Abstract
Animal models of prenatal ethanol exposure are necessary to more fully understand the effects of ethanol on the developing embryo/fetus. However, most models employ procedures that may produce additional maternal stress beyond that produced by ethanol alone. We employed a daily limited-access ethanol intake model called Drinking in the Dark (DID) to assess the effects of voluntary maternal binge-like ethanol intake on the developing mouse. Evidence suggests that binge exposure may be particularly harmful to the embryo/fetus, perhaps due to the relatively higher blood ethanol concentrations achieved. Pregnant females had mean daily ethanol intakes ranging from 4.2-6.4 g/kg ethanol over gestation, producing blood ethanol concentrations ranging from 115-182 mg/dl. This level of ethanol intake produced behavioral alterations among adolescent offspring that disappeared by adulthood, including altered sensitivity to ethanol’s hypnotic actions. The DID model may provide a useful tool for studying the effects of prenatal ethanol exposure in mice.
Keywords: in utero, binge, ethanol, intake, behavior, mouse, Drinking in the Dark
Introduction
Ingestion of alcohol (ethanol) by the pregnant mother can result in a variety of neurodevelopment consequences in offspring, including mental retardation and deficits in cognitive ability, attention, executive function, motor control, and social behavior (Kelly et al., 2000; Mattson and Riley, 1998). Referred to as Fetal Alcohol Spectrum Disorders (FASD), it is estimated that as many as 1 in 100 live births are seen with such prenatal alcohol-related impairments (Sampson et al., 1997). Moreover, it is estimated that Americans spent $2 million for the medical care, special education, residential care, and the lost productivity associated with each individual diagnosed with the most severe form of FASD, fetal alcohol syndrome, in 2002 (Lupton et al., 2004). Thus, FASD would appear to pose a major public health concern, and continued investigations into the actions of prenatal ethanol exposure on brain development/function seem warranted. The use of animal models of fetal alcohol exposure will be necessary to more fully understand how such exposure impairs brain development/function.
The mechanisms by which ethanol produces FAS/FASD are not fully understood. However, most animal studies looking at the effects of prenatal alcohol exposure on the developing embryo/fetus have required that pregnant dams consume an ethanol-containing liquid diet (Chernoff, 1977; Middaugh et al., 1988; Zhou et al., 2003), or that researchers administer ethanol via intraperitoneal injection (Kronick, 1976; Dunty et al., 2002) or intragastric intubation (Boehm II et al., 1997; Gilliam et al., 1989). These procedures may produce additional stress for the mother, making it more difficult to elucidate the mechanisms by which prenatal ethanol exposure produces its effects. Recently, Allan et al. (2003) described a new paradigm in which C57BL/6J mice were provided with a saccharin-sweetened ethanol solution in a voluntary drinking paradigm. Offspring from dams exposed to ethanol in this paradigm were found to spend more time inspecting a novel object in their environment as well as reduced contextual fear when trained in a delay fear conditioning procedure (Allan et al., 2003).
A new mouse model of binge-like ethanol intake has been described by Rhodes et al. (2005; 2007), and more recently Moore et al. (2007), called drinking in the dark (DID). Allan and colleagues (2003) noted that their pregnant female mice consumed most of their 10% ethanol/saccharin solution during the dark phase of the light-dark cycle. The DID model takes advantage of the nocturnal nature of mice by allowing the them a limited access (2 or 4 hours) to a 20% unsweetened ethanol solution at their peak period of arousal, 3 hours into the dark cycle. Using the DID model, C57BL/6J mice consistently drink to pharmacologically relevant BECs that produce behavioral impairment (Moore et al., 2007; Rhodes et al., 2005, 2007). Indeed, a mere 2-hour access produces average ethanol intakes of 5.5 g/kg with corresponding blood ethanol concentrations approaching 70 mg/dl in non-pregnant females (Moore et al., 2007). Thus, DID may prove useful for investigating the effects of prenatal ethanol exposure in mice.
DID may also provide a useful means by which to model the effects of prenatal binge ethanol exposure in humans. Such exposure has been shown to increase the odds (two fold) of six psychiatric disorders and traits in adults exposed to one or more binge alcohol episodes in utero (Barr et al., 2006). Moreover, Bailey et al. (2004) has shown that children who were exposed to binge drinking in utero were 1.7 times more likely to have IQ scores in the mentally retarded range, and 2.5 times more likely to have clinically significant levels of delinquent behavior. Maier and West (2001) have suggested that binge ethanol exposure may be more deleterious to the developing fetal brain than more continuous ethanol exposure, perhaps due to the relatively higher blood ethanol concentrations achieved when considerable amounts of ethanol are consumed over a short time interval. Indeed, prenatal binge-like ethanol exposure was shown to produce relatively greater reductions in hippocampal and cerebellar cell counts in fetal rat brain than more continuous ethanol intake (Bonthius and West, 1990). Thus, the binge-like pattern of the ethanol intake in the DID model may better approximate the binge pattern of ethanol exposure in some human alcoholics, and yield a pattern of prenatal effects not seen with other continuous ethanol access models.
The goal for the present work was to develop the DID model for exposure of pregnant C57BL/6J dams to daily binge-like ethanol intake. We predicted that pregnant mice would consume high amounts of ethanol, and that their adolescent and adult offspring would exhibit deficits in a variety of behavioral tasks as suggested by studies using other prenatal ethanol exposure paradigms, including those measuring anxiety-like behavior (Dursun et al., 2006), motor coordination (Meyer et al., 1990; Riley, 1990; Hannigan and Riley, 1988), and general locomotion (Carneiro et al., 2005; Dursun et al., 2006; Riley, 1990). Based on data suggesting that prenatal ethanol exposure might alter behavioral sensitivity to the ethanol in offspring (Fulginiti et al., 1989; Reyes et al., 1993), we also assessed hypnotic sensitivity to the drug in adolescents and adults using the loss of righting reflex assay.
Materials and Methods
Animals
Male and female C57BL/6J inbred mice purchased from Jackson Laboratory (Bar Harbor, ME) and shipped to our animal facility at Binghamton University were used as breeders for this study. Mice were individually housed in standard shoebox mouse cages and were approximately 9 weeks old at the start of breeding. Lighting prior to weaning was maintained on a 12-hour reverse light-dark cycle with lights out at 1 PM. However, at weaning litters were moved to an adjacent room on a normal 12-hour light-dark cycle with lights out at 7PM. All experimental procedures (accept maternal DID) were carried out during the light phase. For maternal DID, dim red lighting throughout the 12-hr dark phase allowed the technician to navigate the procedure room in the dark. A dark anti-room immediately adjacent the procedure room was used to enter and exit; this ensured that no light was allowed into the procedure room upon opening the door. The temperature of all procedure rooms was always maintained at 21 ± 1 degrees Celsius.
Food was freely available to sires, dams, and offspring at all times except during offspring behavioral testing. Water was freely available from normal water bottles at all times except during offspring behavioral testing and maternal DID. For maternal DID, pregnant dams had free access to water in their normal water bottles for 22 hrs per day; during the remaining two hrs each day the normal water bottles were replaced with sipper tubes (see details below) containing either ethanol or plain tap water. The two-hour access to these sipper tubes was provided throughout each dam’s 21 day gestational period, and for gradually shorter intervals during a post-gestational three-day ethanol weaning period (see details below). All procedures were approved by the Binghamton University Institutional Animal Care and Use Committee and conformed to the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (The National Academies Press, 2003).
