Abstract
Objectives
We investigated the effects of an arginase inhibitor on bladder overactivity and measured bladder arginase I and II mRNA levels in rats with chronic spinal cord injury (SCI).
Methods
Awake cystometrograms were performed 3–4 weeks after spinal cord transection in female rats. Cystometric parameters such as mean amplitudes and number of non-voiding contractions (NVCs), voided volume, voiding efficiency, and micturition pressure were evaluated before and after intravenous (i.v.) injection of an arginase inhibitor (nor-NOHA: Nω-Hydroxy-nor-L-arginine) in SCI rats. The effects of a NOS inhibitor (L-NAME: Nω-Nitro-L-arginine methyl ester hydrochloride) were also examined to determine whether suppression of bladder overactivity by arginase inhibition is mediated by increased production of NO. In addition, mRNA levels of arginase I and II in SCI bladders were measured using quantitative real–time polymerase chain reaction (qRT-PCR).
Results
nor-NOHA (10 mg/kg, i.v.) significantly decreased the amplitude and number of NVCs. There were no significant changes in other parameters before and after administration of vehicle or nor-NOHA at any dose. When L-NAME (20 mg/kg, i.v.) was administered prior to nor-NOHA injection (10 mg/kg, i.v.), nor-NOHA–induced inhibition of NVCs was prevented. The relative levels of both arginase I and II mRNA in the bladder were significantly higher in SCI rats compared to spinal cord intact rats.
Conclusions
These results suggest that arginase inhibition can suppress SCI-induced bladder overactivity as indicated by a reduction in NVCs. Thus, arginase inhibition could be an effective treatment for neurogenic bladder overactivity in pathological conditions such as SCI.
Keywords: Arginase inhibitor, NO, Bladder overactivity, Spinal cord injury
INTRODUCTION
Nitric oxide (NO) has been recognized to play an important physiologic role during micturition and possibly in the pathophysiology of lower urinary tract dysfunctions. NO serves as a neurotransmitter and cell mediator with a broad range of effects in the lower urinary tract, including smooth muscle relaxation.1 It has been reported that NO in the bladder can inhibit bladder afferent activity to suppress bladder overactivity and nociceptive responses. NO donors applied intravesically suppressed bladder overactivity induced by cyclophosphamide,2 and the NO scavenger oxyhemoglobin induced bladder overactivity.3 It was also shown that NO can directly suppress N-type Ca2+ channels in bladder afferent neurons.4 Thus, enhancement of NO expression in the bladder could be beneficial to suppress bladder overactivity.
NO production can be controlled not only by activities of constitutive and inducible NO synthase (NOS) isozymes but also by activities of either of the two arginase isozymes.5–10 Notably, these results include demonstrations that inhibition of arginase activity can enhance NO-dependent relaxation of isolated smooth muscle tissue.6,7 However, little is known about the contribution of arginase activity to bladder function although it has recently been reported that activation of arginase pathways that increases polyamine biosynthesis contributes to muscle injury and remodeling of the rabbit bladder following ischemia.11 Therefore, we investigated the effects of an arginase inhibitor on bladder overactivity and measured bladder arginase I and II mRNA levels using quantitative real–time polymerase chain reaction (qRT-PCR) in rats with spinal cord injury (SCI). In addition, the effect of a NOS inhibitor was also examined to determine whether suppression of bladder overactivity by arginase inhibition is mediated by increased production of NO.
MATERIAL AND METHODS
Adult female Sprague-Dawley rats (250–300 gm) were used. All experiments were conducted in accordance with institutional guidelines and approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Drugs
Nω-Hydroxy-L-arginine dihydrochloride (nor-NOHA), an arginase inhibitor (Alexis Biochemicals, San Diego, CA), and Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME), a NOS inhibitor (Sigma Chemical Co., St. Louis, MI), were dissolved in distilled water (dH2O).
