Abstract
Purpose
To determine the contribution of tumor necrosis factor-alpha (TNFα)e pathogenesis of experimental Bacillus cereus endophthalmitis.
Methods
Experimental B. cereus endophthalmitis was induced in wild-type control (B6.129F1) or age-matched homozygous TNFα knockout mice (TNFα−/−, B6.129S6-Tnftm1Gk1/J). At various times postinfection, eyes were analyzed by electroretinography, and harvested for quantitation of bacteria, myeloperoxidase, proinflammatory cytokines and chemokines, and histological analysis.
Results
B. cereus replicated more rapidly in eyes of TNFα−/− mice compared to that of B6.129F1 eyes. Retinal function decreased more rapidly in eyes of TNFα−/− mice compared to that of B6.129F1 eyes. Retinal layers were not as structurally intact at 6 and 12 h postinfection in TNFα−/− eyes compared to that of B6.129F1 eyes. Histological analysis suggested less PMN infiltration in the vitreous of TNFα−/− mice than in B6.129F1 mice. B6.129F1 eyes also had greater myeloperoxidase concentrations than did eyes of TNFα−/− mice. In general, concentrations of proinflammatory cytokines and chemokines (IL-1β IL-6, and MIP-1α) were greater in eyes of TNFα−/− mice than in B6.129F1 eyes.
Conclusions
TNFα is important to intraocular pathogen containment by PMN during experimental B. cereus endophthalmitis. In the absence of TNF α, less PMN migrated into the eye, facilitating faster bacterial replication and retinal function loss. Although greater concentrations of proinflammatory cytokines were synthesized in the absence of TNFα, the resulting inflammation was less, and an equally devastating course of infection occurred.
Keywords: Bacillus cereus, endophthalmitis, inflammation, mouse, retina
INTRODUCTION
Bacillus cereus is feared as an ocular pathogen because of its ability to blind rapidly during endophthalmitis.1–4 B. cereus endophthalmitis often results in significant vision loss or loss of globe architecture in 1 to 2 days. Several reports have credited the pathogenesis of B. cereus and other severe forms of bacterial endophthalmitis to toxins produced by the offending strain.5–11 However, the intraocular inflammatory response can be just as hazardous. Intraocular inflammation during endophthalmitis can be transient, as in the case of infection with avirulent organisms, or can evolve rapidly, as occurs during B. cereus endophthalmitis.1
The primary function of innate immunity is to detect invading pathogens and clear them as quickly as possible. During an acute intraocular infection, a primary and essential component of this response is neutrophil influx. Cellular infiltration in human endophthalmitis cases has been described as vitritis, the presence of a hypopyon, and corneal ring abscess formation. Experimental models have identified polymorphonuclear leukocytes (PMN) as the primary infiltrating cell type during bacterial endophthalmitis.12–15 The recruitment and activation of neutrophils within an infected eye is a biological dilemma. PMN infiltration is necessary for bacterial clearance, but the generation of toxic reactive oxygen intermediates and other inflammatory mediators by PMN may result in bystander damage to delicate tissues of the retina. Robust inflammation is a hallmark of endophthalmitis caused by B. cereus and other types of virulent bacteria. In experimental B. cereus endophthalmitis, inflammatory cells were observed in the posterior chamber in close proximity to the optic nerve head as early as 4 h postinfection.13 Further analysis confirmed that the primary infiltrating cell was the PMN. The numbers of CD18+/Gr-1+ PMN were minimal at 4 and 6 h postinfection, but increased significantly thereafter. The influx of CD18+/Gr-1+ PMN into the posterior segment occurred simultaneously with the increase of TNFα in the eye at approximately 4–6 h postinfection.13 Despite their potential importance, the roles of TNFα and several other cytokines in endophthalmitis remain unexplored.
TNFα is a potent mediator of acute inflammatory reactions via activation of proinflammatory signaling cascades. TNFα is a cytokine secreted by macrophages and neutrophils, and is important in upregulating cell adhesion expression on vascular endothelial cells. TNFα also stimulates mononuclear phagocytes to produce cytokines, such as IL-1, IL-6 and itself. 16 In an experimental rat model of S. aureus endophthalmitis, TNFα, IL-1β, and CINC (rat homologue of CXCL8) were detected in the vitreous within 6 h of intravitreal inoculation.14 The authors hypothesized that upregulation of proinflammatory cytokines may have contributed to the breakdown of the blood-retina barrier and the recruitment of neutrophils into the eye. Upregulation of TNFα, IL-1β, and IFNγ; has also been detected in experimental S. epidermidis endophthalmitis.17 Injection of TNFα into the vitreous of rabbits18 and rats19 induced vascular permeability and cellular infiltration. Studies have also demonstrated upregulation of TNFα and other proinflammatory cytokines in experimental autoimmune uveoretinitis.20 No studies have quantified cytokines or chemokines in the human eye during endophthalmitis, but based on experimental studies, it is reasonable to hypothesize that proinflammatory cytokines are key mediators of acute inflammation during this infection.
The inflammatory pathways involved in B. cereus-induced intraocular inflammation remain to be fully elucidated. However, such a rapid response strongly suggests that acute phase mediators and immune cells are involved. As stated above, TNFα is upregulated in the eye in parallel with influx of PMN under experimental conditions13, but the contribution of TNFα to the pathogenesis of endophthalmitis has not been determined. We hypothesized that TNFα is an important proinflammatory cytokine that contributes to recruitment of PMN into the eye during experimental endophthalmitis. To test this hypothesis, we analyzed infection in wild-type control and homozygous TNFα knockout mice. The results demonstrated that TNFα was important in bacterial growth control via the acute inflammatory response to B. cereus endophthalmitis. In the absence of TNFα, inflammation was muted, resulting in more rapid bacterial replication and retinal function loss. Compensating proinflammatory cytokines and chemokines were synthesized in the eye in the absence of TNFα, resulting in less inflammation, but an equally devastating course of infection.
METHODS
Mice and Infections
Breeding pairs of background mice (B6.129F1) and homozygous TNFα−/− mice (B6.129S6-Tnftm1Gk1/J)21 were obtained from Jackson Laboratories (Bar Harbor, ME). C57BL/6J mice were also used for comparisons with B6.129F1 mice for some experiments. Mice were bred and cared for in housing facility conditions according to institutional guidelines and guidelines provided by the Association for Research in Vision and Ophthalmology. Male and female mice from the breeding colonies were used between 6–8 weeks of age, with the appropriate age-matched controls. Polymerase chain reaction (PCR) was performed to confirm the homozygosity of littermates (data not shown).
