Abstract
Valproate (VPA) treatment in pregnancy leads to congenital anomalies, possibly by disrupting folate or homocysteine metabolism. Since methylenetetrahydrofolate reductase (MTHFR) is a key enzyme of folate interconversion and homocysteine metabolism, we addressed the possibility that VPA might have different teratogenicity in Mthfr+/+ and Mthfr+/− mice and that VPA might interfere with folate metabolism through MTHFR modulation. Mthfr+/+ and Mthfr+/− pregnant mice were injected with VPA on gestational day 8.5; resorption rates and occurrence of neural tube defects (NTDs) were examined on gestational day 14.5. We also examined the effects of VPA on MTHFR expression in HepG2 cells and on MTHFR activity and homocysteine levels in mice. Mthfr+/+ mice had increased resorption rates (36%) after VPA treatment, compared to saline treatment (10%), whereas resorption rates were similar in Mthfr+/− mice with the 2 treatments (25–27%). NTDs were only observed in one group (VPA-treated Mthfr +/+). In HepG2 cells, VPA increased MTHFR promoter activity and MTHFR mRNA and protein (2.5- and 3.7-fold, respectively). Consistent with cellular MTHFR up-regulation by VPA, brain MTHFR enzyme activity was increased and plasma homocysteine was decreased in VPA-treated pregnant mice compared to saline-treated animals. These results underscore the importance of folate interconversion in VPA-induced teratogenicity, since VPA increases MTHFR expression and has lower teratogenic potential in MTHFR deficiency.
Keywords: folate, methylenetetrahydrofolate reductase, neural tube, congenital defects, valproic acid
Valproic acid (VPA), also known as valproate or 2-propylpentanoic acid, is a widely used drug for multiple conditions including epilepsy [Perucca, 2002], migraine headaches [Frediani, 2004] and bipolar disorders [Macritchie et al., 2003]. However, women who are administered anti-epileptic drugs such as VPA during pregnancy are at an increased risk for congenital anomalies in their offspring. Studies have suggested that the incidence of congenital malformations with anti-epileptic drug exposure is 6 – 7% compared to 2 – 3% in the general population [Dieterich et al., 1980; Claytonsmith and Donnai, 1995]. In particular, offspring of pregnant mothers administered VPA have 1–2% frequency of spina bifida [Lammer et al., 1987]. Thus VPA- induced malformations are of concern considering that neural tube defects (NTDs) in the general population occur at a rate of approximately 0.2–3.5 per 1000 births [Greene and Copp, 2005]. Although there are alternatives to VPA, it is still one of the most prescribed antiepileptic agents [Perucca, 2002], and it is therefore important to elucidate the deleterious effects caused by VPA so that they can be controlled or prevented.
Several mechanisms for VPA induction of congenital anomalies have been proposed. One study suggested that toxic metabolites of VPA were responsible for the fetal malformations caused by VPA treatment during pregnancy [Jurima-Romet et al., 1996]. This mechanism was supported by studies which demonstrated that co-administration of the antioxidant vitamin E with VPA decreased fetal malformations in mice compared to VPA treatment alone [Al Deeb et al., 2000]. More recent studies have identified a role for VPA in modulating histone deacetylase and activating transcription from diverse promoters [Chen et al., 1999; Gottlicher et al., 2001; Phiel et al., 2001; Arinze and Kawai, 2003; Menegola et al., 2005].
Work in mice [Wegner and Nau, 1992, Padmanabhan and Shafiullah, 2003] and clinical studies have suggested that VPA interferes with folate metabolism, since folate levels are decreased after administration of VPA [Karabiber et al., 2003]. In fact, rescue experiments in mice, involving folic acid supplementation in females, significantly reduced VPA-induced NTDs in their offspring [Padmanabhan and Shafiullah, 2003; Dawson et al., 2006]. Reduced folate can compromise DNA synthesis since N5,N10-methylenetetrahydrofolate (N5,N10-methyleneTHF) and N10-formyltetrahydrofolate (N10-formylTHF) are required for the synthesis of thymidine and purines, respectively. In addition, reduced folate levels may result in hyperhomocysteinemia, since N5-methyltetrahydrofolate (N5-methylTHF) is required for remethylation of homocysteine to methionine and S-adenosylmethionine. Homocysteine has been considered a mediator of the teratogenic potential of VPA, although there are conflicting reports on the association between VPA and disruption of homocysteine metabolism. Some studies have shown that the levels of homocysteine in humans taking VPA were significantly higher than those in controls [Verrotti et al., 2000; Karabiber et al., 2003], while other studies have shown a decrease in homocysteine [Apeland et al., 2000; Gidal et al., 2005]. Decreased intake of folate is a well-recognized risk factor for NTDs in the general population and the associated hyperhomocysteinemia in NTD families has also been reported [Steegers-Theunissen et al., 1994]. Although it is unclear whether hyperhomocysteinemia is itself teratogenic or whether it is simply a biomarker for disturbances in folate or methionine metabolism, mild maternal hyperhomocysteinemia has been considered to be a risk factor for NTDs [van der Put and Blom, 2000].
Methylenetetrahydrofolate reductase (MTHFR) affects the distribution of folate forms, since it reduces N5,N10-methyleneTHF to N5-methylTHF. MTHFR maintains the delicate balance between folate utilized for nucleotide synthesis and folate utilized for methionine synthesis, since its substrate is required for thymidine synthesis and its product is required for homocysteine remethylation to methionine (Figure 1). Mild MTHFR deficiency, due to homozygosity for a C→T substitution at bp 677, occurs in approximately 10% of many North American and European populations [Frosst et al., 1995]. The reduced activity of MTHFR results in hyperhomocysteinemia, reduced methylation and an increase in non-methylated folates [Rozen, 2005]. Mild MTHFR deficiency is associated with higher risk of NTDs, complications during pregnancy, and possibly other birth defects [Rozen, 2005]. Similar risk estimates have been obtained for the maternal 677TT genotype and for case genotype in association studies examining this variant and risk for NTDs [Vollset and Botto, 2005]. Reduced maternal erythrocyte folate concentration is a particularly strong risk factor for NTD (Amorim et al, 2007) but may act in conjunction with other risk factors; cellular processes involved in neurulation and possible roles for folate in this process have been reviewed in Sadler (2005).
Figure 1.

Schematic representation of the role of MTHFR in folate and homocysteine metabolism. TS = thymidylate synthase, MTR = methionine synthase, DHF = dihydrofolate, THF = tetrahydrofolate, SHMT = serine hydroxymethyltransferase. Pathway provided by Andrea Lawrance, McGill University.
In light of studies that suggest VPA may disturb folate metabolism, the interaction between VPA and MTHFR requires investigation. One small study on fetal anticonvulsant syndrome, consisting of 57 cases and 152 controls, suggested that mild MTHFR deficiency in the mothers might influence risk for the syndrome, but the women were taking several different anti-epileptic drugs and the risk for specific anomalies, such as NTDs, could not be examined in this small cohort [Dean et al., 1999]. A study on children taking VPA did not identify changes in folate or homocysteine that were related to MTHFR genotype [Vilaseca et al., 2000].