Spinal transaction
Spinal cord transection was performed between Th8 to Th9 with the rat under isoflurane anesthesia. After Th8 laminectomy the dura and spinal cord were cut with scissors and a sterile Gelform sponge was placed between the cut ends of the spinal cord. Postoperatively the animals were treated with ampicillin (50 mg/kg intramuscularly) for 7 days. The bladder of spinalized rats was manually emptied twice daily after spinalization until performing the experiments. Bladders were harvested from normal spinal cord intact rats as control and SCI rats 3–4 weeks after spinalization and then frozen in dry ice and stored at liquid nitrogen until further processing.
Cystometry
Three to four weeks after spinalization, cystometry in conscious rats was performed. Under isoflurane anesthesia, a PE-90 catheter was inserted via a midline abdominal incision into the bladder through the bladder dome. Intravenous (i.v.) injections were made through a cannula (PE-10) inserted into the right jugular vein. After surgery, rats were placed in a restraining cage and allowed to recover from anesthesia for 1 to 2 hours. The intravesical catheter was connected via a 3-way stopcock to a pressure transducer and a syringe pump for recording intravesical bladder pressure and infusing saline into the bladder, respectively. Saline at room temperature was infused at a rate of 0.08 ml per minute to elicit bladder contraction. Saline voided from the urethral meatus was collected and measured to determine voided volume (VV). After every bladder contraction, infusion was stopped and post-void residual volume (RV) was measured. Residual saline was withdrawn through the intravesical catheter by gravity, and then the bladder was confirmed to be emptied by palpation through the abdominal wall. Bladder capacity (BC) was calculated as BC= VV + RV. Voiding efficiency (VE) was calculated as VE = ((VV/BC) × 100)). Maximum voiding pressure and pressure threshold for voiding were also measured. The number and mean amplitudes of non-voiding contractions (NVCs) per voiding cycle were also measured during 4 min prior to micturition. NVCs were defined as rhythmic intravesical pressure increases greater than 7 cm water from baseline pressure without a release of fluid from the urethra. These cystometric parameters were evaluated before and after i.v. injection of nor-NOHA. nor-NOHA at doses of 1.0, 5.0, and 10.0 mg/kg was administered in a separate groups of SCI rats (n=6 each), and L-NAME (20 mg/kg, i.v.) after i.v. injection of nor-NOHA (10.0 mg/kg) was administered in 6 SCI rats. Vehicle (dH2O) was administered in 6 SCI rats as controls.
qRT-PCR of Arginase I and II mRNA
Measurement of Arginase I and II mRNA levels in the whole bladder was performed using 7 spinal intact rats and 7 SCI rats 3–4 weeks after spinalization. Total RNA was extracted from frozen tissues using TRIZOL (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. The concentration of the RNA was quantified by determination of optical density at 260 nm (OD 260). Reverse transcription (RT) was performed as previously described.12 The resulting cDNA was stored at −20°C until assayed by real-time PCR. Sequences of oligonucleotides used as primers for arginase I and arginase II mRNAs are Arginase I forward: GGCAATTGGAAGCATCTCTGG; Arginase I reverse: CTGTGATGTAGAGACCTTCTC; Arginase II forward: ACAGCCTGGCAATCGGTACC; Arginase II reverse: GACCTTCTGGATACCAAGTCG. Primers for mRNA of 18s rRNA, which was used as the internal control, were purchased from Qiagen (Valencia, CA). Real time PCR was performed using Mx3000P (Stratagene, La Jolla, CA) and carried out with QuantiTect SYBR Green PCR Master Mix (Qiagen). Each PCR amplification was performed in duplicate, using the following profile: one cycle at 95°C for 15 minutes, 35 cycles at 95°C for 1 minute, at 55°C for 1 minute, and at 72°C for 1 minute. Specificity of arginase I and II PCR products was confirmed by melting curve analysis. Standard curves constructed from serial dilution of cDNA in each tissue were analyzed with the Mx3000P Multiplex Quantitative PCR Systems (Stratagene). The quantitative values for arginase I and II were normalized to that for 18S rRNA obtained from the same samples.
Statistics
All data values are expressed as mean ± S.E. Statistical significance was determined with paired or Mann Whitney test for analysis of cystometry parameters and arginase mRNA, respectively. P-values less than 0.05 are considered to be significant.
