Abstract
A critical process for vascular endothelial growth factor (VEGF)- and fibroblast growth factor 2 (FGF2)-regulated cellular function is reversible protein phosphorylation, which is tightly controlled by a balance of protein kinases and phosphatases. We have reported that in ovine fetoplacental artery endothelial (OFPAE) cells, VEGF and FGF2 stimulate cell proliferation in part via activation of mitogen-activated protein kinase kinase 1/2 (MAP2K1/2)/mitogen-activated protein kinase 3/1 (MAPK3/1) and phosphoinositide 3-kinase (PI3K)/v-akt murine thymoma viral oncogene homolog 1 (AKT1) pathways. In the present study, we examined if protein phosphatase 3 (PPP3) mediated VEGF- and FGF2-stimulated OFPAE cell proliferation via modulating activation of MAPK3/1 and AKT1. Small interfering RNA (siRNA) targeting human PPP3 catalytic subunit alpha (PPP3CA) was used to suppress PPP3CA protein expression in OFPAE cells. Compared with the scrambled siRNA, PPP3CA siRNA decreased PPP3CA protein levels by approximately 97% without altering protein levels of protein phosphatase 2 catalytic subunit alpha, total MAPK3/1, total AKT1, or glyceraldehyde-3-phosphate dehydrogenase. Knockdown of PPP3CA protein expression enhanced VEGF-stimulated, but not FGF2-stimulated, cell proliferation. Knockdown of PPP3CA protein expression did not significantly affect VEGF-induced MAPK3/1 and AKT1 phosphorylation but attenuated FGF2-induced MAPK3/1 and AKT1 phosphorylation. Thus, to our knowledge, the present study is the first to demonstrate successful knockdown of PPP3CA protein expression in any cell model using a single pair of double-strained siRNA. Moreover, specific knockdown of PPP3CA protein expression enhances VEGF-stimulated, but not FGF2-stimulated, OFPAE cell proliferation and attenuates FGF2-induced, but not VEGF-induced, MAPK3/1 and AKT1 activation. Thus, PPP3CA differentially modulates the VEGF- and FGF2-stimulated cell proliferation and signaling cascades in OFPAE cells. These data also suggest that signaling molecules other than MAPK3/1 and AKT1 play an important role in VEGF- and FGF2-stimulated cell proliferation after knockdown of PPP3CA in OFPAE cells.
Keywords: AKT1, cell proliferation, FGF2, growth factors, kinases, MAPK3/1, phosphatases, placenta, placental endothelial cells, PPP3, pregnancy, VEGF
PPP3 mediates the VEGF-stimulated placental endothelial cell proliferation
INTRODUCTION
During pregnancy, angiogenesis and vasodilatation are two key processes essential for increasing placental blood flows, which are directly correlated with fetal growth and survival as well as with neonatal weights and survivability [1, 2]. Vascular endothelial growth factor (VEGF) and fibroblast growth factor 2 (FGF2) are two of the most potent angiogenic factors [3, 4]. Expression of VEGF and FGF2 in placentas is positively associated with the placental vascular growth and blood flows during pregnancy [1, 2, 5, 6]. We and other researchers have provided solid evidence showing that VEGF and FGF2 promote placental angiogenesis and production of the potent vasodilator nitric oxide, implicating critical roles of VEGF and FGF2 in placental angiogenesis and vasodilatation [1–7].
It is well established that the biological actions of VEGF and FGF2 are initiated by binding to their specific receptors, thereby activating the tyrosine kinase domains. On activation, these tyrosine kinases activate multiple downstream protein kinases, including mitogen-activated protein kinase 3/1 (MAPK3/1; also termed as ERK1/2) and v-akt murine thymoma viral oncogene homolog 1 (AKT1), via phosphorylation [8–10]. A threonine/tyrosine protein kinase, MAPK3/1 is the major downstream target of mitogen-activated protein kinase kinase 1/2 (MAP2K1/2; also known as MEK1/2). The MAP2K1/2/MAPK3/1 is a key signaling pathway for regulating cell proliferation and differentiation [11]. Similarly, AKT1, a serine/threonine protein kinase, is involved in regulating cell survival, proliferation, and migration [12, 13]. Recently, we have reported that the VEGF- and FGF2-stimulated ovine fetoplacental artery endothelial (OFPAE) cell proliferation is mediated at least in part via the MAP2K1/2/MAPK3/1 and phosphoinositide 3-kinase (PI3K)/AKT1 signaling pathways [14–16].
After activation, these protein kinases must undergo inactivation, returning to an unphosphorylated status and ready for the next stimulus. Protein dephosphorylation is the primary mechanism for protein kinase inactivation. For example, dephosphorylation of either threonine or tyrosine residue of MAPK3/1 results in complete inactivation of MAPK3/1 [17]. Dephosphorylation of protein kinase is catalyzed by multiple families of protein phosphatases, including serine/threonine phosphoprotein phosphatases [18, 19]. Protein phosphatase 3 (PPP3; formally termed PP2B or calcineurin) is composed of catalytic and regulatory subunits [18–20]. To date, three PPP3 catalytic (α, β, and γ) and two regulatory (B1 and B2) subunits have been identified. Expression of α, β, and B1 subunits is ubiquitous in mammalian tissues, whereas γ and B2 subunits are mainly distributed in the testis and brain [20]. In mouse T cells, the PPP3 catalytic subunit α (PPP3CA) constitutes 70–80%, whereas the catalytic β subunit accounts for 20–30% of total phosphatase activity of PPP3 [21]. The PPP3 actively regulates immune responses, cardiac hypertrophy, and neuronal and muscle development, which could be mediated in part via directly dephosphorylating members of nuclear factor of activated T cell (NFAT) transcriptional factors [18, 19]. Of note, the interaction between PPP3 and NFAT is tightly regulated by multiple signaling pathways, including MAPK3/1 [18, 19].
Mouse embryos with disrupted Ppp3r1, Nfatc3, and Nfatc4 genes die in early embryogenesis, largely because of abnormal vascular development, implicating a role of PPP3 in vascular development [22]. Suppression of PPP3 activity by its pharmacological inhibitor cyclosporin A (CsA; a widely used immunosuppressant) inhibits VEGF-induced, but not FGF2-induced, angiogenesis in human umbilical vein endothelial [23] and intestinal microvascular endothelial cells [24]. This inhibition is associated with blockade or attenuation of MAPK3/1 activation [24, 25]. Similar reciprocal relationships between PPP3 and MAPK3/1 also have been observed in cardiomyocytes [26] and in B cells [27]. In contrast, CsA is capable of promoting MAPK3/1 activation, as reported in human first-trimester trophoblasts [28] and canine kidney epithelial cells [29]. Little is known about the role of PPP3 in the PI3K/AKT1 pathway, but one recent study showed that PPP3 inhibition by FK506 (another selective PPP3 inhibitor) had no effect on activation of the PI3K/AKT pathway in A549 cells [30].