Our murine model of mild MTHFR deficiency, due to a single knockout allele of the Mthfr gene (Mthfr+/−), has been very useful in studying the biochemical and clinical consequences of the 677TT genotype in human populations [Chen et al., 2001]. Similarly to the human deficiency, the mice are hyperhomocysteinemic and have reduced DNA methylation [Chen et al., 2001], tissue-specific altered distribution of folate derivatives with increased non-methylfolates [Ghandour et al., 2004], and increased incidence of fetal loss, intrauterine growth retardation, and heart defects [Li et al., 2005; Leclerc and Rozen, 2005]. In this study, we used our animal model to investigate the influence of VPA on resorption rates and NTDs in MTHFR deficiency. We also examined the possibility that VPA might regulate MTHFR directly, by assessing MTHFR levels in HepG2 cells treated with VPA as well as in brain of treated mice.
MATERIALS AND METHODS
Animal studies
Animal experimentation was approved by the Montreal Children’s Hospital Animal Care Committee, according to the guidelines of the Canadian Council on Animal Care. At 4 weeks of age, Mthfr+/+ and Mthfr+/− female mice of a BALB/c background were placed on amino acid-defined diets (Harlan Teklad, Indianapolis) containing all the necessary components recommended by the American Institute of Nutrition, including the recommended amount of folic acid, 2mg/kg diet. Between 10 and 14 weeks of age, females were mated with Mthfr+/− males between 4 and 6 p.m. (11 p.m. of that night was designated as day 0). The next morning, females were inspected for vaginal plugs. At gestational day 8.5, pregnant mice were injected once intraperitoneally with either 300mg/kg VPA or a saline solution. At gestational day 14.5, the females were sacrificed and embryos were inspected for any apparent anomalies. Failure of anterior or posterior neural tube closure was designated as NTDs; however, only cases of exencephaly, failure of anterior neural tube closure, were observed in this study. Resorption rates were calculated as follows: (Number of implantation sites – number of viable embryos)/Number of implantation sites. Dead embryos were designated as an implantation site with a non-viable embryo which had not yet resorbed. Mthfr genotyping was performed for dams (liver sections and toes) and embryos (yolk sac) by previously-reported methods [Chen et al., 2001].
For determination of plasma homocysteine, blood was collected from pregnant female mice at gestational day 8.5, one hour after injection of 300 mg/kg VPA or a saline solution; these mice had been fasted overnight. Tubes containing EDTA were used for blood collection. Homocysteine and methionine levels were determined as described in Wang et al. (2005).
Generation of promoter constructs
In previous work, we had characterized the human and mouse genomic MTHFR structures and identified 2 clusters of transcription sites corresponding to 2 Mthfr promoters in the mouse genome, located within a 2.7 kb region 5′ to the first coding exon; these are referred to as the upstream and downstream promoters, respectively. These 2 promoters lead to the synthesis of 2 protein isoforms (77 and 70 kDa depending on splicing into the first exon) [Tran et al., 2002]. For functional analyses of the mouse promoters, we had created 11 promoter constructs, 6 from the upstream and 5 from the downstream promoter [Pickell et al., 2005]. For the present study, we generated sets of human promoter constructs based on orthologous regions of the afore-mentioned mouse promoter constructs (Figure 2).
Figure 2.
Schematic representation of upstream and downstream promoter deletion constructs and their activities in HepG2 cells. (A) Location of constructs in the 5 ′ UTR of the human MTHFR gene. The diagram at the top represents a segment of genomic DNA encompassing a portion of the MTHFR gene, upstream of MTHFR exon 2. D1 to D6 are donor splice sites and A1 to A3 are acceptor splice sites. Representative MTHFR cDNAs, that have the potential to encode the short or long MTHFR protein isoforms, are aligned under the complex genomic structure. Promoter deletion constructs hupA through hupF comprise the designated upstream promoter region and deletion constructs hdownA through hdownE comprise the designated downstream promoter region. The human promoter schematic is adapted from that of Tran et al. [2002] and the representation of human promoter deletion constructs is adapted from Pickell et al. [2005]. The relative numbers at the boundaries of the promoter segments (hupA-hupF and hdownA-hdownE) are derived from GenBank Accession no. AF398930, with the base A of the most upstream MTHFR translational start site as the +1 position. (B and C) Activity profile of upstream and downstream promoter deletion constructs in HepG2 cells. Activities for hupA and hdownA show a relative value of 1.0 since upstream promoter deletion constructs are normalized against promoter construct hupA activity whereas downstream promoter deletion constructs are normalized against construct hdownA. Each value represents the mean ± SEM of 3 experiments performed in duplicate.
*, p<0.05 compared to the preceding promoter construct.
A PAC clone encompassing the 5′ region of the MTHFR gene, as well as additional non-MTHFR sequences, was isolated. A specific 4.6 kb XbaI segment was subcloned into Bluescript. This plasmid (pX5) was used as a template for PCR-dependent generation of MTHFR promoter sequences. Primers used for amplification of the upstream promoter contained a KpnI restriction site in the forward primers and a BglII restriction site in the reverse primer. Primer sequences were as follows: hupAFOR, 5′-CAGGTACCGCAGGGTAGACGCTTCGAGAGC-3′; hupBFOR, 5′-ACGGTACCCCCCTGCACCCCGCCATCTT-3′; hupCFOR, 5′-TCGGTACCTGCCACTCTGGACCCCTCTAC-3′; hupDFOR, 5′-GGGGTACCGTCACATGACGATAAAGGCACG-3′; hupEFOR, 5′-GTGGTACCCGGGGCTTCCGGTCACCCG-3′; hupFFOR, 5′-CTGGTACCCAACCTGACACCTGCGCCGC-3′; hupREV, 5′-CCAGATCTTCCCTCCCGGCGACCCCGG-3′; hdownAFOR, 5′-GATTCTCGGCCACCTGGGCGC-3′; hdownBFOR, 5′-GCGATTCTCCTGCCTCAGCCTC-3′; hdownCFOR, 5′-CTGAACTTGGGTCTGGCTATTTTT-3′; hdownDFOR, 5′-CAGAGTGAGCTGTTCCTTCTCTG-3′; hdownEFOR, 5′-CTTCCTTTGTCGCAGCTCCGC-3′; hdoREV, 5′CTCTGTCAGCTCAGGCCCAGAG-3′. Primers hupAFOR, hupBFOR, hupCFOR, hupDFOR, hupEFOR and hupFFOR were paired with hupREV and primers hdownAFOR, hdownBFOR, hdownCFOR, hdownDFOR and hdownEFOR were paired up with hdoREV. High-specificity and high-fidelity PCR amplifications were performed in solutions containing 2X PCR buffer, 48 μM each dNTP, 1mM MgSO4, 20ng pX5 DNA template, 1X PCRx Enhancer Solution, 15 pmol of each relevant primer and 1.5 units Platinum Pfx DNA polymerase (Invitrogen). PCR program steps were as follows: 94°C 5 min; 94°C 15 sec, 68°C 2.5 min, repeat 30 cycles; 72°C 5 min.
