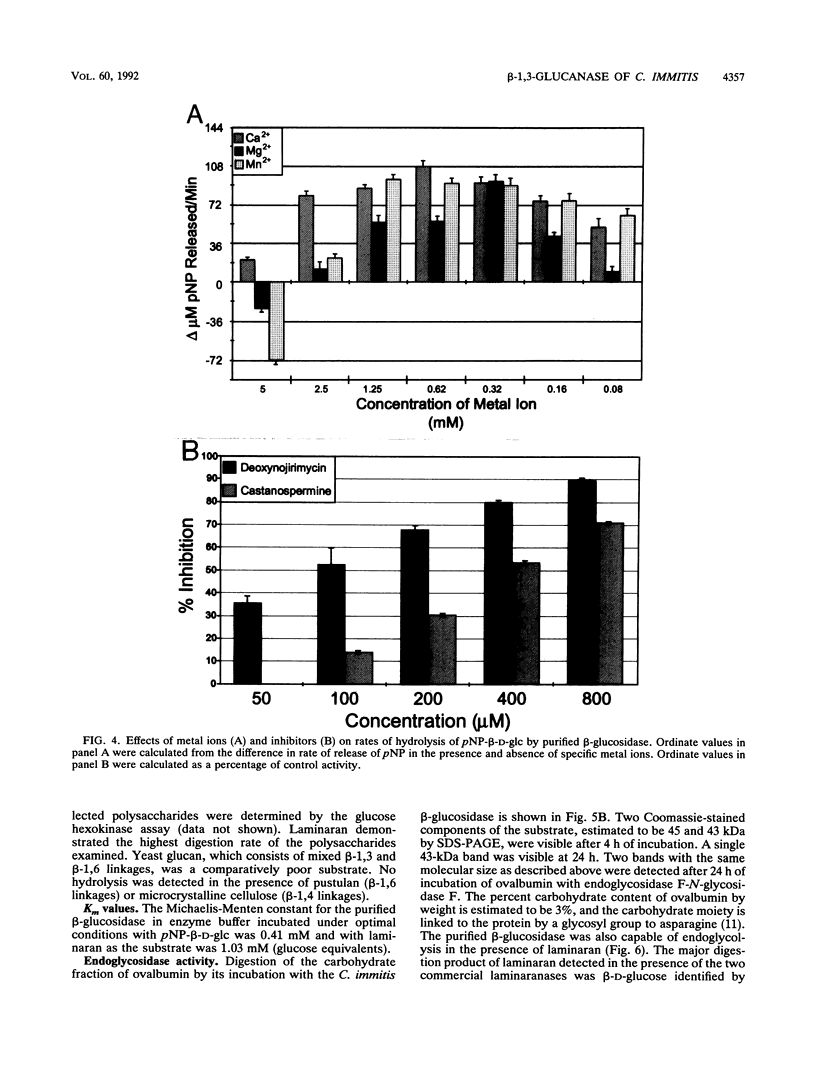
Abstract
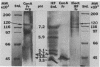
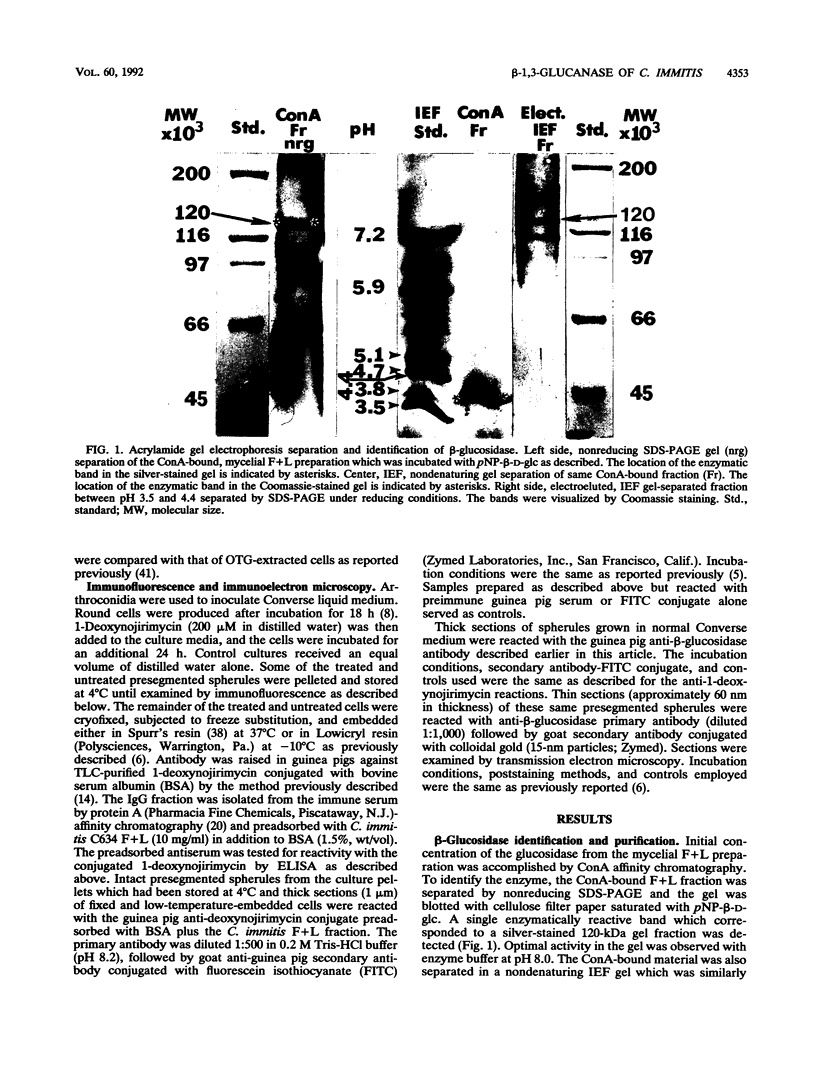
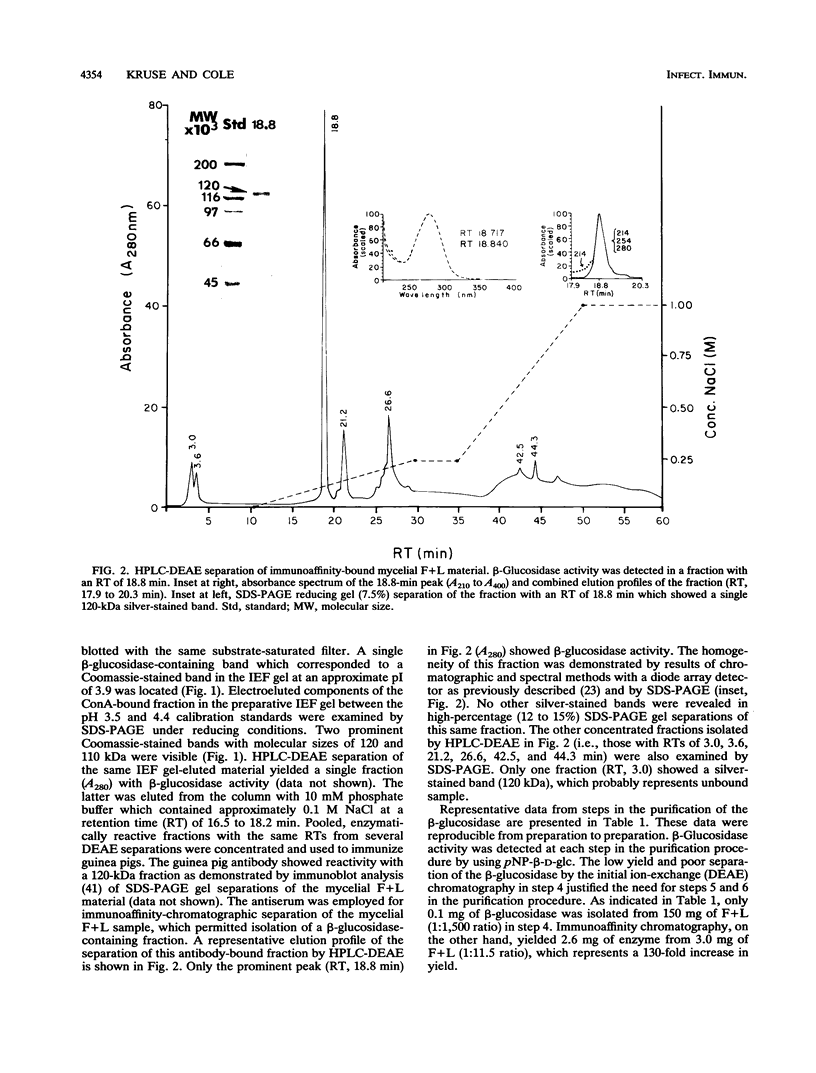
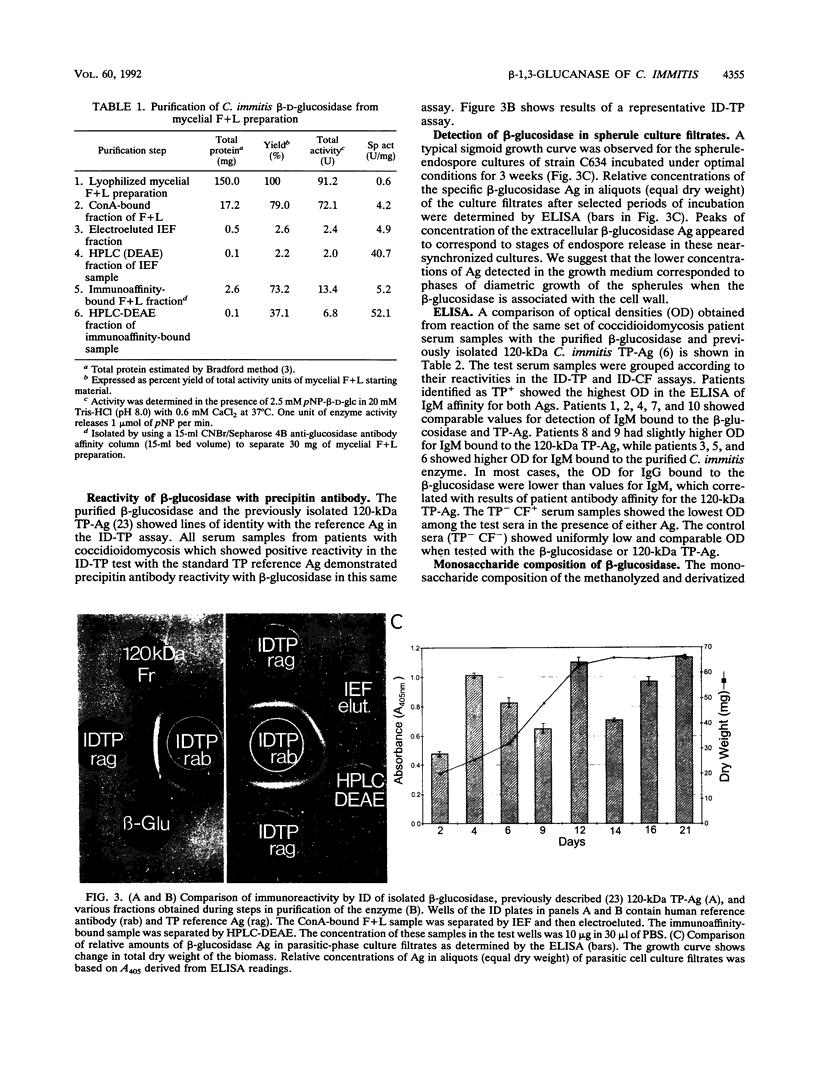
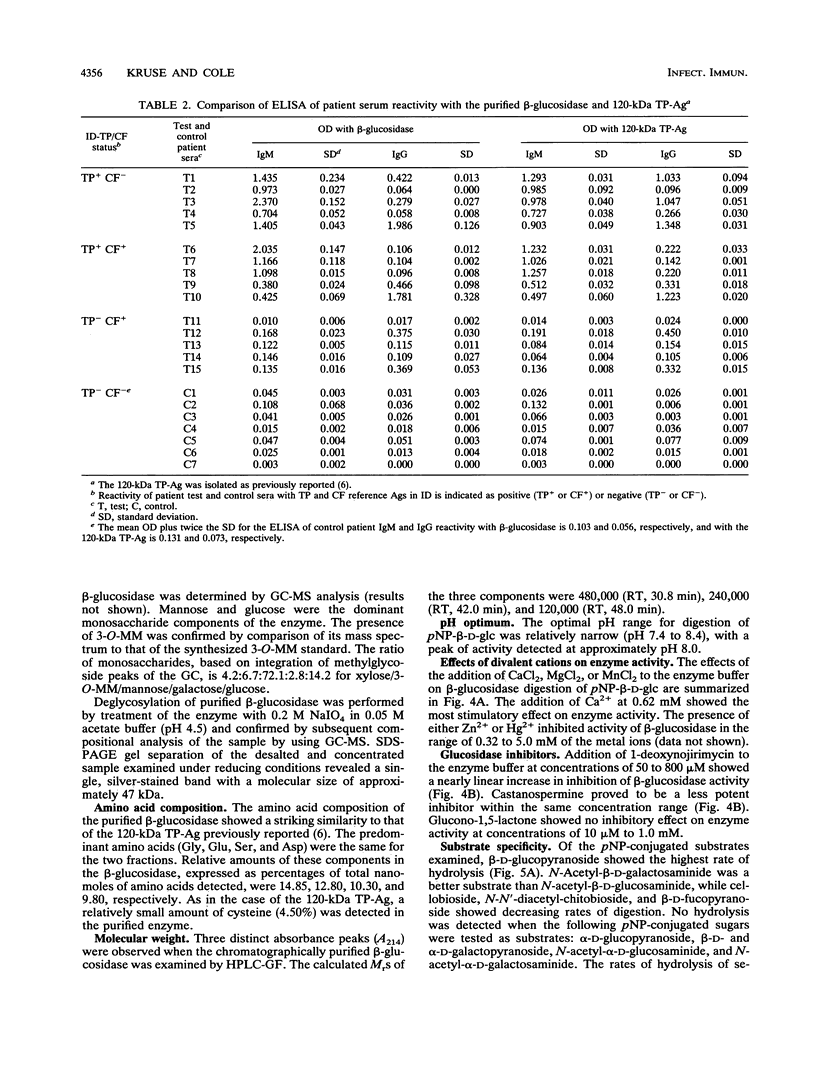
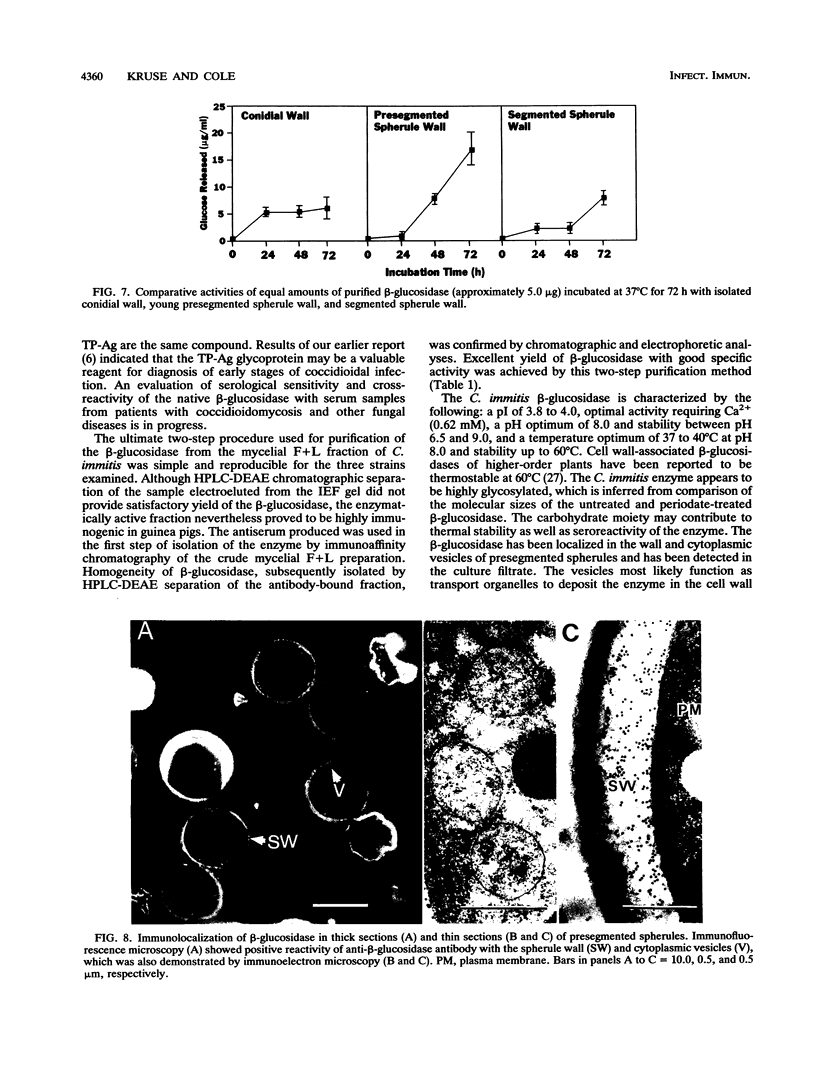
A beta-glucosidase of Coccidioides immitis was identified in electrophoresis gel separations of the concanavalin A-bound mycelial culture-filtrate-plus-lysate preparation. p-Nitrophenol-beta-D-glucopyranoside was used as the substrate to visualize the enzymatically active fraction in nonreducing gels. The gel-isolated, chromatographically purified enzyme has an optimal pH of 8.0 and cleaves beta-1,3-glycosyl linkages. The alkaline beta-glucosidase was further characterized by a pI of 3.8 to 4.0, optimal activity at 37 to 40 degrees C, and molecular size of 120 kDa as identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The purified beta-glucosidase is identical to a previously reported 120-kDa antigen (Ag) which reacts with immunoglobulin M (IgM) tube precipitin (TP) antibody in sera from patients with coccidioidomycosis. The TP-Ag was described as a valuable serodiagnostic reagent for detection of specific IgM in patients with early coccidioidal infections. The beta-glucosidase, like the