Abstract
Repeated cocaine exposure enhances glutamatergic output from the medial prefrontal cortex to subcortical brain regions. Loss of inhibitory control of cortical pyramidal neurons may partly account for this augmented cortical glutamate output. Recent research indicated that repeated cocaine exposure reduced the ability of cortical Group II metabotropic glutamate receptors to modulate behavioral and neurochemical responses to cocaine. Thus, experiments described below examined whether repeated cocaine exposure alters metabotropic glutamate receptor regulation of mesocorticolimbic glutamatergic transmission using in vivo microdialysis. Infusion of the Group II metabotropic glutamate receptor antagonist LY341495 into the medial prefrontal cortex enhanced glutamate release in this region, the nucleus accumbens and the ventral tegmental area in sensitized animals, compared to controls, following short-term withdrawal but not after long-term withdrawal. Additional studies demonstrated that vesicular (K+-evoked) and non-vesicular (cystine-evoked) glutamate release in the medial prefrontal cortex was enhanced in sensitized animals, compared to controls, that resulted in part from a reduction in Group II metabotropic glutamate receptor modulation of these pools of glutamate. In summary, these findings indicate that the expression of sensitization to cocaine is correlated with an altered modulation of mesocorticolimbic glutamatergic transmission via reduction of Group II metabotropic glutamate receptor function.
Keywords: Behavioral Sensitization, Locomotion, Microdialysis, Nucleus Accumbens, Ventral Tegmental Area
Repeated cocaine exposure enhances glutamatergic output from the medial prefrontal cortex (mPFC) to subcortical brain regions, including the nucleus accumbens and ventral tegmental area (VTA). This enhanced glutamatergic output is associated with augmented behavioral responses to cocaine challenge, which is referred to as behavioral sensitization (Pierce et al. 1996; Wolf 1998; Tzschentke 2000; Steketee 2003; Kalivas 2004). It has been suggested that enhanced glutamatergic output may result from increased excitatory drive in the mPFC. For example, a transient increase of glutamate overflow was observed in cocaine-sensitized animals after 1 day and 7 days, but not 30 days of withdrawal (Williams and Steketee 2004). Repeated cocaine administration increased membrane excitability of pyramidal neurons in the rat mPFC via an increase in voltage-sensitive calcium currents (Nasif et al. 2005). Besides enhancement of excitatory drive, it has been hypothesized that a loss of inhibitory control may also be responsible for augmented glutamatergic output from the mPFC (Steketee 2003). Previous studies have indicated a reduction of receptor function including dopamine D2 and GABAB receptors, in the mPFC of cocaine sensitized animals (Beyer and Steketee 2002; Steketee and Beyer 2005). In addition, repeated cocaine administration has been shown to promote long-term potentiation in rat mPFC (Huang et al. 2007). Both GABAA receptor-mediated synaptic currents and GABAA alpha1 subunit expression in mPFC pyramidal neurons were reduced following repeated cocaine exposure (Huang et al. 2007). Thus, it was suggested that cocaine-induced long-term potentiation resulted from a reduction in GABAA receptor-mediated inhibition of mPFC pyramidal neurons (Huang et al. 2007). Consistent with these findings is a recent study that indicated that repeated cocaine exposure reduced the ability of Group II metabotropic glutamate receptors (mGluR), which include mGluR2 and mGluR3 receptors, to modulate the behavioral and neurochemical responses to cocaine after a long period of withdrawal (Xie and Steketee, unpublished observation). These data suggest that functional reduction of these inhibitory receptors, which may enhance mesocorticolimbic glutamate transmission, is correlated with the long-term expression of cocaine-induced behavioral sensitization.
Group II mGluRs have been suggested to play an inhibitory role in regulating glutamate release. Group II mGluRs are found in various combinations of presynaptic, postsynaptic and glial localizations that may reflect differential modulation of excitatory amino acid transmission (Petralia et al. 1996; Conn and Pin 1997). Antagonism of presynaptic Group II mGluRs elevates extracellular glutamate level in the nucleus accumbens (Xi et al. 2002). In addition to acting as an autoreceptor and/or postsynaptic inhibitory receptor, Group II mGluRs can decrease extracellular glutamate levels in the nucleus accumbens by inhibiting the Na+-independent cystine/glutamate antiporter (Baker and Shen 2002; Baker et al. 2002; Baker et al. 2003). It has been shown that repeated cocaine administration reduced the function of Group II mGluRs in the nucleus accumbens that may partly result in enhanced glutamate release within this region associated with cocaine sensitization (Xi et al. 2002). However, few studies have been conducted to reveal the modulation of mesocorticolimbic glutamate transmission by mPFC Group II mGluRs. Thus, studies were designed to examine Group II mGluR modulation of mesocorticolimbic glutamate transmission in both control and cocaine sensitized animals.
Studies have demonstrated a transient cocaine induced increase in mPFC glutamate overflow in sensitized animals (Williams and Steketee 2004). However, the source of the glutamate was not determined. Glutamate can be released from both mPFC afferent terminals and axon collaterals of pyramidal neurons and can include both vesicular and non-vesicular pools (Melendez et al. 2005). It has been shown that K+-stimulated glutamate efflux in the mPFC was enhanced in animals following repeated methamphetamine exposure (Stephans and Yamamoto 1995), suggesting that repeated cocaine exposure may influence the vesicular pool of glutamate. Recent evidence suggested that the non-vesicular pool of glutamate, regulated by the cystine/glutamate antiporter, in the nucleus accumbens was modulated by repeated cocaine administration (Baker et al. 2003). However, it has yet to be determined whether repeated cocaine exposure can alter the release of this pool of glutamate in the mPFC. Intra-mPFC infusion of a Group II mGluR antagonist, but not an agonist, elevated glutamate levels in the mPFC (Melendez et al. 2005), suggesting that these receptors may negatively regulate glutamate release. Thus, additional studies determined whether vesicular and/or non-vesicular pools contribute to enhanced glutamate release seen in the mPFC of cocaine-sensitized animals and whether a reduction in Group II mGluR function may play a role in the enhanced glutamate release from either of these sources.
Based on the discussion above, pharmacological agents of Group II mGluRs, including an agonist, LY379268 [(1R,4R,5S,6R)-4-amino-2-oxabicyclo[3.1.0]hexane-4,6-dicarboxylic acid] or an antagonist, LY341495 [(2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid] were chosen to assess the effects of repeated cocaine exposure on mPFC Group II mGluR modulation of mesocorticolimbic glutamate transmission during the development of cocaine sensitization.
Materials and methods
Animals and surgery
Male Sprague-Dawley rats that weighed 275–300 g at the time of surgery were housed under 12 hr light/dark cycle and had free access to food and water. Rats were housed in groups of four before surgery and individually after surgery. Prior to surgery, rats were anesthetized (ketamine hydrochloride and xylazine: 80 and 12.5 mg/kg respectively; i.p.) and their heads were mounted in a stereotaxic frame (Kopf Instruments). The scalp was incised to expose the skull, and bregma and lambda were aligned in the same horizontal plane. All animals received microdialysis guide cannulae (20 guage, 14 mm) implanted 3.0 mm above the mPFC (A/P, + 3.2 mm; M/L, ± 0.6 mm and D/V, −1.5 mm). For dual probe microdialysis experiments animals also received ipsilateral implants of guide cannulae 3.0 mm above the nucleus accumbens (A/P, + 1.4 mm; M/L, ± 1.4 mm and D/V, −5.0 mm) or VTA (A/P, −4.8 mm; M/L, ± 0.6 mm and D/V, −5.4 mm). The A/P, M/L and D/V coordinates were based on bregma, midline and dura, respectively (Paxinos and Watson 1986). Cannulae were anchored with 3 stainless steel screws and dental acrylic. Obturators (25 gauge, 14 mm) were inserted into the cannulae in order to prevent their occlusion. Animals were allowed at least one week to recover from surgery. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The University of Tennessee Health Science Center Animal Resources Advisory Committee approved all experimental procedures.