Drugs
Ethanol (200 proof) was obtained from Pharmco, Inc (Brookfield, CT). Ethanol solutions for maternal DID were made in tap water (20% v/v). Ethanol (4 g/kg) solutions for the loss of righting reflex assay were made in 0.9% sterile saline and administered intraperitoneally in a volume of 0.2 ml/kg body weight.
Procedure
During the light cycle, female mice were weighed and placed in the male’s home cage to breed for one-hour daily (10-11AM). During this time the female’s food pellets were weighed for determination of food intake. After the breeding period the females were examined for vaginal plugs and returned to their home cages. Mice were later presented with either ethanol or water using the Drinking in the Dark procedure (DID) adapted from Rhodes et al (2005; 2007), and recently detailed by Moore et al. (2007). Three-hours into the dark cycle (4 PM), pregnant females were given fluid access for 2 hours. A 10 ml plastic tube affixed to a ball-bearing sipper filled with the ethanol solution or water was placed in the cage where the water bottle was normally located. To prevent the mice from moving the sipper tube and producing potential leakage, the animals’ water bottle was placed on top of the sipper tube to hold it steady. Sipper tube volumes were recorded both before and after the two hour access period starting the first day of breeding, and continuing through out gestation.
The fluid access period was incrementally reduced over the three days immediately following the birth of litters. Our goals were to avoid the possible negative effects of continued maternal binge-like ethanol intake on litter care while at the same time attempting to avoid any possible negative effects of withdrawal from binge-like ethanol intake on litter care. The period of binge-like ethanol or water access was shortened to 90 min on PD0 (the day of birth), 60 min on PD1, and just 30 min on PD2. DID procedures were no longer employed on PD3, or any day thereafter.
Whole brain weight was determined in a subset of ethanol- and water-exposed litters on PD 0. In an attempt avoid disrupting the interaction between mom and litter, only one pup from such litters was removed, weighed and sacrificed for brain extraction to determine whole brain weight. However, on PD7 all pups were removed from their home cage, counted, and weighed. The pups were individually placed back into the home cage at the furthest point from the nest (almost always an opposite corner of the cage), and the dams’ latency to retrieve each pup was recorded. An average latency to retrieve time was generated for each litter and used in the statistical analyses. On PD 14 pups were again removed from their home cage and weighed. Pups were sexed, weaned, weighed, and moved to a holding room with standard lighting (12-hr light/dark cycle with lights on at 7AM) on PD21. Animals were allowed to acclimate to the new lighting conditions for 10 days prior to subsequent behavioral testing.
Offspring Behavior
From PD31-34 (adolescence), one male and one female from a subset of litters were tested in a battery of behavioral tests which included the elevated plus maze, the accelerating rotarod, the locomotor activity chamber, and ethanol-induced loss of righting reflex test (one test per day, in that order). Given a sufficiently large litter, the behavioral test battery was repeated exactly as indicated above with a different male and female from the same litter at PD91-94 (adulthood). Details about the individual behavioral tests are given in the sections that follow.
Anxiety-Like Behavior: Elevated Plus Maze
At 31 days old, one male and one female from a litter were tested for anxiety-like behavior using a mouse elevated plus maze (Med Associates, Inc., St. Albans, Vermont). The maze comprised two opposing open arms and two opposing closed arms set at 90 degree angles from each other and elevated 74.5 cm above the ground. The end of one arm to the end of the opposing arm measured 76 cm. Whereas the two opposing closed arms had walls extending 20.5 cm vertically from the elevated floor, the two opposing open arms did not. Animals were placed in the center of the plus maze, and the 5 min test was immediately started. Time spent in open arms was recorded. At the conclusion of each test, animals were returned to their home cages and the plus maze was cleaned with a 10% ethanol solution. A separate male and female from the litter were tested for plus maze anxiety-like behavior at PD 91 when available.
Motor Coordination: Accelerating Rotarod
At 32 days old, the same animals were tested for motor coordination using a Rotamex Accelerating Rotorod (Columbus Instruments, Columbus, OH). The rotating rotorod cylinder (10 cm) was elevated 46.5 cm above the floor of the apparatus and was programmed to accelerate at 1 rpm every 3 sec. Woodchip bedding was placed at the base of the rotorod to soften the fall for the animals. The animals were placed on the rotorod beam, and latency to fall was recorded for 6 trials. Between each trial, animals had a minimum of 30 s rest. Immediately following the test animals were returned to their home cages. A separate male and female from each litter was tested for motor coordination on PD 92 (the same male and female that was tested on PD 91 above).
Basal Locomotion
At 33 days old, animals were tested in activity chambers. A VersaMax Animal Activity Monitoring System (Accuscan Instruments, Columbus, OH, USA) was used to monitor locomotor activity over a 30 min period, in 5-min time bins. Movement was detected by eight pairs of intersecting photocell beams (2 cm above the chamber floor) evenly spaced along the walls of the 40x40-cm test chamber. Sound-attenuating box chambers (inside dimensions, 53 cm across × 58 cm deep × 43 cm high) equipped with a house light and fan for ventilation and background noise encased the test chamber. The locomotor activity testing equipment was interfaced with a Dell computer. Animals were placed in the chambers and total distance (cm) was measured every 5 min during the 30 min test. Animals were returned to their home cages following the test, and activity chambers were cleaned with 10% ethanol solution. In addition, a separate male and female from each litter was tested for habituation to a novel environment at PD 93 (the same male and female that was tested on PD 91 and 92 above).
Hypnotic Sensitivity to Ethanol: Loss of Righting Reflex
At 34 days old animals were weighed, challenged with a 4 g/kg dose of ethanol, placed in classic v-shaped sleep troughs, and duration of sleep time was recorded (min). A separate male and female from each litter was weighed and challenged with ethanol on PD 94 (the same male and female that was tested on PD 91, 92, and 93 above). Animals were considered to have lost their righting reflex once they could not right themselves within a 60 s window. An animal was determined to have regained the righting reflex if it could right itself twice within 60 s. The duration of loss of righting reflex was defined as the time between initial loss of the righting response (immediately after ethanol injection) and regain of the righting response at some point thereafter. Immediately upon regain of the righting reflex retro-orbital sinus bloods were collected for determination of blood ethanol concentration (see detailed methods below).
Maternal Blood Ethanol Concentration
A separate study was conducted to determine maternal blood ethanol content. Three different groups of pregnant females were allowed daily access to ethanol using DID procedures and blood ethanol content was determined immediately thereafter on gestational days 7, 14, or 20, respectively. None of the offspring from these dams were used for assessments of brain or body weight or behavioral testing.
Determination of Blood Ethanol Concentration
For determination of blood ethanol content, a retro-orbital sinus blood sample (25μl) was collected immediately after the 2hr ethanol access period (maternal DID) or regain of the righting response, spun down, and the plasma fraction siphoned off and stored in a -20 degree freezer until the time of assay. Determination of blood ethanol content was achieved using an Analox Alcohol Analyzer (Analox Instruments, Lunenburg, MA).
Data and Statistics
Only one male and one female from a litter were used for behavioral testing. Thus, the group sizes given in the figure captions represent the number of litters used. There were not always enough litter mates of a given sex to satisfy all measures (one pup for determination of pup and brain weight, one male and one female for behavioral testing during adolescence, one male and one female for testing during adulthood). However, pups were always randomly selected from those available in each litter for the weight or behavioral measures. Finally, in some instances equipment malfunction (rotarod and locomotor activity chambers) or scheduling conflicts (maternal weight gain, maternal care on PD 7, litter size on PD 7, litter weights on PD 7, 14, and 21) prevented collection of data.