TP-Ag, was localized in the cell wall and cytoplasmic vesicles of parasitic cells (spherules) by immunofluorescence and immunoelectron microscopy with specific antiserum raised against the purified enzyme. The boiled cell wall fraction isolated from these same young (presegmented) spherules was partially digested by the beta-glucosidase. Addition of a potent beta-glucosidase inhibitor, 1-deoxynojirimycin, to the parasitic-phase culture medium at a concentration of 200 microM blocked or retarded conversion of arthroconidia to spherules. Antibody was raised in guinea pigs against chromatographically purified 1-deoxynojirimycin which was conjugated with bovine serum albumin. The inhibitor was localized by immunofluorescence in the wall of the 1-deoxynojirimycin-treated cells. We suggest that the spherule wall-associated, alkaline hydrolase functions as a beta-1,3-glucanase to provide for wall plasticity as well as intussusception of newly synthesized wall polymers during the period of rapid diametric growth of parasitic cells of C. immitis.
Full text
PDF


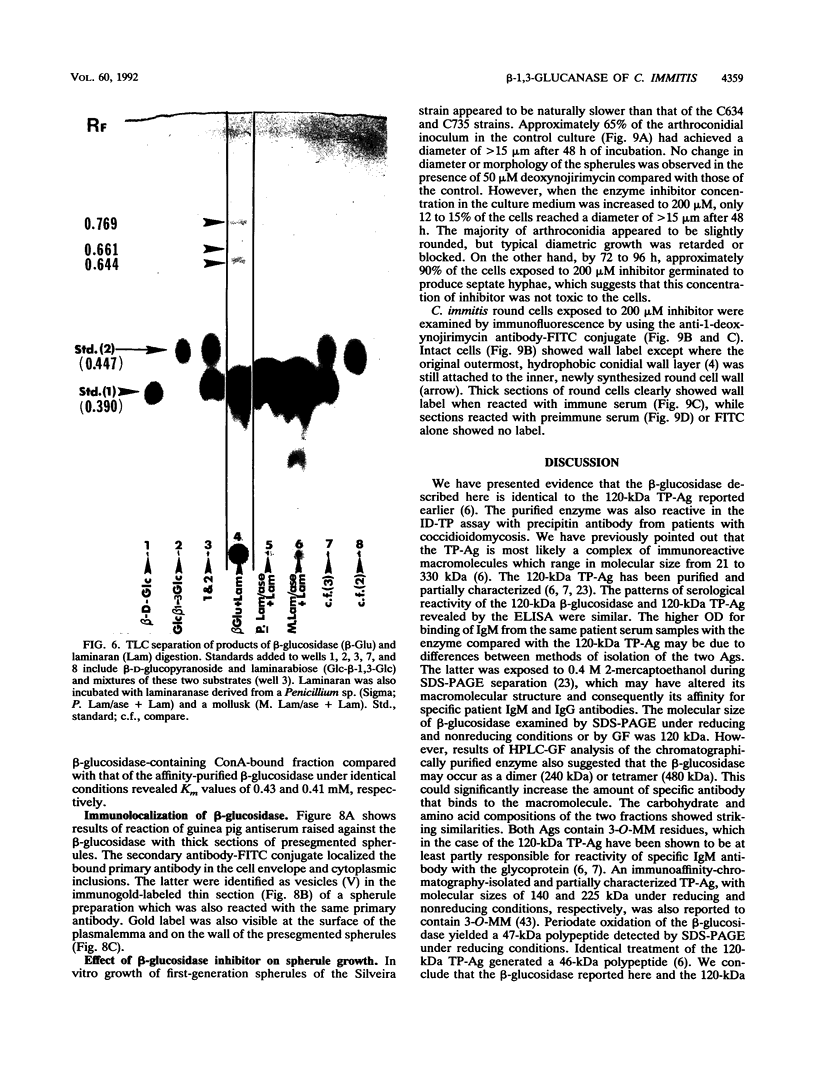


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cole G. T., Kirkland T. N., Franco M., Zhu S., Yuan L., Sun S. H., Hearn V. M. Immunoreactivity of a surface wall fraction produced by spherules of Coccidioides immitis. Infect Immun. 