Mice were infected intravitreally with wildtype B. cereus as previously described.13 Briefly, mice were anesthetized generally with a ketamine/xylazine cocktail (85 mg/kg body weight, Ketaved [Phoenix Scientific, St. Joseph MO]/14 mg/kg body weight, Rompun [Bayer Corp., Shawnee Mission KS]) and topically with 0.5% proparacaine HCl (Ophthetic, Allergan, Hormigueros, Puerto Rico). Bacteria were injected into the mid-vitreous with a sterile glass capillary needle containing 100 CFU B. cereus strain ATCC 14579 in 0.5 μL brain heart infusion (BHI) medium. At various times postinfection, endophthalmitis was analyzed by biomicroscopy, quantitation of intraocular bacterial growth, proinflammatory cytokines and chemokines, and myeloperoxidase (MPO, to estimate PMN infiltration), and electroretinography, as described below.
Electroretinography
Retinal function was assessed by electroretinography as previously described.13 Following injection of B. cereus, mice were dark-adapted for at least 6 h. Prior to ERG, mice were then anesthetized with ketamine/xylazine as described above and pupils were dilated with 10% topical phenylephrine (Akorn, Inc., Buffalo Grove IL). Gold-wire electrodes were placed on each cornea and a reference electrode was placed in the mouth. The stimulus used to evoke the response was delivered by a white sphere that mimicked a Ganzfeld. The interval between 2 flashes (10-ms duration) was 60 s to prevent light adaptation. A-wave and B-wave amplitudes were measured from the initiation of the light flash to the trough of the A-wave, and the trough of the A-wave to the peak of the B-wave, respectively. A total of five readings were recorded and averaged. Percentages of retinal function retained compared with controls were calculated as described previously.13 Values represent the mean ± standard error of the mean (SEM) for N ≥ 6 samples per time point.
Bacterial Growth
Globes were homogenized with 1-mm sterile glass beads (BioSpec Products, Inc., Bartlesville OK) in 400 μl of PBS. Bacteria were quantified by track plating serial 10-fold dilutions onto BHI agar.13,22 Values represent the mean ± SEM for N ≥ 5 eyes per time point.
Cytokines and Chemokines
Eyes were analyzed for the presence of representative proinflammatory cytokines and chemokines shown to be upregulated in various experimental models of ocular infection and inflammation.13,14,17,23–27 Globes were removed and homogenized with 1-mm glass beads in a protease inhibitor cocktail (Triton X-100, 0.5 M EDTA, 10 mM sodium orthovanadate [Sigma, St. Louis MO] and Protease Inhibitor [Calbiochem, La Jolla, CA] in PBS, pH 7.4). Supernatants were then analyzed for IL-1β, MIP-1α, KC, and IL-6 by ELISA (Quantikine Kits, R&D Systems, Minneapolis MN) according to manufacturers instructions. Concentrations in supernatants of tissue homogenates were compared to that of a standard curve. Values represent the mean ± SEM for N=6 eyes per time point.
PMN and Myeloperoxidase
To compare the numbers of circulating PMN, whole blood was harvested and PMN were quantified with a hemocytometer. Values represent the mean ± SEM for N=3 mice per genotype.
To estimate the extent of PMN infiltration into the eye, MPO was quantified.13 Mouse eyes were removed, homogenized with 1-mm glass beads in a lysis buffer (200 mM NaCl, 5 mM EDTA, 10 mM Tris, 10% glycine [vol/vol], 1 mM PMSF, 1 μg/ml leupeptide, 28 μg/ml aprotinine) and supernatants were analyzed for MPO levels by sandwich ELISA (Mouse MPO ELISA Test Kit, Cell Sciences, Canton MA). Values represent mean ± SEM for N ≥ 6 eyes per timepoint.
Histology
Globes were harvested and eyes were fixed in Perfix and incubated for 24 h. Globes were embedded in paraffin, sectioned, and stained with hematoxylin and eosin stain by standard procedures. Histology sections were scored by a masked observer and graded on a scale of 0 to 4+ in terms of severity.13 Sections presented are representative of N=3 eyes per time point.
Anti-TNFα and B. cereus Endophthalmitis
A pilot study was undertaken to analyze the potential anti-inflammatory effects of anti-TNFα (infliximab, Remicade®, Centocor Inc.). 50 ng/0.5 μl of anti-TNFα was injected immediately prior to B. cereus infection. MPO concentrations (N=3) were analyzed at 10 h postinfection.
Statistics
Student’s t-test was used for statistical comparisons between mouse strains at each time point. Wilcoxon’s rank sum test was used for statistical comparison between infection groups. A P ≤ value 0.05 was considered significant.
RESULTS
Bacterial Growth
B. cereus grew logarithmically in eyes of B6.129F1 wild-type controls and TNFα−/− mice, as well as in eyes of C57BL/6J background control mice (Figure 1). The rates of growth in eyes of C57BL/6J and B6.129F1 wild-type controls were similar (P>0.5 at all time points). However, B. cereus grew faster in eyes of the TNFα−/− strain, with greater numbers of viable B. cereus recovered per eye at 6, 8, and 12 h postinfection compared to that recovered from B6.129F1 wild-type control eyes (P<0.05). In the absence of TNFα, faster intraocular bacterial replication occurred.
Figure 1. Bacterial growth during experimental B. cereus endophthalmitis.
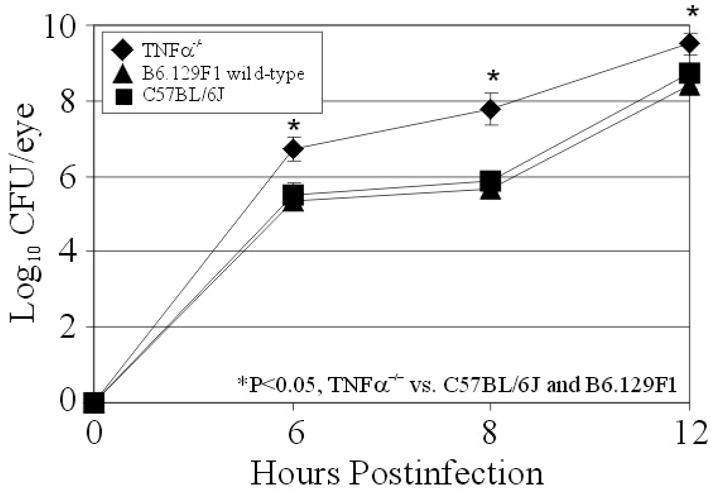
C57BL/6J, B6.129F1 wild-type, and TNFα−/− mouse eyes were injected with 100 CFU of B. cereus. Eyes were harvested, homogenized, and analyzed for bacterial growth. B. cereus grew to higher concentrations in infected eyes of TNFα−/− mice than in eyes of wild type or background mice (P<0.05 at all time points). Values represent the mean ± SEM of N ≥ 5 eyes per time point.