Maternal food intake and weight gain data were analyzed by two-way repeated measures analysis of variance (ANOVA) with treatment as the between groups factor and day as the within groups factor. Maternal blood ethanol concentration data were analyzed by one-way between subjects ANOVA. Maternal fluid intake on PD0-2 were analyzed by two-way ANOVA with treatment as the between groups factor and postnatal day as the within groups factor. Pup body and brain weights on PD0 were analyzed by two-tailed t-test, as were litter sizes assessed on PD7. Although the sex of offspring was determined on PD21, this information was not available on PD7 or 14, so sex was not included as factor in the analysis of average litter weights. Pre-weanling litter weights on PD7, 14, and 21 were analyzed by two-way repeated measures ANOVA with treatment as the between groups factor and PD as the within groups factor. Elevated plus maze and loss of righting reflex data were analyzed by two-way ANOVAs with sex and treatment as between groups factors. Accelerating rotarod and locomotor activity data were analyzed by three-way repeated measures ANOVAs with sex and treatment as the between groups factors and trial or activity testing bin as within groups factors, and where appropriate (locomotor activity), by two-way repeated measures ANOVA with treatment as the between groups factor and test bin as the within groups factor. Adolescent and adult offspring behavioral data were always analyzed separately, as were offspring weights determined immediately prior to assessment of ethanol-induced loss of righting reflex. Significant results were followed up by Newman-Keuls post-hoc test or planned contrasts where appropriate. Maternal intake, pre-weanling, and offspring behavioral data were considered significant at p<0.05.
Results
Maternal intake
Maternal data over the course of gestation are shown in Figure 1 and Table 1. Pregnant females given access to an unsweetened 20% ethanol solution for 2hrs each day consumed 4.5-6.7 g/kg ethanol (Figure 1A, see Table 1 for ml/kg ethanol solution consumed). Corresponding blood ethanol concentrations on days 7, 14, and 20 did not significantly differ (p>0.05), although consistent with the intake data tended to be lower on gestational day 20 (Figure 1B). Pregnant females given similar access to plain tap water consumed 28.4-48.2 ml/kg during the 2hr access period each day (Table 1). Twenty-four hour food intake differed from day to day (main effect of time, F(18,33)=4.2, p<0.001), but ethanol-and water-exposed dams did not significantly differ (no main effect of treatment or interaction of treatment and time, p’s>0.05; Figure 1C). Compared to water-exposed counterparts, ethanol-exposed dams appeared to gain less weight over gestation (main effect of treatment, F(1,32)=4.5, p<0.05; main effect of time, F(19,608)=634.0, p<0.001; interaction, F(19,608)=4.8, p<0.001; Table 1). However, when maternal weight gain was adjusted for initial weight by calculating a percent maternal weight gain, the treatment effect was no longer evident. Although percent maternal weights increased over gestation as evidenced by a significant main effect of time (F(19,608)=521.8, p<0.001), and a significant interaction of treatment and time indicated that the ethanol- and water-exposed dams might have differed at some point over gestation (F(19,608)=1.9, p<0.05), subsequent Newman-Keuls post-hoc tests failed to detect any specific differences in percent maternal weight on any gestational day (p’s>0.05; Figure 1D).
Figure 1.
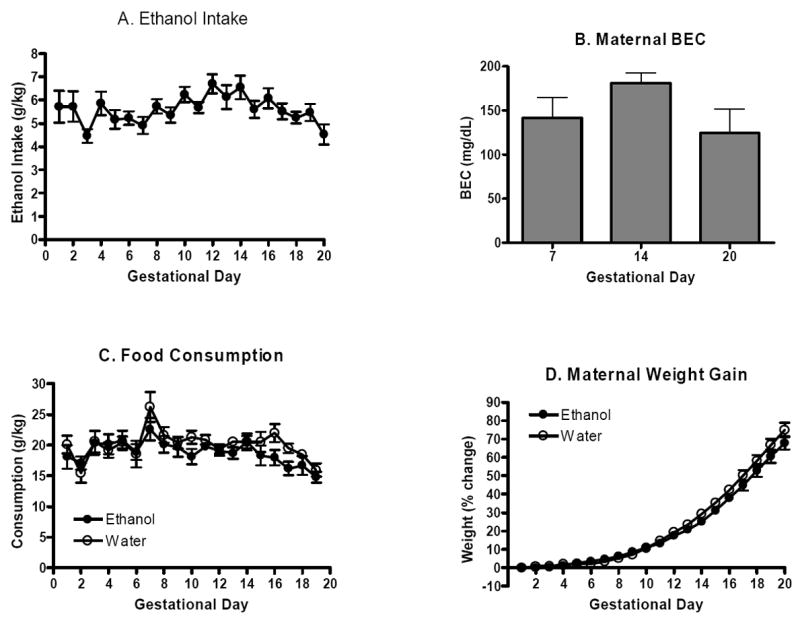
Maternal intake. A. Mean (±SEM) ethanol intake in pregnant dams given daily 2hr access to an unsweetened 20% ethanol solution using DID procedures from gestational days 1-20 (n=18). B. Mean (±SEM) maternal blood ethanol concentrations immediately following ethanol access at 7, 14, and 20 days gestation. Blood ethanol concentrations were determined in separate groups of pregnant females (n’s=4-5). C. Mean (±SEM) 24hr food intake for ethanol-and water-exposed dams from gestational days 1-20 (n’s=17-18). D. Mean (±SEM) percent maternal weight gain from gestational days 1-20 (n’s=16-18). Percentages were determined compared to initial weight at initiation of pregnancy (gestational day 1).
Table 1.
Daily maternal weights (g±SEM) and 2hr intakes (ml/kg±SEM) in pregnant C57BL/6J dams allowed access to either ethanol or water using DID procedures.
| Gestational
|
Ethanol-Exposed† |
Water-Exposed‡ |
||
|---|---|---|---|---|
| Day
|
weight (g)
|
intake (ml/kg)
|
weight (g)
|
intake (ml/kg)
|
| 1 | 21.6±0.6 | 28.6±3.4 | 22.6±0.6 | 45.2±4.0 |
| 2 | 21.6±0.6 | 28.6±3.3 | 22.8±0.6 | 33.7±3.8 |
| 3 | 21.7±0.6 | 22.3±1.5 | 22.8±0.6 | 39.5±3.5 |
| 4 | 21.9±0.6 | 29.3±2.5 | 23.1±0.7 | 42.0±2.6 |
| 5 | 22.2±0.6 | 25.9±2.0 | 23.1±0.6 | 43.5±3.9 |
| 6 | 22.4±0.6 | 26.2±1.4 | 23.1±0.6 | 38.8±3.6 |
| 7 | 22.6±0.6 | 24.6±1.8 | 23.4±0.6 | 45.0±3.5 |
| 8 | 23.0±0.6 | 28.7±1.6 | 23.8±0.6 | 39.1±3.1 |
| 9 | 23.4±0.5 | 26.8±1.6 | 24.2±0.6 | 39.6±3.7 |
| 10 | 23.9±0.5 | 31.2±1.6 | 25.0±0.6 | 40.6±3.3 |
| 11 | 24.4±0.5 | 28.4±1.2 | 25.9±0.6 | 45.4±3.8 |
| 12 | 25.3±0.5 | 33.5±2.1 | 26.9±0.6 | 47.7±3.5 |
| 13 | 26.0±0.5 | 30.7±2.5 | 27.9±0.6 | 48.8±3.1 |
| 14 | 26.9±0.6 | 32.8±2.6 | 29.2±0.7 | 41.3±3.0 |
| 15 | 28.2±0.5 | 28.0±1.8 | 30.5±0.7 | 43.5±2.9 |
| 16 | 30.0±0.5 | 30.4±2.1 | 32.1±0.7 | 46.2±3.2 |
| 17 | 31.1±0.6 | 27.6±1.7 | 33.9±0.8 | 43.5±3.2 |
| 18 | 32.8±0.6 | 26.3±1.2 | 35.6±0.8 | 39.2±3.4 |
| 19 | 34.4±0.6 | 27.4±1.9 | 37.6±0.9 | 36.0±3.3 |
| 20 | 36.0±0.7 | 22.7±2.2 | 39.4±1.0 | 28.4±4.1 |
Dams were allowed access to an unsweetened 20% ethanol solution for 2 hours each day, 3 hours intor the dark cycle.