RESULTS
Cystometry
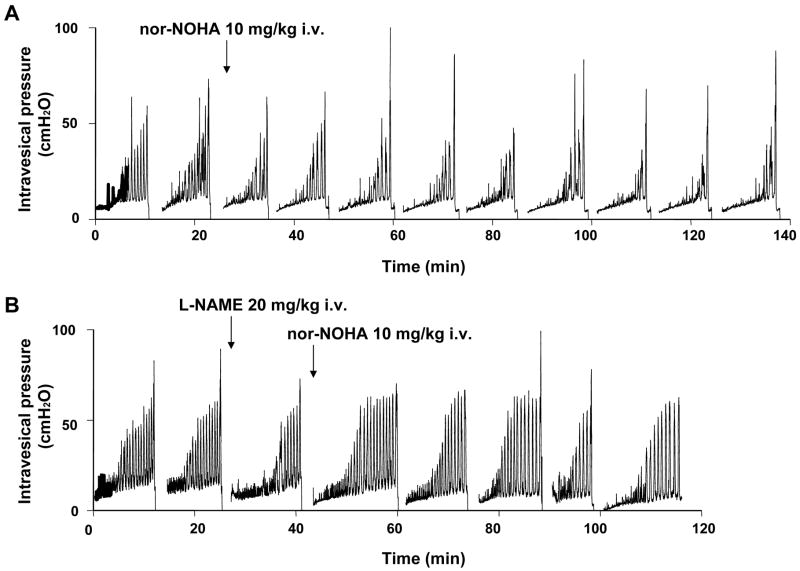
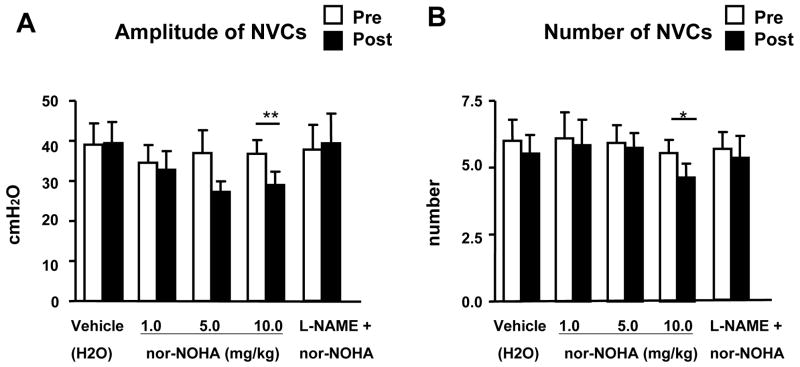
During awake cystometry, all spinalized rats showed NVCs before large amplitude voiding bladder contractions occurred (fig. 1). The amplitude of NVCs increased as the bladder was filled with infused saline. The mean amplitude and number of NVCs were 39.0 ± 5.3 cm H2O and 6.0 ± 0.8 per voiding cycle, respectively (table 1). The highest dose (10mg/kg, i.v.) of nor-NOHA significantly decreased the amplitudes of NVCs from 36.8 ± 3.4 cm H2O to 29.0 ± 3.3 cm H2O (P < 0.01, n=6) and number of NVCs from 5.6 ± 0.4 to 4.7 ± 0.5 (P < 0.05, n=6), whereas vehicle (n=6) or any lower dose of nor-NOHA (1.0 and 5.0 mg/kg, i.v., n=6 each) did not affect the amplitudes and number of NVCs (fig. 2). The inhibitory effects of nor-NOHA on NVCs were seen 20–30 min after the drug administration and lasted for 90–120 min, followed by partial recovery. There were no significant changes in other cystometric parameters before and after administration of vehicle or nor-NOHA at any dose (table 1, fig. 2). When L-NAME (20 mg/kg, i.v.) was injected one voiding-cycle before nor-NOHA (10 mg/kg, i.v.) application, nor-NOHA-induced reductions in the number and mean amplitude of NVCs were prevented (table 1, fig. 1, 2).
Figure 1.
Effects of i.v. administration of nor-NOHA (A) alone and nor-NOHA after i.v. injection of L-NAME (B) on NVCs in SCI rats. Arrows indicate the timing of drug administration.