Upstream promoter sequences were digested with KpnI and BglII enzymes, and cloned into the pGL3-Enhancer (Promega) vector. Ligation reactions were carried out overnight. Downstream promoters were subcloned into the PCR2.1 vector (Invitrogen), digested with KpnI and XhoI, and cloned into the pGL3-Enhancer vector. All plasmids were grown in DH5α E. coli cells (Invitrogen) and isolated using Plasmid Purification Midi Prep kits (Qiagen). All inserts and cloning junctions were verified by fluorescent-based sequencing on an ABI-310 PRISM Genetic Analyzer (Applied Biosystems) according to the manufacturer’s protocol.
Cell culture
HepG2 cells were grown in DMEM/F12 media with 10% FBS. SK-N-SH cells were grown in MEM/EBSS media with 10% FBS, 0.1mM non-essential amino acids and 1 mM sodium pyruvate.
For transfection of promoter constructs, 125 000 HepG2 cells/well were plated into 24-well plates and grown overnight. The next morning, HepG2 cells were transfected using Lipofectamine 2000 reagent (Invitrogen), according to the manufacturer’s protocol.
For measurement of endogenous levels of MTHFR transcripts and protein, 600 000 HepG2 cells/well and 300 000 SK-N-SH cells/well were plated into 6-well plates and grown overnight. The next morning, media was replaced with media supplemented with 1mM VPA. Cells were harvested 24 hours later.
Transfections and promoter assays
500ng of pGL3-Enhancer vector containing promoter sequences and 500ng β-gal vector were used for each transfection. Twenty hours after transfection, cells were lysed using Reporter Lysis Buffer (Promega) and assayed for luciferase activity with the Luciferase Activity Kit (Promega) and for β-gal activity with a β-Galactosidase Activity Kit (Applied Biosystems). Each experiment was performed three times in duplicate.
For promoter activity in cells exposed to VPA, media was replaced with 1mM VPA-supplemented media 4 hours after transfection. Twenty hours later, cells were lysed and assayed for β-gal and luciferase activities as above. VPA was found to have an effect on the activity of the CMV promoter of the pCMVβ-gal vector. This problem has been encountered in a previous study where VPA was shown to induce several promoters that regulated β-gal, rendering it inefficient as a normalizing agent for transfection efficiency [Arinze and Kawai, 2003]. Our smallest construct from the downstream promoter, hdownE, was only mildly stimulated by VPA. To overcome the VPA-mediated effect on our reporter vector, we constructed a novel β-gal vector, by first removing the CMV promoter from the pCMVβ-gal by digestion with restriction enzymes KpnI and BamHI. We then excised our hdownE promoter region from the pGL3-Enhancer vector with the same restriction enzymes and ligated our promoter with the β-gal coding sequence. A milder VPA-mediated effect (approximately 1.6 fold increase in activity) was still obtained with our new β-gal vector. Therefore, the effect of VPA was calculated by averaging the β-gal activity increase in the 12 wells treated with VPA and dividing it by the average of β-gal activity in the 12 wells treated with saline for each 24-well plate experiment; the β-gal-normalized luciferase activities following VPA treatment were multiplied by the resulting ratio. Each experiment was performed three times in duplicate.
RNA isolation and quantitative real time RT-PCR
RNA was isolated from HepG2 cells with the Trizol reagent (Invitrogen). DNAse treatment, reverse transcription and quantitative real time RT-PCR were carried out as previously reported [Mikael et al., 2006]. Primers for MTHFR amplification were MTHFRFOR, 5 ′-GAAGAACATCATGGCGCTGC-3 ′ and MTHFRREV, 5 ′-TTCGGATGTGCTTCACCAGG-3 ′. Oligonucleotides for GAPDH amplification were GAPDHFOR, 5 ′-CAATATGATTCCACCCATGGCAAA-3′ and GAPDHREV, 5 ′-GAAGATGGTGATGGGATTTC-3 ′. MTHFR real time PCR runs were carried out as follows: 50°C 2 min, 94°C 3 min, 94°C 1 min, 61°C 1 min, 72°C 1 min, repeat 39 cycles. GAPDH PCR runs were as follows: 50°C 2 min, 94°C 3 min, 94°C 30 sec, 60°C 30 sec, 72°C 30 sec, repeat 39 cycles. Experiments were performed three times.
Protein isolation and Western immunoblotting
Cell protein extracts were isolated and quantified as previously reported [Mikael et al., 2006]. Thirty and 60 μg of extracts from HepG2 and SK-N-SH cells, respectively, were run on 8 % SDS-polyacrylamide gels. Gels used to quantify actin or MTHFR were run for 1 hour at 160V or 16 hrs at 20V, respectively. Proteins were transferred to a nitrocellulose membrane at 56 V for 2 hrs at 4°C. The membrane was blocked overnight at 4°C in Tris-buffered saline/Tween buffer with 2% skim milk powder. The primary antibodies were rabbit anti-MTHFR (as previously reported [Frosst et al., 1995]) and rabbit anti-β-Actin (Sigma). The secondary antibody was a horseradish peroxidase-linked anti-rabbit IgG (Amersham Biosciences). Signal intensity was obtained and quantification was expressed as intensity of the MTHFR band/intensity of the actin band, as previously reported [Mikael et al., 2006]. Experiments were performed three times.
MTHFR enzyme assays
Cytosolic extracts were prepared and enzymatic assays were performed as in Tran et al (2002).
Statistical analyses
A non-parametric Kruskal-Wallis test was performed for resorption rates, NTDs and number of viable and dead embryos. Independent sample t-tests were used for luciferase data from promoter activity profiles and one sample t-tests were performed on promoter activity data with VPA exposure. Independent sample t-tests were carried out on quantitative RT-PCR data and on enzyme activity data. Two-factor ANOVA was performed to examine differences in homocysteine levels between genotype and treatment groups.
RESULTS
VPA and teratogenicity in Mthfr+/+ and Mthfr+/− dams
Four groups of dams (Mthfr+/+ and Mthfr+/−, injected with VPA or saline) were studied to determine if Mthfr-deficient mice responded differently from wild type mice to VPA injection. A minimum of 10 litters was examined in each group (Table 1). VPA-treated Mthfr+/+ dams had significantly increased resorption rates and more dead embryos compared to saline-treated Mthfr+/+ dams (p<0.05). These rates were higher than those in the VPA-treated Mthfr+/− group, but statistical significance was not achieved. Resorption rates in saline-treated Mthfr+/− dams were already quite high (27%) compared to the saline-treated Mthfr+/+ genotype group (10%; p<0.05), similar to the rates reported in our earlier study (27.9% vs 13.4%) [Li et al., 2005], and VPA did not affect this rate, nor did it affect the numbers of dead embryos in this genotype group. There did not appear to be any selective loss of genotypes, based on genotyping of live embryos from 4 or 5 litters from each group of dams (data not shown).