Information regarding the interactions between PPP3 and protein kinases in angiogenesis and other endothelial functions is limited. In the present study, we tested, using OFPAE cells as a model, the hypothesis that suppression of PPP3CA expression inhibited VEGF- and FGF2-stimulated angiogenesis via attenuating activations of the MAP2K1/2/MAPK3/1 and PI3K/AKT1 pathways.
MATERIALS AND METHODS
Primary OFPAE Cell Preparation
Primary OFPAE cells were established in our laboratory [7]. All OFPAE cells used in the present study were at passages 8–10. Protocols for animal uses and experimental procedures were approved by the Research Animal Care Committees of both the Medical and Public Health School and the College of Agriculture and Life Sciences and by the Institutional Review Board, University of Wisconsin-Madison.
Small Interfering RNA Design and Transfection
The small interfering RNA (siRNA) duplexes against human PPP3CA were designed based on the human gene coding sequence (GenBank accession no. NM_000944) using an online siRNA design program (Dharmacon, Inc., Chicago, IL) and synthesized with 3′-overhanging thymidine dimers (Integrated DNA Technologies, Coralville, IA). In the preliminary studies, we generated three pairs of double-strained siRNA and found that only one pair (sense, 5′-AUAUACGCGUUCUGAAUACTT-3′; antisense, 5′-GUAUUCAGAACGCGUAUAUTT-3′) at 20 and 40 nM significantly inhibited PPP3CA protein expression as compared to scrambled control siRNA (sense, 5′-AGUUUGACCUGCUCUCCAUTT-3′; antisense, 3′-TTUCAAACUGGACGAGAGGUA-5′). This scrambled siRNA was conjugated with Cy3 at 5′ end, which also was used to monitor transfection efficiency.
Cells were cultured in 60-mm culture dishes in Dulbecco modified Eagle medium (DMEM) containing 5% fetal bovine serum, 5% calf serum, and 1% penicillin-streptomycin. After reaching 50–60% of confluence, cells were washed with DMEM and cultured in 1 ml of DMEM. The PPP3CA siRNA duplexes were mixed with siLentFect Lipid Reagent (Bio-Rad, Hercules, CA), diluted in DMEM, and incubated for 20 min at room temperature. This siRNA transfection complex (200 μl) was added to cell cultures to give a final siRNA concentration at 20 nM. After 5 h of transfection, the medium was supplemented with serum and antibiotics, followed by another 48 h of culture. Cells were harvested, and proteins were prepared for Western blot analysis as described below. Additional cells were transfected with the scrambled siRNA at 20 nM as controls.
Western Blot Analysis
Western blot analysis was performed as described previously [14–16, 31]. After 48 h of transfection, cells were washed twice with cold PBS, harvested, and lysed by sonication in buffer (20 mM imidazole-HCl, 2 mM EGTA, 2 mM EDTA [pH 7.0], 1 mM benzamidine, 1 mM PMSF, 1% Triton X-100, 5 μg/ml of leupeptin, and 5 μg/ml of aprotinin). The lysates were centrifuged (16110 × g, 10 min, 4° C), and protein concentrations of the supernatant were determined. Proteins were separated on 10% SDS-PAGE gels, electroblotted onto an Immobilon-P membrane (Millipore, Bedford, MA), and probed with an antibody against PPP3CA (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA). The proteins were detected using the enhanced chemiluminescence detection system (Amersham Biosciences, Piscataway, NJ). The membranes were reprobed with protein phosphatase 2 (PPP2) catalytic subunit α (PPP2CA; 1:2000; Santa Cruz Biotechnology, Santa Cruz, CA) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:4000; Research Diagnostics, Concord, MA) antibodies. PPP2CA shares approximately 40% identity in amino acid sequence with PPP3CA [32]. Changes in PPP3CA, PPP2CA, and GAPDH protein levels were quantified by scanning densitometry. Data from three independent experiments on PPP3CA and PPP2CA were normalized to GAPDH.
To determine changes in total and phosphorylated MAPK3/1 and AKT1 protein levels, cells, after 16 h of serum deprivation, were treated with 10 ng/ml of VEGF (recombinant human VEGF165; PeproTech, Rocky Hill, NJ) or FGF2 (R&D Systems, Minneapolis, MN) for 0–60 min. Cells were washed twice with cold PBS, harvested, and then lysed by sonication in buffer (4 mM sodium pyrophosphate, 50 mM Hepes [pH 7.5], 100 mM NaCl, 10 mM EDTA, 10 mM sodium fluoride, 2 mM sodium orthovanadate [Na3VO4], 1 mM PMSF, 1% Triton X-100, 5 mg/ml of leupeptin, and 5 mg/ml of aprotinin). The protein concentrations in supernatants of the lysates were determined. Proteins (15–20 μg/lane) were subjected to Western blot analysis as described above. Proteins on the membranes were probed with an antibody against total or phospho-specific MAPK3/1 (1:2000; Cell Signaling Technology, Beverly, MA), total AKT1 (1:2000; Cell Signaling Technology), or phospho-specific AKT1 (1:1000; Cell Signaling Technology). The membranes were reprobed for GAPDH as described above. Changes in total and phosphorylated MAPK3/1 and AKT1 protein levels were quantified. Data regarding phosphorylated MAPK3/1 and AKT1 were normalized to total MAPK3/1 and AKT1, respectively. Data regarding total MAPK3/1 and AKT1 were normalized to GAPDH. The kinase studies were run in at least five independent experiments.
Cell Proliferation
Cell proliferation was assayed as described previously [14–16, 31]. After transfection with the PPP3CA or scrambled siRNA, cells were cultured in 96-well plates (4000–6000 cells/well), followed by 16 h of serum deprivation. Cells were treated without (control) or with VEGF or FGF2 at 0.01, 0.1, 1, 10, and 100 ng/ml (n = 6 wells/dose). After another 48 h of culture, the number of cells was determined. Briefly, wells were rinsed with PBS, fixed in methanol, air-dried, and stained with 0.1% (w/v) crystal violet. Wells were rinsed with distilled water and then air-dried again. Once dry, cells were lysed with 2% (w/v) sodium deoxycholate solution with gentle agitation. Absorbance was measured at 570 nm on a microplate reader (BioTek Instruments, Winooski, VT). Wells containing known cell numbers (0, 1000, 2000, 5000, 10 000, 20 000, or 40 000 cells/well; n = 6 wells/cell density) were treated in a similar fashion to establish standard curves. Cell proliferation studies were run in eight independent experiments.