Figure 2.
Amplitudes (A) and the number of NVCs (B) in SCI rats before (Pre) and after (Post) i.v. administration of vehicle (dH2O), nor-NOHA (1.0–10.0 mg/kg) alone, and nor-NOHA after i.v. injection of L-NAME. Note that the highest dose of nor-NOHA significantly suppressed the number and amplitude of NVCs. Each histogram represents mean ± S.E. **P <0.01, *P <0.05 compared with pre-drug values.
qRT-PCR
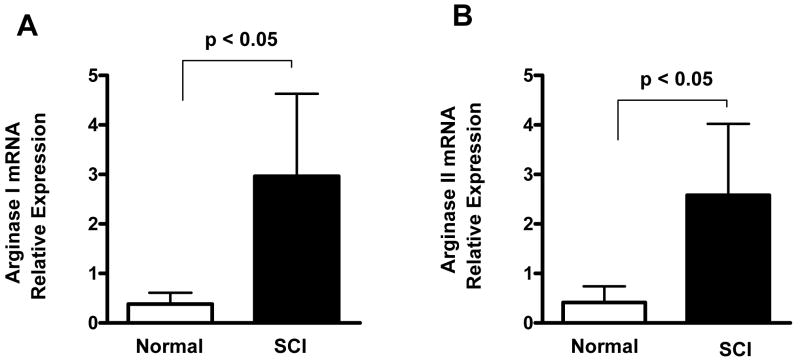
The relative levels of arginase I and II mRNAs in the rat bladder are shown in fig. 3. Levels of arginase I mRNA averaged 9.4-fold higher in SCI bladder than in spinal cord intact rats, and levels of arginase II mRNA averaged 4.2-fold higher in SCI bladder (p < 0.05 for both arginase mRNAs, n=7).
Figure 3.
Relative expression of arginase I (A) and arginase II (B) mRNAs in the bladder of normal rats (n=7) and SCI rats (n=7). Relative expression of arginase mRNAs was normalized against β-actin mRNA. Each bar represents mean ± S.E.
COMMENT
In the present study, inhibition of arginase suppressed bladder overactivity as evidenced by a reduction in the number and mean amplitude of NVCs in chronic SCI rats. In addition, because nor-NOHA–induced inhibition of NVCs was prevented by an NOS inhibitor, suppression of bladder overactivity by arginase inhibition seems to be mediated at least in part by increased production of NO. Furthermore, the levels of arginase I and II mRNAs in the bladder are significantly increased in chronic SCI rats compared with spinal cord intact rats.
Overactive voiding can develop days or weeks after spinal cord injury. Damage to the spinal cord above the sacral level results in bladder overactivity. This type of neurogenic bladder overactivity after spinal cord injury is mediated by the emergence of a capsaicin-sensitive C-fiber-mediated spinal micturition reflex due to C-fiber hyperexcitability and reorganization of synaptic connections in the spinal cord.13 In chronic SCI rats, Aδ bladder afferents trigger the voiding reflex, but C-fiber afferents appear to induce bladder overactivity (i.e., NVCs) during the filling phase. Increased NOS expression in bladder afferent pathways has been reported in rats with SCI.14 NO has only minimal relaxing effects on rat bladder smooth muscle,15 and it was suggested that exogenous NO depressed reflex bladder activity by suppressing the excitability and/or the release of transmitters from bladder afferent nerves.2 The present study showed that i.v. administration of an arginase inhibitor significantly suppressed the NVCs induced by hyperexcitable C-fiber afferents in chronic SCI rats in the awake state, and a NOS inhibitor prevented nor-NOHA–induced inhibition of NVCs. These results suggest that increased production of NO in the bladder induced by arginase inhibition can suppress bladder activity induced by spinal cord injury. In addition, because other cystometric parameters that are related to Aδ-fiber-dependent voiding function were not altered after arginase inhibition, it is likely that the effect of the arginase inhibitor was mediated by the action on C-fiber bladder afferent pathways, but not on Aδ-fiber afferent pathways.