Table 1.
Effect of valproic acid on offspring of Mthfr-deficient (+/−) and normal (+/+) mice
| Genotype and treatment | Implantation Sites | Viable embryos¶ | Dead embryos* | % Resorption*¶ | % NTD*† | Total NTDs | Litters | Total live embryos | |
|---|---|---|---|---|---|---|---|---|---|
| Mthfr+/+ | 300mg/kg VPA | 8.5 ± 0.43 | 5.6 ± 0.72 | 1.0 ± 0.37 | 35.5 ± 6.8 | 21.1 ± 8.64 | 11 | 10 | 56 |
| Mthfr+/− | 300mg/kg VPA | 7.7 ± 0.44 | 5.7 ± 0.47 | 0.38 ± 0.18 | 25.2 ± 4.6 | 0 ± 0 | 0 | 13 | 74 |
| Mthfr+/+ | Saline | 8.8 ± 0.25 | 7.9 ± 0.35 | 0.00 ± 0 | 10.4 ± 2.6 | 0 ± 0 | 0 | 10 | 79 |
| Mthfr+/− | Saline | 7.9 ± 0.58 | 5.6 ± 0.62 | 0.09 ± 0.09 | 27.0 ± 6.7 | 0 ± 0 | 0 | 11 | 62 |
Implantation sites, viable embryos, dead embryos, % resorption and % NTD are presented as averages per litter ± SEM.
, p<0.05, significant difference between treatments in Mthfr+/+ dams
, p<0.05 , significant difference between genotypes treated with VPA
, p<0.05 , significant difference between genotypes treated with saline
A total of 271 viable embryos from 44 different litters were screened for NTDs in the 4 groups. There were no significant differences in embryonic weights or lengths between groups, based on measurements in 5 –7 litters from each group of dams (data not shown). Eleven NTDs were observed, all of which were characterized as exencephaly. All of these were from VPA-treated Mthfr +/+ dams; this amounts to an average of 21.1% NTDs per litter, which is significantly higher than that in the other 3 groups which had no NTDs. VPA did not elicit NTDs in offspring of Mthfr+/− mice and did not induce any other type of observable anomaly in this study. Of the 11 embryos with NTDs, 5 were Mthfr+/+ and 6 were Mthfr+/−. This almost equal distribution of NTDs in the 2 embryonic genotype groups is indicative of a maternal effect of VPA rather than an embryonic effect.
Expression profiles of MTHFR promoter deletion constructs in HepG2 cells
Eleven constructs for the 2 human MTHFR promoters (6 upstream, 5 downstream promoter constructs) were generated based on our previous constructs of the mouse promoters (Fig. 2a). There is 47% identity between human and mouse sequences covered by these constructs. The plasmids were transfected and assayed in HepG2 cells to identify key regions of MTHFR promoter activity. Upstream MTHFR promoter constructs (Fig. 2b) revealed several possible regulatory regions. A significant increase in activity was observed between the largest construct (hupA) and deleted construct hupB (p<0.05). Gradual and significant decreases were observed between deletion constructs hupC and hupD, hupD and hupE, and between hupE and hupF. Activity of the smallest deletion construct (hupF) was almost completely abolished.
Expression profiles from the downstream MTHFR promoter (Fig. 2c) also revealed several possible regulatory regions. An increase in activity, although not statistically significant, was observed between construct hdownA and deletion construct hdownB. A significant 5-fold decrease in activity was observed between deletion construct hdownD and the smallest construct hdownE. These observations provide a preliminary delineation of the characteristics of human MTHFR promoters.
Modulation of MTHFR promoters by VPA in HepG2 cells
MTHFR promoter activity in HepG2 cells exposed to VPA was assessed, in order to determine whether VPA could regulate MTHFR. Both upstream and downstream MTHFR promoter constructs exhibited increases in activity when cells were treated with VPA, compared to saline controls. VPA significantly (p<0.05) increased upstream promoter activity of 5 of the 6 constructs by approximately 2-fold; the smallest construct (hupF) did not show a significant increase in activity (Fig. 3a). The low activity of the hupF construct alludes to a possible VPA-regulated region in the 56 bp located between the 5 ′ boundaries of hupE and hupF.
Figure 3.

Effect of VPA on activity of (A) upstream promoter deletion constructs and (B) downstream promoter deletion constructs in HepG2 cells. Promoter activities in VPA-supplemented cells are normalized against the same construct activity in saline-supplemented cells. The promoter activities were corrected for the VPA effect on the normalizing β-gal vector as described in Materials and Methods. Each value represents the mean ± SEM of 3 experiments performed in duplicate.
*, p<0.05 compared to the saline control.
Downstream constructs hdownA, hdownB and hdownC showed significant (p<0.05) increases in activity (between 3- and 4-fold) in the presence of VPA compared to saline (Fig. 3b); the mean increase in construct hdownD was not statistically significant due to greater variability and construct hdownE appeared to be only mildly influenced by VPA. These findings point to a regulatory region sensitive to VPA treatment in the 195 bp deleted from hdownD.
Modulation of MTHFR mRNA and protein levels by VPA in HepG2 cells
To confirm that VPA regulated MTHFR expression, we assessed the levels of MTHFR mRNA and protein in HepG2 cells treated with VPA or saline. Quantitative real time RT-PCR demonstrated a significant (p<0.01) increase of MTHFR transcripts in VPA-treated cells compared to saline-treated cells; the mean increase was 2.45± 0.25 in 3 experiments (Figure 4a).
Figure 4.
Effect of VPA on MTHFR mRNA and protein levels. (A) Quantitative real time RT-PCR measurement of MTHFR transcripts in HepG2 cells treated with VPA or saline. RNA quantity was normalized through GAPDH expression. MTHFR transcript levels in VPA experiments were normalized against MTHFR transcript levels in saline controls. Value represents mean ± SEM for 3 experiments performed in duplicate. *, p<0.01 for comparison of VPA treatment with saline treatment. (B) Representative Western immunoblotting analysis of MTHFR in HepG2 and SK-N-SH cells treated with either VPA or saline. β-actin was used as a loading control. Lanes 1 and 2 are SK-N-SH cell extracts treated with saline and VPA, respectively; lanes 3 and 4 are HepG2 cells treated with saline and VPA, respectively. Lanes 5 and 6 are HepG2 cells untreated and treated with homocysteine, respectively, which are presented as reference bands for the phosphorylated and unphosphorylated MTHFR 70 kDa isoform.
Analysis of MTHFR immunoreactive protein by Western immunoblotting (Fig. 4b) indicated a 4-fold increase in MTHFR protein in HepG2 cells treated with VPA compared to saline-treated cells. A second cell line, neuronal SK-N-SH cells, was also treated with VPA to measure MTHFR protein following VPA treatment; this line showed a similar increase (3.7-fold) in MTHFR levels.