Retinal Function
Retinal function analysis of endophthalmitis in eyes of TNFα−/− and B6.129F1 wild-type mice is summarized in Figure 2. A- and B-wave amplitudes declined at significantly greater rates in infected eyes of TNFα−/− mice compared to that observed in eyes of B6.129F1 mice (P<0.01 at 6 and 8 h postinfection). Taken together, retinal function loss in eyes of the TNFα−/− mice was approximately 3-fold greater at 6 h than that of eyes of wild-type B6.129F1 mice. By 12 h postinfection, retinal function was < 5% in all infected eyes of either mouse strain. In the absence of TNFα, retinal function declined more rapidly, likely due to increased intraocular bacterial replication.
Figure 2. Retinal function analysis during B. cereus endophthalmitis.

B6.129F1 wild-type and TNFα−/− mouse eyes were injected with 100 CFU of B. cereus. Retinal function was assessed by electroretinography. At 6 h and 8 h postinfection, the A- and B-wave amplitudes retained were significantly less in TNFα−/− eyes than that of B6.129F1 eyes. By 12 h postinfection, retinal function was nearly abolished in all infected eyes. Values represent the mean ± SEM of N ≥ 6 eyes per time point.
Whole Blood PMN and Intraocular Inflammation
PMN were quantified in whole blood of TNFα−/− and B6.129F1 wild-type mice. Manual counts detected similar numbers of PMN from the blood of TNFα−/− mice (6.92 ± 0.05 log10 PMN/ml) and wild type mice (6.84 ± 0.1 log10 PMN/ml; P=0.052).
Intraocular inflammation was analyzed by quantifying MPO of infiltrating PMN (Figure 3) and proinflammatory cytokines and chemokines (Figure 4) in eyes during endophthalmitis. At 0 h and 4 h postinfection, MPO concentrations were similar in TNFα−/− and B6.129F1 eyes (P>0.05). MPO concentrations of TNFα−/− and B6.129F1 eyes were greater at 4 h postinfection than at 0 h postinfection (P<0.01), with significant increases within each group at each time point thereafter. MPO concentrations were significantly less in eyes of TNFα−/− mice compared to that of eyes of B6.129F1 mice at 6, 8, and 12 h postinfection (P<0.05 at all time points). In the absence of TNFα, PMN influx into the infected eyes of TNFα−/− mice was less than that of infected wild-type eyes.
Figure 3. Infiltration of PMN into mouse eyes during B. cereus endophthalmitis.

B6.129F1 wild-type and TNFα−/− mouse eyes were injected with 100 CFU of B. cereus. PMN infiltration was analyzed by quantifying myeloperoxidase (MPO) in whole eyes. MPO concentrations were greater in B6.129F1 eyes than in TNFα−/− eyes at 6, 8, and 12 h postinfection (P<0.05), suggesting greater numbers of PMN in wild-type eyes than in TNFα−/− eyes. Values represent the mean ± SEM for N ≥ 6 per group.
Figure 4. Proinflammatory cytokine expression during B. cereus endophthalmitis.

B6.129F1 wild-type and TNFα−/− mouse eyes were injected with 100 CFU of B. cereus. Whole eyes were analyzed for proinflammatory cytokines and chemokines by ELISA. In general, levels of MIP1α, IL-6, KC, and IL-1β were greater in TNFα−/− mouse eyes than in B6.129F1 eyes during infection. Values represent the mean ± SEM of N = 6 eyes per time point.
In general, proinflammatory cytokine and chemokine concentrations were greater in TNFα−/− eyes than in wild-type B6.129F1 eyes. In TNFα−/− eyes, concentrations of KC were greater than in eyes of B6.129F1 at 4, 8, and 12 h postinfection (P<0.05 at all time points). Concentrations of MIP-1α and IL6 were greater in TNFα−/− eyes than that of B6.129F1 eyes at 8 and 12 h postinfection (P<0.05). Concentrations of IL-1βwere greater in TNFα−/− eyes than in B6.129F1 eyes at 12 h only (P<0.05). Upregulation of other proinflammatory cytokines and chemokines in the absence of TNFα resulted in lower numbers of infiltrating PMN in infected eyes of TNFα−/− mice.
Histology
Whole eye and retinal histology of infected TNFα−/− and B6.129F1 wild-type eyes demonstrated evolving endophthalmitis similar to that previously reported in this model13 (Figure 5). At 6 h postinfection in B6.129F1 wild-type eyes, the majority of infiltrating PMN observed were located in close proximity to the optic nerve head (histology score ranging from 1+ to 2+). At this time, retinal architecture was slightly disrupted (histology score of 1+). At 12 h postinfection, significant loss of retinal architecture was observed and significant numbers of PMN were seen in the posterior segment, near the ciliary body, and in the anterior chamber (histology scores ranging from 2+ to 3+). At 6 h postinfection in TNFα−/− mouse eyes, very few PMN were seen in the posterior segment (histology score ranging from 0 to 1+), and retinal architecture was disrupted to a greater degree compared to that of wild-type eyes (histology score of 2+). By 12 h postinfection, retinas of infected eyes of TNFα−/− mice were disrupted and detached, with great numbers of B. cereus accumulating near the retina and optic nerve, and PMN were present in both segments (histology score ranging from 3+ to 4+). In TNFα−/− mice, retinal disruption evolved more rapidly, but PMN influx was less than that seen in B6.129F1 wild-type mice.
Figure 5. Whole organ (top) and retinal (bottom) histology of B. cereus endophthalmitis.

