Abstract
The tea polyphenol (−)-epigallocatechin-3-gallate (EGCG) has been reported to act as a cancer preventive agent through folate pathway inhibition in experimental studies. We hypothesized that if folate pathway inhibition is the mechanism of cancer preventive activities of EGCG, then the protective effect against breast cancer would be stronger among women with low dietary folate intake and the high-activity methylenetetrahydrofolate reductase (MTHFR) and thymidylate synthase (TYMS) genotypes. In a nested case–control study of 380 women with incident breast cancer and 662 controls within the Singapore Chinese Health Study, we found no association between either green tea intake or gene polymorphisms of MTHFR (C677T and A1298C) and TYMS (1494 ins/del) and breast cancer risk. However, among women with low folate intake (<133.4 μg/day), weekly/daily green tea intake was inversely associated with breast cancer risk compared with less green tea intake [odds ratio (OR) = 0.45, 95% confidence interval (CI) = 0.26–0.79, P for interaction = 0.02]. Among women with high folate intake (≥133.4 μg/day), green tea intake was not associated with breast cancer. Similarly, among women possessing the high-activity MTHFR/TYMS genotypes (0–1 variant allele), weekly/daily versus less frequent green tea intake was associated with lower breast cancer risk (OR = 0.66, 95% CI = 0.45–0.98), which was observed even more strongly among those who also had low folate intake (OR = 0.44, 95% CI = 0.22–0.89) than high folate intake (OR = 0.92, 95% CI = 0.55–1.54). This association was not observed among women possessing the low-activity genotypes (2–4 variant alleles). Our findings suggest that folate pathway inhibition may be one mechanism through which green tea protects against breast cancer in humans.
Introduction
Tea, brewed from dried leaves of the plant Camellia sinensis, is one of the most widely consumed beverages in the world. Although black tea is the most commonly consumed tea in Western countries, green tea is favored in Asian countries, especially in Japan and China. Green tea differs from other types of tea in the abundant presence of tea polyphenols, known as catechins and its major compound, (−)-epigallocatechin-3-gallate (EGCG), which have been reported to act as a cancer chemoprotective agent. Although the exact mechanism by which green tea polyphenols may protect against cancers in human is not completely understood, there have been extensive in vitro and animal studies of possible mechanisms (1–4). One suggested mechanism is the inhibition of dihydrofolate reductase by EGCG, which stimulates apoptosis and cell cycle arrest of cancer cells. In cell culture and animal models, EGCG has been shown to inhibit cell growth in a variety of cancers including skin, prostate, breast, lung and colon (5).
To date, the few epidemiologic studies have shown inconsistent results regarding the association between green tea intake and risk of breast cancer (6–12). Given inconsistent findings of epidemiologic studies, we were interested in evaluating whether genetic variation modifies the relationship between green tea intake and risk of breast cancer. In a case–control study with Asian-American women, green tea catechin protected against breast cancer among carriers of low-activity catechol-O-methyltransferase alleles but not among those who possessed high-activity catechol-O-methyltransferase alleles (13). We reported previously a low risk of breast cancer among women with higher green tea intake and the low-activity genotype of angiotensin-converting enzyme gene among Singapore Chinese women (14). In the present study, we were specifically interested in determining whether the association between green tea intake and risk of breast cancer differs among women in terms of genetic variation of the folate pathway enzymes: methylenetetrahydrofolate reductase (MTHFR) and thymidylate synthase (TYMS).
MTHFR catalyzes the irreversible conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, whereas TYMS uses the 5,10-methylenetetrahydrofolate as a methyl group donor for conversion of uracil to thymidine for DNA synthesis. Two common polymorphisms, C677T and A1298C, in the coding region of the MTHFR gene are associated with reduced enzyme activity of MTHFR in vitro (15–18). Compared with the wild-type 677 CC genotype, the activity level of MTHFR is ∼30 and 60% in the homozygous variant 677 TT and the heterozygous variant 677 CT genotypes, respectively (17). The A1298C polymorphism also decreases MTHFR activities especially accompanied with the C677T polymorphism (19). Similarly, one potentially functional polymorphism, a 6 bp deletion in the TYMS 3′-untranslated region, has been shown to decrease TYMS expression (20,21). We hypothesized that if methylation inhibition in the folate pathway is the mechanism for cancer preventive effects of EGCG, then the preventive effect of green tea would be stronger among women with high-activity MTHFR and TYMS compared with women with low activity of these enzymes. We also hypothesized that the effect of EGCG on breast cancer risk would be modified by dietary folate intake. In other words, women with fewer variant alleles in MTHFR and TYMS genes who drink green tea regularly with less folate intake would have the lowest risk of breast cancer.