The shorter MTHFR isoform is post-translationally modified by phosphorylation [Yamada et al., 2005]. We previously reported that HepG2 cells express mainly the short (70kDa) phosphorylated MTHFR isoform [Mikael et al., 2006] , although there is a higher molecular weight isoform of 77kDa in some tissues (Tran et al, 2002). In both HepG2 and SK-N-SH cell lines, upregulation by VPA was observed for the phosphorylated and unphosphorylated 70kDa MTHFR isoform, although the signal for the latter protein was quite weak in SK-N-SH cells. For size standardization of the phosphorylated and unphosphorylated 70kDa MTHFR isoform in HepG2 cells, lanes 5 and 6 of Figure 4b show untreated and homocysteine-treated cells, respectively.
Plasma homocysteine and MTHFR activity in mice following VPA injection
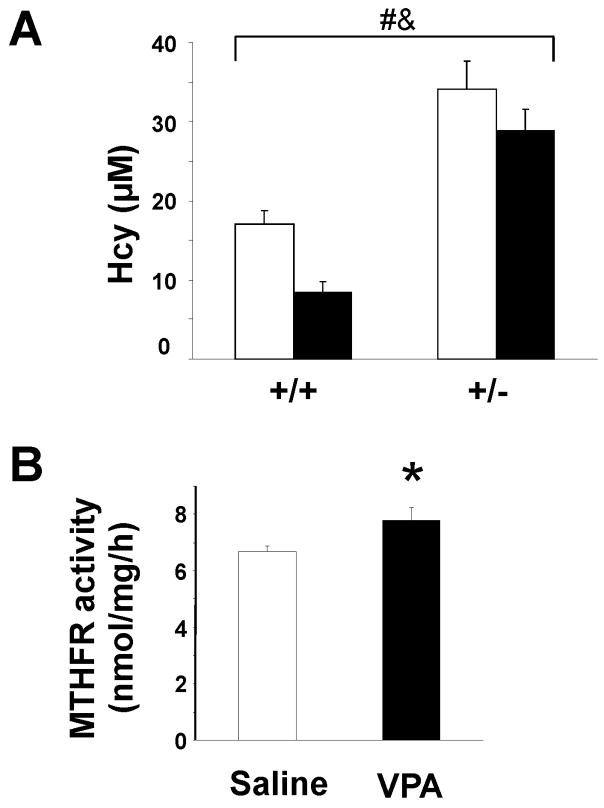
VPA increased MTHFR promoter activity, transcript levels and protein levels in cells. To determine whether VPA also regulated MTHFR expression in vivo, we measured MTHFR enzyme activity in brain extracts of pregnant mice one hour after VPA injection, since brain is a target tissue of this anticonvulsant. We also measured plasma homocysteine levels at this time point, since an increase in MTHFR expression could enhance the conversion of homocysteine to methionine through homocysteine remethylation by methionine synthase. As shown in Figure 5A, plasma homocysteine was significantly decreased in VPA-treated pregnant mice compared to saline-treated animals. Homocysteine levels were also significantly higher in Mthfr+/− mice compared to Mthfr+/+ (Figure 5A), as previously observed [Chen et al., 2001]. MTHFR enzymeactivity was increased in brain extracts (borderline significant; p=0.052) in VPA-treated Mthfr+/+ pregnant mice compared to saline-treated controls (Figure 5B; 7.8 ± 0.4 vs 6.7 ± 0.2 nmol/mg/h, respectively). These results are consistent with the in vitro increase in MTHFR expression and the decrease in plasma homocysteine in pregnant Mthfr+/+ mice.
Figure 5. Plasma homocysteine concentrations and MTHFR enzyme activity after VPA injection.
(A) Plasma homocysteine concentrations were determined for Mthfr+/+ and Mthfr+/−pregnant females injected with VPA (black bars) or saline (white bars). Values represent the mean ± SEM of 7 mice in each group. #,&, p<0.05 for comparison of genotype and treatment groups, respectively (two-factor ANOVA) (B) MTHFR activity in brain extracts of pregnant Mthfr+/+ mice injected with either VPA or saline. Values represent the mean ± SEM of 5 mice in each group. *, Treatment effect showed borderline significance (p=0.052) (independent sample t-test).
DISCUSSION
Our findings suggest that VPA elicits a different response in pregnant mice depending on MTHFR activity. VPA injections into Mthfr-deficient pregnant mice failed to elicit NTDs in their offspring whereas wild type mice had an average of 21.1% NTDs per litter. Injection of mice with saline did not result in NTDs in the offspring. Although mild MTHFR deficiency in humans due to the 677 variant is considered to be a risk factor for NTD, other genetic factors and maternal folate status also contribute to the risk for this multifactorial disorder. NTD in human populations occur with variable frequencies; 1 per 1000 births is frequently quoted. The lack of obvious NTD in offspring of saline-treated Mthfr-deficient mice may be due to the multifactorial nature of the disorder or to the small sample number.
VPA also significantly increased resorption rates and the number of dead embryos in wild type mice, compared to saline-injected mice. In contrast, VPA did not increase rates of resorption or dead embryos in Mthfr+/− mice. It is noteworthy that MTHFR deficiency in this study, as first demonstrated in our previous report [Li et al., 2005], was associated with increased resorptions (27% in this study, 27.9% in our previous study [Li et al., 2005]). Since this rate is quite high compared to that of Mthfr+/+ mice in this study (10.4%) or compared to that seen in BALB/c mice in other reports (12.2%) [Matalon et al., 2003], it is possible that VPA cannot induce an additional increase in resorptions when the rate is already quite high. The increased pregnancy loss associated with MTHFR deficiency has also been observed in clinical studies of the 677bp variant [Lissak et al., 1999]. The influence of MTHFR deficiency on fetal loss and congenital defects may relate to reduced homocysteine conversion to methionine and decreased methylation potential, or to a toxic effect of homocysteine [Rozen, 2005]. However, some experimental observations argue against homocysteine being a causative agent, since two independent studies have demonstrated that injection of homocysteine into pregnant mice or treatment of postimplantation rat embryo cultures with homocysteine failed to produce NTDs in the developing embryos (Vanaerts et al., 1994, Bennet et al., 2006).
Paradoxically, our study suggests that increased activity of MTHFR, following treatment with VPA, may also increase resorption rates, dead embryos and NTDs. These results highlight the importance of MTHFR in both folate interconversion and methionine metabolism, since we propose that the altered distribution of folates by increased MTHFR activity may contribute to VPA-induced teratogenicity. The substrate of MTHFR is also the substrate for the thymidylate synthase-catalyzed conversion of dUMP to dTMP, the rate-limiting step in DNA synthesis. N5,N10-methyleneTHF is also converted to N10-formylTHF which is required for purine synthesis. Studies of MTHFR-deficient mice as well as homozygous MTHFR 677TT human volunteers have demonstrated a decrease in methylated folate and an increase in non-methylated folate [Bagley and Selhub, 1998; Ghandour et al., 2004]. We hypothesize that a VPA-induced increase in MTHFR would drive the balance of N5,N10-methyleneTHF towards N5-methylTHF and the homocysteine remethylation cycle (Fig. 1). Decreased availability of N5,N10-methyleneTHF would cause decreased conversion of dUMP to dTMP which results in DNA instability via increased uracil misincorporation into DNA and chromosome strand breakage leading to apoptosis [Blount et al., 1997; Koury et al., 1997]. Decreased availability of thymidine for DNA synthesis would also result from this folate imbalance. Our hypothesis is supported by studies of NTDs in splotch mice, which have been reported to be deficient in thymidine synthesis. Thymidine or folic acid supplementation in this strain resulted in decreased NTD development [Fleming and Copp, 1998]. NTDs are associated with apoptosis in neuroepithelial tissue [Phelan et al., 1997]; p53 mutated splotch embryos did not exhibit NTDs suggesting that the NTDs may occur through increased apoptosis [Pani et al., 2002].