Dams were allowed access to plain tap water for 2 hours each day, 3 hours into the dark cycle.
Maternal Care
Upon birth of litters, dams were gradually weaned from DID over a 3 day period. Ethanol intake was reduced to 1.5 hrs on PD0, 1 hr on PD1, and 0.5 hr on PD2 with the goal of incrementally minimizing the effects of continued binge-like ethanol intake on maternal care. Although intakes were assessed, we did not determine maternal weights on these days so as not to disturb the dam and litter. Thus, g/kg intakes could not be calculated. Mean ethanol intakes were 0.66±0.08, 0.59±0.06, and 0.48±0.05 ml on PD0-2 respectively. Mean water intakes were 0.97±0.14, 0.82±0.16, and 0.86±0.13 ml on PD0-2, respectively. Two-way ANOVA indicated a significant main effect of treatment (F(1,66)=11.4, p<0.01), but not postnatal day or interaction of these factors (p’s>0.05). Thus, although ethanol-exposed dams consumed less of the ethanol solution than water-exposed dams consumed water, the weaning procedure did not appear to significantly reduce the amount of ethanol or water consumed over PD0-2 in either group.
Maternal care was assessed on PD7 by examining the time it took a dam to retrieve her pups when they were moved from the nest to an opposite corner of the cage. Mean retrieval times (18.5±2.2 and 15.6±2.8 s for dams that had access to ethanol or water, respectively) did not significantly differ (t[17]=0.82, p=0.43) indicating that maternal binge-like ethanol intake did not alter maternal care.
Offspring Weight and Litter Size
In a subset of litters one pup was weighed and brain weight assessed on PD0, but neither parameter was significantly affected by prenatal ethanol exposure (p’s>0.05). Pup weights averaged 1.4±0.05 and 1.4±0.05 grams in ethanol- and water-exposed pups respectively (t[10]=0.07, p=0.95), whereas brain weights averaged 0.08±0.002 and 0.09±0.002 grams in ethanol- and water-exposed pups, respectively (t[12]=0.07, p=0.95). To minimize disruption to maternal care of litters, litter weight or size was not determined on PD0. However, litter size was determined on PD7. Average litter size on PD7 averaged 5.8±0.5 and 6.7±0.6 for prenatal ethanol- and water-exposed litters, respectively, but this difference was not significant (t[20]=1.01, p=0.29). Average litter weights were also determined on PD7, and subsequently on PD14 and PD21. Two-way repeated measures ANOVA indicated that prenatal binge-like ethanol exposure reduced average litter weights (main effect of treatment, F(1,18)=5.8, p<0.05) and that average litter weights increased over time (main effect of day, F(2,36)=75.8, p<0.001). The interaction of these effects was not significant (p’s>0.05). Newman-Keuls post-hoc tests indicated that although average litter weights did not significantly differ on either PD7 or PD14 (p’s>0.05), prenatal ethanol-exposed litters exhibited significant reductions in average litter weight on PD21 (p<0.05; Table 2). No other obvious gross morphological effects of prenatal ethanol exposure using the DID paradigm were observed.
Table 2.
Effects of binge-like ethanol exposure in utero: Etimates of average pup weights across ontogeny.
|
Average pup weight at birth*
|
PD0
|
||
| prenatal ethanol-exposed | 1.2 ± 0.1 | ||
| prenatal water-exposed | 1.2 ± 0.1 | ||
|
Pre-weaning average litter weight**
|
PD7
|
PD14
|
PD21
|
| prenatal ethanol-exposed | 3.3 ± 0.2 | 6.1 ± 0.3 | 6.9 ± 0.3† |
| prenatal water-exposed | 3.6 ± 0.2 | 6.4 ± 0.2 | 7.6 ± 0.2 |
|
Average weight***
|
PD34‡
|
PD94
|
|
| Prenatal ethanol-exposed | |||
| male | 15.1 ± 0.5 | 26.2 ± 0.2 | |
| female | 11.9 ± 0.7 | 21.9 ± 0.5 | |
| Prenatal water-exposed | |||
| male | 17.1 ± 0.6 | 26.6 ± 0.3 | |
| female | 14.6 ± 0.7 | 22.3 ± 0.4 |
Weights are indicated in grams.
Average calculated from one pup per litter on PD0.
Average calculated by determining the mean weights for all pups in a litter on PD7, 14, and 21.
Average caclulated from one male and one female mouse per litter.
Significant at p<0.05.
Prenatal ethanol-exposed < prenatal water-exposed, p<0.001.
Mice were also weighed prior to assessment of ethanol’s hypnotic sensitivity during adolescence on PD34 and adulthood on PD94. Consistent with the overall reduced average litter weight observed prior to weaning on PD21, prenatal ethanol-exposed offspring exhibited reduced weights on PD34 as evidenced by a significant main effect of treatment (F(1,26)=14.2, p<0.001; Table 2). Whereas the main effect of sex was also significant (males > females, F(1,26)=21.2, p<0.001; Table 2), the interaction of sex and treatment was not (p>0.05). However, by PD94 ethanol-exposed offspring no longer exhibited reduced weight compared to controls (no main effect of treatment or the interaction of sex and treatment, p’s>0.05), although male offspring continued to weigh more than female offspring (significant main effect of sex, F(1,32)=128.7, p<0.001; Table 2).
Offspring behavior
Elevated Plus Maze Anxiety
On postnatal days 31 and 91 the percentage of time spent on the open arms of the elevated plus maze was assessed in the male and female offspring of ethanol- and water-exposed dams. Two-way ANOVA did not indicate any significant main effects or interactions (p’s>0.05). Thus, binge-like ethanol intake during pregnancy does not appear to alter anxiety-like behavior in adolescent offspring (Figure 2A). For adult offspring, two-way ANOVA indicated a significant main effect of sex (F(1,37)=4.6, p<0.05), but not prenatal treatment or interaction of these factors (p’s>0.05). Thus, although the results are similar to those for adolescent offspring suggesting that maternal binge-like ethanol intake during pregnancy does not alter anxiety-like behavior as assessed by the elevated plus maze, they indicate that adult female C57BL/6J mice are generally less anxious than their adult male counterparts (Figure 2B).
Figure 2.
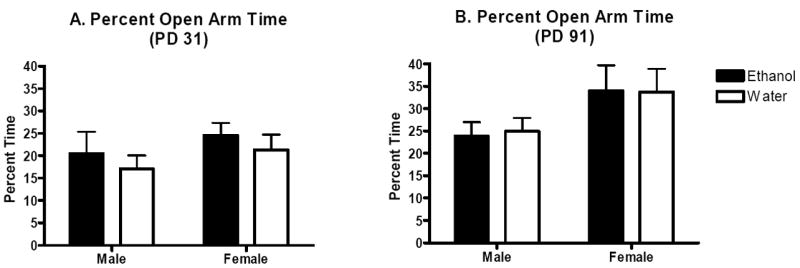
Elevated plus maze anxiety-like behavior in the adolescent and adult offspring of dams allowed daily binge-like access to ethanol or water over gestation. Mean (±SEM) percentage time spent on the open arm during a 5-min test. A. Male and female adolescent offspring (n’s=10 per group). B. Male and female adult offspring (n’s=8-13).