In vivo Microdialysis
Microdialysis experiments were conducted in conscious, freely moving rats. Animals received daily injections of saline (saline-pretreated, 1.0 ml/kg) or cocaine (cocaine-pretreated, 15 mg/kg) over 4 consecutive days in their home cages. In vivo microdialysis experiments were conducted 1, 7 or 30 days following the last of the daily injections. Dialysis probes (2 mm active membrane) were introduced into the guide cannulae 16 hr prior to the beginning of the dialysis experiments in order to minimize damage-induced release of neurotransmitters and metabolites. Each animal participated in only 1 microdialysis experiment.
Experiment 1: Group II mGluRs and mesocorticolimbic glutamate transmission
The probe was continuously perfused with dialysis buffer (KCl 2.7 mM, NaCl 140 mM, CaCl2 1.2 mM, MgCl2 1.2 mM, plus 0.2 mM phosphate-buffered saline to achieve a pH=7.4). The flow rate was reduced to 0.003 μl/min during the overnight period and was adjusted back to 2 μl/min at least 1 hr before the beginning of sample collection. Following collection of four baseline samples LY341495, a Group II mGluR antagonist, was infused into the mPFC in escalating concentrations (0.1, 1 and 10 μM) via reverse dialysis. Four samples were collected from the mPFC, nucleus accumbens and/or VTA after each change in concentration. Samples were collected into 10 μl of 0.05 mM HCl in 0.5 ml microcentrifuge tubes. Samples either immediately underwent high performance liquid chromatography (HPLC) analysis of glutamate or were stored at −80°C. Samples were stored no more than 2 weeks.
Experiment 2: Group II mGluRs and mPFC glutamate release
All animals received a single probe in the mPFC. Procedures were the same as above except that animals received infusions of KCl (80 mM) or cystine (50 μM) following collection of baseline samples. After collection of 4 additional 20 min samples, animals received intra-mPFC co-infusions of LY379268, an agonist of Group II mGluRs, with KCl or cystine via reverse dialysis and an additional 4 samples were collected. Animals then received infusions of dialysis buffer as a washout for 80 min.
High Performance Liquid Chromatography
The glutamate HPLC system consisted of a 25 cm (5 μm) octadecasilane column, a Shimadzu LC-10AD solvent delivery system (Shimadzu Corporation, Kyoto, Japan), a Shimadzu SIL-10AD autosampler and a fluorescence spectrophotometer (Shimadzu RF-10AXL). Samples underwent derivatization (20 μl sample + 20 μl fluoraldehyde) before being injected onto the column via the autosampler. The flow rate of the mobile phase (62 mM NaH2PO4, 0.5% v/v tetrahydrofuran and 40% v/v methanol, pH =6.3 with 6 N NaOH) was 1.0 ml/min. The fluorescence spectrophotometer detected glutamate with an excitation wavelength of 260 nm and an emission wavelength of 455 nm. The external standard curve ranged from 0.5–25 pmol for quantification.
Behavior
Locomotor activity was measured in a Micromax monitoring system (Accuscan Instruments, Columbus, OH, USA) as previously described (Beyer and Steketee 2002) to verify the treatment regimen produced sensitization. Within 3 days after the in vivo microdialysis experiments, animals were adapted to activity boxes (45 × 24 × 19 cm), which were placed in individual sound attenuating chambers, for 60 min. All animals received systemic injections of cocaine (15 mg/kg, i.p.) and locomotor activity was monitored for 2 h following injection.
Histology
After completion of studies animals were deeply anesthetized with sodium pentobarbital and were perfused by intracardiac infusion of phosphate-buffered saline (50 ml) and 10% formaldehyde (50 ml). Brains were stored in vials with 10% formaldehyde for at least 3 days before sectioning. Brains were sectioned (100 μm) on a vibratome and sections were mounted onto gelatin-coated slides. Sections were stained with cresyl violet and dialysis probe placements were visualized by light microscopy and verified according to Paxinos and Watson (1986).
Statistics
The microdialysis data were converted to percentage of baseline in order to avoid variability associated with differences in probe placement and probe recovery. In studies where the influence of the Group II mGluR agonist LY379268 on cystine- or K+-evoked glutamate release was determined, the percent change from baseline was averaged for each infusion period, (i.e. baseline, cystine or KCL, cystine or KCL + LY379268 and washout). A two-way analysis of variance (ANOVA) with one repeated measure (time) was used to analyze neurochemical data. Multiple comparisons were made with a modified least significant differences test (Milliken and Johnson 1984). Total motor activity data and differences in baseline neurotransmitter levels between saline- and cocaine-pretreated animals were analyzed using an unpaired Student’s t-test.
Drugs
Cocaine hydrochloride was purchased from Sigma Chemical Company (St Louis, MO). LY379268 and LY341495 were purchased from TOCRIS Bioscience (Ellisville, MO). LY379268 was diluted with isotonic saline. (0.9% sodium chloride) and LY341495 was diluted with 1.2 eq. NaOH. KCl (80 mM) buffer was prepared as followed: KCl 82.7 mM, NaCl 60 mM, CaCl2 1.2 mM, MgCl2 1.2 mM, plus 0.2 mM phosphate-buffered saline to achieve a pH=7.4. Cystine (50 μM) was diluted with dialysis buffer.
