via protein kinase A-independent pathway. Biochim Biophys Acta 1758: 1100–1105, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Yamada T, Terada Y, Homma MK, Nonoguchi H, Sasaki S, Yuasa Y, Tomita K, Marumo F. AVP inhibits EGF-stimulated MAP kinase cascade in Madin-Darby canine kidney cells. Kidney Int 48: 745–752, 1995. [DOI] [PubMed] [Google Scholar]
- 40.Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem 279: 40419–40430, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Yip KP Coupling of vasopressin-induced intracellular Ca2+ mobilization and apical exocytosis in perfused rat kidney collecting duct. J Physiol 538: 891–899, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu MJ, Pisitkun T, Wang G, Shen RF, Knepper MA. LC-MS/MS analysis of apical and basolateral plasma membranes of rat renal collecting duct cells. Mol Cell Proteomics 5: 2131–2145, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B). Science 286: 1741–1744, 1999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.