Rotarod Motor Performance
Separate three-way repeated measure ANOVAs were conducted for adolescent and adult offspring. The analysis for adolescent mice indicated a significant main effect of trial (F(5,190)=12.0, p<0.001), but no other main effects or interactions of these factors (p’s>0.05, Figures 3A and 3B). Thus, although performance improved over trials, the improvement did not depend upon sex or prenatal ethanol exposure. Similar results were seen when rotarod performance was analyzed for adult offspring. The three-way repeated measures ANOVA indicated a significant main effect of trial (F(5,190)=13.7, p<0.001), but not sex or treatment or interaction of these factors (p’s>0.05, Figures 3C and 3D). Thus, prenatal ethanol exposure using the DID paradigm does not appear to alter accelerating rotarod performance.
Figure 3.
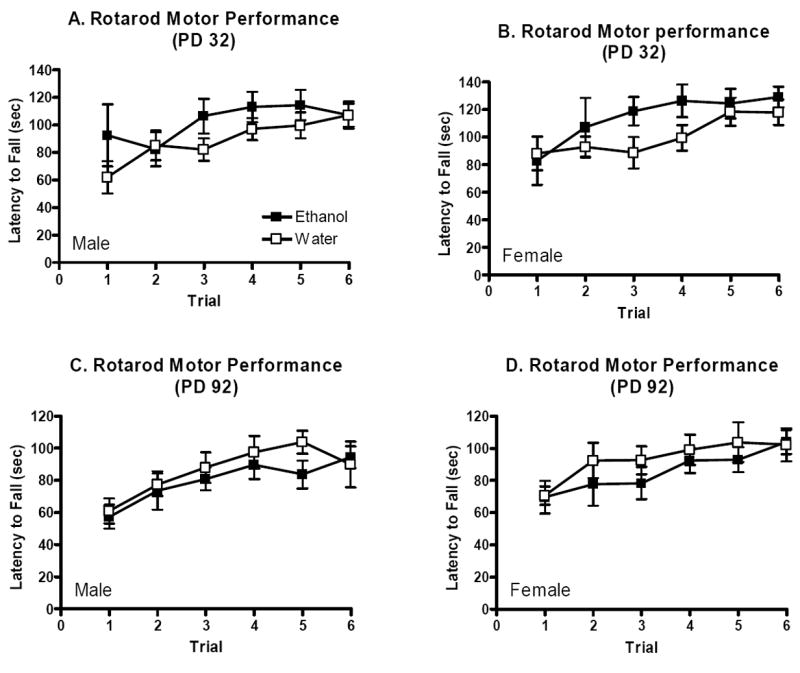
Accelerating rotarod motor performance in adolescent and adult offspring of dams allowed daily binge-like access to ethanol or water over gestation. Mean (±SEM) latencies to fall from the accelerating rotarod over 6 successive practice trials. A. Male adolescent offspring (n’s=10-11). B. Female adolescent offspring (n’s=10). C. Male adult offspring (n’s=10-13). D. Female adult offspring (n’s=8-11).
Locomotor Activity
The overall three-way repeated measures analysis for adolescent offspring indicated a marginally significant interaction of sex and treatment (F(1,27)=3.9, p=0.06), so subsequent two-way repeated measures analyses were performed for male and female adolescent offspring separately. In male adolescents, this analysis detected a main effect of bin for distance traveled (F(5,70)=7.0, p<0.001), but no other main effect or interaction (p’s>0.05, Figure 4A). However, in female adolescents, the two-way analysis revealed a significant main effect of treatment (F(1,13)=8.1, p<0.001), bin for distance traveled (F(5,65)=10.2, p<0.001), and a significant interaction of these factors (F(5,65)=2.8, p<0.05). Planned contrasts indicated that whereas adolescent female water-exposed controls exhibited a clear novelty-induced enhancement of locomotor activity upon placement in the chamber during the first 15 min of the test (bins 1, 2, and 3), female adolescents that had been exposed to binge-like ethanol in utero did not (p’s<0.05; Figure 4B).
Figure 4.
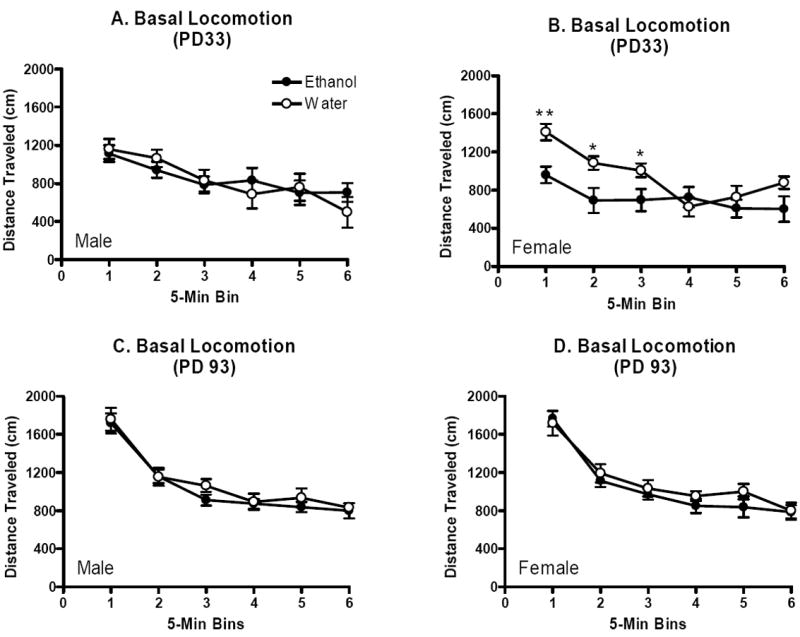
Basal locomotion in adolescent and adult offspring of dams allowed daily binge-like access to ethanol or water over gestation. Mean (±SEM) distance traveled in cm in 12 consecutive 5-min bins. A. Male adolescent offspring (n’s=8). B. Female adolescent offspring (n’s=7-8). C. Male adult offspring (n’s=10-13). D. Female adult offspring (n’s=9-11).
For the adult offspring the overall three-way analysis indicated a main effect of bin for distance traveled (F(5,195)=100.5, p<0.001), but not other main effects or interactions (p’s>0.05, Figure 4C and 4D). Thus, locomotor activity decreased to a similar degree over the 30 min test for both prenatal ethanol-exposed and control offspring.
Loss of righting reflex
Exposure to binge-like ethanol intake in utero resulted in reduced sensitivity to ethanol’s hypnotic actions (4 g/kg dose) in adolescent male and female offspring as indicated by shorter durations of ethanol-induced loss of the righting reflex. Two-way ANOVA with sex and treatment as factors indicated a significant main effect of treatment (F(1, 73)=4.1, p<0.05), but not sex or interaction of these factors (p’s>0.05, Figure 5A). However, this effect was gone by adulthood as the two-way analysis for adult offspring failed to detect any significant main effects or interaction (p’s>0.05, Figure 5C). There were no significant differences in blood ethanol concentration at regain of the righting reflex for either adolescent or adult offspring (p’s>0.05, Figure 5B and D).
Figure 5.