Subjects and methods
Study subjects
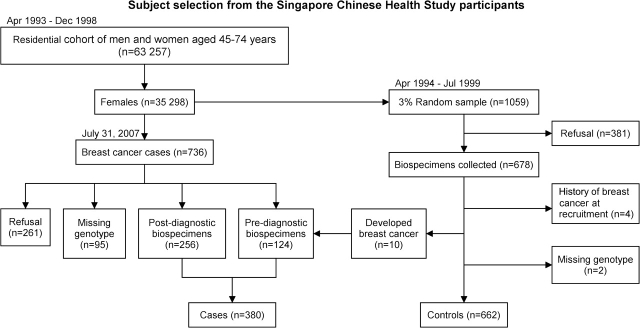
The subjects were selected from participants of the Singapore Chinese Health Study (SCHS), a population-based prospective cohort investigation to elucidate the role of diet and its interaction with genetic factors in risk of cancer. The study design and subject recruitment of the SCHS have been described elsewhere (22). Briefly, 63 257 Chinese women and men aged 45–74 years belonging to the Hokkien or Cantonese dialect group in Singapore were enrolled in the study between April 1993 and December 1998. At recruitment, information on lifestyle factors, usual diet and reproductive history was obtained through in-person interviews. The dietary component of the questionnaire was validated through a series of 24 h food recalls (22). Each subject was asked to estimate his or her usual intake frequencies and portion sizes for 165 food and beverage items during the past 12 months. The frequency of green tea intake was defined as nine categories: never or hardly ever, one to three times a month, once a week, two to three times a week, four to six times a week, once a day, two to three times a day, four to five times a day and six or more times a day. This study was approved by the Institutional Review Boards at the University of Minnesota, the University of Southern California and the National University of Singapore.
Between April 1994 and July 1999, we attempted to collect blood and single-void urine specimens from a random 3% sample of study enrollees (Figure 1). Details of the biospecimen collection, processing and storage procedures have been described previously (23). If the subject refused to donate blood, buccal cell samples were requested and collected if the subject consented. Out of 1059 female cohort participants contacted for biospecimen donation, blood (n = 514) or buccal cells (n = 164) were collected from 678 subjects, representing a participation rate of 64%.
Fig. 1.
Subject selection from the SCHS cohort.
The control group for the present study was comprised of this subcohort of women who were free of a history of breast cancer as of 31 July 2007. Six hundred and sixty-two subjects satisfied this criterion (4 had positive history at enrollment, 2 had missing genotype and 10 developed breast cancer during the follow-up). In January 2000, eligibility of the biospecimen subcohort was extended to all surviving cohort enrollees.
Post-diagnostic biospecimen samples were requested from all incident cases of female breast cancer starting from April 1994. Incident cases of breast cancer were identified through linkage with the population-based cancer registry in Singapore. As of 31 July 2007, 736 cases of incident breast cancer had been identified among the female cohort subjects via this linkage. Histological and staging information on all breast cancer diagnoses were confirmed by manual review of the pathology reports and clinical charts. The number of incident breast cancer cases within the cohort is comparable with the expected number of cases based on age- and sex-specific incidence rates of breast cancer for all Chinese in Singapore (24,25). Genotype information was determined for 380 (51.6%) incident cases of breast cancer with blood or buccal cell samples (124 pre-diagnostic and 256 post-diagnostic biospecimens). Compared with women with breast cancer who did not participate due to lack of biospecimens or genotype information, the study participants who were enrolled as cases were more likely to be of Hokkien (54.2 versus 44.5%, P < 0.01) and attained regular period at an earlier age (55.8 versus 43.4% at 14 years old or earlier, P = 0.02). The two breast cancer groups were otherwise similar.
Genotyping methods
For the genotype determination, blood and buccal cell samples were shipped on dry ice to the University of Southern California. Genomic DNA was purified from buffy coats of peripheral blood and buccal cell specimens using a PureGene Blood Kit (Gentra System, Minneapolis, MN) or a QIAamp 96 DNA Blood Kit (Qiagen, Valencia, CA). Genotyping assays were developed for the MTHFR C677T (rs1801133), MTHFR A1298C (rs1801131) and the TYMS3′-untranslated region polymorphism (1494 ins/del6) (rs16430). Genotype frequencies were determined using TaqMan assays as reported previously (26).
Statistical analysis
The t-tests (for continuous variables) or chi-square tests (for categorical variables) were used to compare the cases and controls in baseline characteristics and risk factors for breast cancer. Data were analyzed by standard methods for unmatched case–control studies (27). Unconditional logistic regression models were used to examine the associations between green tea intake frequency and risk of breast cancer and the possible modifying effect of MTHFR/TYMS gene polymorphism and folate intake on the tea–breast cancer association. The associations were estimated by odds ratios (ORs), 95% confidence intervals (CIs) and P values. The P values for trend were calculated based on the logistic regression model with ordinal value for the exposure variables.
Relevant demographic factors including age (continuous, years) at recruitment, year of recruitment (1993–1998), dialect group (Cantonese and Hokkien), level of education (no formal education, primary school, secondary school or higher), black tea intake (continuous, number of cups per month) and established risk factors for breast cancer including body mass index (continuous, kilogram per square meter), age when period became regular (<12, 13–14, 15–16, 17+ years or period never became regular) and number of live births (none, 1–2, 3–4 and 5+) were adjusted for in the analysis (23,28). Statistical analysis was carried out using the SAS software Version 9.1 (SAS Institute, Cary, NC). All reported P values were calculated by two sided and P < 0.05 was considered statistically significant.