Statistics Procedures
Data were analyzed using one-way ANOVA (SigmaStat; Jandel Co., San Rafael, CA). When an F-test was significant, data were compared with their respective control by the Bonferroni multiple comparison test or Student t-test.
RESULTS
PPP3CA siRNA Blocked PPP3CA but not PPP2CA or GAPDH Protein Expression in OFPAE cells
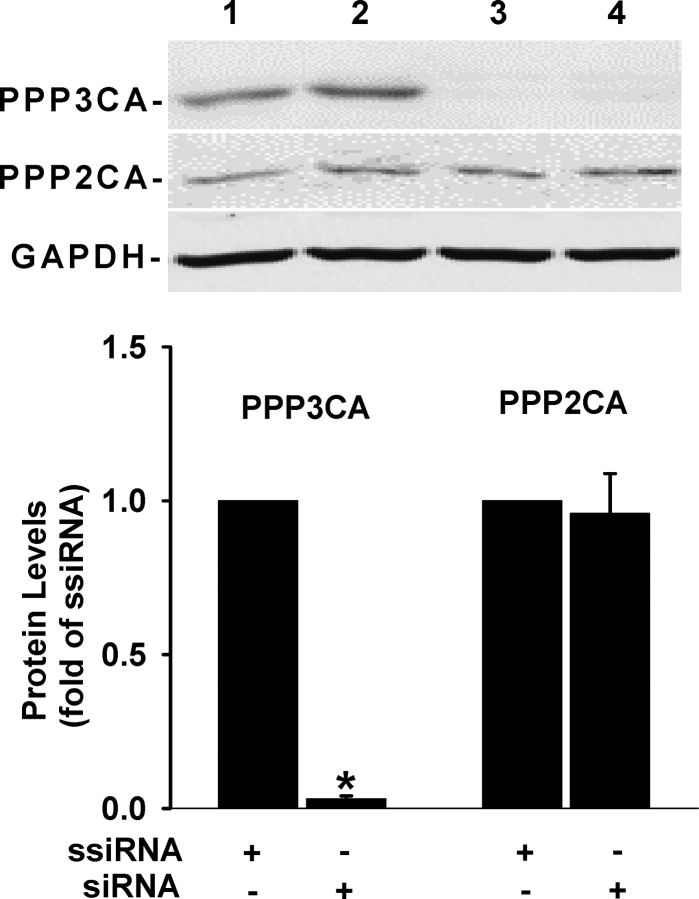
After 48 h of transfection, the majority of cells exhibited normal endothelial morphology (Fig. 1). In cells transfected with the Cy3-labeled scrambled siRNA, the majority of cells showed reddish fluorescence under fluorescence microscopy, indicating high siRNA transfection efficiency in OFPAE cells (Fig. 1). As compared with the scrambled siRNA, the PPP3CA siRNA at 20 or 40 nM decreased (P < 0.05) PPP3CA protein levels by approximately 97%, but protein levels of PPP2CA and GAPDH were unaltered (Fig. 2), indicating specific knockdown of PPP3CA protein expression by the PPP3CA siRNA. This suppressive effect of PPP3CA siRNA on PPP3CA protein expression was maintained for at least 3 days after the transfection (data not shown).
FIG. 1.
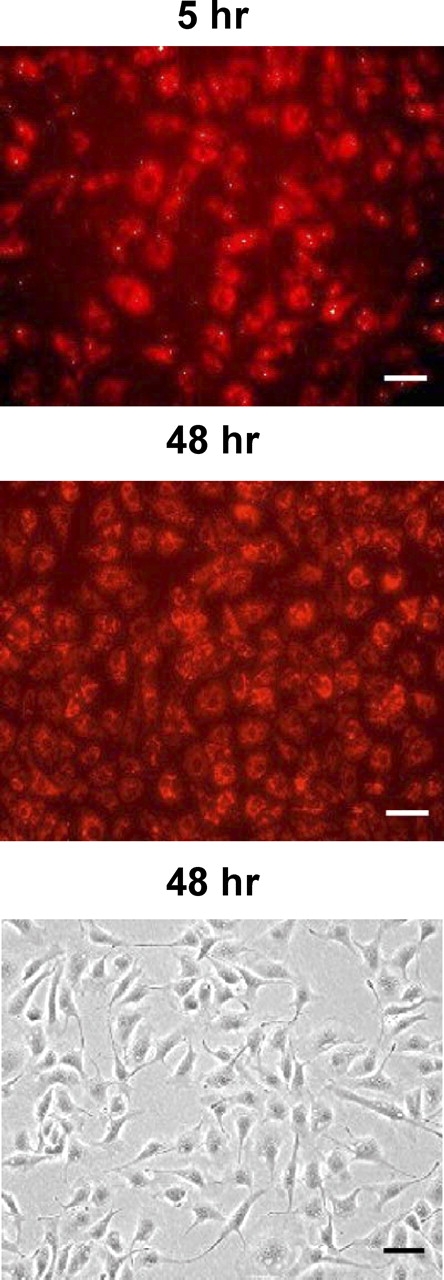
Transfection of OFPAE cells with the scrambled siRNA conjugated with Cy3. Cells were cultured in 60-mm culture dishes in DMEM containing 5% fetal bovine serum, 5% calf serum, and 1% penicillin-streptomycin. After reaching 50–60% of confluence, cells were transfected with the scrambled siRNA (20 nM). After 5 and 48 h of transfection, cells were examined. Top) 5 h of transfection. Middle and Bottom) 48 h of transfection. Reddish fluorescence indicates presence of Cy3 inside cells. Bar = 50 μm.
FIG. 2.
Effects of PPP3CA siRNA on protein expression of PPP3CA, PPP2CA, and GAPDH in OFPAE cells. Cells at 50–60% confluence were transfected with the scrambled or PPP3CA siRNA (20 and 40 nM) for 48 h. Proteins (20 μg) were used for Western blot analysis for PPP3CA, PPP2CA, and GAPDH. Lanes 1 and 2: scrambled siRNA at 20 and 40 nM, respectively; lanes 3 and 4: PPP3CA siRNA at 20 and 40 nM, respectively. Data normalized to GAPDH protein levels are expressed as the fold-value (mean ± SEM) of the scrambled siRNA from three independent experiments. Means with an asterisk differ significantly (P < 0.05) from the scrambled siRNA control. ssiRNA, scrambled siRNA; siRNA, PPP3CA siRNA.