Results
Experiment 1: Group II mGluRs and mesocorticolimbic glutamate transmission
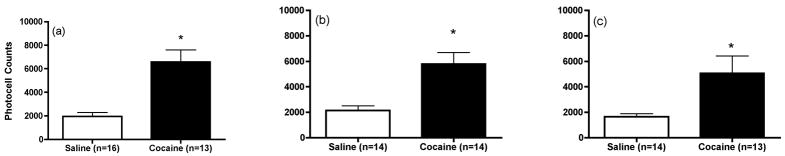
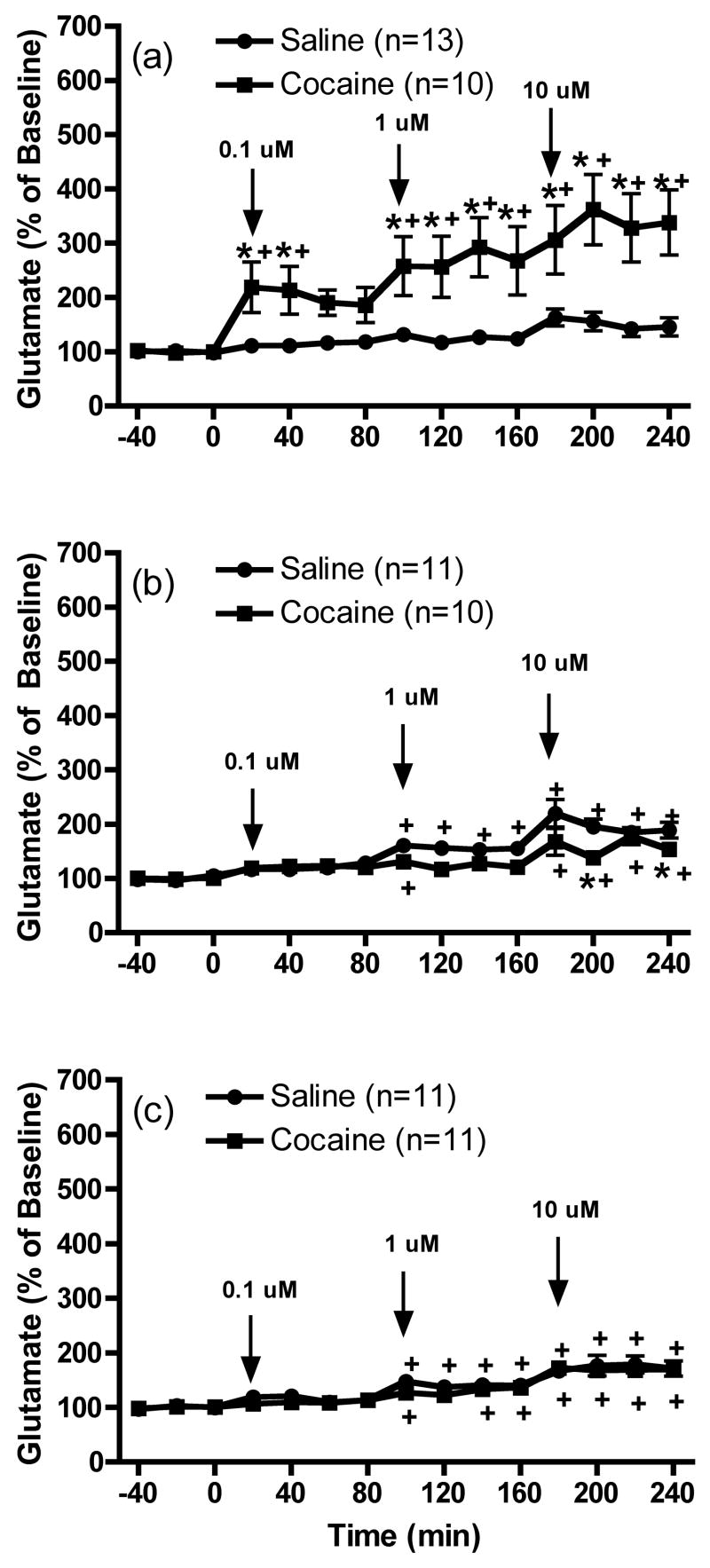
Figure 1 shows that the motor response to cocaine was significantly enhanced in animals that had previously received daily repeated cocaine injections compared to repeated saline [1 day: t(27)=4.699, p<0.05; 7 days: t(26)=3.703, p<0.05 and 30 days t(25)=2.573, p<0.05]. Figure 2 shows the effects of the infusion of LY341495 (0.1, 1 and 10 μM) on extracellular glutamate levels within the mPFC in animals that previously received repeated cocaine exposure. The data were analyzed by two-way ANOVA with repeated-measures followed by a modified least significant differences test (Milliken and Johnson 1984) and F scores were as follows: 1 day: treatment F(1,21)=10.078, p=0.0046; time F(14,294)=12.387, p<0.0001 and interaction F(14,294)=6.352, p<0.0001; 7 days: treatment F(1,19)=3.748, p=0.0679; time F(14,266)=21.419, p<0.0001 and interaction F(14,266)=2.753, p=0.0008 and 30 days: treatment F(1,20)=0.8, p=0.3819; time F(14,280)=25.431, p<0.0001 and interaction effect F(14,280)=0.41, p=0.9713. LY341495 produced a trend towards increases above baseline glutamate levels in saline control animals following 1 day of withdrawal. This effect achieved significance in control animals following prolonged withdrawal (7 days and 30 days). After 1 day of withdrawal from repeated cocaine injections, infusions of three different concentrations of LY341495 into the mPFC produced significantly enhanced extracellular glutamate levels in this region in animals previously exposed to repeated cocaine as compared to saline (Figure 2a). LY341495 failed to induce an enhancement of glutamate levels in daily cocaine animals as compared to daily saline animals following 7 or 30 days of withdrawal (Figures 2b and 2c respectively). In fact, the response to LY341495 (10 μM) was significantly reduced in sensitized animals compared to controls following 7 days of withdrawal. Finally, no significant differences in basal extracellular glutamate levels (pmol/20 min; mean ± SEM) were observed between saline and cocaine animals [1 day: Cocaine, 3.6 ± 0.8; Saline, 4.6 ± 0.6; t(21)=1.048, p=0.3067; 7 days: Cocaine, 5.8 ± 1.5; Saline, 6.6 ± 0.8; t(19)=0.4350, p=0.6685 and 30 days: Cocaine, 8.0 ± 1.3; Saline, 6.2 ± 0.6; t(20)=1.338, p=0.1958].
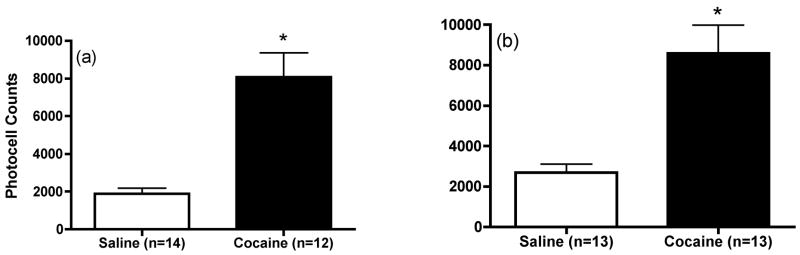
Figure 1.
Motor stimulant response to cocaine in animals that had previously received daily repeated saline or cocaine injections for microdialysis studies in Experiment 1 following (a) 1 day, (b) 7 days or (c) 30 days of withdrawal. Data represented as mean ± SEM photocell counts. *p< 0.05 compared to saline.
Figure 2.
The effects of the intra-mPFC infusion of LY341495 (0.1, 1 and 10 μM) on extracellular glutamate levels within the mPFC (a) 1 day, (b) 7 days or (c) 30 days following repeated saline or cocaine exposure. *p < 0.05 compared to saline animals and +p < 0.05 compared to baseline.