B6.129F1 wild-type and TNFα−/− mouse eyes were injected with 100 CFU of B. cereus. Eyes were harvested and processed for hematoxylin and eosin staining. In B6.129F1 eyes, retinas remained essentially intact but inflammation was significant during infection. In TNFα−/− eyes, retina were disrupted to a significant degree by 6 h postinfection, but inflammation was less than that observed in B6.129F1 eyes. Sections are representative of N = 3 per group. CH, choroid; RPE, retinal pigment epithelium; PCL, photoreceptor layer; ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; VIT, vitreous.
Magnification, ×20 (top) and ×200 (bottom).
Anti-TNFα and B. cereus Endophthalmitis
At 10 h postinfection, MPO concentrations were decreased by approximately 40% in eyes injected with 50 ng anti-TNFαalone compared to that of uninjected control eyes.
DISCUSSION
Pathogen recognition and a well-regulated inflammatory response to infection are essential in clearing invading organisms with minimal damage to surrounding tissue. A tightly controlled response is even more critical in the eye, where non-regenerative cells and tissues responsible for vision reside. Experimental models of bacterial endophthalmitis have demonstrated that once a pathogen is introduced into the posterior segment, an acute response occurs, including synthesis of proinflammatory cytokines and influx of PMN into the eye.12–14 In the case of virulent pathogens such as S. aureus or B. cereus, low numbers of bacteria can be cleared effectively by an adequate inflammatory response.13,28 Once an inoculum threshold is passed, bacterial growth and toxin production overwhelm the inflammatory response. In an exhaustive attempt to clear the infection, PMN fill the posterior and anterior segments.
Regulation of inflammation is the key to removing the pathogen without harming the eye, but bystander damage from infiltrating cells can occur. For S. aureus endophthalmitis, depletion of neutrophils early in the inflammatory response reduced the severity of host inflammation, but severely hampered bacterial clearance, resulting in a more severe infection.12 In the present study, depression of initial cellular influx in the absence of TNFα resulted in higher bacterial numbers and faster retinal function loss. Explosive inflammation is characteristic of B. cereus endophthalmitis. However, due to rapid bacterial growth, migration, and toxin production by B. cereus in the eye1, attempts at infection control by the host are typically futile.
We reported that one of the earliest cytokines detected during experimental B. cereus endophthalmitis was TNFα.13 TNFα was detected in whole eyes during experimental B. cereus endophthalmitis as early as 4 h postinfection, a time when PMN were first observed infiltrating into the posterior segment. During many different types of infections, TNFα initiates a cascade of proinflammatory cytokine synthesis and contributes to increased vascular permeability and upregulation of cell adhesins, effectively recruiting macrophages and neutrophils to the infection site. Because TNFα levels increased in parallel with increasing numbers of intraocular PMN during experimental B. cereus endophthalmitis, we sought to determine to what extent TNFα contributed to this initial inflammatory response by comparing infections in wild-type and TNFα−/− mice. Studies of non-ocular infections in TNFα−/− or TNFα-receptor knockout mouse strains have demonstrated the value of TNFα in containment of a wide range of ocular pathogens, including S. aureus29,30, Candida31, and pneumococcus32,33.
To ensure that experimental endophthalmitis was reproducible in mouse strain B6.129F1, the wild-type strain used in these studies, bacterial growth rates and pathology were compared to that of C57BL/6J mice. Ocular TNFα concentrations were similar in these two mouse strains during experimental infection (data not shown). Retinal function loss and MPO levels in B6.129F1 eyes were comparable to that of C57BL/6J eyes, as reported previously.13 Intraocular B. cereus growth and clinical signs of infection were similar at all time points, further validating the use of this genetic background for these studies.
B. cereus grew more rapidly and to greater numbers in eyes of TNFα−/− mice than in eyes of B6.129F1 wild-type mice. As expected, retinal function declined more rapidly in TNFα−/− mice than in B6.129F1 mice. Histological evidence demonstrated that retinas were damaged and detached to a greater degree in TNFα−/− eyes than in eyes of B6.129F1 mice. These results suggested that in the absence of TNFα bacterial growth was unimpeded, facilitating greater retinal damage and function loss. Greater numbers of bacteria likely resulted in higher concentrations of toxins produced in the eye, resulting in faster retinal damage and loss of function. The importance of toxins to the intraocular virulence of B. cereus endophthalmitis has been well-documented.1,7–9
In infected TNFα−/− mouse eyes, inflammation was muted compared to that of eyes of B6.129F1 mice. PMN migrated in fewer numbers into the eyes in TNFα−/− mice than in B6.129F1 mice, as demonstrated by MPO assay and histology. Taken together, these data indicated that in the absence of TNFα, fewer PMN migrating into the posterior segment resulted in higher intraocular bacterial loads and subsequently, more significant retinal damage. This result would suggest that TNFα contributed to the early recruitment of PMN into the eye and subsequent pathogen control during endophthalmitis. By virtue of its role in affecting blood-retinal barrier integrity34,35, the absence of TNFα may have resulted in less barrier permeability and migration of fewer PMN into the eye. The absence of TNFα has been demonstrated to decrease tight junction-associated permeability36,37 and result in less PMN infiltration36 in experimental models of acute lung inflammation and restraint stress (small intestine analyzed). Manual counts detected similar numbers of PMN from the blood of TNFα−/− and B6.129F1 mice, so differences in intraocular PMN quantities were not the result of differences in whole blood PMN between the mouse strains. Our PMN numbers in TNFα−/− mice were similar to those reported by Kuprash et al.38 who detected statistically higher whole blood white blood cells and neutrophils in TNFα−/− mice compared to that of C57BL/6 mice. Since the numbers of PMN were greater at 4 h postinfection in eyes of both mouse strains than that of freshly-infected eyes at 0 h, PMN quantities at 4 h likely represent cells that were recruited to the eye as a result of infection.
In the absence of TNFα, other proinflammatory cytokines and chemokines were synthesized during infection to facilitate recruitment of PMN into the eye. In this study, KC was detected at 4 h postinfection, and higher concentrations of KC were detected in TNFα−/− eyes than in B6.129F1 eyes. Because KC and MPO levels paralleled one another and increased beginning at 4 h postinfection in a manner similar to that of TNFα in C57BL/6J mice13, KC may also be an important recruiting cytokine in the eye during the initial stages of experimental B. cereus endophthalmitis. KC and its homologs have been detected in experimental and human ocular infections, including keratitis caused by fungi39, Adenovirus40, Pseudomonas24,41, and Staphylococcus42, acute bacterial conjunctivitis43, and in human uveitis cases44. Further studies in transgenic mice deficient in KC can confirm the contribution of this cytokine to the pathogenesis of bacterial endophthalmitis.