PPP3CA siRNA Promoted VEGF-Stimulated, but not FGF2-Stimulated, Cell Proliferation
In OFPAE cells transfected with the scrambled siRNA, both VEGF and FGF2 dose-dependently stimulated (P < 0.05) cell proliferation (Fig. 3), similar to results in untransfected OFPAE cells reported previously [15, 16]. As compared with the scrambled siRNA, the PPP3CA siRNA promoted (P < 0.05) VEGF-stimulated, but not FGF2-stimulated, OFPAE cell proliferation (Fig. 3). This PPP3CA siRNA-enhanced cell proliferation was dose-dependent, occurring at 1, 10, and 100 ng/ml of VEGF but not at 0.01 and 0.1 ng/ml of VEGF.
FIG. 3.
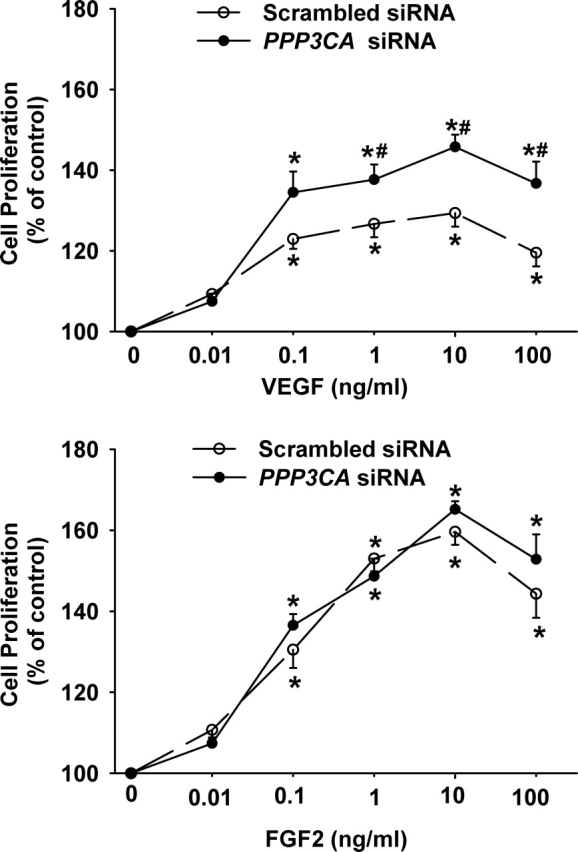
Effects of PPP3CA siRNA on VEGF- and FGF2-stimulated OFPAE cell proliferation. Cells after transfection with the scrambled or PPP3CA siRNA were seeded in 96-well plates (4000 cells/well). After serum starvation, cells were treated without (control) or with VEGF or FGF2 for 48 h. Data are expressed as a percentage (mean ± SEM) of the control from eight independent experiments. Numbers of cells per well in the control were 6036 ± 544. Asterisks indicate significant (P < 0.05) differences from the control. Number symbols denote significant (P < 0.05) differences from the scrambled siRNA at the corresponding dose of growth factor.
Effects of PPP3CA siRNA on VEGF- and FGF2-Induced Phosphorylation of MAPK3/1 and AKT1
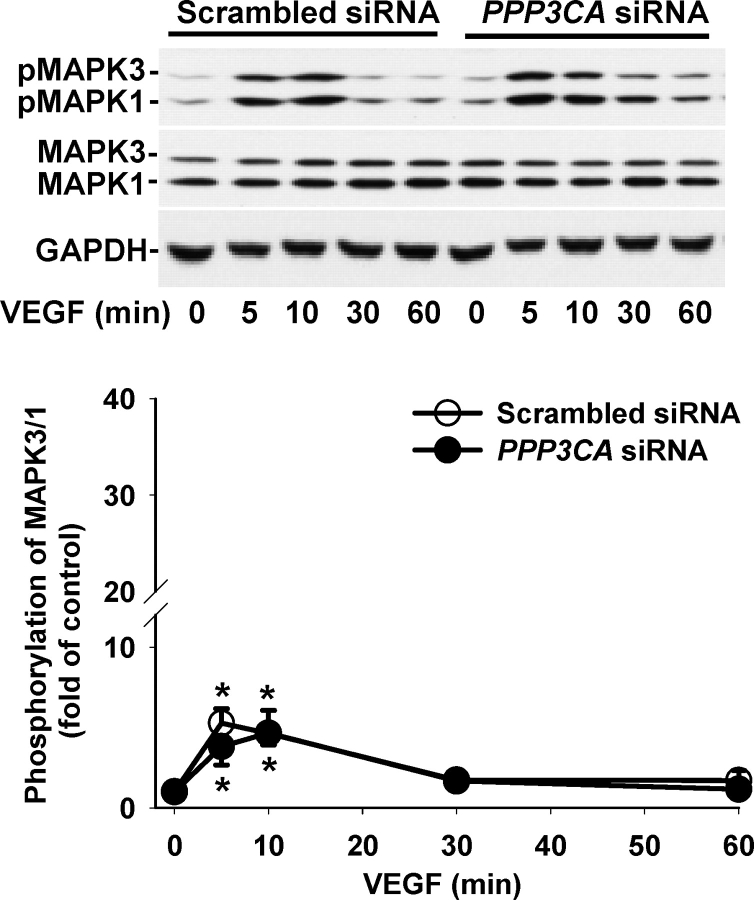
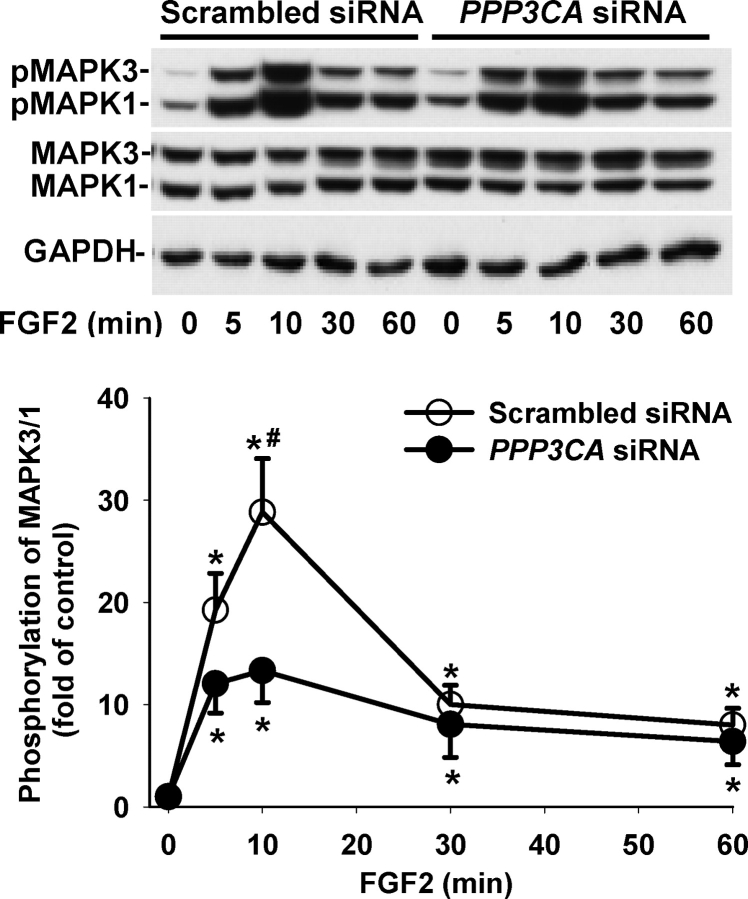
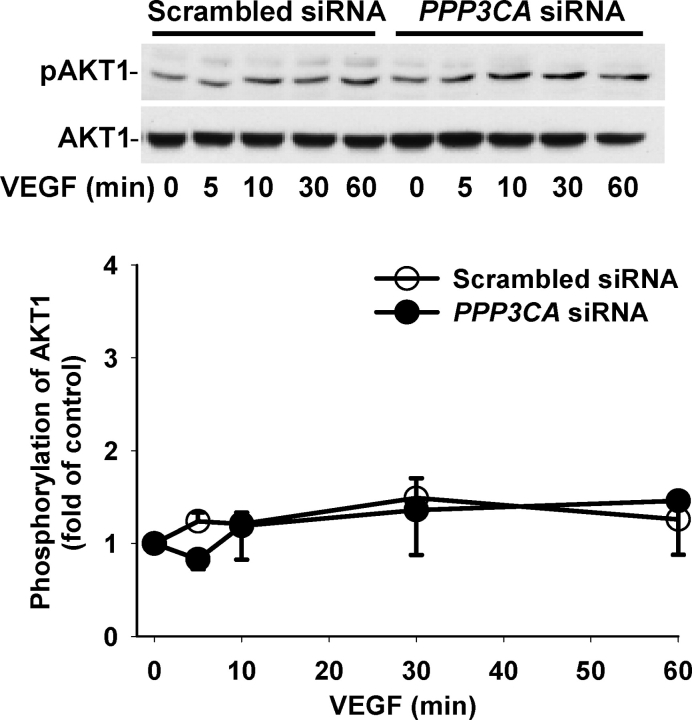
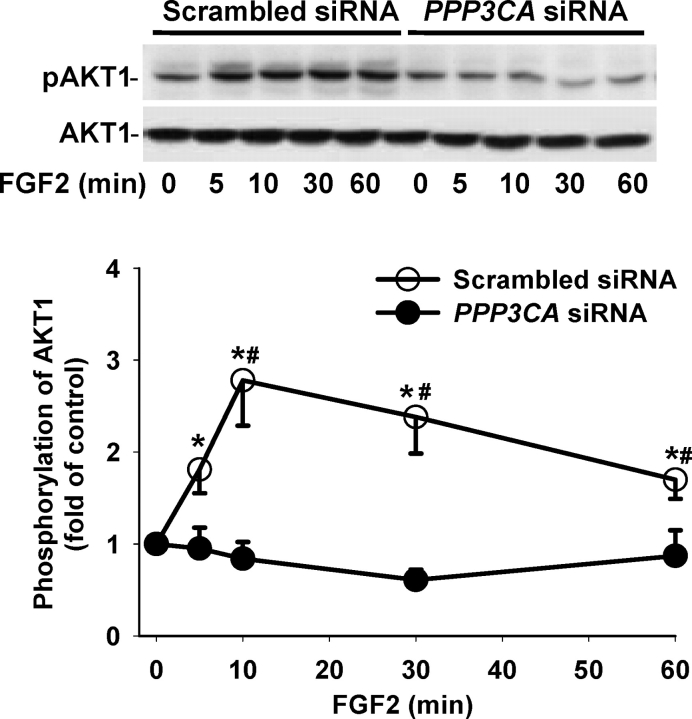