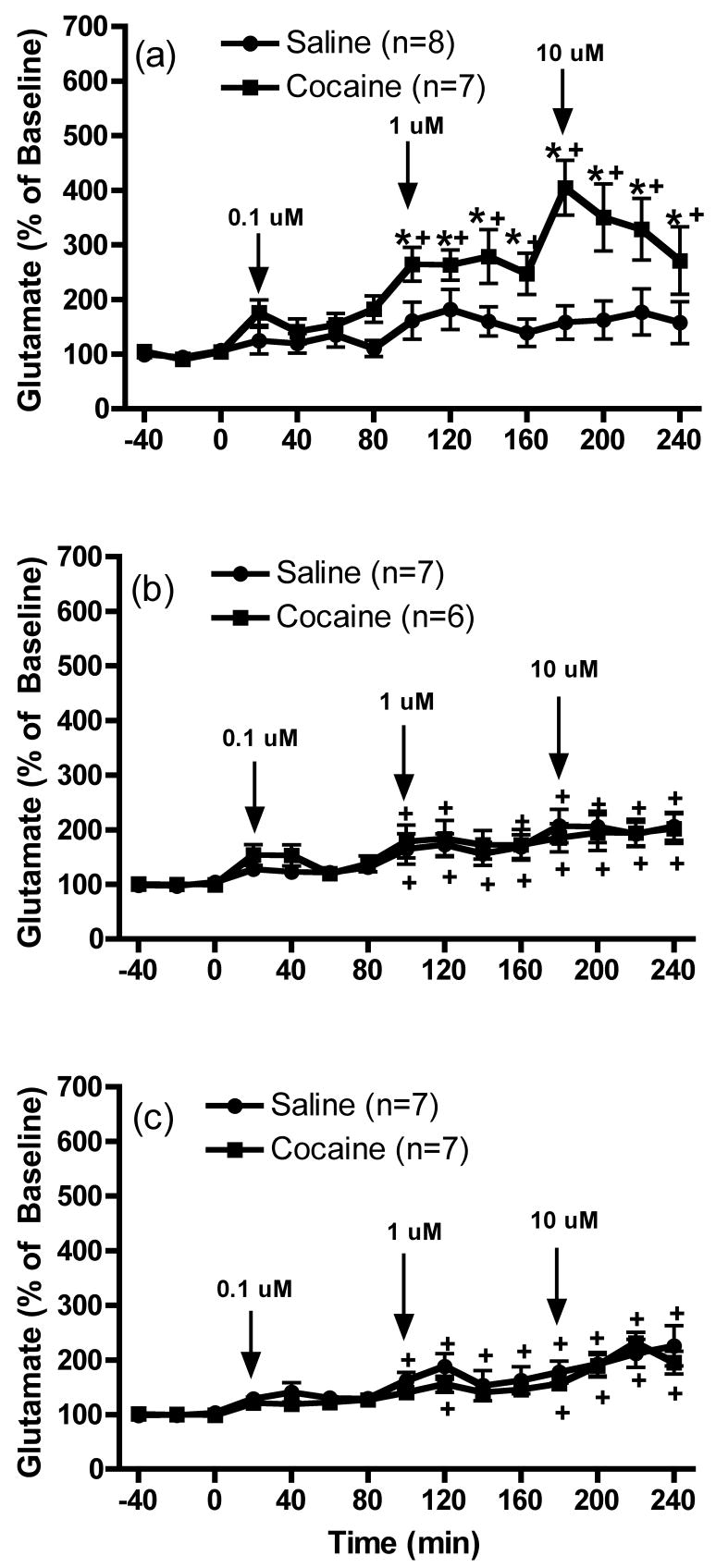
The effects of the infusion of LY341495 (0.1, 1 and 10 μM) into the mPFC on extracellular glutamate levels in the nucleus accumbens of animals that previously received repeated cocaine exposure are shown in Figure 3. The data were analyzed by two-way ANOVA with one repeated-measures followed by a modified least significant differences test (Milliken and Johnson 1984) and F scores were as follows: 1 day: treatment F(1,12)=10.416, p=0.0073; time F(14,168)=9.104, p<0.0001 and interaction F(14,168)=3.812, p<0.0001; 7 days: treatment F(1,11)=0.06, p=0.811; time F(14,154)=10.719, p<0.0001 and interaction F(14,154)=0.335, p=0.9884 and 30 days treatment F(1,12)=0.756, p=0.4016; time F(14,168)=14.574, p<0.0001; and interaction F(14,168)=0.508, p=0.9264. Intra-mPFC infusions of LY341495 produced a trend towards increased baseline glutamate levels in the nucleus accumbens of saline animals that achieved significance following prolonged withdrawal (7 or 30 days). Infusions of LY341495 into the mPFC produced a significant increase of extracellular glutamate concentrations in the nucleus accumbens of daily cocaine animals after 1 day of withdrawal, compared with daily saline animals (Figure 3a). In contrast to results in mPFC shown in Figure 2a, this significant effect was only observed at the 2 highest concentrations of LY341495. However, following 7 or 30 days of withdrawal (Figures 3b and 3c respectively), cocaine animals no longer showed a significant increase in glutamate levels following intra-mPFC infusion of LY341495 compared with control animals. Finally, significant differences in basal extracellular glutamate levels (pmol/20 min; mean ± SEM) were not observed between saline and cocaine animals [1 day: Cocaine, 3.2 ± 0.7; Saline, 4.7 ± 0.8; t(13)=1.396, p= 0.1860; 7 days: Cocaine, 5.3 ± 2.3; Saline, 2.9 ± 0.7; t(11)=1.081, p= 0.3030 and 30 days: Cocaine, 7.2 ± 1.8; Saline, 4.1 ± 1.3; t(12)=1.392, p=0.1891].
Figure 3.
The effects of the intra-mPFC infusion of LY341495 (0.1, 1 and 10 μM) on extracellular glutamate levels within the nucleus accumbens (a) 1 day, (b) 7 days or (c) 30 days following repeated saline or cocaine exposure. *p < 0.05 compared to saline animals and +p < 0.05 compared to baseline.
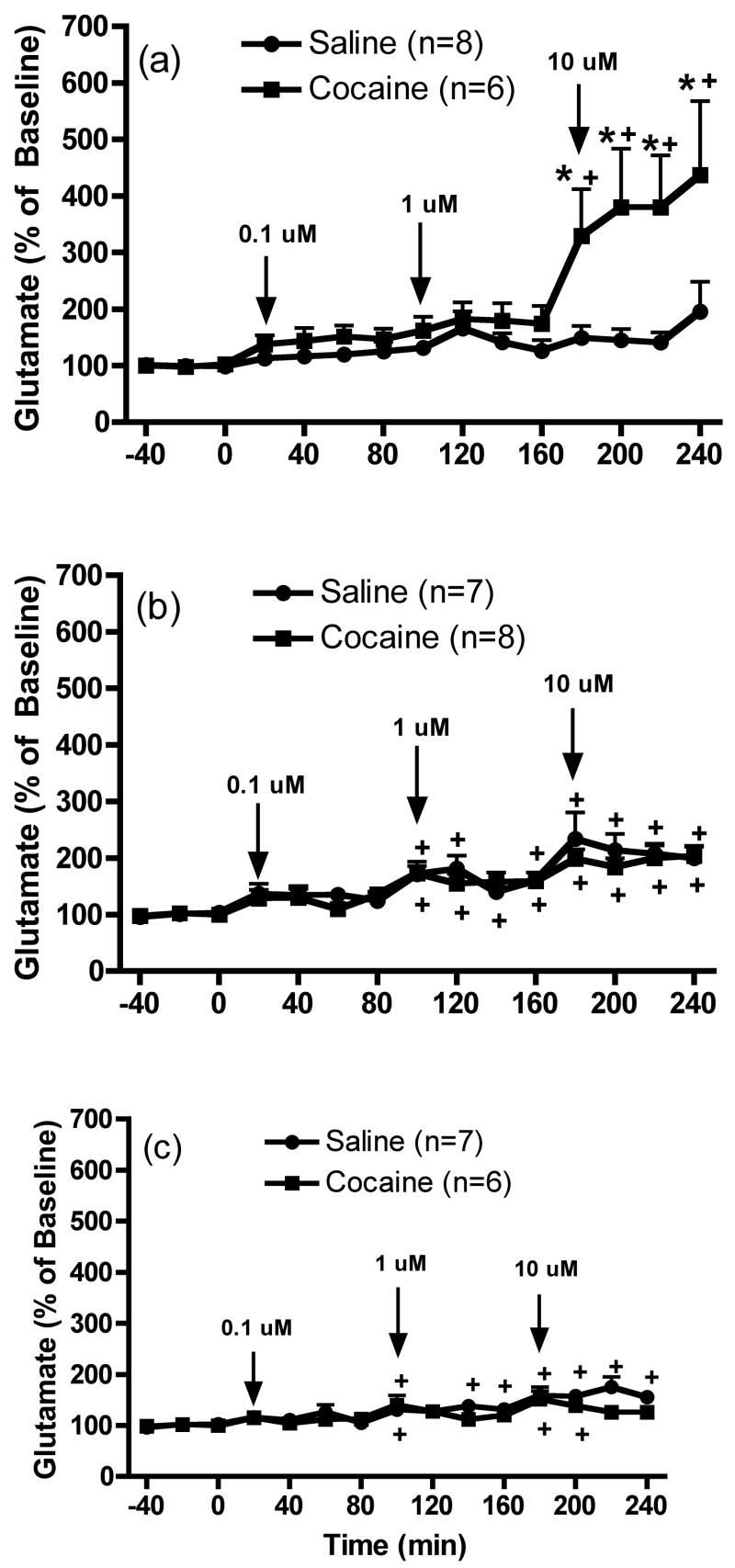
Figure 4 shows the effects of the intra-mPFC infusion of LY341495 (0.1, 1 and 10 μM) on extracellular glutamate levels in the VTA after withdrawal from repeated cocaine injections. The data were analyzed by two-way ANOVA with one repeated-measures followed by a modified least significant differences test (Milliken and Johnson 1984) and F scores were as follows: 1 day: treatment F(1,12)=6.689, p=0.0239; time F(14,168)=6.978, p<0.0001 and interaction effect F(14,168)=4.041, p<0.0001; 7 days: treatment F(1,13)=0.379, p=0.549; time F(14,182)=13.269, p<0.0001 and interaction F(14,182)=0.49, p=0.9363 and 30 days: treatment F(1,11)=1.136, p=0.3094; time F(14,154)=11.093, p<0.0001 and interaction F(14,154)=1.842, p=0.0371. Similar to the results in the mPFC and nucleus accumbens, intra-mPFC infusions of LY341495 induced a trend towards increased basal extracellular glutamate concentrations in the VTA in saline animals following 1 day withdrawal, which achieved significance in control animals following prolonged withdrawal (7 or 30 days). Intra-mPFC infusions of LY341495 produced a significant increase of glutamate levels in the VTA of cocaine animals after 1 day of withdrawal, compared with saline animals, but only at the highest concentration (10 μM) (Figure 4a). Furthermore, daily cocaine animals did not show a significant increase in VTA glutamate levels following intra-mPFC infusion of LY341495 after 7 or 30 days of withdrawal (Figures 4b and 4c, respectively) compared to controls. Finally, significant differences in basal extracellular glutamate levels (pmol/20 min; mean ± SEM) were not observed between saline and cocaine animals [1 day: Cocaine, 3.5 ± 0.8; Saline, 3.7 ± 0.8; t(12)=0.1386, p=0.8921; 7 days: Cocaine, 6.3 ± 2.2; Saline, 4.1 ± 1.2; t(13)=0.8804, p=0.3946 and 30 days: Cocaine, 6.7 ± 1.3; Saline, 4.4 ± 0.6; t(11)=1.599, p=0.1381].