Results
Table I shows the distributions of baseline demographic characteristics and potential risk factors between case and control subjects. Cases and controls were comparable with respect to age at enrollment, dialect group, body mass index and current smoking status. Similar to previous reports from the SCHS (14,23), the cases were more educated, attained regular period at an earlier age, had fewer numbers of live births and reached menopause later than the controls. As use of hormone replacement and family history of breast cancer are well-established risk factors of breast cancer, the proportion of hormone replacement use and family history of breast cancer were both slightly higher among cases than controls in the present study population, as expected. However, the differences did not reach statistical significance. It is probably because that breast cancer incidence and the rate of hormone replacement use are relatively low in Singapore. None of the green tea, black tea and coffee intake frequencies were different between cases and controls. The frequency of green tea intake only was not associated with the risk of breast cancer with or without adjustment for covariates (Table II).
Table I.
Demographic characteristics and risk factors in breast cancer cases and control subjects
| Cases (%), n = 380 | Controls (%), n = 662 | P | |
| Age (mean ± SD) | 55.8 ± 7.6 | 55.8 ± 8.0 | 0.99 |
| Dialect group | |||
| Cantonese | 55.5 | 50.0 | 0.09 |
| Hokkien | 44.5 | 50.0 | |
| Highest level of education | |||
| No formal education | 30.8 | 38.5 | 0.04 |
| Primary school | 41.1 | 37.5 | |
| Secondary school or higher | 28.1 | 24.0 | |
| Body mass index (kg/m2) | |||
| <20 | 11.1 | 14.2 | 0.19 |
| 20 to <24 | 56.3 | 55.9 | |
| 24 to <28 | 22.9 | 23.3 | |
| 28+ | 9.7 | 6.6 | |
| Age at period became regular | |||
| <13 | 14.7 | 13.6 | 0.05 |
| 13–14 | 41.0 | 36.0 | |
| 15–16 | 30.3 | 29.9 | |
| 17+/never became regular | 14.0 | 20.5 | |
| Number of live births | |||
| 0 | 12.1 | 7.2 | <0.01 |
| 1–2 | 35.5 | 28.0 | |
| 3–4 | 34.2 | 39.3 | |
| 5+ | 18.2 | 25.5 | |
| Age at menopausea | |||
| <50 | 28.7 | 36.1 | <0.01 |
| 50–54 | 53.0 | 54.8 | |
| 55+ | 18.3 | 9.1 | |
| Use of hormone replacement | |||
| Never | 92.4 | 94.3 | 0.45 |
| Former users | 1.3 | 1.2 | |
| Current users | 6.3 | 4.5 | |
| Family history of breast cancerb | |||
| No | 97.9 | 98.8 | 0.26 |
| Yes | 2.1 | 1.2 | |
| Cigarette smoking | |||
| Never smokers | 93.4 | 93.7 | 0.88 |
| Ever smokers | 6.6 | 6.3 | |
| Black tea intake | |||
| None or <weekly | 76.8 | 79.6 | 0.30 |
| ≥Weekly | 23.2 | 20.4 | |
| Coffee intake | |||
| <Daily | 28.7 | 28.0 | 0.80 |
| Daily | 71.3 | 72.0 | |
| Dietary folate intakec (μg/day) | |||
| <133.4 | 45.3 | 48.3 | 0.34 |
| ≥133.4 | 54.7 | 51.7 |
Including only subjects who were 55 years or older at recruitment (202 cases and 332 controls).
Family history of breast cancer among first-degree relatives.
Median dietary folate intake for all female participants in the SCHS = 133 μg/day.
Table II.
Green tea intake, MTHFR/TYMS genotype and risk of breast cancer
| Cases (n = 380) | Controls (n = 662) | Adjusted ORa (95% CI) | P | |
| Green tea intake | ||||
| None or <weekly | 279 | 467 | 1.00 | 0.41 |
| Weekly to <daily | 50 | 119 | 0.65 (0.45–0.94) | |
| Daily | 51 | 76 | 1.00 (0.82–1.22) | |
| MTHFR677 genotypes | ||||
| CC | 239 | 393 | 1.00 | 0.24 |
| CT | 120 | 226 | 0.87 (0.66–1.15) | |
| TT | 21 | 43 | 0.79 (0.45–1.38) | |
| MTHFR1298 genotypes | ||||
| AA | 225 | 387 | 1.00 | 0.54 |
| AC | 139 | 234 | 1.02 (0.77–1.34) | |
| CC | 16 | 41 | 0.70 (0.38–1.30) | |
| Sum of MTHFR variant alleles | ||||
| 0 | 119 | 182 | 1.00 | 0.27 |
| 1 | 226 | 416 | 0.83 (0.62–1.11) | |
| 2 | 35 | 64 | 0.84 (0.70–1.36) | |
| TYMS genotypes | ||||
| −6 bp/−6 bp | 193 | 328 | 1.00 | 0.54 |
| −6 bp/+6 bp | 157 | 273 | 0.98 (0.75–1.29) | |
| +6 bp/+6 bp | 30 | 61 | 0.83 (0.51–1.35) | |
| Sum of MTHFR and TYMS variant alleles | ||||
| 0–1 | 224 | 366 | 1.00 | 0.25 |
| 2–4 | 156 | 296 | 0.86 (0.66–1.12) |
Adjusted for age, year of enrollment, education, dialect, body mass index, age when period became regular, number of live births and black tea intake.