To determine whether the PPP3CA siRNA affected VEGF- and FGF2-induced activation of MAPK3/1 and AKT1, the phosphorylation of MAPK3/1 (Figs. 4 and 5) and AKT1 (Figs. 6 and 7) was assessed by Western blot analysis. As compared with the scrambled siRNA, the PPP3CA siRNA did not significantly change basal levels (at time zero) of total MAPK3/1 (total MAPK3/1/GAPDH: 1.55 ± 0.613 vs. 1.32 ± 0.224 [ratio of PPP3CA siRNA to scrambled siRNA]) and total AKT1 (total AKT1/GAPDH: 2.29 ± 0.705 vs. 2.41 ± 0.398) and basal phosphorylation levels of MAPK3/1 (phospho/total MAPK3/1: 0.20 ± 0.030 vs. 0.14 ± 0.019) and AKT1 (phospho/total AKT1: 0.54 ± 0.093 vs. 0.49 ± 0.092) as well as total MAPK3/1 and AKT1 at any time point examined (Figs. 4–7) (quantitative data not shown). In OFPAE cells transfected with the PPP3CA and scrambled siRNA, VEGF and FGF2 induced (P < 0.05) rapid MAPK3/1 phosphorylation starting at 5 min. The VEGF-induced MAPK3/1 phosphorylation started to decline at 10 min and returned to basal levels at 30 min (Fig. 4). By contrast, the FGF2-induced MAPK3/1 phosphorylation was maintained at relatively high levels up to 60 min (Fig. 5). In comparison to the scrambled siRNA, the PPP3CA siRNA did not significantly alter VEGF-induced MAPK3/1 phosphorylation but markedly inhibited (P < 0.05) FGF2-induced MAPK3/1 phosphorylation at 10 min (Figs. 4 and 5). In OFPAE cells transfected with the scrambled and PPP3CA siRNA, VEGF induced a slight increase (1.2- to 1.5-fold of control) in AKT1 phosphorylation; however, this stimulatory effect did not reach statistical significance (Fig. 6). In OFPAE cells transfected with the scrambled siRNA, FGF2 time-dependently induced (P < 0.05) AKT1 phosphorylation, which started at 5 min (Fig. 7), was maximized at 10 min and then declined, but was maintained at relatively higher levels up to 60 min. In OFPAE cells transfected with the PPP3CA siRNA, FGF2 did not induce significant AKT1 phosphorylation (Fig. 7).