To further characterize the mechanism by which VPA and MTHFR interact, we first investigated the effect of VPA on MTHFR promoter activity. We therefore generated human MTHFR promoter constructs based on our studies of the murine promoters. Activity profiles of human promoter constructs in HepG2 cells identified several key regions which could potentially regulate MTHFR.
The largest upstream and downstream promoter constructs, hupA and hdownA, revealed 1.8- and 3.5- fold increases in activity in the presence of VPA. A similar increase was seen in 4 deletion constructs (hupB through hupE) of the upstream promoter and in 3 deletion constructs of the downstream promoter (hdownB through hdownD). The lowest increases in activity were seen with constructs hupF and hdownE, suggesting the presence of VPA-regulatable sites in regions 5 ′ to these constructs.
We verified that VPA increased MTHFR expression by demonstrating an increase in MTHFR mRNA and protein following VPA addition to HepG2 cells. A similar increase in MTHFR protein was observed following VPA treatment of a neuroblastoma line, SK-N-SH, indicating that the VPA effect is not restricted to liver cells.
We also obtained evidence for MTHFR activation by VPA in vivo. The increased MTHFR enzymatic activity in brain as well as the decreased plasma homocysteine in pregnant mice after VPA injection is consistent with the VPA-induced increase in MTHFR expression in vitro. Clinical studies have yielded conflicting results on homocysteine levels during VPA treatment [Apeland et al., 2000; Verrotti et al., 2000; Karabiber et al., 2003; Gidal et al., 2005]. A murine study reported that NTD occurrence is increased with co-administration of homocysteine and VPA compared to VPA treatment alone; however, these mice received injections of homocysteine which increased their plasma homocysteine to very high levels [Padmanabhan et al., 2006].
We also measured methionine levels in plasma of treated mice, but, as previously reported (Mudd et al, 1972), plasma methionine is not a good indicator of remethylation activity, since, even in patients with severe MTHFR deficiency, methionine levels can be in the normal range. In this study, methionine levels in plasma were not related to the observed changes in homocysteine levels (data not shown).
All NTD embryos in this study were observed in the VPA-treated Mthfr+/+ mice and these mice have lower plasma homocysteine levels than saline-treated mice. On the other hand, several clinical reports have reported that elevation of homocysteine is associated with increased frequency of NTD. We suggest that an alteration of the delicate balance between folate utilized for nucleotide synthesis and folate utilized for methionine synthesis can contribute to NTD. We hypothesize that higher levels of MTHFR, as observed upon treatment with VPA, alter the levels of folates required for nucleotide synthesis and increase risk for NTD. However, a lower level of MTHFR activity may also contribute to NTD risk through a disruption of homocysteine remethylation and decreased methylation potential.
We suggest that VPA administration to pregnant mothers increases MTHFR levels which inevitably results in decreased dUMP conversion to dTMP and leads to misincorporation of dUTP during DNA synthesis within the developing embryo, resulting in DNA damage. This might result in apoptosis of the embryological neuroepithelial tissue and in failure of neural tube closure. MTHFR upregulation would also decrease availability of nucleotides for DNA synthesis. We recently demonstrated that transgenic mice overexpressing MTHFR have altered distributions of folates, as expected given that MTHFR converts nonmethylated folates, used for thymidine and purine synthesis, to 5-methyltetrahydrofolate, used in homocysteine remethylation to methionine [Celtikci et al, 2008]. Therefore lower expression of Mthfr in the pregnant Mthfr+/− dams would protect the developing embryos against VPA-mediated NTDs through enhanced flux of folate toward dTMP synthesis. Supplementation of folic acid would rescue this phenotype by augmenting levels of N5,N10- methyleneTHF, as well as the levels of other critical folates involved in development. Thymidine supplementation in pregnant mothers taking VPA might also protect against NTDs by providing adequate levels of nucleotides for DNA synthesis during embryonic development. The results from this study suggest that pregnant women receiving VPA who are homozygous for the 677C→T mutation may be less likely to have children suffering from neural tube defects compared to those without the polymorphism. Large-scale clinical studies of MTHFR deficiency in pregnant women on VPA therapy are required to properly assess the pharmacogenetic effects of the 677C→T variant on embryonic development. In any event, folate supplementation prior to pregnancy is still deemed beneficial for healthy development of the embryo.
Acknowledgments
We thank Sébastien Dubé and Andrea Lawrance for advice with the statistical analyses.
Grant sponsor: Canadian Institutes of Health Research Grant number 43232 (R.R.); National Institute of Health HL057299, core grant CA06927, and an appropriation from the Commonwealth of Pennsylvania (W.D.K.); Studentship from the Montreal Children’s Hospital Research Institute (M.R.).