Controls were in Hardy–Weinberg equilibrium for both MTHFR C677T and A1298C (P = 0.16 and 0.48, respectively). The MTHFR C677T and A1298C sites were in strong linkage disequilibrium (r2 = 0.10, P < 0.01) among controls, as others have reported previously (29). These findings suggest a founder effect in which each alteration evolved on separate wild-type alleles. On the other hand, TYMS 1494 ins/del and MTHFR C677T or A1298C were not in linkage disequilibrium. Neither the MTHFR 677 variant genotypes that have high/low (CT) or low/low (TT) activity alleles nor the MTHFR1298 variant genotypes with high/low (AC) or low/low (CC) activity alleles were associated with the risk of breast cancer (Table II). Similarly, the TYMS gene polymorphism, i.e. 6bp ins/del, was not associated with the risk of breast cancer. The total number of variant alleles in MTHFR and TYMS genes was not associated with the risk of breast cancer. However, risk of breast cancer was statistically significantly lower among women who drank green tea weekly or more compared with non-green tea drinkers among women with 0 or 1 variant allele in MTHFR and TYMS genes after adjustment (OR = 0.66, 95% CI = 0.45–0.98). There were no associations between green tea intake and risk of breast cancer for any single genotype of MTHFR and TYMS (Table III).
Table III.
Green tea intake and risk of breast cancer by MTHFR/TYMS genotype
| Green tea intake |
Adjusted ORa (95% CI) | ||||
| None or <weekly |
≥Weekly |
||||
| Cases | Controls | Cases | Controls | ||
| MTHFR677 genotypes | |||||
| CC | 177 | 272 | 62 | 121 | 0.70 (0.48–1.03) |
| CT | 86 | 158 | 34 | 68 | 0.79 (0.47–1.33) |
| TT | 16 | 37 | 5 | 6 | 5.03 (0.64–39.47) |
| MTHFR1298 genotypes | |||||
| AA | 170 | 286 | 55 | 101 | 0.86 (0.58–1.28) |
| AC | 96 | 155 | 43 | 79 | 0.83 (0.51–1.36) |
| CC | 13 | 26 | 3 | 15 | 0.39 (0.07–2.23) |
| TYMS genotypes | |||||
| −6 bp/−6 bp | 144 | 227 | 49 | 101 | 0.72 (0.47–1.08) |
| −6 bp/+6 bp | 112 | 193 | 45 | 80 | 0.89 (0.56–1.40) |
| +6 bp/+6 bp | 23 | 47 | 7 | 14 | 0.49 (0.12–1.90) |
| Number of variant allelesb | |||||
| 0–1 | 172 | 256 | 52 | 110 | 0.66 (0.45–0.98) |
| 2–4 | 107 | 211 | 49 | 85 | 0.96 (0.61–1.50) |
OR for ≥weekly green tea intake group with none or <weekly green tea intake group as a reference group; adjusted for age, year of enrollment, education, dialect, body mass index, age when period became regular, number of live births and black tea intake.
Total number of variant alleles in MTHFR677, MTHFR1298 and TYMS.
Since our hypothesized mechanism for cancer preventive activity of EGCG involves folate metabolism, we considered that dietary folate intake might modify the association between green tea intake and risk of breast cancer. Overall, the average daily dietary folate intake among the study population was 149.2 μg/day (range = 31.2–590.2 μg/day), which is significantly lower than the recommended daily allowance both in Singapore (200 μg/day) and the USA (400 μg/day). Dietary folate intake was not statistically significantly different between cases and controls (Table I). However, among all female participants in the SCHS, dietary folate intake was ∼30% higher among women who drank green tea weekly or daily (mean ± SD = 174.6 ± 75.2 μg/day) than non-drinkers (135.2 ± 62.7 μg/day). All subjects were categorized as high (≥133.4 μg/day) or low (<133.4 μg/day) folate consumers with a cut point at the median folate intake for all female participants in the SCHS. Among women whose dietary folate intake was low, risk of breast cancer was statistically significantly lower in women who drank green tea weekly or more (OR = 0.45, 95% CI = 0.26–0.79) than those with less frequent green tea intake (Table IV). This association was not observed among women with high folate intake. The inverse association between green tea intake and risk of breast cancer among women with high-activity alleles was even stronger among those who also had low dietary folate intake (OR = 0.44, 95% CI = 0.22–0.89, P for interaction = 0.02) (Table IV). This inverse association between green tea intake and risk of breast cancer was not observed among women with high folate intake.