FIG. 4.
Effects of PPP3CA siRNA on VEGF-induced MAPK3/1 phosphorylation in OFPAE cells. Cells were transfected with the scrambled or PPP3CA siRNA for 48 h. After serum starvation, cells were treated with 10 ng/ml of VEGF. Proteins (15 μg/lane) were subjected to Western blot analysis. Data normalized to total MAPK3/1 are expressed as the fold-value (mean ± SEM) of the controls (time zero) from seven independent experiments. Asterisks indicate significant (P < 0.05) differences from the corresponding time-zero control.
FIG. 5.
Effects of PPP3CA siRNA on FGF2-induced MAPK3/1 phosphorylation in OFPAE cells. Cells were transfected with the scrambled or PPP3CA siRNA for 48 h. After serum starvation, cells were treated with 10 ng/ml of FGF2. Proteins (15 μg/lane) were subjected to Western blot analysis. Data normalized to total MAPK3/1 are expressed as the fold-value (mean ± SEM) of the controls (time zero) from seven independent experiments. Asterisks indicate significant (P < 0.05) differences from the corresponding time-zero control. Number symbols denote significant (P < 0.05) differences from the scrambled siRNA.
FIG. 6.
Effects of PPP3CA siRNA on VEGF-induced AKT1 phosphorylation in OFPAE cells. Cells were transfected with the scrambled or PPP3CA siRNA for 48 h. After serum starvation, cells were treated with 10 ng/ml of VEGF. Proteins (15 μg/lane) were subjected to Western blot analysis. Data normalized to total AKT1 are expressed as the fold-value (mean ± SEM) of the controls (time zero) from five independent experiments. Asterisks indicate significant (P < 0.05) differences from the corresponding time-zero control.
FIG. 7.
Effects of PPP3CA siRNA on FGF2-induced AKT1 phosphorylation in OFPAE cells. Cells were transfected with the scrambled or PPP3CA siRNA for 48 h. After serum starvation, cells were treated with 10 ng/ml of FGF2. Proteins (15 μg/lane) were subjected to Western blot analysis. Data normalized to total AKT1 are expressed as the fold-value (mean ± SEM) of the controls (time zero) from five independent experiments. Asterisks indicate significant (P < 0.05) differences from the corresponding time-zero control. Number symbols denote significant (P < 0.05) differences from the scrambled siRNA.
DISCUSSION
Both VEGF- and FGF2-induced cellular responses are mediated by a complex signaling network involving their activation of multiple protein kinases via phosphorylation and their deactivation via dephosphorylation by protein phosphatases. The signaling network mediating VEGF- and FGF2-regulated placental angiogenesis, particularly for the role of protein phosphatases, is poorly defined. Herein, for the first time in any cell model, we have demonstrated a successful use of a single pair of double-strained siRNA to specifically knock down PPP3CA protein expression. This inhibitory effect of siRNA on PPP3CA protein expression was much more potent than that reported in any cell model, including human 293T cells, in which 50% reduction of PPP3CA protein expression was achieved using a commercially available, pooled PPP3CA siRNA [33]. We have also demonstrated, for the first time, that knockdown of PPP3CA protein expression promotes VEGF-stimulated, but not FGF2-stimulated, OFPAE cell proliferation. These data implicate a direct link between PPP3CA and VEGF-stimulated cell proliferation. In contrast to our hypothesis, however, the PPP3CA knockdown-increased VEGF-stimulated cell proliferation was independent on the VEGF-induced activation of MAPK3/1 and AKT1. Meanwhile, failure of PPP3CA knockdown to affect FGF2-stimulated OFPAE cell proliferation was coupled with a decrease in the FGF2-induced MAPK3/1 and AKT1 activation. Thus, our data suggest that PPP3CA plays an important role in the VEGF-stimulated, but not the FGF2-stimulated, OFPAE cell proliferation as well as in the FGF2-induced, but not the VEGF-induced, MAPK3/1 and AKT1 activation. These data also confirmed our recent studies showing that different regulatory mechanisms were involved in the VEGF- and FGF2-induced OFPAE cell responses [16]. The exact mechanisms by which PPP3CA differentially regulates the VEGF- and FGF2-induced cell proliferation and kinase activation in OFPAE cells, however, remain to be elucidated.
Our current observations that PPP3 mediated VEGF-stimulated, but not FGF2-stimulated, endothelial proliferation are consistent with previous reports using other endothelial cell types [23, 24]. However, in contrast to these previous reports showing that inhibition of PPP3 activity attenuates VEGF-induced angiogenesis [23, 24], knockdown of PPP3CA protein expression enhances OFPAE cell proliferation. The reason that PPP3CA displays a completely opposite role in regulating proliferation of endothelial cells with different origins is currently unknown. It is noteworthy, however, that in both previous studies [23, 24], inhibition of PPP3 activity was induced by the pharmacological inhibitor CsA, which executes its action via binding to the interface of the catalytic and regulatory subunits of PPP3 [34]. Generally considered to be a selective inhibitor of PPP3 and widely used in investigating the roles of PPP3 [18, 19, 21, 26], this drug also can function independent of PPP3 [21, 26, 35]. For example, CsA inhibition of the opening of mitochondrial inner membrane permeability transition pore is not mediated via PPP3 [35]. Thus, PPP3 might not be the only major target in the CsA-inhibited, VEGF-stimulated, in vitro and in vivo angiogenic responses [23, 24]. On the other hand, OFPAE cells were exposed to VEGF after PPP3CA protein expression was suppressed for at least 16 h (the serum starvation period). This relatively chronic knockdown of PPP3CA might lead to extensive changes in signaling network that differ to those with the relatively acute (∼30 min to 1 h) inhibition of PPP3 by CsA before VEGF stimulation [23, 24]. This notion is supported by a recent study showing that chronic and acute inhibition of PPP2 caused opposite regulation of MAPK3/1 and AKT1 activation [36]. Moreover, in the present study, the siRNA was designed to target only PPP3CA, which might contribute only a portion of the total PPP3 activity in OFPAE cells, whereas CsA inhibits the total PPP3 activity derived from all catalytic subunits of PPP3 expressed in the cells. Thus, it is plausible that inhibition of the total PPP3 activity is required for attenuating VEGF-induced angiogenesis [23, 24]. Whether the catalytic β subunit is expressed in OFPAE cells is still unclear. However, because the catalytic β subunit is expressed ubiquitously in mammalian cells and, even though accounting for only 20–30% of the total PPP3 activity in mouse T cells [21], its activity is essential for T-cell activation, our present results cannot exclude the possibility that the catalytic β subunit plays a role in the VEGF-stimulated endothelial proliferation.