NO has a dual effect, possibly dependent on concentration or different sites of action (i.e., central or peripheral), to suppress or amplify nociceptive mechanisms.16 Some studies indicated that NO might have a depressant effect on afferent activity. Reduced nitrite levels in the urine of patients with interstitial cystitis suggested that decreased NO release in the bladder might contribute to the disorder.16 Patients treated with L-arginine to increase the synthesis of NO exhibited some reduction in symptom, raising the possibility that NO might have an antinociceptive action.17,18 In rats, intravesical administration of an NO donor depressed bladder hyperactivity associated with cyclophosphamide- induced cystitis,2 and intravesical oxyhemoglobin, a NO scavenger, induced detrusor overactivity in normal rats,3 suggesting a local inhibitory effect of NO on bladder activity. It has also been reported that inhibition of the L-arginine/NO pathway by NOS inhibitors resulted in detrusor overactivity and decreases bladder capacity.19,20 By contrast, NO centrally released in the spinal cord can induce detrusor overactivity because cystitis-induced detrusor overactivity was reportedly suppressed by intrathecal injection with a NOS inhibitor in rats.21
Cellular production of NO absolutely depends on the availability of arginine, the nitrogenous substrate of NOS. A second major pathway of arginine metabolism is via the arginases, which catalyze the conversion of L-arginine to L-ornithine plus urea. L-arginine catabolism via arginase can act as an endogenous negative control system to regulate overall NO production.5–9,11 However, the arginases may be responsible not only for regulating NO synthesis by modulating intracellular arginine availability, but also for providing ornithine for the synthesis of polyamines controlling cell proliferation and differentiation.8,22 Consequently, increased polyamine synthesis via arginase activity may play a role in bladder hypertrophy, which is often an end stage situation in various pathological conditions such as benign prostatic hyperplasia and neurogenic bladder dysfunction including SCI. The present study found significant upregulation of arginase I and II mRNAs in SCI bladders. Therefore, arginine and arginine metabolites may also exert specific effects on the regulation of cell proliferation and hypertrophy; however, further studies are needed to clarify this point.
CONCLUSIONS
These results suggest that upregulation of arginase I and II is involved in the mechanism inducing bladder overactivity in chronic SCI rats and that arginase inhibition can suppress SCI-induced bladder overactivity as indicated by a reduction in NVCs. Thus, arginase inhibition could be an effective treatment for neurogenic bladder overactivity in pathological conditions such as spinal cord injury.
Acknowledgments
This work was supported by grants from NIH (DK057267, DK068557 and P01 DK044935).
References
- 1.Ho MH, Bhatia NN, Khorram O. Physiologic role of nitric oxide and nitric oxide synthase in female lower urinary tract. Curr Opin Obstet Gynecol. 2004;16:423–9. doi: 10.1097/00001703-200410000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Ozawa H, Chancellor MB, Jung SY, et al. Effect of intravesical nitric oxide therapy on cyclophosphamide-induced cystitis. J Urol. 1999;162:2211–6. doi: 10.1016/S0022-5347(05)68161-X. [DOI] [PubMed] [Google Scholar]
- 3.Pandita RK, Mizusawa H, Andersson KE. Intravesical oxyhemoglobin initiates bladder overactivity in conscious, normal rats. J Urol. 2000;164:545–50. [PubMed] [Google Scholar]
- 4.Yoshimura N, Seki S, de Groat WC. Nitric oxide modulates Ca(2+) channels in dorsal root ganglion neurons innervating rat urinary bladder. J Neurophysiol. 2001;86:304–11. doi: 10.1152/jn.2001.86.1.304. [DOI] [PubMed] [Google Scholar]
- 5.Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336(Pt 1):1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baggio R, Emig FA, Christianson DW, et al. Biochemical and functional profile of a newly developed potent and isozyme-selective arginase inhibitor. J Pharmacol Exp Ther. 1999;290:1409–16. [PubMed] [Google Scholar]