Table IV.
Green tea intake, combined MTHFR/TYMS genotype and risk of breast cancer by folate intake
| Green tea intake | Low folate intakea |
High folate intakea |
||||
| Cases | Controls | Adjusted ORb (95% CI) | Cases | Controls | Adjusted ORb (95% CI) | |
| None or <weekly | 152 | 252 | 1.00 | 127 | 215 | 1.00 |
| ≥Weekly | 20 | 68 | 0.45 (0.26–0.79) | 81 | 127 | 1.04 (0.72–1.50) |
| 0–1 variant alleles | ||||||
| None or <weekly | 100 | 134 | 1.00 | 72 | 122 | 1.00 |
| ≥Weekly | 13 | 39 | 0.44 (0.22–0.89) | 39 | 71 | 0.92 (0.55–1.54) |
| 2–4 variant alleles | ||||||
| None or <weekly | 52 | 118 | 1.00 | 55 | 93 | 1.00 |
| ≥Weekly | 7 | 29 | 0.50 (0.19–1.30) | 42 | 56 | 1.18 (0.67–2.07) |
Low and high folate intake categories use the cutoff point at 133.4 μg/day (median of all female participants in the cohort).
Adjusted for age, year of enrollment, education, dialect, body mass index, age when period became regular, number of live births and black tea intake.
Discussion
The present study suggests a possible protective effect of green tea against breast cancer among women with high-activity genotypes of the MTHFR and TYMS genes. This effect was even stronger among those who were low consumers of dietary folate. To our knowledge, this is the first epidemiologic study that examines a gene–environment interaction between MTHFR and TYMS gene polymorphisms and green tea intake on its potential preventive activity against breast cancer development. The findings of the present study support the hypothesis that green tea polyphenols may influence the folate pathway and that genetic polymorphisms of enzymes in the folate pathway may modify the tea–breast cancer association.
EGCG inhibits dihydrofolate reductase, which catalyzes the conversion of dihydrofolate to tetrahydrofolate in the folate pathway. The folate pathway is vital for nucleotide synthesis, especially for rapidly replicating cells such as cancer cells. In fact, cancer chemopreventive agents such as antifolates induce apoptosis and thus affect the steady cell population. The mechanism of anticarcinogenic activity of EGCG was recently found to be similar to antifolate chemotherapeutic agents in vivo (30).
Epidemiologic studies have not shown consistent results regarding the association between tea intake and breast cancer risk. Majority of studies in the USA or Europe, where black tea is preferred to green tea, have reported no association between tea intake and breast cancer risk, probably because of the extremely small amount of tea polyphenol intake (31–34). Although a few studies have evaluated the association in an Asian population, where green tea consumption is relatively high, study findings have again been inconsistent (6–8,14). One possible explanation is that gene–nutrient interaction may modify the anticarcinogenic activity of green tea polyphenols in humans. Numerous studies have investigated the effect of MTHFR gene polymorphisms on risk of breast cancer; however, results have been discordant (35–46). In the present study, the risk of breast cancer was not associated with MTHFR and TYMS genotypes.
Considering MTHFR as a regulating enzyme in the folate pathway, the other possible explanation for inconsistency among previous study results is the nutrient–nutrient interaction between folate and tea polyphenols. We found a lower risk of breast cancer associated with regular green tea intake only among women with low folate intake. Furthermore, the inverse association between risk of breast cancer and regular green tea intake was the strongest among women possessing high-activity MTHFR and TYMS genotypes whose dietary folate intake was low. Our results correspond with a limited number of previous studies that examined the interaction among folate intake, genotype and breast cancer risk (47–49). Folate depletion increases the sensitivity of cancer cell lines to EGCG in vitro (30). Thus, EGCG does not have to compete with folate and can effectively inhibit dihydrofolate reductase. Findings from the present study lend support to the hypothesis that green tea polyphenols may have anticarcinogenic properties through competing with folate in the folate pathway.
The present study has several strengths. Singapore is a small city-state where all citizens have good access to specialized medical care. The nation-wide cancer registry has been available since 1968 and has been shown to be comprehensive in its recording of cancer cases (24). Thus, the ascertainment of incidence cases of breast cancer can be assumed to be comprehensive. Secondly, our study population is Asian with a relatively high frequency of green tea intake compared with the USA or European study cohorts. Consequently, we were able to collect a relatively large number of green tea drinkers for cases and controls. Thirdly, green tea intake was assessed prior to breast cancer diagnosis and therefore can be presumed to be free of recall bias. Furthermore, average folate intake among the current study population was significantly lower compared with the average daily dietary folate intake for adult women in the USA (300–350 μg/day) (50), which offered the opportunity to evaluate antifolate effects of EGCG in relative absence of opposition from folate.