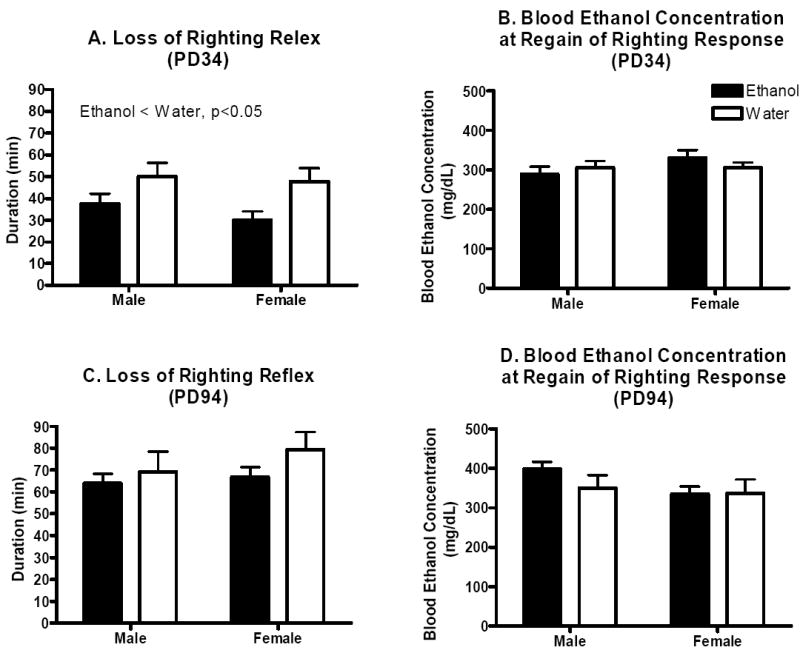
Hypnotic sensitivity to ethanol in the adolescent and adult offspring of dams allowed daily binge-like access to ethanol or water over gestation. A. Mean (±SEM) duration of loss of righting reflex in male and female adolescent offspring (n’s=8-10). B. Mean (±SEM) blood ethanol concentration at regain of the righting reflex in male and female adolescent offspring (n’s=7-9). C. Mean (±SEM) duration of loss of righting reflex in male and female adult offspring (n’s=8-13). D. Mean (±SEM) blood ethanol concentration at regain of the righting reflex in male and female adult offspring (n’s=6-11).
Discussion
Since the discovery that maternal ethanol consumption may be harmful to the developing embryo/fetus, a number of different animal models have been developed to investigate mechanisms by which the drug produces these effects (Cudd, 2005). However, the majority of these animal models involve procedures that may impart additional stress on the pregnant dam, possibly further compromising the health status of the offspring. In the present study we have utilized a new limited ethanol access mouse model to expose pregnant dams to ethanol. Called drinking in the dark or DID, naïve female C57BL/6J mice of mating age will consume stable intoxicating amounts of an unsweetened 20% ethanol solution during a two hour exposure period each day (Moore et al., 2007). The procedure is relatively simple, eliminating the need for lengthy access periods or the addition of sweeteners to promote ethanol intake, and bypasses the potential stress associated with other routs of maternal ethanol administration. Moreover, the DID procedure mimics the type of binge-like ethanol intake observed in many women of childbearing age. Indeed, approximately 28.5% of women in this age group reported frequent binges involving consumption of 5 or more drinks on a typical drinking day, and about 21.4% of the women reported consuming 45 or more drinks within a month (Tsai et al., 2007). Among pregnant women, whereas 59.1% reported having consumed alcohol over the past year, 19.6% reported having actually engaged in binge drinking during that time frame (Caetano et al., 2006). Thus, the DID model may also better approximate the type and pattern of ethanol intake characteristic of at least some pregnant women.
Pregnant dams given daily access to ethanol consumed 4.5-6.7 g/kg ethanol per day throughout gestation, producing estimated blood ethanol concentrations ranging from 124-180 mg/dl per day. This level of ethanol intake did not appear to substitute for calories derived from the mouse chow as ethanol-exposed dams did not differ from their water-exposed controls in food intake or maternal weight gain. In offspring, this level of intake did not appear to alter litter size as assessed on PD7. However, some caution is warranted as litter size was not assessed at birth. Although prenatal ethanol exposure did not alter offspring body or brain weights assessed on PD0, analysis of average litter weights on PD7, 14, and 21 indicated an overall reduction in the weights of pups prenatally exposed to ethanol. Ethanol-exposed pups continued to exhibit reduced weight compared to water-exposed controls on PD34, but this difference was no longer apparent by PD94. No other gross morphological deficits were observed in offspring.
Our finding that prenatal exposure to ethanol reduced the weight of offspring is consistent with previously published reports (Hannigan, 1995, Nathaniel et al., 1986). However, the deficits in weight we observed did not appear to emerge until at least weaning (PD21), and were gone by adulthood (PD94). A number of studies have also indicated that brain weights are reduced in the offspring of dams exposed to ethanol during gestation (Nathaniel et al., 1986). Although brain weights following prenatal exposure to binge-like ethanol intake did not differ at birth, it is unclear whether any such deficits might have emerged as the pups matured. Indeed, if brain weight parallels pup weight, one might expect to see a deficit in this parameter emerge toward weaning, extending through adolescence, and then declining by adulthood. Future work will examine this possibility.
Several studies have indicated that prenatal exposure to ethanol alters stress and/or anxiety measures in offspring. Rats prenatally exposed to ethanol exhibited greater anxiety-like behavior (reduced time on the open arms) as assessed by the elevated plus maze (Dursun et al., 2006). Moreover, prenatal ethanol exposure resulted in higher basal serum corticosterone levels in female mice (Allan et al., 2003), and testing on the elevated plus maze was associated with elevated serum corticosterone levels in prenatal ethanol exposed mice suggesting greater stress responsivity (Osborn et al., 1998). Indeed, maternal ethanol intake has been demonstrated to increase hypothalamic-pituitary-adrenal (HPA) axis activity in both the consuming mother and the offspring, alterations that may underlie the hyperresponsiveness to stressors (such as the elevated plus maze) seen in offspring (Glavas et al., 2007; Zhang et al., 2005).
We did not detect any change in anxiety-like behavior among prenatal ethanol-exposed mice as assessed by the elevated plus maze. However, there are several notable differences between our study and those listed above. For example, Osborn et al. (1998) administered ethanol via liquid diet, perhaps producing a more continuous level of ethanol in blood. Although Dursun et al. (2006) administered once daily 6 g/kg ethanol via intubation from gestational days 7-20 which should have better approximated the type of binge-like ethanol exposure in the present work, the ethanol doses in that study at times exceeded our daily estimated intakes for DID (4.5-6.7 g/kg) producing higher blood ethanol concentrations ranging from 334-349 mg/dl (versus our range of 124-180 mg/dl). We have not yet assessed serum corticosterone levels or any other measures of HPA tone in our mice. The current data nevertheless suggest that voluntary binge-like ethanol intake in pregnant C57BL/6J mice does not alter anxiety-like behavior in offspring as assessed by the elevated plus maze.
Prenatal ethanol exposure using DID procedures also did not alter motor coordination as assessed by the accelerating rotarod apparatus. Human studies of patients diagnosed with FASD have indicated relative deficits in balance and fine motor control compared to non-affected controls (Connor et al., 2006). The rat literature is consistent with these findings having shown that prenatal ethanol exposure alters patterned gait (Riley, 1990). Nevertheless, a recent report using rats is consistent with our mouse data indicating that accelerating rotarod motor performance is not altered by prenatal ethanol exposure (Dursun et al., 1996). Future work will continue to address whether prenatal ethanol exposure using the DID paradigm alters motor coordination by assessing patterned gait (Meyer et al., 1990; Hannigan and Riley, 1988).