It is noteworthy that compared with untransfected OFPAE cells, the scrambled siRNA appeared to decrease phosphorylation levels of the VEGF- and FGF2-induced MAPK3/1 and AKT1, although we did not observe significant changes in VEGF- and FGF2-stimulated cell proliferation between transfected and untransfected OFPAE cells [16]. For example, we previously observed an average increase of approximately threefold in VEGF-induced AKT1 phosphorylation in untransfected OFPAE cells [16], whereas in the present study, we observed only a 1.2- to 1.5-fold increase in OFPAE cells transfected with the scrambled siRNA (Fig. 6). This change is likely to have resulted from the interference of siRNA transfection system, because it is well known that the routinely used siRNA transfection reagents, such as lipofectin and oligofectamine, can modulate expression of many genes, including protein kinases and phosphatases, critical for cell proliferation, differentiation, and apoptosis [37].
It is well established that MAPK3/1 and AKT1 are actively involved in the regulation of endothelial function [7, 16, 31, 38]. Recently, we also have demonstrated in OFPAE cells that activation of the MAP2K1/2/MAPK3/1 and PI3K/AKT1 pathways is critical for the VEGF- and FGF2-stimulated cell proliferation [16]. The major findings of our present study are that PPP3CA knockdown potentiated the VEGF-stimulated, but not the FGF2-stimulated, cell proliferation but decreased the FGF2-induced, but not the VEGF-induced, MAPK3/1 and AKT1 activation in OFPAE cells. These data suggest that after knockdown of PPP3CA, additional, yet-to-be-defined signaling cascades other than the MAP2K1/2/MAPK3/1 and PI3K/AKT1 might emerge as major players in mediating the VEGF- and FGF2-stimulated cell proliferation. Indeed, in addition to MAPK3/1, MAPK9/8 (also termed as JNK2/1) and MAPK11 (also termed as p38 MAPK) have been implicated in such regulation in human intestinal microvascular endothelial cells [24]. Alternatively, differential regulation of other protein phosphatases (i.e., MAPK phosphatase as well as phosphatase and tensin homolog [PTEN]) might contribute to differential activation of MAPK3/1 and AKT1 by VEGF and FGF2 in OFPAE cells transfected with PPP3CA siRNA.
Our present findings that knockdown of PPP3CA attenuated the FGF2-induced MAPK3/1 and AKT1 activation in OFPAE cells contradict those of a previous report showing that inhibition of PPP3 by FK506 did not alter activation of the PI3K/AKT1 pathway in A549 cells [30]. These data suggest, again, that the pharmacological inhibitors- and siRNA-mediated PPP3 downregulation may have different effects on MAPK3/1 and AKT1 activation. The mechanisms underlying the findings that suppression of PPP3CA and PPP2CA attenuated the FGF2-induced MAPK3/1 and AKT1 activation in OFPAE cells remain to be explored. It has been postulated, however, that siRNA-mediated, partial suppression of PPP2 activity induces chronic MAPK3/1 and AKT1 hyperphosphorylation, which can downregulate signaling molecules upstream of Ras, leading to attenuation of the MAPK3/1 and AKT1 activation in response to growth factors, including FGF2 [36]. A similar mechanism might not be involved in PPP3CA modulation of MAPK3/1 and AKT1 activation in OFPAE cells, because in the present study, the basal levels (at time zero) of MAPK3/1 and AKT1 phosphorylation in the PPP3CA siRNA transfected cells were similar to those in the scrambled siRNA-transfected cells.
Taken together, the present results show for the first time, that PPP3CA differentially modulates VEGF and FGF2 regulation of placental endothelial cell proliferation and signaling. Our current finding that PPP3 is critical for the VEGF-stimulated cell proliferation and the FGF2-induced MAPK3/1 and AKT1 activation further advances our understanding of the complex signaling mechanism controlling placental endothelial function. Future studies are needed to dissect these signaling networks, which may provide fundamental information for modulating placental vasculature and blood flows by altering activation of signaling cascades by angiogenic factors.
Acknowledgments
We thank Dr. Ronald R. Magness from the Perinatal Research Laboratories, Department of Obstetrics and Gynecology, University of Wisconsin, Madison, for critically reading this manuscript.
Footnotes
1Supported in part by NIH grants HL64703 and HD38843 (J.Z.) and HL74947 and HL70562 (D.-B.C.).
REFERENCES
- Zygmunt M, Herr F, Munstedt K, Lang U, Liang OD. Angiogenesis and vasculogenesis in pregnancy. Eur J Obstet Gynecol Reprod Biol 2003; 110: S10 S18 [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Borowicz PP, Vonnahme KA, Johnson ML, Grazul-Bilska AT, Wallace JM, Caton JS, Redmer DA. Animal models of placental angiogenesis. Placenta 2005; 26: 689 708 [DOI] [PubMed] [Google Scholar]
- Magness RR, Zheng J. Circulatory changes during gestation. Gluckman PD, Heymann MA. Scientific Basis of Pediatric and Perinatal Medicine. London: Edward Arnold Publishers; 1996: 762 772 [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003; 9: 669 676 [DOI] [PubMed] [Google Scholar]
- Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocrine-Related Cancer 2000; 7: 165 197 [DOI] [PubMed] [Google Scholar]
- Bogic LV, Brace RA, Cheung CY. Developmental expression of vascular endothelial growth factor (VEGF) receptors and VEGF binding in ovine placenta and fetal membranes. Placenta 2001; 22: 265 275 [DOI] [PubMed] [Google Scholar]
- Zheng J, Bird IM, Chen DB, Magness RR. Angiotensin II regulation of fetoplacental artery endothelial functions. J Physiol (London) 2005; 565: 59 69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse B, Thisse C. Functions and regulations of fibroblast growth factor signaling during embryonic development. Dev Biol 2005; 287: 390 402 [DOI] [PubMed] [Google Scholar]
- Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J Cell Mol Med 2005; 9: 777 794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey L, Ambrosetti D, Mansukhani A, Basilico C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev 2005; 16: 233 247 [DOI] [PubMed] [Google Scholar]