The major limitation of the present study is lack of detailed data on green tea intake. Although green tea drinking is more popular in Singapore compared with Western countries, Singapore, having been a major port for foreign trade, has developed the unique mixture of Asian and Western cultures, which has also had impacts on beverage drinking habits. As shown in Table II, the number of daily green tea drinkers was relatively small in the present study population. Therefore, daily green tea drinkers were combined into the category of weekly or more frequently drinkers in the final analyses. Our findings require confirmation in other large cohorts with high exposures to green tea. Moreover, the EGCG content in green tea varies depending on how it is prepared; in the current study, information on the methods used to brew the tea (infusion time and strength) was unavailable. In addition, we assessed tea intake only at a single time point (baseline). However, non-differential misclassification of exposure status tends to minimize the underlying relative risk toward the null. It is unlikely that such exposure misclassification leads to a spurious association between exposure and disease risk. Next, biospecimens for genotype determination were donated by only 64% of controls and 51.6% of cases. However, refusal to donate biospecimens occurred before genotypes were determined; therefore, it is unlikely that genotypes could affect subjects’ decisions to donate their biospecimens. Thus, it is unlikely that refusal to donate biospecimens led to selection bias regarding genotype. Lastly, our result that dietary folate intake was significantly higher among women with breast cancer who drank green tea more frequently suggests that green tea drinking habits may be associated with a healthier lifestyle. If so, there might be some unmeasured factors that is reflected by green tea intake and decreased breast cancer risk.
In summary, the present study provides supportive epidemiologic evidence to the previously suggested hypothesis that EGCG stimulates apoptosis and cell cycle arrest through inhibition of the folate cycle. Genetic polymorphisms in MTHFR and TYMS were found to modify the preventive activities of green tea polyphenols against breast cancer. Our findings were demonstrated in a population with low average folate intake. These results need to be confirmed by future studies with large sample sizes, considering folate intake as a potential effect modifier in the interaction among tea polyphenols, MTHFR/TYMS genotypes and breast cancer risk.
Funding
National Cancer Institute, Bethesda, MD (R01-CA55069, R35-CA53890 and R01-CA80205).
Acknowledgments
We thank Siew-Hong Low of the National University of Singapore for supervising the fieldwork of the SCHS and Kazuko Arakawa for the development and management of the cohort study database. We also thank the Ministry of Health in Singapore for assistance with the identification of cancer cases via database linkages.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- CI
confidence interval
- EGCG
(−)-epigallocatechin-3-gallate
- MTHFR
methylenetetrahydrofolate reductase
- OR
odds ratio
- SCHS
Singapore Chinese Health Study
- TYMS
thymidylate synthase
References
- 1.Yang CS, et al. Possible mechanisms of the cancer-preventive activities of green tea. Mol. Nutr. Food Res. 2006;50:170–175. doi: 10.1002/mnfr.200500105. [DOI] [PubMed] [Google Scholar]
- 2.Mukhtar H, et al. Tea polyphenols: prevention of cancer and optimizing health. Am. J. Clin. Nutr. 2000;71:1698S–1702S. doi: 10.1093/ajcn/71.6.1698S. discussion 1703S–1704S. [DOI] [PubMed] [Google Scholar]
- 3.Lambert JD, et al. Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am. J. Clin. Nutr. 2005;81:284S–291S. doi: 10.1093/ajcn/81.1.284S. [DOI] [PubMed] [Google Scholar]
- 4.Stuart EC, et al. Role of epigallocatechin gallate (EGCG) in the treatment of breast and prostate cancer. Life Sci. 2006;79:2329–2336. doi: 10.1016/j.lfs.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 5.Mukhtar H, et al. Green tea in chemoprevention of cancer. Toxicol. Sci. 1999;52:111–117. doi: 10.1093/toxsci/52.2.111. [DOI] [PubMed] [Google Scholar]
- 6.Wu AH, et al. Green tea and risk of breast cancer in Asian Americans. Int. J. Cancer. 2003;106:574–579. doi: 10.1002/ijc.11259. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki Y, et al. Green tea and the risk of breast cancer: pooled analysis of two prospective studies in Japan. Br. J. Cancer. 2004;90:1361–1363. doi: 10.1038/sj.bjc.6601652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagano J, et al. A prospective study of green tea consumption and cancer incidence, Hiroshima and Nagasaki (Japan) Cancer Causes Control. 2001;12:501–508. doi: 10.1023/a:1011297326696. [DOI] [PubMed] [Google Scholar]