1988 Oct;56(10):2695–2701. doi: 10.1128/iai.56.10.2695-2701.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G. T., Kruse D., Seshan K. R. Antigen complex of Coccidioides immitis which elicits a precipitin antibody response in patients. Infect Immun. 1991 Jul;59(7):2434–2446. doi: 10.1128/iai.59.7.2434-2446.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G. T., Kruse D., Zhu S. W., Seshan K. R., Wheat R. W. Composition, serologic reactivity, and immunolocalization of a 120-kilodalton tube precipitin antigen of Coccidioides immitis. Infect Immun. 1990 Jan;58(1):179–188. doi: 10.1128/iai.58.1.179-188.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G. T., Zhu S. W., Hsu L. L., Kruse D., Seshan K. R., Wang F. Isolation and expression of a gene which encodes a wall-associated proteinase of Coccidioides immitis. Infect Immun. 1992 Feb;60(2):416–427. doi: 10.1128/iai.60.2.416-427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G. T., Zhu S. W., Pan S. C., Yuan L., Kruse D., Sun S. H. Isolation of antigens with proteolytic activity from Coccidioides immitis. Infect Immun. 1989 May;57(5):1524–1534. doi: 10.1128/iai.57.5.1524-1534.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conchie J., Hay A. J., Strachan I., Levvy G. A. The enzymic degradation of ovalbumin and its glycopeptides. Biochem J. 1969 Dec;115(4):717–723. doi: 10.1042/bj1150717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERLANGER B. F., BEISER S. M. ANTIBODIES SPECIFIC FOR RIBONUCLEOSIDES AND RIBONUCLEOTIDES AND THEIR REACTION WITH DNA. Proc Natl Acad Sci U S A. 1964 Jul;52:68–74. doi: 10.1073/pnas.52.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann U., Bause E., Ploegh H. Inhibitors of oligosaccharide processing. Biochim Biophys Acta. 1985 Jun 24;825(2):95–110. doi: 10.1016/0167-4781(85)90095-8. [DOI] [PubMed] [Google Scholar]
- Galgiani J. N., Dugger K. O., Ampel N. M., Sun S. H., Law J. H. Extraction of serologic and delayed hypersensitivity antigens from spherules of Coccidioides immitis. Diagn Microbiol Infect Dis. 1988 Oct;11(2):65–80. doi: 10.1016/0732-8893(88)90075-2. [DOI] [PubMed] [Google Scholar]
- Gow N. A. Control of extension of the hyphal apex. Curr Top Med Mycol. 1989;3:109–152. doi: 10.1007/978-1-4612-3624-5_6. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Use of protein A-bearing staphylococci for the immunoprecipitation and isolation of antigens from cells. Methods Enzymol. 1981;73(Pt B):442–459. doi: 10.1016/0076-6879(81)73084-2. [DOI] [PubMed] [Google Scholar]