In terms of proinflammatory cytokine synthesis, IL-6, KC, MIP-1α, and IL-1β were synthesized to higher levels in TNFα−/− mice than in B6.129F1 mice. IL-1β and IL-6 were below the limits of detection at 4 h postinfection, but were detected at 8 h in eyes of both mouse strains, 4 h later than the initial influx of PMN into the posterior segment. MIP-1α levels were just above the limit of detection at 4 h postinfection, but levels increased therefter. IL-1β levels were significantly higher in TNFα−/− eyes than in B6.129F1 eyes at 12 h only. Higher concentrations of cytokines/chemokines may be synthesized to compensate for the absence of the most potent recruiting cytokine, TNFα. However, greater cytokine concentrations may not necessarily translate to greater numbers of PMN in the eye, as was demonstrated in this model, particularly if the absence of TNFα resulted in a greater impermeability of the blood-ocular barrier. Studies have demonstrated the production of increased levels of other proinflammatory cytokines/chemokines in the absence of TNFα in experimental models of infection and inflammation.29,45–47 Because IL-6, MIP-1α, and IL-1β were produced at a later stage of infection when significant numbers of PMN were already present in the eye, these cytokines could be a product of the infiltrating inflammatory cells themselves and perhaps played only a minor role in initial PMN recruitment. However, PMN are not the only potential source of cytokines in the eye during intraocular inflammation. Other resident ocular cells, such as retinal or optic nerve head glia and microglia, may also synthesize cytokines/chemokines. These particular cell types have been shown to synthesize proinflammatory cytokines during various states of infection, inflammation, and retinal stress.48–53 The specific cells involved in cytokine and chemokine synthesis during intraocular bacterial infection are yet to be identified.
Using single-gene knockouts in mediators of the host response is sufficient for analyzing a deficiency in one mediator, but this approach has its disadvantages. For example, the same types of cells synthesize IL-1β and TNFαThese cytokines act on comparable cell types during inflammation and also signal through the NFκB pathway. Hypothetically, the absence of one cytokine may induce synthesis of other compensating cytokines (in a manner similar to that seen in this study), confounding the role of the original cytokine of interest in infection. Functional redundancy has been reported for TNFα and IL-1 in experimental autoimmune uveitis, where deletion of receptors for both cytokines were more effective in reducing infiltrating cell numbers than deleting each receptor alone.54 In the context of experimental B. cereus endophthalmitis, IL-1β was first detected well after TNFα and KC were initially detected and PMN were present the posterior segment, suggesting a minimal role for IL-1β during the initial stages of inflammation.
Because the absence of TNFα was demonstrated in this infection model to dampen the initial inflammatory response during B. cereus endophthalmitis, it was of interest to analyze whether therapy targeting TNFα would effectively attenuate inflammation. Our preliminary data demonstrated the anti-inflammatory potential of anti-TNFα when injected immediately prior to B. cereus infection. Infliximab has attenuated intraocular inflammation in experimental models of choroidal neovascularization55,56 and endotoxin-induced uveitis57, and in human uveitis patients.58–62 Infliximab was recently shown to be non-toxic at levels up to 1.7 mg in rabbit eyes.63 These findings suggest the potential for attenuation of inflammation during endophthalmitis by targeting TNFα and perhaps other cytokines, but this sort of therapy would likely be best suited for the initial stages of infection.64 Continuing studies will determine the therapeutic potential of cytokine targeting in conjunction with early antibiotic treatment in reducing inflammation during bacterial endophthalmitis.
Acknowledgments
This work was presented at the Association for Research in Vision and Ophthalmology Annual Meeting 2007 and was funded by a Lew R. Wasserman Award from Research to Prevent Blindness, Inc. (M.C.C.). This research was also supported in part by National Institutes of Health Grants R01EY12985 (M.C.C.), P30EY012190 (NIH CORE grant for Dr. Robert E. Anderson, OUHSC), P20RR017703 (NCRR COBRE grant for Dr. Robert E. Anderson, OUHSC), and an unrestricted research grant to the Dean A. McGee Eye Institute from Research to Prevent Blindness, Inc. The authors gratefully acknowledge Billy D. Novosad (OUHSC Microbiology/Immunology), Dustin Woods (OU School of Medicine), and Mark Dittmar (Dean A. McGee Eye Institute Animal Research Facility) for their invaluable technical assistance. The authors would also like to thank Paula Pierce (Excalibur Pathology, Moore OK) for histology expertise. We also appreciate the helpful comments of Brandt Wiskur (OK Center for Neuroscience), and Drs. Muayyad Al-Ubaidi, John Ash, James Chodosh, Eric Howard (OUHSC), and Michael Gilmore (Schepens Eye Research Institute, Boston, MA).
Footnotes
Commercial Relationships: N for Ramadan, Moyer, and Callegan.