- 9.Sun CL, et al. Green tea, black tea and breast cancer risk: a meta-analysis of epidemiological studies. Carcinogenesis. 2006;27:1310–1315. doi: 10.1093/carcin/bgi276. [DOI] [PubMed] [Google Scholar]
- 10.Nakachi K, et al. Influence of drinking green tea on breast cancer malignancy among Japanese patients. Jpn. J. Cancer Res. 1998;89:254–261. doi: 10.1111/j.1349-7006.1998.tb00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue M, et al. Regular consumption of green tea and the risk of breast cancer recurrence: follow-up study from the Hospital-based Epidemiologic Research Program at Aichi Cancer Center (HERPACC), Japan. Cancer Lett. 2001;167:175–182. doi: 10.1016/s0304-3835(01)00486-4. [DOI] [PubMed] [Google Scholar]
- 12.Seely D, et al. The effects of green tea consumption on incidence of breast cancer and recurrence of breast cancer: a systematic review and meta-analysis. Integr. Cancer Ther. 2005;4:144–155. doi: 10.1177/1534735405276420. [DOI] [PubMed] [Google Scholar]
- 13.Wu AH, et al. Tea intake, COMT genotype, and breast cancer in Asian-American women. Cancer Res. 2003;63:7526–7529. [PubMed] [Google Scholar]
- 14.Yuan JM, et al. Green tea intake, ACE gene polymorphism and breast cancer risk among Chinese women in Singapore. Carcinogenesis. 2005;26:1389–1394. doi: 10.1093/carcin/bgi080. [DOI] [PubMed] [Google Scholar]
- 15.Yamada K, et al. Effects of common polymorphisms on the properties of recombinant human methylenetetrahydrofolate reductase. Proc. Natl Acad. Sci. USA. 2001;98:14853–14858. doi: 10.1073/pnas.261469998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weisberg I, et al. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol. Genet. Metab. 1998;64:169–172. doi: 10.1006/mgme.1998.2714. [DOI] [PubMed] [Google Scholar]
- 17.Frosst P, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat. Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 18.de Bree A, et al. Effect of the methylenetetrahydrofolate reductase 677C–>T mutation on the relations among folate intake and plasma folate and homocysteine concentrations in a general population sample. Am. J. Clin. Nutr. 2003;77:687–693. doi: 10.1093/ajcn/77.3.687. [DOI] [PubMed] [Google Scholar]
- 19.Lievers KJ, et al. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J. Mol. Med. 2001;79:522–528. doi: 10.1007/s001090100253. [DOI] [PubMed] [Google Scholar]
- 20.Ulrich CM, et al. Searching expressed sequence tag databases: discovery and confirmation of a common polymorphism in the thymidylate synthase gene. Cancer Epidemiol. Biomarkers Prev. 2000;9:1381–1385. [PubMed] [Google Scholar]
- 21.Mandola MV, et al. A 6 bp polymorphism in the thymidylate synthase gene causes message instability and is associated with decreased intratumoral TS mRNA levels. Pharmacogenetics. 2004;14:319–327. doi: 10.1097/00008571-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Hankin JH, et al. Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr. Cancer. 2001;39:187–195. doi: 10.1207/S15327914nc392_5. [DOI] [PubMed] [Google Scholar]
- 23.Koh WP, et al. Angiotensin I-converting enzyme (ACE) gene polymorphism and breast cancer risk among Chinese women in Singapore. Cancer Res. 2003;63:573–578. [PubMed] [Google Scholar]
- 24.Parkin DM, et al. Cancer Incidence in Five Continents. Lyon: IARC Scientific Publications; 2002. [PubMed] [Google Scholar]
- 25.Rastogi T, et al. Cancer incidence rates among South Asians in four geographic regions: India, Singapore, UK and US. Int. J. Epidemiol. 2008;37:147–160. doi: 10.1093/ije/dym219. [DOI] [PubMed] [Google Scholar]
- 26.Yuan JM, et al. Genetic polymorphisms in the methylenetetrahydrofolate reductase and thymidylate synthase genes and risk of hepatocellular carcinoma. Hepatology. 2007;46:749–758. doi: 10.1002/hep.21735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breslow NE, et al. Statistical methods in cancer research. Volume I—the analysis of case-control studies. IARC Sci. Publ. 1980;32:5–338. [PubMed] [Google Scholar]
- 28.Yuan JM, et al. Risk factors for breast cancer in Chinese women in Shanghai. Cancer Res. 1988;48:1949–1953. [PubMed] [Google Scholar]
- 29.Stegmann K, et al. Linkage disequilibrium of MTHFR genotypes 677C/T-1298A/C in the German population and association studies in probands with neural tube defects(NTD) Am. J. Med. Genet. 1999;87:23–29. [PubMed] [Google Scholar]