- Kirkland T. N., Zhu S. W., Kruse D., Hsu L. L., Seshan K. R., Cole G. T. Coccidioides immitis fractions which are antigenic for immune T lymphocytes. Infect Immun. 1991 Nov;59(11):3952–3961. doi: 10.1128/iai.59.11.3952-3961.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse D., Cole G. T. Isolation of tube precipitin antibody-reactive fractions of Coccidioides immitis. Infect Immun. 1990 Jan;58(1):169–178. doi: 10.1128/iai.58.1.169-178.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE H. B. Purification of the spherule-endospore phase of Coccidioides immitis. Sabouraudia. 1961 Jun;1:112–115. doi: 10.1080/00362176285190231. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Van Keuren M. L. Gel protein stains: silver stain. Methods Enzymol. 1984;104:441–447. doi: 10.1016/s0076-6879(84)04111-2. [DOI] [PubMed] [Google Scholar]
- Notario V. beta-Glucanases from Candida albicans: purification, characterization and the nature of their attachment to cell wall components. J Gen Microbiol. 1982 Apr;128(4):747–759. doi: 10.1099/00221287-128-4-747. [DOI] [PubMed] [Google Scholar]
- Pappagianis D. Epidemiology of coccidioidomycosis. Curr Top Med Mycol. 1988;2:199–238. doi: 10.1007/978-1-4612-3730-3_6. [DOI] [PubMed] [Google Scholar]