References
- 1.Callegan MC, Gilmore MS, Gregory M, Ramadan RT, Wiskur BJ, Moyer AL, Hunt JJ, Novosad BD. Bacterial endophthalmitis: therapeutic challenges and host-pathogen interactions. Prog Retin Eye Res. 2007;26(2):189–203. doi: 10.1016/j.preteyeres.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Das T, Choudhury K, Sharma S, Jalali S, Nuthethi R Endophthalmitis Research Group. Clinical profile and outcome in Bacillus endophthalmitis. Ophthalmology. 2001;108(10):1819–1825. doi: 10.1016/s0161-6420(01)00762-x. [DOI] [PubMed] [Google Scholar]
- 3.David DB, Kirkby GR, Noble BA. Bacillus cereus endophthalmitis. Br J Ophthalmol. 1994;78(7):577–580. doi: 10.1136/bjo.78.7.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller JJ, Scott IU, Flynn HW, Jr, Smiddy WE, Murray TG, Berrocal A, Miller D. Endophthalmitis caused by Bacillus species. Am J Ophthalmol. 2008;145(5):883–888. doi: 10.1016/j.ajo.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 5.Booth MC, Cheung AL, Hatter KL, Jett BD, Callegan MC, Gilmore MS. Staphylococcal accessory regulator (sar) in conjunction with agr contributes to Staphylococcus aureus virulence in endophthalmitis. Infect Immun. 1997;65(4):1550–1556. doi: 10.1128/iai.65.4.1550-1556.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Booth MC, Atkuri RV, Nanda SK, Iandolo JJ, Gilmore MS. Accessory gene regulator controls Staphylococcus aureus virulence in endophthalmitis. Invest Ophthalmol Vis Sci. 1995;36(9):1828–1836. [PubMed] [Google Scholar]
- 7.Callegan MC, Jett BD, Hancock LE, Gilmore MS. Role of hemolysin BL in the pathogenesis of extraintestinal Bacillus cereus infection assessed in an endophthalmitis model. Infect Immun. 1999;67(7):3357–3366. doi: 10.1128/iai.67.7.3357-3366.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callegan MC, Cochran DC, Kane ST, Gilmore MS, Gominet M, Lereclus D. Contribution of membrane-damaging toxins to Bacillus endophthalmitis pathogenesis. Infect Immun. 2002;70(10):5381–5389. doi: 10.1128/IAI.70.10.5381-5389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callegan MC, Kane ST, Cochran DC, Gilmore MS, Gominet M, Lereclus D. Relationship of plcR-regulated factors to Bacillus endophthalmitis virulence. Infect Immun. 2003;71(6):3116–3124. doi: 10.1128/IAI.71.6.3116-3124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jett BD, Jensen HG, Nordquist RE, Gilmore MS. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect Immun. 1992;60(6):2445–2452. doi: 10.1128/iai.60.6.2445-2452.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jett BD, Jensen HG, Atkuri RV, Gilmore MS. Evaluation of therapeutic measures for treating endophthalmitis caused by isogenic toxin-producing and toxin-nonproducing Enterococcus faecalis strains. Invest Ophthalmol Vis Sci. 1995;36(1):9–15. [PubMed] [Google Scholar]
- 12.Giese MJ, Rayner SA, Fardin B, Sumner HL, Rozengurt N, Mondino BJ, Gordon LK. Mitigation of neutrophil infiltration in a rat model of early Staphylococcus aureus endophthalmitis. Invest Ophthalmol Vis Sci. 2003;44(7):3077–3082. doi: 10.1167/iovs.02-1250. [DOI] [PubMed] [Google Scholar]
- 13.Ramadan RT, Ramirez R, Novosad BD, Callegan MC. Acute inflammation and loss of retinal architecture and function during experimental Bacillus endophthalmitis. Curr Eye Res. 2006;31(11):955–965. doi: 10.1080/02713680600976925. [DOI] [PubMed] [Google Scholar]
- 14.Giese MJ, Sumner HL, Berliner JA, Mondino BJ. Cytokine expression in a rat model of Staphylococcus aureus endophthalmitis. Invest Ophthalmol Vis Sci. 1998;39(13):2785–2790. [PubMed] [Google Scholar]
- 15.Ravindranath RM, Hasan SA, Mondino BJ. Immunopathologic features of Staphylococcus epidermidis-induced endophthalmitis in the rat. Curr Eye Res. 1997;16(10):1036–1043. doi: 10.1076/ceyr.16.10.1036.9015. [DOI] [PubMed] [Google Scholar]
- 16.Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996;334(26):1717–1725. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- 17.Petropoulos IK, Vantzou CV, Lamari FN, Karamanos NK, Anastassiou ED, Pharmakakis NM. Expression of TNF-alpha, IL-1beta, and IFN-gamma in Staphylococcus epidermidis slime-positive experimental endophthalmitis is closely related to clinical inflammatory scores. Graefes Arch Clin Exp Ophthalmol. 2006;244(10):1322–1328. doi: 10.1007/s00417-006-0261-2. [DOI] [PubMed] [Google Scholar]
- 18.Luna JD, Chan CC, Derevjanik NL, Mahlow J, Chiu C, Peng B, Tobe T, Campochiaro PA, Vinores SA. Blood-retinal barrier (BRB) breakdown in experimental autoimmune uveoretinitis: comparison with vascular endothelial growth factor, tumor necrosis factor alpha, and interleukin-1beta-mediated breakdown. J Neurosci Res. 1997;49(3):268–280. doi: 10.1002/(sici)1097-4547(19970801)49:3<268::aid-jnr2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 19.De Vos AF, Van Haren MA, Verhagen C, Hoekzema R, Kijlstra A. Tumour necrosis factor-induced uveitis in the Lewis rat is associated with intraocular interleukin 6 production. Exp Eye Res. 1995;60(2):199–207. doi: 10.1016/s0014-4835(95)80011-5. [DOI] [PubMed] [Google Scholar]
- 20.Dick AD, Forrester JV, Liversidge J, Cope AP. The role of tumour necrosis factor (TNF-alpha) in experimental autoimmune uveoretinitis (EAU) Prog Retin Eye Res. 2004;23(6):617–637. doi: 10.1016/j.preteyeres.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184(4):1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jett BD, Hatter KL, Huycke MM, Gilmore MS. Simplified agar plate method for quantifying viable bacteria. Biotechniques. 1997;23(4):648–650. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- 23.Ohta K, Yamagami S, Wiggert B, Dana MR, Streilein JW. Chemokine gene expression in iris-ciliary body during experimental autoimmune uveoretinitis. Curr Eye Res. 2002;24(6):451–457. doi: 10.1076/ceyr.24.6.451.8595. [DOI] [PubMed] [Google Scholar]