- 7.Kim NN, Cox JD, Baggio RF, et al. Probing erectile function: S-(2-boronoethyl)-L-cysteine binds to arginase as a transition state analogue and enhances smooth muscle relaxation in human penile corpus cavernosum. Biochemistry. 2001;40:2678–88. doi: 10.1021/bi002317h. [DOI] [PubMed] [Google Scholar]
- 8.Li H, Meininger CJ, Hawker JR, Jr, et al. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am J Physiol Endocrinol Metab. 2001;280:E75–82. doi: 10.1152/ajpendo.2001.280.1.E75. [DOI] [PubMed] [Google Scholar]
- 9.Lee J, Ryu H, Ferrante RJ, et al. Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox. Proc Natl Acad Sci U S A. 2003;100:4843–8. doi: 10.1073/pnas.0735876100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryoo S, Lemmon CA, Soucy KG, et al. Oxidized low-density lipoprotein-dependent endothelial arginase II activation contributes to impaired nitric oxide signaling. Circ Res. 2006;99:951–60. doi: 10.1161/01.RES.0000247034.24662.b4. [DOI] [PubMed] [Google Scholar]
- 11.Kawano K, Masuda H, Yano M, et al. Altered nitric oxide synthase, arginase and ornithine decarboxylase activities, and polyamine synthesis in response to ischemia of the rabbit detrusor. J Urol. 2006;176:387–93. doi: 10.1016/S0022-5347(06)00515-5. [DOI] [PubMed] [Google Scholar]
- 12.Afiatpour P, Latifpour J, Takahashi W, et al. Developmental changes in the functional, biochemical and molecular properties of rat bladder endothelin receptors. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:462–72. doi: 10.1007/s00210-003-0715-6. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimura N. Bladder afferent pathway and spinal cord injury: possible mechanisms inducing hyperreflexia of the urinary bladder. Prog Neurobiol. 1999;57:583–606. doi: 10.1016/s0301-0082(98)00070-7. [DOI] [PubMed] [Google Scholar]
- 14.Vizzard MA. Increased expression of neuronal nitric oxide synthase in bladder afferent and spinal neurons following spinal cord injury. Dev Neurosci. 1997;19:232–46. doi: 10.1159/000111212. [DOI] [PubMed] [Google Scholar]
- 15.Andersson KE, Persson K. Nitric oxide synthase and the lower urinary tract: possible implications for physiology and pathophysiology. Scand J Urol Nephrol Suppl. 1995;175:43–53. [PubMed] [Google Scholar]
- 16.Colasanti M, Suzuki H. The dual personality of NO. Trends Pharmacol Sci. 2000;21:249–52. doi: 10.1016/s0165-6147(00)01499-1. [DOI] [PubMed] [Google Scholar]
- 17.Smith SD, Wheeler MA, Foster HE, Jr, et al. Urinary nitric oxide synthase activity and cyclic GMP levels are decreased with interstitial cystitis and increased with urinary tract infections. J Urol. 1996;155:1432–5. [PubMed] [Google Scholar]
- 18.Smith SD, Wheeler MA, Foster HE, Jr, et al. Improvement in interstitial cystitis symptom scores during treatment with oral L-arginine. J Urol. 1997;158:703–8. doi: 10.1097/00005392-199709000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Persson K, Igawa Y, Mattiasson A, et al. Effects of inhibition of the L-arginine/nitric oxide pathway in the rat lower urinary tract in vivo and in vitro. Br J Pharmacol. 1992;107:178–84. doi: 10.1111/j.1476-5381.1992.tb14483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masuda H, Ogawa T, Kihara K, et al. Effects of anaesthesia on the nitrergic pathway during the micturition reflex in rats. BJU Int. 2007;100:175–80. doi: 10.1111/j.1464-410X.2007.06872.x. [DOI] [PubMed] [Google Scholar]
- 21.Kakizaki H, de Groat WC. Role of spinal nitric oxide in the facilitation of the micturition reflex by bladder irritation. J Urol. 1996;155:355–60. [PubMed] [Google Scholar]
- 22.Ignarro LJ, Buga GM, Wei LH, et al. Role of the arginine-nitric oxide pathway in the regulation of vascular smooth muscle cell proliferation. Proc Natl Acad Sci U S A. 2001;98:4202–8. doi: 10.1073/pnas.071054698. [DOI] [PMC free article] [PubMed] [Google Scholar]