- Pappagianis D., Zimmer B. L. Serology of coccidioidomycosis. Clin Microbiol Rev. 1990 Jul;3(3):247–268. doi: 10.1128/cmr.3.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pócsi I., Kiss L. Kinetic studies on the broad-specificity beta-D-glucosidase from pig kidney. Biochem J. 1988 Nov 15;256(1):139–146. doi: 10.1042/bj2560139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram S. P., Romana L. K., Shepherd M. G., Sullivan P. A. Exo-(1----3)-beta-glucanase, autolysin and trehalase activities during yeast growth and germ-tube formation in Candida albicans. J Gen Microbiol. 1984 May;130(5):1227–1236. doi: 10.1099/00221287-130-5-1227. [DOI] [PubMed] [Google Scholar]
- Resnick S., Pappagianis D., McKerrow J. H. Proteinase production by the parasitic cycle of the pathogenic fungus Coccidioides immitis. Infect Immun. 1987 Nov;55(11):2807–2815. doi: 10.1128/iai.55.11.2807-2815.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridruejo J. C., Muñoz M. D., Andaluz E., Larriba G. Inhibition of yeast exoglucanases by glucosidase inhibitors. Biochim Biophys Acta. 1989 Dec 8;993(2-3):179–185. doi: 10.1016/0304-4165(89)90161-x. [DOI] [PubMed] [Google Scholar]
- Saul R., Chambers J. P., Molyneux R. J., Elbein A. D. Castanospermine, a tetrahydroxylated alkaloid that inhibits beta-glucosidase and beta-glucocerebrosidase. Arch Biochem Biophys. 1983 Mar;221(2):593–597. doi: 10.1016/0003-9861(83)90181-9. [DOI] [PubMed] [Google Scholar]