References
- Al Deeb S, Al Moutaery K, Arshaduddin M, Tariq M. Vitamin E decreases valproic acid induced neural tube defects in mice. Neurosci Lett. 2000;292:179–182. doi: 10.1016/s0304-3940(00)01457-9. [DOI] [PubMed] [Google Scholar]
- Amorim MR, Lima MA, Castilla EE, Orioli IM. Non-Latin European descent could be a requirement for association of NTDs and MTHFR variant 677C > T: A meta-analysis. Am J Med Genet. 2007 doi: 10.1002/ajmg.a.31812. (in press) [DOI] [PubMed] [Google Scholar]
- Apeland T, Mansoor MA, Strandjord RE, Kristensen O. Homocysteine concentrations and methionine loading in patients on antiepileptic drugs. Acta Neurol Scand. 2000;101:217–223. doi: 10.1034/j.1600-0404.2000.101004217x./. [DOI] [PubMed] [Google Scholar]
- Arinze IJ, Kawai Y. Sp family of transcription factors is involved in valproic acid-induced expression of G alpha(i2) J Biol Chem. 2003;278:17785–17791. doi: 10.1074/jbc.M209430200. [DOI] [PubMed] [Google Scholar]
- Bagley PJ, Selhub J. A common mutation in the methylenetetrahydrofolate reductase gene is associated with an accumulation of formylated tetrahydrofolates in red blood cells. Proc Natl Acad Sci USA. 1998;95:13217–13220. doi: 10.1073/pnas.95.22.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GD, Vanwaes J, Moser K, Chaudoin T, Starr L, Rosenquist TH. Failure of homocysteine to induce neural tube defects in a mouse model. Birth Defects Res B Dev Reprod Toxicol. 2006;77:89–94. doi: 10.1002/bdrb.20071. [DOI] [PubMed] [Google Scholar]
- Blount BC, Mack MM, Wehr CM, MacGregor JT, Hiatt RA, Wang G, Wickramasinghe SN, Everson RB, Ames BN. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: Implications for cancer and neuronal damage. Proc Natl Acad Sci USA. 1997;94:3290–3295. doi: 10.1073/pnas.94.7.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celtikci B, Leclerc D, Lawrance AK, Deng L, Friedman HC, Krupenko NI, Krupenko SA, Melnyk S, James SJ, Peterson AC, Rozen R. Altered expression of methylenetetrahydrofolate reductase modifies response to methotrexate in mice. Pharmacogenetics and Genomics. 2008 doi: 10.1097/FPC.0b013e32830058aa. in press. [DOI] [PubMed] [Google Scholar]
- Chen G, Yuan PX, Jiang YM, Huang LD, Manji HK. Valproate robustly enhances AP-1 mediated gene expression. Molec Brain Research. 1999;64:52–58. doi: 10.1016/s0169-328x(98)00303-9. [DOI] [PubMed] [Google Scholar]
- Chen ZT, Karaplis AC, Ackerman SL, Pogribny IP, Melnyk S, Lussier-Cacan S, Chen MF, Pai A, John SW, Smith RS, Bottiglieri T, Bagley P, Selhub J, Rudnicki MA, James SJ, Rozen R. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Human Molec Genet. 2001;10:433–443. doi: 10.1093/hmg/10.5.433. [DOI] [PubMed] [Google Scholar]
- Claytonsmith J, Donnai D. Fetal Valproate Syndrome. J Med Genet. 1995;32:724–727. doi: 10.1136/jmg.32.9.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson JE, Raymond AM, Winn LM. Folic acid and pantothenic acid protection against valproic acid-induced neural tube defects in CD-1 mice. Toxicol Applied Pharmacol. 2006;211:124–132. doi: 10.1016/j.taap.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Dean JCS, Moore SJ, Osborne A, Howe J, Turnpenny PD. Fetal anticonvulsant syndrome and mutation in the maternal MTHFR gene. Clin Genet. 1999;56:216–220. doi: 10.1034/j.1399-0004.1999.560306.x. [DOI] [PubMed] [Google Scholar]
- Dieterich E, Steveling A, Lukas A, Seyfeddinipur N, Spranger J. Congenital Anomalies in Children of Epileptic Mothers and Fathers. Neuropediatrics. 1980;11:274–283. doi: 10.1055/s-2008-1071396. [DOI] [PubMed] [Google Scholar]
- Fleming A, Copp AJ. Embryonic folate metabolism and mouse neural tube defects. Science. 1998;280:2107–2109. doi: 10.1126/science.280.5372.2107. [DOI] [PubMed] [Google Scholar]
- Frediani F. Anticonvulsant drugs in primary headaches prophylaxis. Neurol Sci. 2004;25(Suppl 3):S161–166. doi: 10.1007/s10072-004-0278-4. [DOI] [PubMed] [Google Scholar]
- Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP, Rozen R. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- Ghandour H, Chen Z, Selhub J, Rozen R. Mice deficient in methylenetetrahydrofolate reductase exhibit tissue-specific distribution of folates. J Nutr. 2004;134:2975–2978. doi: 10.1093/jn/134.11.2975. [DOI] [PubMed] [Google Scholar]
- Gidal BE, Tamura T, Hammer A, Vuong A. Blood homocysteine, folate and Vitamin B-12 concentrations in patients with epilepsy receiving lamotrigine or sodium valproate for initial monotherapy. Epilepsy Res. 2005;64:161–166. doi: 10.1016/j.eplepsyres.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG, Heinzel T. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20:6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene NDE, Copp AJ. Mouse models of neural tube defects: Investigating preventive mechanisms. Amer J Med Genet Part C-Seminars Med Genet. 2005;135C:31–41. doi: 10.1002/ajmg.c.30051. [DOI] [PubMed] [Google Scholar]
- Jurima-Romet M, Abbott FS, Tang W, Huang HS, Whitehouse LW. Cytotoxicity of unsaturated metabolites of valproic acid and protection by vitamins C and E in glutathione-depleted rat hepatocytes. Toxicology. 1996;112:69–85. doi: 10.1016/0300-483x(96)03352-5. [DOI] [PubMed] [Google Scholar]
- Karabiber H, Sommezgoz E, Ozerol E, Yakinci C, Otlu B, Yologlu S. Effects of valproate and carbamazepine on serum levels of homocysteine, vitamin B12, and folic acid. Brain & Development. 2003;25:113–115. doi: 10.1016/s0387-7604(02)00163-8. [DOI] [PubMed] [Google Scholar]
- Koury MJ, Horne DW, Brown ZA, Pietenpol JA, Blount BC, Ames BN, Hard R, Koury ST. Apoptosis of late-stage erythroblasts in megaloblastic anemia: Association with DNA damage and macrocyte production. Blood. 1997;89:4617–4623. [PubMed] [Google Scholar]
- Lammer EJ, Sever LE, Oakley GP. Teratogen Update - Valproic Acid. Teratology. 1987;35:465–473. doi: 10.1002/tera.1420350319. [DOI] [PubMed] [Google Scholar]
- Leclerc D, Rozen R. Molecular biology of methylenetetrahydrofolate reductase (MTHFR) and overview of mutations/polymorphisms. In: Ueland PM, Rozen RR, editors. MTHFR polymorphisms and disease. Georgetown: Landes Bioscience; 2005. pp. 1–20. [Google Scholar]
- Li DQ, Pickell L, Liu Y, Wu Q, Cohn JS, Rozen R. Maternal methylenetetrahydrofolate reductase deficiency and low dietary folate lead to adverse reproductive outcomes and congenital heart defects in mice. Amer J Clin Nutr. 2005;82:188–195. doi: 10.1093/ajcn.82.1.188. [DOI] [PubMed] [Google Scholar]