- 30.Navarro-Peran E, et al. The antifolate activity of tea catechins. Cancer Res. 2005;65:2059–2064. doi: 10.1158/0008-5472.CAN-04-3469. [DOI] [PubMed] [Google Scholar]
- 31.Zheng W, et al. Tea consumption and cancer incidence in a prospective cohort study of postmenopausal women. Am. J. Epidemiol. 1996;144:175–182. doi: 10.1093/oxfordjournals.aje.a008905. [DOI] [PubMed] [Google Scholar]
- 32.Goldbohm RA, et al. Consumption of black tea and cancer risk: a prospective cohort study. J. Natl Cancer Inst. 1996;88:93–100. doi: 10.1093/jnci/88.2.93. [DOI] [PubMed] [Google Scholar]
- 33.Michels KB, et al. Coffee, tea, and caffeine consumption and breast cancer incidence in a cohort of Swedish women. Ann. Epidemiol. 2002;12:21–26. doi: 10.1016/s1047-2797(01)00238-1. [DOI] [PubMed] [Google Scholar]
- 34.Adebamowo CA, et al. Dietary flavonols and flavonol-rich foods intake and the risk of breast cancer. Int. J. Cancer. 2005;114:628–633. doi: 10.1002/ijc.20741. [DOI] [PubMed] [Google Scholar]
- 35.Zintzaras E. Methylenetetrahydrofolate reductase gene and susceptibility to breast cancer: a meta-analysis. Clin. Genet. 2006;69:327–336. doi: 10.1111/j.1399-0004.2006.00605.x. [DOI] [PubMed] [Google Scholar]
- 36.Stevens VL, et al. Association of polymorphisms in one-carbon metabolism genes and postmenopausal breast cancer incidence. Cancer Epidemiol. Biomarkers Prev. 2007;16:1140–1147. doi: 10.1158/1055-9965.EPI-06-1037. [DOI] [PubMed] [Google Scholar]
- 37.Lewis SJ, et al. Meta-analyses of observational and genetic association studies of folate intakes or levels and breast cancer risk. J. Natl Cancer Inst. 2006;98:1607–1622. doi: 10.1093/jnci/djj440. [DOI] [PubMed] [Google Scholar]
- 38.Martin YN, et al. Methylenetetrahydrofolate reductase haplotype tag single-nucleotide polymorphisms and risk of breast cancer. Cancer Epidemiol. Biomarkers Prev. 2006;15:2322–2324. doi: 10.1158/1055-9965.EPI-06-0318. [DOI] [PubMed] [Google Scholar]
- 39.Justenhoven C, et al. One-carbon metabolism and breast cancer risk: no association of MTHFR, MTR, and TYMS polymorphisms in the GENICA study from Germany. Cancer Epidemiol. Biomarkers Prev. 2005;14:3015–3018. doi: 10.1158/1055-9965.EPI-05-0592. [DOI] [PubMed] [Google Scholar]
- 40.Le Marchand L, et al. MTHFR polymorphisms, diet, HRT, and breast cancer risk: the multiethnic cohort study. Cancer Epidemiol. Biomarkers Prev. 2004;13:2071–2077. [PubMed] [Google Scholar]
- 41.Xu X, et al. Polymorphisms of one-carbon-metabolizing genes and risk of breast cancer in a population-based study. Carcinogenesis. 2007;28:1504–1509. doi: 10.1093/carcin/bgm061. [DOI] [PubMed] [Google Scholar]
- 42.Lissowska J, et al. Genetic polymorphisms in the one-carbon metabolism pathway and breast cancer risk: a population-based case-control study and meta-analyses. Int. J. Cancer. 2007;120:2696–2703. doi: 10.1002/ijc.22604. [DOI] [PubMed] [Google Scholar]
- 43.Chen J, et al. One-carbon metabolism, MTHFR polymorphisms, and risk of breast cancer. Cancer Res. 2005;65:1606–1614. doi: 10.1158/0008-5472.CAN-04-2630. [DOI] [PubMed] [Google Scholar]
- 44.Langsenlehner U, et al. The common 677C>T gene polymorphism of methylenetetrahydrofolate reductase gene is not associated with breast cancer risk. Breast Cancer Res. Treat. 2003;81:169–172. doi: 10.1023/A:1025752420309. [DOI] [PubMed] [Google Scholar]
- 45.Campbell IG, et al. Methylenetetrahydrofolate reductase polymorphism and susceptibility to breast cancer. Breast Cancer Res. 2002;4:R14. doi: 10.1186/bcr457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Macis D, et al. Methylenetetrahydrofolate reductase (MTHFR) and breast cancer risk: a nested-case-control study and a pooled meta-analysis. Breast Cancer Res. Treat. 2007;106:263–271. doi: 10.1007/s10549-006-9491-6. [DOI] [PubMed] [Google Scholar]
- 47.Chou YC, et al. Genetic polymorphisms of the methylenetetrahydrofolate reductase gene, plasma folate levels and breast cancer susceptibility: a case-control study in Taiwan. Carcinogenesis. 2006;27:2295–2300. doi: 10.1093/carcin/bgl108. [DOI] [PubMed] [Google Scholar]
- 48.Friso S, et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc. Natl Acad. Sci. USA. 2002;99:5606–5611. doi: 10.1073/pnas.062066299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shrubsole MJ, et al. MTHFR polymorphisms, dietary folate intake, and breast cancer risk: results from the Shanghai Breast Cancer Study. Cancer Epidemiol. Biomarkers Prev. 2004;13:190–196. doi: 10.1158/1055-9965.epi-03-0273. [DOI] [PubMed] [Google Scholar]
- 50.Ervin RB, et al. Dietary intake of selected vitamins for the United States population: 1999–2000. Adv. Data. 2004;339:1–4. [PubMed] [Google Scholar]