When basal locomotion was assessed in our activity chambers, adolescent female mice that had been prenatally exposed to ethanol exhibited a blunted locomotor response during the first 15 min of the 60 min test; no such difference was observed in male adolescents, or in adult mice. This result stands in contrast to a number of studies that have demonstrated hyperlocomotion in rodents prenatally exposed to ethanol (Riley, 1990). However, not all studies have agreed. For example, the present results are consistent with at least two recent reports showing that prenatal ethanol exposure results in attenuated locomotor activity during the first 5 min of testing in an open field (Carneiro et al., 2005; Dursun et al., 2006). As both studies employed a once-daily maternal ethanol administration procedure, these results may indicate that prenatal exposure to binge-like ethanol intake blunts the normal elevated locomotion in response to a novel environment.
A number of studies have suggested that prenatal ethanol exposure reduces behavioral sensitivity to ethanol in rats (Abel et al., 1981; Fulginiti et al., 1989; Reyes et al., 1993). One of our goals of the present work was therefore to determine if binge-like ethanol exposure in utero alters ethanol behavioral phenotypes in C57BL/6J offspring. When hypnotic sensitivity to ethanol (4 g/kg) was assessed, we found a similar developmental pattern as described for offspring weight and locomotor behavior; whereas prenatal ethanol-exposed adolescent mice were significantly less sensitive to ethanol-induced loss of the righting reflex (exhibiting a shorter duration of loss of righting reflex compared to prenatal water-exposed controls), prenatal ethanol-exposed adult mice did not differ. Prenatal ethanol-exposed adolescent mice also exhibited near equal blood ethanol concentrations at regain of the righting response compared to water-exposed controls, perhaps indicating that they metabolize ethanol more rapidly. Nevertheless, these results are generally supportive of the previously published reports suggesting that prenatal ethanol exposure reduces behavioral sensitivity to ethanol in offspring and are particularly interesting in light of studies indicating that prenatal ethanol exposure increases propensity to self-administer ethanol (Chotro et al., 2007). It may be that reduced behavioral sensitivity to ethanol predisposes offspring to self-administer larger amounts of the drug, particularly during adolescence. Indeed, at least one study has suggested that in utero ethanol exposure may indeed be associated with early alcohol use problems prior to age 21 in human adolescents (Alati et al., 2006).
Several important caveats to interpretation of the present data warrant mentioning. First, presentation of ethanol or water using DID procedures was in some cases initiated prior to actual pregnancy. Ethanol or water access was always initiated upon detection of a vaginal plug. However, females did not always end up pregnant. In such cases daily breeding had to be re-started, leaving open the possibility that prior DID exposure, particularly to ethanol, may have influenced our results. Second, we attempted to control for the effects of continued maternal binge-like ethanol intake on care for offspring by weaning the dams from ethanol over a three day period. However, intakes were not significantly reduced by this approach, and we consequently cannot rule out the possibility that maternal care was impacted by the continued high binge-like ethanol intake on PD0-2, or its abrupt withdrawal on the days immediately following. It is, however, notable that we did not detect any differences in maternal care on PD7. Third, we did not randomize the order of tasks in our behavioral test battery and therefore cannot be certain that testing order did not influence our results. Our primary goal was to avoid the possible confound of prior ethanol injection on subsequent behavior, so the loss of righting reflex task was placed last in the test sequence. We were also concerned that previous testing might interfere with assessment of anxiety-like behavior using the elevated plus maze, so this assay was placed first in the test sequence. However, McIlwain et al. (2001) has demonstrated that prior behavioral testing may influence task performance, particularly when using the elevated plus maze or locomotor activity chambers. We therefore cannot rule out the possibility that the order of the tasks might have influenced the present results.
In the present study we have demonstrated that a new and relatively simple limited access mouse model of binge-like ethanol intake called drinking in the dark (DID) can be adapted to examine the prenatal effects of the drug on a number of behavioral outcomes in offspring. Prenatal exposure to ethanol using DID procedures avoids stress that may be associated with other routs of prenatal ethanol exposure, and may more precisely mimic the effects of maternal binge ethanol drinking on offspring. Similar to that seen in non-pregnant females, pregnant C57BL/6J mice will consume considerable amounts of daily ethanol throughout pregnancy. This level of ethanol intake produces physiologically relevant blood ethanol concentrations and quantifiable effects in offspring. Prenatal ethanol exposure using the DID paradigm alters offspring weight, reduces basal locomotion in females, and reduces sensitivity to ethanol’s hypnotic actions in both males and females. However, developmental stage appears to be important as all of these effects, while present during adolescence, disappear by adulthood. Although much work remains to fully characterize the effects of prenatal ethanol exposure using DID, it should prove useful in the continued study of mechanisms underlying ethanol’s deleterious effects in utero.
Acknowledgments
This work was supported in part by grants from NIAAA (AA015434) and the Center for Development and Behavioral Neuroscience at Binghamton University.
References
- Abel EL, Bush R, Dintcheff BA. Exposrue of rats to alcohol in utero alters drug sensitivity in adulthood. Science. 1981;212:1531–1533. doi: 10.1126/science.7233243. [DOI] [PubMed] [Google Scholar]
- Alati R, Al Mamun A, Williams GM, O’Callaghan M, Najman JM, Bor W. In utero alcohol exposure and prediction of alcohol disorders in early adulthood: a birth cohort study. Archives of General Psychiatry. 2006;63:1009–1016. doi: 10.1001/archpsyc.63.9.1009. [DOI] [PubMed] [Google Scholar]
- Allan AM, Chynoweth J, Tyler LA, Caldwell KK. A mouse model of prenatal ethanol exposure using a voluntary drinking paradigm. Alcoholism Clinical and Experimental Research. 2003;27:2009–2016. doi: 10.1097/01.ALC.0000100940.95053.72. [DOI] [PubMed] [Google Scholar]
- Bailey BN, Delaney-Black V, Covington CY, Ager J, Janisse J, Hannigan JH, Sokol RJ. Prenatal exposure to binge drinking and cognitive and behavioral outcomes at age 7 years. American Journal of Obstetrics and Gynecology. 2004;191:1037–1043. doi: 10.1016/j.ajog.2004.05.048. [DOI] [PubMed] [Google Scholar]
- Barr HM, Bookstein FL, O’Malley KD, Connor PD, Huggins JE, Streissguth AP. Binge drinking during pregnancy as a predictor of psychiatric disorders on the Structured Clinical Interview for DSM-IV in young adult offspring. American Journal of Psychiatry. 2006;163:1061–1065. doi: 10.1176/ajp.2006.163.6.1061. [DOI] [PubMed] [Google Scholar]