- Saunier B., Kilker R. D., Jr, Tkacz J. S., Quaroni A., Herscovics A. Inhibition of N-linked complex oligosaccharide formation by 1-deoxynojirimycin, an inhibitor of processing glucosidases. J Biol Chem. 1982 Dec 10;257(23):14155–14161. [PubMed] [Google Scholar]
- Spielman L. L., Mowshowitz D. B. A specific stain for alpha-glucosidases in isoelectric focusing gels. Anal Biochem. 1982 Feb;120(1):66–70. doi: 10.1016/0003-2697(82)90318-9. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Yuan L., Cole G. T. Isolation and characterization of an extracellular proteinase of Coccidioides immitis. Infect Immun. 1987 Sep;55(9):1970–1978. doi: 10.1128/iai.55.9.1970-1978.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L., Cole G. T., Sun S. H. Possible role of a proteinase in endosporulation of Coccidioides immitis. Infect Immun. 1988 Jun;56(6):1551–1559. doi: 10.1128/iai.56.6.1551-1559.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer B. L., Pappagianis D. Immunoaffinity isolation and partial characterization of the Coccidioides immitis antigen detected by the tube precipitin and immunodiffusion-tube precipitin tests. J Clin Microbiol. 1989 Aug;27(8):1759–1766. doi: 10.1128/jcm.27.8.1759-1766.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]