- Lissak A, Sharon A, Fruchter O, Kassel A, Sanderovitz J, Abramovici H. Polymorphism for mutation of cytosine to thymine at location 677 in the methylenetetrahydrofolate reductase gene is associated with recurrent early fetal loss. Amer J Obstetrics Gynecol. 1999;181:126–130. doi: 10.1016/s0002-9378(99)70447-3. [DOI] [PubMed] [Google Scholar]
- Macritchie K, Geddes JR, Scott J, Haslam D, de Lima M, Goodwin G. Valproate for acute mood episodes in bipolar disorder. Cochrane Database Syst Rev. 2003:CD004052. doi: 10.1002/14651858.CD004052. [DOI] [PubMed] [Google Scholar]
- Matalon ST, Blank M, Matsura E, Inagaki J, Nomizu M, Levi Y, Koike T, Shere Y, Ornoy A, Shoenfeld Y. Immunization of naïve mice with mouse laminin-1 affected pregnancy outcome in a mouse model. Am J Reprod Immunol. 2003;50:159–165. doi: 10.1034/j.1600-0897.2003.00068.x. [DOI] [PubMed] [Google Scholar]
- Menegola E, Di Renzo F, Broccia ML, Prudenziati M, Minucci S, Massa V, Giavini E. Inhibition of histone deacetylase activity on specific embryonic tissues as a new mechanism for teratogenicity. Birth Defects Res B Dev Reprod Toxicol. 2005;74:392–398. doi: 10.1002/bdrb.20053. [DOI] [PubMed] [Google Scholar]
- Mikael LG, Genest J, Rozen R. Elevated homocysteine reduces apolipoprotein A–I expression in hyperhomocysteinemic mice and in males with coronary artery disease. Circulation Res. 2006;98:564–571. doi: 10.1161/01.RES.0000204825.66410.0b. [DOI] [PubMed] [Google Scholar]
- Mudd SH, Uhlendorf BW, Freeman JM, Finkelstein JD, Shih VE. Homocystinuria associated with decreased methylenetetrahydrofolate reductase activity. Biochem Biophys Res Commun. 1972;46:905–912. doi: 10.1016/s0006-291x(72)80227-4. [DOI] [PubMed] [Google Scholar]
- Padmanabhan R, Shafiullah MM. Amelioration of sodium valproate-induced neural tube defects in mouse fetuses by maternal folic acid supplementation during gestation. Congenit Anom (Kyoto) 2003;43:29–40. doi: 10.1111/j.1741-4520.2003.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Padmanabhan R, Shafiullah M, Benedict S, Nagelkerke N. Effect of maternal exposure to homocystine on sodium valproate-induced neural tube defects in the mouse embryos. Eur J Nutr. 2006;45:311–319. doi: 10.1007/s00394-006-0600-4. [DOI] [PubMed] [Google Scholar]
- Pani L, Horal M, Loeken MR. Rescue of neural tube defects in Pax-3-deficient embryos by p53 loss of function: implications for Pax-3-dependent development and tumorigenesis. Genes Development. 2002;16:676–680. doi: 10.1101/gad.969302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucca E. Pharmacological and therapeutic properties of valproate: a summary after 35 years of clinical experience. CNS Drugs. 2002;16:695–714. doi: 10.2165/00023210-200216100-00004. [DOI] [PubMed] [Google Scholar]
- Phelan SA, Ito M, Loeken MR. Neural tube defects in embryos of diabetic mice: role of the Pax-3 gene and apoptosis. Diabetes. 1997;46:1189–1197. doi: 10.2337/diab.46.7.1189. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer and teratogen. J Biol Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- Pickell L, Tran P, Leclerc D, Hiscott J, Rozen R. Regulatory studies of murine methylenetetrahydrofolate reductase reveal two major promoters and NF-kappa B sensitivity. Biochim Biophys Acta. 2005;1731:104–114. doi: 10.1016/j.bbaexp.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Rozen R. Methylenetetrahydrofolate Reductase Gene Polymorphism - Clinical Implications. Encyclopedia of Medical Genomics and Proteomics. 2005 doi: 10.1081/E-EDGP-120030861. [DOI] [Google Scholar]
- Sadler TW. Embryology of neural tube development. Am J Med Genet C Semin Med Genet. 2005;135:2–8. doi: 10.1002/ajmg.c.30049. [DOI] [PubMed] [Google Scholar]
- Steegers-Theunissen RP, Boers GH, Trijbels FJ, Finkelstein JD, Blom HJ, Thomas CM, Borm GF, Wouters MG, Eskes TK. Maternal hyperhomocysteinemia: a risk factor for neural-tube defects? Metabolism. 1994;43:1475–1480. doi: 10.1016/0026-0495(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Tran P, Leclerc D, Chan M, Pai A, Hiou-Tim F, Wu Q, Goyette P, Artigas C, Milos R, Rozen R. Multiple transcription start sites and alternative splicing in the methylenetetrahydrofolate reductase gene result in two active enzyme isoforms. Mamm Genome. 2002;13:483–492. doi: 10.1007/s00335-002-2167-6. [DOI] [PubMed] [Google Scholar]
- van der Put NM, Blom HJ. Neural tube defects and a disturbed folate dependent homocysteine metabolism. Eur J Obstet Gynecol Reprod Biol. 2000;92:57–61. doi: 10.1016/s0301-2115(00)00426-7. [DOI] [PubMed] [Google Scholar]
- Vanaerts LA, Blom HJ, Deabreu RA, Trijbels FJ, Eskes TK, Copius Peereboom-Stegeman JH, Noordhoek J. Prevention of neural tube defects by and toxicity of L-homocysteine in cultured postimplantation rat embryos. Teratology. 1994;50:348–360. doi: 10.1002/tera.1420500506. [DOI] [PubMed] [Google Scholar]
- Verrotti A, Pascarella R, Trotta D, Giuva T, Morgese G, Chiarelli F. Hyperhomocysteinemia in children treated with sodium valproate and carbamazepine. Epilepsy Res. 2000;41:253–257. doi: 10.1016/s0920-1211(00)00150-9. [DOI] [PubMed] [Google Scholar]
- Vilaseca MA, Monros E, Artuch R, Colome C, Farre C, Valls C, Cardo E, Pineda M. Anti-epileptic drug treatment in children: hyperhomocysteinaemia, B-vitamins and the 677C-->T mutation of the methylenetetrahydrofolate reductase gene. Eur J Paediatr Neurol. 2000;4:269–277. doi: 10.1053/ejpn.2000.0379. [DOI] [PubMed] [Google Scholar]
- Vollset SE, Botto LD. Neural tube defects, other congenital malformations and single nucleotide polymorphisms in the 5,10 methylenetetrahydrofolate reductase (MTHFR) gene: a meta-analysis. In: Ueland PM, Rozen R, editors. MTHFR polymorphisms and disease. Georgetown: Landes Bioscience; 2005. pp. 125–143. [Google Scholar]
- Wang L, Chen X, Tang B, Hua X, Klein-Szanto A, Kruger WD. Expression of mutant human cystathionine beta-synthase rescues neonatal lethality but not homocystinuria in a mouse model. Hum Mol Genet. 2005;14:2201–2208. doi: 10.1093/hmg/ddi224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner C, Nau H. Alteration of embryonic folate metabolism by valproic acid during organogenesis: implications for mechanism of teratogenesis. Neurology. 1992;42(4 Suppl 5):17–24. [PubMed] [Google Scholar]
- Yamada K, Strahler JR, Andrews PC, Matthews RG. Regulation of human methylenetetrahydrofolate reductase by phosphorylation. Proc Natl Acad Sci USA. 2005;102:10454–10459. doi: 10.1073/pnas.0504786102. [DOI] [PMC free article] [PubMed] [Google Scholar]