- Boehm SL, II, Lundahl KR, Caldwell J, Gilliam DM. Ethanol teratogenesis in the C57BL/6J, DBA/2J, and A/J inbred mouse strains. Alcohol. 1997;14:389–395. doi: 10.1016/s0741-8329(97)87950-5. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: incrased brain damage with binge exposure. Alcohol Clin Exp Res. 1990;14:107–118. doi: 10.1111/j.1530-0277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Carneiro LM, Diórgenes JP, Vasconcelos SM, Aragão GF, Noronha EC, Gomes PB, Viana GS. Behavioral and neurochemical effects on rat offspring after prenatal exposure to ethanol. Neurotoxicololgy and Teratology. 2005;27:585–592. doi: 10.1016/j.ntt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Caetano R, Ramisetty-Mikler S, Floyd LR, McGrath C. The epidemiology of drinking among women of child-bearing age. Alcohol Clin Exp Res. 2006;30:1023–1030. doi: 10.1111/j.1530-0277.2006.00116.x. [DOI] [PubMed] [Google Scholar]
- Chernoff GF. The fetal alcohol syndrome in mice: an animal model. Teratology. 1977;15:223–229. doi: 10.1002/tera.1420150303. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Arias C, Laviola G. Increased ethanol intake after prenatal ethanol exposure: studies with animals. Neuroscience and Biobehavioral Reviews. 2007;31:181–191. doi: 10.1016/j.neubiorev.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Connor PD, Sampson PD, Streissguth AP, Bookstein FL, Barr HM. Effects of prenatal alcohol exposure on fine motor coordination and balance: A study of two adult samples. Neuropsychologia. 2006;44:744–751. doi: 10.1016/j.neuropsychologia.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Cudd TA. Animal model systems for the study of alcohol teratology. Experimental Biology and Medicine. 2005;230:389–393. doi: 10.1177/15353702-0323006-06. [DOI] [PubMed] [Google Scholar]
- Dunty WC, Jr, Zucker RM, Sulik KK. Hindbrain and cranial nerve dysmorphogenesis result from acute maternal ethanol administration. Dev Neurosci. 2002;24:328–342. doi: 10.1159/000066748. [DOI] [PubMed] [Google Scholar]
- Dursun I, Jakubowska-Doğtru E, Uzbay T. Effects of prenatal exposure to alcohol on activity, motor coordination, and memory in young adult Wistar rats. Pharmacology Biochemistry and Behavior. 2006;85:345–355. doi: 10.1016/j.pbb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Fulginiti S, Artinian J, Cabrera R, Contreras P. Response to an ethanol challenge dose on sleep time and blood alcohol level in Wistar rats prenatally exposed to ethanol during gestational day 8. Alcohol. 1989;6:253–256. doi: 10.1016/0741-8329(89)90028-1. [DOI] [PubMed] [Google Scholar]
- Gilliam DM, Dudek BC, Riley EP. Responses to ethanol challenge in long- and short-sleep mice prenatally exposed to alcohol. Alcohol. 1990;7:1–5. doi: 10.1016/0741-8329(90)90051-d. [DOI] [PubMed] [Google Scholar]
- Gilliam DM, Kotch LE, Dudek BC, Riley EP. Ethanol teratogenesis in selectively bred long-sleep and short-sleep mice: a comparison to inbred C57BL/6J mice. Alcohol Clin Exp Res. 1989;13:667–672. doi: 10.1111/j.1530-0277.1989.tb00402.x. [DOI] [PubMed] [Google Scholar]
- Glavas MM, Ellis L, Yu WK, Weinberg J. Effects of prenatal ethanol exposure on basal limbic hypothalamic-pituitary-adrenal regulation: role of corticosterone. Alcohol Clin Exp Res. 2007;31:1598–1610. doi: 10.1111/j.1530-0277.2007.00460.x. [DOI] [PubMed] [Google Scholar]
- Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. The National Academies Press; 2003. [PubMed] [Google Scholar]
- Hannigan JH. Effects of prenatal exposure to alcohol plus caffeine in rats: Pregnancy outcome and early offspring development. Alcoholism: Clinical and Experimental Research. 1995;19:238–246. doi: 10.1111/j.1530-0277.1995.tb01498.x. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, Riley EP. Prenatal ethanol alters gait in rats. Alcohol. 1988;5:451–454. doi: 10.1016/0741-8329(88)90081-x. [DOI] [PubMed] [Google Scholar]
- Kelly JL, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behaviorl in humans and other species. Neurotoxicology and Teratology. 2000;22:143–149. doi: 10.1016/s0892-0362(99)00073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronick JB. Teratogenic effects of ethyl alcohol administered to pregnant mice. Am J Obstet Gynecol. 1976;124:676–680. doi: 10.1016/s0002-9378(16)33334-8. [DOI] [PubMed] [Google Scholar]
- Lupton C, Burd L, Harwood R. Cost of fetal alcohol spectrum disorders. Am J Med Genet C Semin Med Genet. 2004;127:42–50. doi: 10.1002/ajmg.c.30015. [DOI] [PubMed] [Google Scholar]
- Maier SE, West JR. Drinking patterns and alcohol-related birth defects. Alcohol Res Health. 2001;25:168–174. [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcoholism Clinical and Experimental Research. 1998;22:279–294. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R. The use of bhaivoral test batteries: Effects of training history. Physiol Behav. 2001;73:705–717. doi: 10.1016/s0031-9384(01)00528-5. [DOI] [PubMed] [Google Scholar]
- Meyer LS, Kotch LE, Riley EP. Alterations in gait following ethanol exposure during the brain growth spurt in rats. Alcohol Clin Exp Res. 1990;14:23–27. doi: 10.1111/j.1530-0277.1990.tb00440.x. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Randall CL, Favara JP. Prenatal ethanol exposure in C57 mice: effects on pregnancy and offspring development. Neurotoxicol Teratol. 1988;10:175–180. doi: 10.1016/0892-0362(88)90082-7. [DOI] [PubMed] [Google Scholar]
- Moore EM, Serio KM, Goldfarb KJ, Stepanovska S, Linsenbardt DN, Boehm SL., II GABAergic modulation of binge-like ethanol intake in C57BL/6J mice. Pharmacology, Biochemistry and Behavior. 2007;88:105–113. doi: 10.1016/j.pbb.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathaniel EJ, Nathaniel DR, Mohamed SA, Nahnybida L, Nathaniel L. Growth patterns of rat body, brain, and cerebellum in fetal alcohol syndrome. Experimental Neurology. 1986;93:610–620. doi: 10.1016/0014-4886(86)90180-9. [DOI] [PubMed] [Google Scholar]
- Osborn JA, Kim CK, Steiger J, Weinberg J. Prenatal ethanol exposure differentially alters behavior in males and females on the elevated plus maze. Alcoholism: Clinical and Experimental Research. 1998;22:685–696. [PubMed] [Google Scholar]
- Reyes E, Duran E, Switzer SH. Effects of in utero administration of alcohol on alcohol sensitivity in adult rats. Pharmacology Biochemistry and Behavior. 1993;44:307–312. doi: 10.1016/0091-3057(93)90466-7. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiology and Behavior. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu C-H, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain and Behavior. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Riley EP. The long-term behavioral effects of prenatal alcohol exposure in rats. Alcoholism: Clinical and Experimental Research. 1990;14:670–673. doi: 10.1111/j.1530-0277.1990.tb01225.x. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM., Jr Incidence of fetal alcohol sysdrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56:317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Tsai J, Floyd RL, Green PP, Boyle CA. Patterns of average volume of alcohol use among women of childbearing age. Maternal Child Health Journal. 2007;11:437–445. doi: 10.1007/s10995-007-0185-4. [DOI] [PubMed] [Google Scholar]
- Zhang X, Sliwowska JH, Weinberg J. Prenatal alcohol exposure and fetal programming: effects on neuroendocrine and immune function. Exp Biol Med. 2005;230:376–388. doi: 10.1177/15353702-0323006-05. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Powrozek T, Goodlett CR, Li TK. Moderate alcohol exposure compromises neural tube midline development in prenatal brain. Brain Res Dev Brain Res. 2003;144:43–55. doi: 10.1016/s0165-3806(03)00158-5. [DOI] [PubMed] [Google Scholar]