Figure 4.
The effects of the intra-mPFC infusion of LY341495 (0.1, 1 and 10 μM) on extracellular glutamate levels within the VTA (a) 1 day, (b) 7 days or (c) 30 days following repeated saline or cocaine exposure. *p < 0.05 compared to saline animals and +p < 0.05 compared to baseline.
Experiment 2: Group II mGluR and mPFC glutamate release
After at least 1 week of recovery from surgery, animals received once daily systemic injections of saline or cocaine (15 mg/kg) over 4 consecutive days. After 1 or 7 days withdrawal, saline and cocaine animals were subjected to reverse dialysis with either KCl or cystine into the mPFC. Studies were conducted at those time points since previous studies have shown that repeated cocaine exposure enhanced cocaine induced glutamate overflow in the mPFC after 1 and 7, but not 30 days of withdrawal (Williams and Steketee 2004). Within 3 days following microdialysis experiments, animals were challenged with a systemic injection of cocaine. Figure 5 shows that the motor-stimulant response to cocaine was significantly enhanced in animals that had previously received daily cocaine injections [1 day: t(24)=5.064, p<0.05 and 7 days: t(23)=4.206, p<0.05].
Figure 5.
Motor stimulant response to cocaine in animals that had previously received daily repeated saline or cocaine injections for microdialysis studies in Experiment 2 following (a) 1 day or (b) 7 days withdrawal from repeated cocaine exposure. Data represented as mean ± SEM photocell counts. *p < 0.05 compared to saline.
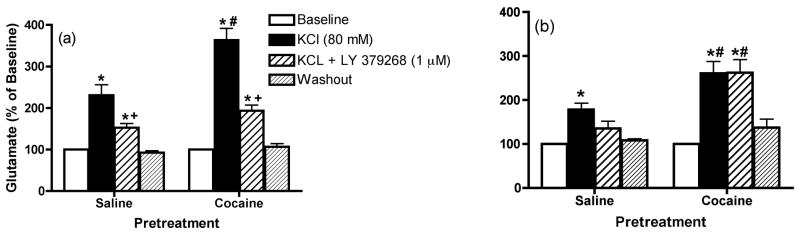
Figure 6 shows the effects infusion of KCl (80 mM) into the mPFC on glutamate levels in this region. The data were analyzed by two-way ANOVA with one repeated-measures followed by a modified least significant differences test (Milliken and Johnson 1984) and the F scores for these data were as follows: 1 day: treatment F(1,11)=12.581, p=0.0046; time F(14,154)=68.227, p<0.0001 and interaction F(14,154)=7.501, p<0.0001 and 7 days: treatment F(1,10)=15.88, p=0.0026; time F(14,140)=8.987, p<0.0001 and interaction F(14,140)=2.725, p=0.0014. These data show that KCl significantly increased extracellular glutamate concentrations above baseline in this region to a greater extent in cocaine-sensitized animals than controls (Figure 6a and 6b). In control animals, this effect was inhibited by intra-mPFC co-infusion of LY379268 (1 μM), an agonist of Group II mGluRs (Figures 6a and 6b). When KCl and LY379268 were co-infused into mPFC of sensitized animals, LY379268 significantly reduced the K+-induced glutamate release following 1 day withdrawal (Figure 6a) but not 7 days withdrawal (Figure 6b). Finally, no significant differences in basal extracellular glutamate levels (pmol/20 min; mean ± SEM) were observed between saline and cocaine animals [1 day: Cocaine, 4.4 ± 0.6; Saline, 5.7 ± 0.7; t(11)=1.409, p=0.1866 and 7 days Cocaine, 3.6 ± 0.7; Saline, 3.0 ± 0.9; t(10)=0.5576, p=0.5894].
Figure 6.
The effects of infusion of KCl (80 mM) into the mPFC on extracellular glutamate levels within this region (a) 1 day or (b) 7 days following repeated saline or cocaine exposure. *p < 0.05 compared to saline animals, +p < 0.05 compared with baseline and #p < 0.05 compared with comparable saline treatment.