- 24.Xue ML, Thakur A, Willcox M. Gene expression of pro-inflammatory cytokines and chemokines in mouse eye infected with Pseudomonas aeruginosa. Clin Experiment Ophthalmol. 2002;30(3):196–199. doi: 10.1046/j.1442-9071.2002.00510.x. [DOI] [PubMed] [Google Scholar]
- 25.Nishida T, Miyata S, Itoh Y, Mizuki N, Ohgami K, Shiratori K, Ilieva IB, Ohno S, Taylor AW. Anti-inflammatory effects of alpha-melanocyte-stimulating hormone against rat endotoxin-induced uveitis and the time course of inflammatory agents in aqueous humor. Int Immunopharmacol. 2004;4(8):1059–1066. doi: 10.1016/j.intimp.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Cole N, Hume E, Khan S, Madigan M, Husband AJ, Garthwaite L, Willcox M. Contribution of the cornea to cytokine levels in the whole eye induced during the early phase of Pseudomonas aeruginosa challenge. Immunol Cell Biol. 2005;83(3):301–306. doi: 10.1111/j.1440-1711.2005.01324.x. [DOI] [PubMed] [Google Scholar]
- 27.Lin M, Carlson E, Diaconu E, Pearlman E. CXCL1/KC and CXCL5/LIX are selectively produced by corneal fibroblasts and mediate neutrophil infiltration to the corneal stroma in LPS keratitis. J Leukoc Biol. 2007;81(3):786–792. doi: 10.1189/jlb.0806502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelbert M, Gilmore MS. Fas ligand but not complement is critical for control of experimental Staphylococcus aureus endophthalmitis. Invest Ophthalmol Vis Sci. 2005;46(7):2479–2486. doi: 10.1167/iovs.04-1139. [DOI] [PubMed] [Google Scholar]
- 29.Stenzel W, Soltek S, Miletic H, Hermann MM, Körner H, Sedgwick JD, Schlüter D, Deckert M. An essential role for tumor necrosis factor in the formation of experimental murine Staphylococcus aureus-induced brain abscess and clearance. J Neuropathol Exp Neurol. 2005;64(1):27–36. doi: 10.1093/jnen/64.1.27. [DOI] [PubMed] [Google Scholar]
- 30.Kielian T, Bearden ED, Baldwin AC, Esen N. IL-1 and TNF-alpha play a pivotal role in the host immune response in a mouse model of Staphylococcus aureus-induced experimental brain abscess. J Neuropathol Exp Neurol. 2004;63(4):381–396. doi: 10.1093/jnen/63.4.381. [DOI] [PubMed] [Google Scholar]
- 31.Marino MW, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, Jungbluth A, Wada H, Moore M, Williamson B, Basu S, Old LJ. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci U S A. 1997;94(15):8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wellmer A, Gerber J, Ragheb J, Zysk G, Kunst T, Smirnov A, Brück W, Nau R. Effect of deficiency of tumor necrosis factor alpha or both of its receptors on Streptococcus pneumoniae central nervous system infection and peritonitis. Infect Immun. 2001;69(11):6881–6886. doi: 10.1128/IAI.69.11.6881-6886.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirby AC, Raynes JG, Kaye PM. The role played by tumor necrosis factor during localized and systemic infection with Streptococcus pneumoniae. J Infect Dis. 2005;191(9):1538–1547. doi: 10.1086/429296. [DOI] [PubMed] [Google Scholar]
- 34.Derevjanik NL, Vinores SA, Xiao WH, Mori K, Turon T, Hudish T, Dong S, Campochiaro PA. Quantitative assessment of the integrity of the blood-retinal barrier in mice. Invest Ophthalmol Vis Sci. 2002;43(7):2462–2467. [PubMed] [Google Scholar]
- 35.Vinores SA, Xiao WH, Shen J, Campochiaro PA. TNF-alpha is critical for ischemia-induced leukostasis, but not retinal neovascularization nor VEGF-induced leakage. J Neuroimmunol. 2007;182(1–2):73–79. doi: 10.1016/j.jneuroim.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazzon E, Cuzzocrea S. Role of TNF-alpha in lung tight junction alteration in mouse model of acute lung inflammation. Respir Res. 2007;8:75. doi: 10.1186/1465-9921-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazzon E, Cuzzocrea S. Role of TNF-alpha in ileum tight junction alteration in mouse model of restraint stress. Am J Physiol Gastrointest Liver Physiol. 2008;294(5):G1268–1280. doi: 10.1152/ajpgi.00014.2008. [DOI] [PubMed] [Google Scholar]
- 38.Kuprash DV, Tumanov AV, Liepinsh DJ, Koroleva EP, Drutskaya MS, Kruglov AA, Shakhov AN, Southon E, Murphy WJ, Tessarollo L, Grivennikov SI, Nedospasov SA. Novel tumor necrosis factor-knockout mice that lack Peyer’s patches. Eur J Immunol. 2005;35(5):1592–1600. doi: 10.1002/eji.200526119. [DOI] [PubMed] [Google Scholar]
- 39.Vasanthi M, Prajna NV, Lalitha P, Mahadevan K, Muthukkaruppan V. A pilot study on the infiltrating cells and cytokine levels in the tear of fungal keratitis patients. Indian J Ophthalmol. 2007;55(1):27–31. doi: 10.4103/0301-4738.29491. [DOI] [PubMed] [Google Scholar]
- 40.Chintakuntlawar AV, Astley R, Chodosh J. Adenovirus type 37 keratitis in the C57BL/6J mouse. Invest Ophthalmol Vis Sci. 2007;48(2):781–788. doi: 10.1167/iovs.06-1036. [DOI] [PubMed] [Google Scholar]
- 41.Cole N, Bao S, Thakur A, Willcox M, Husband AJ. KC production in the cornea in response to Pseudomonas aeruginosa challenge. Immunol Cell Biol. 2000;78(1):1–4. doi: 10.1046/j.1440-1711.2000.00860.x. [DOI] [PubMed] [Google Scholar]
- 42.Hume EB, Cole N, Khan S, Garthwaite LL, Aliwarga Y, Schubert TL, Willcox MD. A Staphylococcus aureus mouse keratitis topical infection model: cytokine balance in different strains of mice. Immunol Cell Biol. 2005;83(3):294–300. doi: 10.1111/j.1440-1711.2005.01326.x. [DOI] [PubMed] [Google Scholar]
- 43.Fodor M, Facskó A, Rajnavölgyi E, Hársfalvi J, Bessenyei E, Kardos L, Berta A. Enhanced release of IL-6 and IL-8 into tears in various anterior segment eye diseases. Ophthalmic Res. 2006;38(4):182–188. doi: 10.1159/000093068. [DOI] [PubMed] [Google Scholar]