- Rubinfeld H, Seger R. The ERK cascade: a prototype of MAPK signaling. Mol Biotechnol 2005; 31: 151 174 [DOI] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science 2002; 296: 1655 1657 [DOI] [PubMed] [Google Scholar]
- Du K, Tsichlis PN. Regulation of the Akt kinase by interacting proteins. Oncogene 2005; 24: 7401 7409 [DOI] [PubMed] [Google Scholar]
- Zheng J, Bird IM, Melsaether AN, Magness RR. Activation of the mitogen-activated protein kinase cascade is necessary but not sufficient for basic fibroblast growth factor- and epidermal growth factor-stimulated expression of endothelial nitric oxide synthase in ovine fetoplacental artery endothelial cells. Endocrinology 1999; 140: 1399 1407 [DOI] [PubMed] [Google Scholar]
- Song Y, Zheng J. Establishment of a functional ovine fetoplacental artery endothelial cell line with a prolonged lifespan. Biol Reprod 2007; 76: 29 35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Wen Y, Song Y, Wang K, Chen DB, Magness RR. Activation of multiple signaling pathways is critical for fibroblast growth factor 2- and vascular endothelial growth factor-stimulated ovine fetoplacental endothelial cell proliferation. Biol Reprod 2008; 78: 143 150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J 2000; 14: 6 16 [PubMed] [Google Scholar]
- Aramburu J, Heitman J, Crabtree GR. Calcineurin: a central controller of signaling in eukaryotes. EMBO Rep 2004; 5: 343 348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins BJ, Molkentin JD. Calcium–calcineurin signaling in the regulation of cardiac hypertrophy. Biochem Biophys Res Commun 2004; 322: 1178 1191 [DOI] [PubMed] [Google Scholar]
- Perrino BA, Wilson AJ, Ellison P, Clapp LH. Substrate selectivity and sensitivity to inhibition by FK506 and cyclosporin A of calcineurin heterodimers composed of the alpha or beta catalytic subunit. Eur J Biochem 2002; 269: 3540 3548 [DOI] [PubMed] [Google Scholar]
- Im SH, Rao A. Activation and deactivation of gene expression by Ca2+/calcineurin-NFAT-mediated signaling. Mol Cells 2004; 18: 1 9 [PubMed] [Google Scholar]
- Graef IA, Chen F, Chen L, Kuo A, Crabtree GR. Signals transduced by Ca(2+)/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell 2001; 105: 863 875 [DOI] [PubMed] [Google Scholar]
- Hernandez GL, Volpert OV, Iniguez MA, Lorenzo E, Martinez-MartInez S, Grau S, Fresno M, Redondo JM. Selective inhibition of vascular endothelial growth factor–mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2. J Exp Med 2001; 193: 607 620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiee P, Heidemann J, Ogawa H, Johnson NA, Fisher PJ, Li MS, Otterson MF, Johnson CP, Binion DG. Cyclosporin A differentially inhibits multiple steps in VEGF induced angiogenesis in human microvascular endothelial cells through altered intracellular signaling. Cell Commun Signal 2004; 2: 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farivar AS, Mackinnon-Patterson BC, Barnes AD, McCourtie AS, Mulligan MS. Cyclosporine modulates the response to hypoxia-reoxygenation in pulmonary artery endothelial cells. Ann Thorac Surg 2005; 79: 1010 1016 [DOI] [PubMed] [Google Scholar]
- Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res 2004; 63: 467 475 [DOI] [PubMed] [Google Scholar]
- Gary-Gouy H, Sainz-Perez A, Bismuth G, Ghadiri A, Perrino BA, Dalloul A. Cyclosporin-A inhibits ERK phosphorylation in B cells by modulating the binding of Raf protein to Bcl2. Biochem Biophys Res Commun 2006; 344: 134 139 [DOI] [PubMed] [Google Scholar]
- Du MR, Zhou WH, Yan FT, Zhu XY, He YY, Yang JY, Li DJ. Cyclosporin A induces titin expression via MAPK/ERK signaling and improves proliferative and invasive potential of human trophoblast cells. Hum Reprod 2007; 22: 2528 2537 [DOI] [PubMed] [Google Scholar]
- Kiely B, Feldman G, Ryan MP. Modulation of renal epithelial barrier function by mitogen-activated protein kinases (MAPKs): mechanism of cyclosporin A–induced increase in transepithelial resistance. Kidney Int 2003; 63: 908 916 [DOI] [PubMed] [Google Scholar]
- Wen HC, Huang WC, Ali A, Woodgett JR, Lin WW. Negative regulation of phosphatidylinositol 3-kinase and Akt signaling pathway by PKC. Cell Signal 2003; 15: 37 45 [DOI] [PubMed] [Google Scholar]
- Zheng J, Wen Y, Austin JL, Chen DB. Exogenous nitric oxide stimulates cell proliferation via activation of a mitogen-activated protein kinase pathway in ovine fetoplacental artery endothelial cells. Biol Reprod 2006; 74: 375 382 [DOI] [PubMed] [Google Scholar]
- Cohen PT, Brewis ND, Hughes V, Mann DJ. Protein serine/threonine phosphatases: an expanding family. FEBS Lett 1990; 268: 355 359 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lin D-H, Wang Z, Jin Y, Yang B, Wang W. K-restriction inhibits protein phosphatase 2B (PP2B) and suppression of PP2B decreases ROMK channel activity in the CCD. Am J Physiol Cell Physiol 2008; 294: C765 C773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke H, Huai Q. Structures of calcineurin and its complexes with immunophilins-immunosuppressants. Biochem Biophys Res Commun 2003; 311: 1095 1102 [DOI] [PubMed] [Google Scholar]
- Nicolli A, Basso E, Petronilli V, Wenger RM, Bernardi P. Interactions of cyclophilin with the mitochondrial inner membrane and regulation of the permeability transition pore, and cyclosporin A-sensitive channel. J Biol Chem 1996; 271: 2185 2192 [DOI] [PubMed] [Google Scholar]
- Van Kanegan MJ, Adams DG, Wadzinski BE, Strack S. Distinct protein phosphatase 2A heterotrimers modulate growth factor signaling to extracellular signal-regulated kinases and Akt. J Biol Chem 2005; 280: 36029 36036 [DOI] [PubMed] [Google Scholar]
- Akhtar S, Benter I. Toxicogenomics of non-viral drug delivery systems for RNAi: potential impact on siRNA-mediated gene silencing activity and specificity. Adv Drug Deliv Rev 2007; 59: 164 182 [DOI] [PubMed] [Google Scholar]
- Li Y, Zheng J, Bird IM, Magness RR. Effects of pulsatile shear stress on signaling mechanisms controlling nitric oxide production, eNOS phosphorylation, and expression in ovine fetoplacental artery endothelial cells. Endothelium 2005; 12: 21 39 [DOI] [PubMed] [Google Scholar]