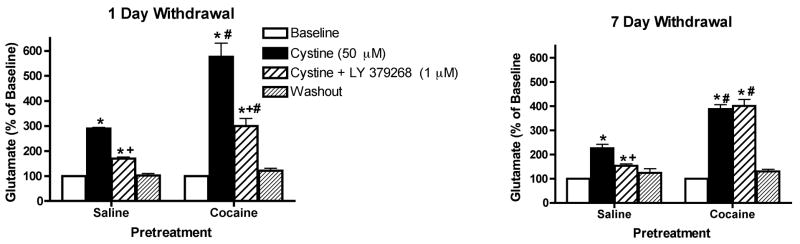
Figure 7 shows that infusion of cystine (50 μM) into the mPFC on glutamate levels in this region. The data were analyzed by two-way ANOVA with one repeated-measures followed by a modified least significant differences test (Milliken and Johnson 1984) and the F scores were as follows: 1 day: treatment F(1,11)=25.504, p=0.0004; time F(14,154)=103.994, p<0.0001 and interaction F(14,154)=20.471, p<0.0001 and 7 days: treatment F(1,12)=51.326, p<0.0001; time F(14,168)=69.644, p<0.0001 and interaction F(14,168)=24.636, p<0.0001. The data demonstrate that intra-mPFC cystine produced a significant increase above baseline in extracellular glutamate concentrations in this region in saline animals, an effect that was reduced by co-infusion of LY379268 (1 μM) into the mPFC (Figures 7a and 7b). Cystine-induced glutamate release was significantly increased in cocaine animals compared with saline animals (Figures 7a and 7b). When cystine and LY379268, were co-infused into mPFC, LY379268 significantly reduced cystine-induced glutamate release in cocaine animals following 1 day withdrawal (Figure 7a), but not 7 days withdrawal (Figure 7b). Finally, significant differences in basal extracellular glutamate levels (pmol/20 min; mean ± SEM) were not observed between saline and cocaine pretreated animals [1 day: Cocaine, 5.9 ±0.4; Saline, 9.1 ± 1.9; t(11)=1.509, p=0.1596 and 7 days: Cocaine, 6.8 ± 1.2; Saline, 4.6 ± 0.3; t(12)=1.797, p=0.0975].
Figure 7.
The effects of the infusion of cystine (50 μM) into the mPFC on extracellular glutamate levels within this region (a) 1 day or (b) 7 days following repeated saline or cocaine exposure. *p < 0.05 compared to saline animals, +p < 0.05 compared with baseline and #p < 0.05 compared with comparable saline treatment.
Histology
Figure 8 shows representative photomicrographs of dialysis probe sites in the mPFC, nucleus accumbens and VTA. Dialysis probes were located medial to the forceps minor corpus callosum with the dialysis membrane encompassing the infralimbic and prelimbic subregions of the mPFC (Figure 8a). In the nucleus accumbens, probe placements were medial to the anterior commissure with the majority of the membrane located in the core of the nucleus accumbens (Figure 8b). The probes in the VTA were in the region of the paranigral nuclei (Figure 8c). Animals with incorrect dialysis probe placements were removed from the study.
Figure 8.
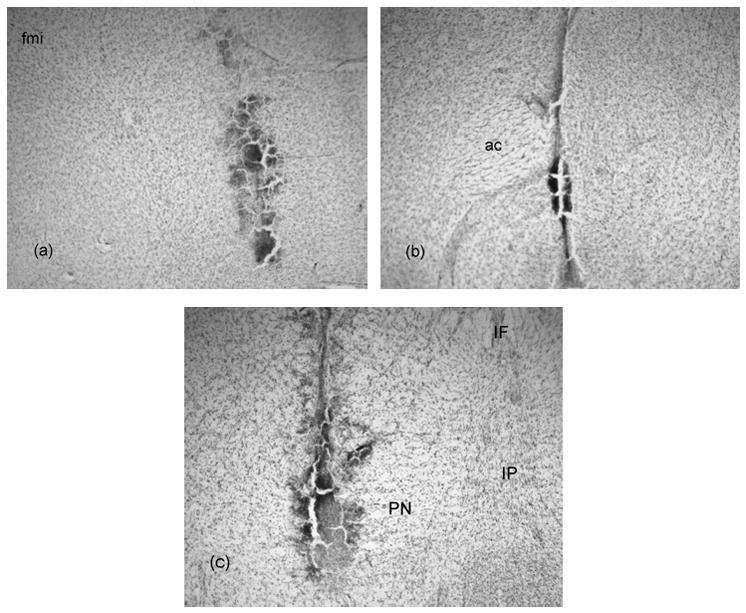
Representative photomicrographs of dialysis probe sites in the a) mPFC, b) nucleus accumbens and c) VTA. fmi= forceps minor corpus callosum, ac= anterior commissure, PBP= parabrachial pigmented nucleus, PN= paranigral nucleus and SN= substantia nigra.
Discussion
The present study demonstrated that intra-mPFC infusions of LY341495, an antagonist of Group II mGluRs increased basal glutamate levels in mesocorticolimbic brain regions including mPFC, nucleus accumbens and VTA. These results suggest that mPFC Group II mGluRs tonically inhibit mesocorticolimbic glutamate transmission. The ability of intra-mPFC LY341495 to increase glutamate levels in these brain regions was further augmented in cocaine-sensitized animals after 1 day, but not 7 or 30 days of withdrawal from repeated cocaine injections. These results indicate that the inhibitory tone maintained by Group II mGluRs in the mPFC on local, as well downstream targets of the mPFC, was transiently increased following short-term, but not long-term, cocaine withdrawal. Furthermore, results in the second experiment of the present study demonstrated that repeated cocaine exposure enhanced vesicular (K+-induced) and non-vesicular (cystine-induced) glutamate release in the mPFC to a greater extent in sensitized animals compared to controls. It was shown that release of glutamate from either of these pools was under the inhibitory influence of Group II mGluRs, an effect that was reduced following 7 days, but not 1 day of withdrawal from repeated cocaine. In general, these data suggest that a functional reduction of mPFC Group II mGluRs caused a loss of inhibitory control of excitatory drive within mPFC thereby resulting in enhanced excitatory output from mPFC to subcortical regions including the nucleus accumbens and VTA.
Previous studies have shown that infusion of a Group II mGluR antagonist into mPFC elevated glutamate levels, while infusion of an agonist was without effect (Melendez et al. 2005), suggesting that there is inhibitory tone regulating mPFC glutamate levels. The present study extends these findings in 2 ways. First it was shown that Group II mGluRs not only maintain inhibitory tone of mPFC glutamate transmission, but also glutamate transmission to down stream targets of the mPFC, including the VTA and nucleus accumbens. Second, this tonic inhibition could be transiently enhanced in sensitized animals as intra-mPFC infusions of LY341495 generated greater glutamate levels in mPFC, nucleus accumbens and VTA following 1 day of withdrawal from repeated cocaine exposure compared to controls. One caveat to these studies requires addressing. Infusion of LY341495 into the mPFC apparently was without effect in control animals tested one day after repeated saline injections. However, when the control data were analyzed with a one-way repeated measures ANOVA intra-mPFC LY341495 did produce a significant dose-dependent increase in glutamate levels in the mPFC, nucleus accumbens and VTA, similar to what was seen in the 7 day and 30 day groups. Thus, it appears that the increased variability contributed by the cocaine group masked the effects of LY341495 at the early time point.
It is unclear what mechanisms underlie the cocaine-induced transient increase in inhibitory tone provided by Group II mGluRs on both local, which involves, in part, altered autoreceptor function, and downstream, which involves altered postsynaptic regulation of pyramidal neurons, glutamate release. However, we have previously shown that cocaine-induced glutamate release in the mPFC is enhanced in sensitized animals (Williams and Steketee 2004). In addition, glutamate releasability was seen to be enhanced following short-term withdrawal from cocaine in the present study. However, these studies do not address whether basal glutamate levels are increased by repeated cocaine exposure. A previous study did demonstrate that basal glutamate levels in the mPFC were unaltered 3 weeks following repeated cocaine exposure (Baker et al. 2003). However, as the current and previous studies show, differences in mPFC neurotransmission exist when determined following short-term versus long-term withdrawal from cocaine (Williams and Steketee 2004; Jayaram and Steketee 2005; Williams and Steketee 2005). Yet, when examining data in the present study, basal glutamate levels never differed between saline and cocaine animals at any of the time points examined. Since these data were not collected using a no-net flux protocol, it is possible that basal glutamate levels are enhanced 24 hrs after repeated cocaine exposure, thereby providing enhanced tonic inhibition via pre- and postsynaptic Group II mGluRs.
Regardless of the underlying mechanisms, the enhancement in Group II mGluR-mediated tonic inhibition produced by repeated cocaine exposure is no longer apparent with longer withdrawals (7–30 days) from cocaine. In fact, following 7 days of withdrawal, the effects of LY341495 on glutamate levels in the mPFC were reduced in cocaine-sensitized animals compared to controls. These data suggested the inhibitory tone on presynaptic Group II mGluRs was reduced. This result is consistent with additional findings that a Group II mGluR agonist lost its ability to inhibit vesicular and non-vesicular release of glutamate in cocaine animals following 7 days of withdrawal. This reduction of inhibitory tone could be due to the functional reduction of Group II mGluR. In support of this hypothesis, previous studies have shown the G protein coupling of Group II mGluRs is reduced in the mPFC of cocaine-sensitized animals (Bowers et al. 2004). Also, injection of APDC, a Group II mGluR agonist, into the mPFC is no longer able to reduce cocaine-stimulated motor activity following longer withdrawals from repeated cocaine exposure (Xie and Steketee, unpublished observation).
It is well known that glutamatergic transmission from the mPFC to the nucleus accumbens and VTA is an important component in the expression of cocaine sensitization (Wolf 1998; Kalivas 2004). The results from the present study demonstrated that repeated cocaine exposure could alter mesocorticolimbic glutamate transmission, following short-term withdrawal, by increasing inhibitory tone on mPFC Group II mGluRs that modulate glutamate transmission to the VTA and nucleus accumbens. However, the effects of this alteration via acting on mPFC Group II mGluRs on specific glutamate transmission pathways within mesocorticolimbic dopamine system appear to be different. This is supported by the results from the present study that intra-mPFC infusions of the Group II mGluR antagonist LY341495 at the highest concentration produced enhanced glutamate levels in the VTA of cocaine sensitized animals, compared to controls, but the enhanced glutamate levels in the nucleus accumbens was initially induced at an intermediate concentration of LY341495. This could be explained by differences in the neuronal circuitry. For example, the glutamatergic projections from the mPFC to the VTA can be both direct and indirect via the pedunculopontine tegmentum (Sesack et al. 1989; Hurley et al. 1991).
While postsynaptic inhibitory tone on glutamatergic projections appears to be enhanced following short-term withdrawal from cocaine, it has returned to normal following longer withdrawals. This finding was surprising as it was hypothesized that inhibitory tone would be reduced as suggested by previous studies (Bowers et al. 2004; Huang et al. 2007; Xie and Steketee 2008). However, it is possible that rather than a long-term change in tone maintained by Group II mGluRs, repeated cocaine reduces the response to increased stimulation of these receptors in the mPFC. If this were true, then infusions of a Group II mGluR agonist into the mPFC should reduce mesocorticolimbic glutamate transmission more effectively in control animals compared to cocaine-sensitized animals. Data in the present study that showed that intra-mPFC infusion of LY379268 reduced evoked-glutamate release in control, but not sensitized animals, support this hypothesis. Also, exposure of mPFC Group II mGluRs to the agonists APDC or DCG-IV were less effective in manipulating behavioral and neurochemical responses to cocaine (Xie and Steketee, unpublished observation) or inducing long-term depression (Huang et al. 2007), respectively.
Extracellular glutamate within the mPFC arises from glutamatergic nerve terminals via vesicular release as well as from glia via cystine/glutamate antiporters (Melendez et al. 2005). It has been suggested that extracellular concentrations of glutamate measured via microdialysis reflect both of these sources of glutamate (Baker et al. 2002). Thus, we were prompted to examine whether repeated cocaine exposure produces specific alterations in the modulation of extracellular glutamate sources within the mPFC. One of the alterations demonstrated in the present study is that both vesicular (K+-induced) and non-vesicular (cystine-induced) glutamate release is enhanced in cocaine-sensitized animals compared to saline animals. In accordance with these findings, K+-induced glutamate release has also been shown to be increased by repeated amphetamine exposure (Stephans and Yamamoto 1995). Although, it was reported in a previous study that repeated cocaine treatment reduced activity of the cystine/glutamate antiporter in the nucleus accumbens (Baker et al. 2003), results from the present study suggested that the function of the mPFC cystine/glutamate antiporter appears to be intact following long term withdrawal from cocaine. In fact, the present study is the first to demonstrate that repeated drug exposure augments cystine-evoked glutamate release in the mPFC. Thus, the enhanced ability of cocaine to increase glutamate levels in the mPFC of sensitized animals (Williams and Steketee 2004) could also be due to the overall enhanced glutamate releasability from vesicular and non-vesicular pools. Importantly, while Group II mGluRs were capable of modulating vesicular and non-vesicular glutamate release in the mPFC, this modulation appears to be lost following prolonged withdrawal from repeated cocaine exposure. These data suggest that cocaine-sensitization might be associated with a reduction in Group II mGluR function in the mPFC.
Group II mGluRs are found in various combinations of presynaptic, postsynaptic and glial localizations that may reflect differential modulation of excitatory amino acid transmission (Petralia et al. 1996; Conn and Pin 1997). Although, the present study did not demonstrate a role of specifically localized Group II mGluRs in the development of cocaine sensitization, previous studies may provide some clues. For example, the functional reduction of Group II mGluRs, acting as autoreceptors in the nucleus accumbens, has been shown in animals that received repeated cocaine treatment (Xi et al. 2002). A more recent study demonstrated that the Group II mGluR-induced postsynaptic LTD was impaired in mPFC pyramidal neurons after repeated cocaine exposure (Huang et al. 2007). Furthermore, the present study also showed a functional reduction of mPFC Group II mGluRs that can inhibit the cystine/glutamate antiporter, which is mainly localized on glia. Taken together, it is reasonable to hypothesize that the functional reduction of mPFC Group II mGluRs is “universal” within this brain region.
To date, few studies that have been conducted to determine the mechanisms underlying the functional reduction of mPFC Group II mGluRs. Recently, expression of AGS3 protein was found to be enhanced in the mPFC of cocaine-sensitized animals (Bowers et al. 2004). AGS3, an activator of G protein signaling has been shown to interact with Giα subunits and disrupt G protein signaling by interfering with receptor coupling (De Vries et al. 2000; Natochin et al. 2000). Hence, this would prevent actions of receptors that couple to Gi proteins, such as Group II mGluRs. It should be noted that previous studies have also suggested that repeated cocaine exposure can reduce the function of other Gi protein coupled receptors, including D2 and GABAB receptors (Beyer and Steketee 2002; Steketee and Beyer 2005). It has been found that repeated cocaine exposure decreased G protein levels such as Giα and Goα but had no effect on the levels of Gsα and Gβ in subcortical regions including nucleus accumbens and VTA (Nestler et al. 1990). Therefore, disruption of Gi protein coupling via increased AGS3 expression could possibly enhance Gs protein coupling to some extent, which may be one of the reasons that repeated cocaine exposure enhances cAMP signaling pathways (Nestler et al. 2001). Therefore, the possible balance for Gi versus Gs coupled receptor signaling might be an important component in modulation of mPFC excitatory transmission in the development of cocaine sensitization.
Previous studies have demonstrated that repeated cocaine exposure is associated with a reduction in basal levels of glutamate in the nucleus accumbens (Baker et al. 2003; Jayaram and Steketee 2004). Thus, it was surprising that in the present study, basal nucleus accumbens glutamate levels did not differ between controls and cocaine sensitized animals. Since we have previously reported a reduction in nucleus accumbens glutamate following repeated cocaine injections, using the same treatment paradigm as the present report, this is unlikely to be the basis of the discrepancy (Jayaram and Steketee 2004). It should be noted that in the present studies no-net flux microdialysis was not used to determine basal glutamate levels. Also, the data were not corrected for differences in probe recovery that might, in part, explain the discrepancy with previous findings.
In conclusion, the present study demonstrated that long-term expression of sensitization to cocaine is associated with altered modulation of mesocorticolimbic glutamatergic transmission by mPFC Group II mGluRs. Thus, following early withdrawal from repeated cocaine exposure (1 day) tonic inhibition of mesocorticolimbic glutamate transmission appears to be enhanced. This result parallels previous results demonstrating that injection of APDC (2R,4R-4-aminopyrrolidine-2,4-dicarboxylate) into the mPFC reduced the expression of sensitization when tested 1 day after repeated cocaine exposure (Xie and Steketee, unpublished observation). More prolonged withdrawal form repeated cocaine (7 days) is associated with a reduction in the inhibitory influence of Group II mGluRs on vesicular and non-vesicular glutamate release in the mPFC. Whether this translates into reduced modulation of downstream (i.e. nucleus accumbens and VTA) glutamate transmission by cortical Group II mGluRs is not yet known. However, intra-mPFC APDC injections that blocked the expression of early sensitization were no longer capable of producing this effect following prolonged withdrawal (Xie and Steketee, unpublished observation). When taken with our previous research, the present findings suggest that sensitization to cocaine is paralleled by a long-term reduction in Group II mGluR function in the mPFC. These data add to a growing body of literature that suggests that sensitization might be associated with time-dependent changes in neurotransmission in the mPFC. Thus, early sensitization is most closely associated with changes in neurotransmitter release while late sensitization is most likely associated with changes in receptor function (see Steketee 2005 for a review).
Acknowledgments
This study was supported by a grant from the National Institute on Drug Abuse DA023215.
Abbreviations
- mGluR
metabotropic glutamate receptor
- mPFC
medial prefrontal cortex
- VTA
ventral tegmental area
References
- Baker DA, Shen HW. Cystine/glutamate exchange serves as the source for extracellular glutamate: modifications by repeated cocaine administration. Amino Acids. 2002;23:161–162. doi: 10.1007/s00726-001-0122-6. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nature Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Beyer CE, Steketee JD. Cocaine sensitization: modulation by dopamine D2 receptors in the rat medial prefrontal cortex. Cereb Cortex. 2002;12:526–535. doi: 10.1093/cercor/12.5.526. [DOI] [PubMed] [Google Scholar]
- Bowers MS, McFarland K, Lake RW, Peterson YK, Lapish CC, Gregory ML, Lanier SM, Kalivas PW. Activator of g protein signaling 3; a gatekeeper of cocaine sensitization and drug seeking. Neuron. 2004;42:269–281. doi: 10.1016/s0896-6273(04)00159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- De Vries L, Fischer T, Tronchere H, Brothers GM, Strockbine B, Siderovski DP, Farquhar MG. Activator of G protein signaling 3 is a guanine dissociation inhibitor for Galpha i subunits. Proc Natl Acad Sci USA. 2000;97:14364–14369. doi: 10.1073/pnas.97.26.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Yang PC, Lin H, Hsu KS. Repeated cocaine administration impairs group II metabotropic glutamate receptor-mediated long-term depression in rat medial prefrontal cortex. J Neurosci. 2007;27:2958–2968. doi: 10.1523/JNEUROSCI.4247-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Jayaram P, Steketee JD. Effects of repeated cocaine on medial prefrontal cortical GABAB receptor modulation of neurotransmission in the mesocorticolimbic dopamine system. J Neurochem. 2004;90:839–847. doi: 10.1111/j.1471-4159.2004.02525.x. [DOI] [PubMed] [Google Scholar]
- Jayaram P, Steketee JD. Effects of cocaine-induced behavioral sensitization on GABA transmission within rat medial prefrontal cortex. Eur J Neurosci. 2005;21:2035–2039. doi: 10.1111/j.1460-9568.2005.04000.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4:23–29. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Vuthiganon J, Kalivas PW. Regulation of extracellular glutamate in the prefrontal cortex: focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. J Pharmacol Exp Ther. 2005;314:139–147. doi: 10.1124/jpet.104.081521. [DOI] [PubMed] [Google Scholar]
- Milliken GA, Johnson DE. Analysis of Messy Data; Vol. 1, Designed Experiments. Lifetime Learning; Toronto, Ontario: 1984. pp. 326–337. [Google Scholar]
- Nasif FJ, Sidiropoulou K, Hu XT, White FJ. Repeated cocaine administration increases membrane excitability of pyramidal neurons in the rat medial prefrontal cortex. J Pharmacol Exp Ther. 2005;312:1305–1313. doi: 10.1124/jpet.104.075184. [DOI] [PubMed] [Google Scholar]
- Natochin M, Lester B, Peterson YK, Bernard ML, Lanier SM, Artemyev NO. AGS3 inhibits GDP dissociation from galpha subunits of the Gi family and rhodopsin-dependent activation of transducin. J Biol Chem. 2000;275:40981–40985. doi: 10.1074/jbc.M006478200. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci USA. 2001;98:11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Terwilliger RZ, Walker JR, Sevarino KA, Duman RS. Chronic cocaine treatment decreases levels of the G protein subunits Giα and Goα in discrete regions of rat brain. J Neurochem. 1990;55:1079–1082. doi: 10.1111/j.1471-4159.1990.tb04602.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2. Academic Press; New York: 1986. [Google Scholar]
- Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Steketee JD. Neurotransmitter systems in the medial prefrontal cortex: potential role in sensitization to psychostimulants. Brain Res Rev. 2003;41:203–228. doi: 10.1016/s0165-0173(02)00233-3. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Beyer CE. Intra-medial prefrontal cortex injections of baclofen blocks the initiation, but not the expression, of cocaine sensitization. Psychoparmacology. 2005;180:352–358. doi: 10.1007/s00213-005-2149-y. [DOI] [PubMed] [Google Scholar]
- Stephans SE, Yamamoto BK. Effect of repeated methampheamine administrations on dopamine and glutamate efflux in rat prefrontal cortex. Brain Res. 1995;700:99–106. doi: 10.1016/0006-8993(95)00938-m. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. The medial prefrontal cortex as a part of the brain reward system. Amino Acids. 2000;19:211–219. doi: 10.1007/s007260070051. [DOI] [PubMed] [Google Scholar]
- Williams JM, Steketee JD. Cocaine increases medial prefrontal cortical glutamate overflow in cocaine-sensitized rats: a time course study. Eur J Neurosci. 2004;20:1639–1646. doi: 10.1111/j.1460-9568.2004.03618.x. [DOI] [PubMed] [Google Scholar]
- Williams JM, Steketee JD. Time dependent effects of repeated cocaine administration on dopamine transmission in the medial prefrontal cortex. Neuropharmacology. 2005;48:51–61. doi: 10.1016/j.neuropharm.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Ramamoorthy S, Baker DA, Shen H, Samuvel DJ, Kalivas PW. Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J Pharmacol Exp Ther. 2002;303:608–615. doi: 10.1124/jpet.102.039735. [DOI] [PubMed] [Google Scholar]
- Xie X, Steketee JD. Repeated exposure to cocaine reduces the ability of medial prefrontal cortical group II metabotropic glutamate receptors to modulate behavioral and neurochemical responses to cocaine in rats. Psychopharmacology. 2008 Submitted. [Google Scholar]