- 44.Sijssens KM, Rijkers GT, Rothova A, Stilma JS, Schellekens PA, de Boer JH. Cytokines, chemokines and soluble adhesion molecules in aqueous humor of children with uveitis. Exp Eye Res. 2007;85(4):443–449. doi: 10.1016/j.exer.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 45.Xu Y, Hunt NH, Bao S. The correlation between proinflammatory cytokines, MAdCAM-1 and cellular infiltration in the inflamed colon from TNF-alpha gene knockout mice. Immunol Cell Biol. 2007;85(8):633–639. doi: 10.1038/sj.icb.7100112. [DOI] [PubMed] [Google Scholar]
- 46.Yimin Kohanawa M. A regulatory effect of the balance between TNF-alpha and IL-6 in the granulomatous and inflammatory response to Rhodococcus aurantiacus infection in mice. J Immunol. 2006;177(1):642–650. doi: 10.4049/jimmunol.177.1.642. [DOI] [PubMed] [Google Scholar]
- 47.Moore TA, Lau HY, Cogen AL, Standiford TJ. Defective innate antibacterial host responses during murine Klebsiella pneumoniae bacteremia: tumor necrosis factor (TNF) receptor 1 deficiency versus therapy with anti-TNF-alpha. Clin Infect Dis. 2005;41(Suppl 3):S213–217. doi: 10.1086/430126. [DOI] [PubMed] [Google Scholar]
- 48.Nakazawa T, Matsubara A, Noda K, Hisatomi T, She H, Skondra D, Miyahara S, Sobrin L, Thomas KL, Chen DF, Grosskreutz CL, Hafezi-Moghadam A, Miller JW. Characterization of cytokine responses to retinal detachment in rats. Mol Vis. 2006;7(12):867–878. [PubMed] [Google Scholar]
- 49.Zeng HY, Zhu XA, Zhang C, Yang LP, Wu LM, Tso MO. Identification of sequential events and factors associated with microglial activation, migration, and cytotoxicity in retinal degeneration in rd mice. Invest Ophthalmol Vis Sci. 2005;46(8):2992–2999. doi: 10.1167/iovs.05-0118. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida S, Yoshida A, Ishibashi T. Induction of IL-8, MCP-1, and bFGF by TNF-alpha in retinal glial cells: implications for retinal neovascularization during post-ischemic inflammation. Graefes Arch Clin Exp Ophthalmol. 2004;242(5):409–413. doi: 10.1007/s00417-004-0874-2. [DOI] [PubMed] [Google Scholar]
- 51.Vinores SA, Derevjanik NL, Shi A, Vinores MA, Klein DA, Whittum-Hudson JA. Vascular endothelial growth factor (VEGF), transforming growth factor-beta (TGFbeta), and interleukin-6 (IL-6) in experimental herpesvirus retinopathy: association with inflammation and viral infection. Histol Histopathol. 2001;16(4):1061–1071. doi: 10.14670/HH-16.1061. [DOI] [PubMed] [Google Scholar]
- 52.Cotinet A, Goureau O, Thillaye-Goldenberg B, Naud MC, de Kozak Y. Differential tumor necrosis factor and nitric oxide production in retinal Müller glial cells from C3H/HeN and C3H/HeJ mice. Ocul Immunol Inflamm. 1997;5(2):111–116. doi: 10.3109/09273949709085059. [DOI] [PubMed] [Google Scholar]
- 53.Drescher KM, Whittum-Hudson JA. Herpes simplex virus type 1 alters transcript levels of tumor necrosis factor-alpha and interleukin-6 in retinal glial cells. Invest Ophthalmol Vis Sci. 1996;37(11):2302–2312. [PubMed] [Google Scholar]
- 54.Brito BE, O’Rourke LM, Pan Y, Anglin J, Planck SR, Rosenbaum JT. IL-1 and TNF receptor-deficient mice show decreased inflammation in an immune complex model of uveitis. Invest Ophthalmol Vis Sci. 1999;40(11):2583–2589. [PubMed] [Google Scholar]
- 55.Olson JL, Courtney RJ, Mandava N. Intravitreal infliximab and choroidal neovascularization in an animal model. Arch Ophthalmol. 2007;125(9):1221–1224. doi: 10.1001/archopht.125.9.1221. [DOI] [PubMed] [Google Scholar]
- 56.Shi X, Semkova I, Müther PS, Dell S, Kociok N, Joussen AM. Inhibition of TNF-alpha reduces laser-induced choroidal neovascularization. Exp Eye Res. 2006;83(6):1325–1334. doi: 10.1016/j.exer.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 57.Diaz-Llopis M, García-Delpech S, Salom D, Udaondo P, Bosch-Morell F, Quijada A, Romero FJ, Amselem L. High-dose infliximab prophylaxis in endotoxin-induced uveitis. J Ocul Pharmacol Ther. 2007;23(4):343–350. doi: 10.1089/jop.2006.0148. [DOI] [PubMed] [Google Scholar]
- 58.Gallagher M, Quinones K, Cervantes-Castañeda RA, Yilmaz T, Foster CS. Biological response modifier therapy for refractory childhood uveitis. Br J Ophthalmol. 2007;91(10):1341–1344. doi: 10.1136/bjo.2007.124081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abu El-Asrar AM, Abboud EB, Aldibhi H, Al-Arfaj A. Long-term safety and efficacy of infliximab therapy in refractory uveitis due to Behçet’s disease. Int Ophthalmol. 2005;26(3):83–92. doi: 10.1007/s10792-006-9006-9. [DOI] [PubMed] [Google Scholar]
- 60.Suhler EB, Smith JR, Wertheim MS, Lauer AK, Kurz DE, Pickard TD, Rosenbaum JT. A prospective trial of infliximab therapy for refractory uveitis: preliminary safety and efficacy outcomes. Arch Ophthalmol. 2005;123(7):903–912. doi: 10.1001/archopht.123.7.903. [DOI] [PubMed] [Google Scholar]
- 61.Galor A, Perez VL, Hammel JP, Lowder CY. Differential effectiveness of etanercept and infliximab in the treatment of ocular inflammation. Ophthalmology. 2006;113(12):2317–2323. doi: 10.1016/j.ophtha.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 62.Rajaraman RT, Kimura Y, Li S, Haines K, Chu DS. Retrospective case review of pediatric patients with uveitis treated with infliximab. Ophthalmology. 2006;113(2):308–314. doi: 10.1016/j.ophtha.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 63.Giansanti F, Ramazzotti M, Vannozzi L, Rapizzi E, Fiore T, Iaccheri B, Degl’ Innocenti D, Moncini D, Menchini U. A pilot study on ocular safety of intravitreal infliximab in a rabbit model. Invest Ophthalmol Vis Sci. 2008;49(3):1151–1156. doi: 10.1167/iovs.07-0932. [DOI] [PubMed] [Google Scholar]
- 64.Wiskur BW, Robinson M, Farrand A, Novosad B, Callegan MC. Improved therapeutic regimens for Bacillus endophthalmitis. Invest Ophthalmol Vis Sci. 2008;49(4):1480–1487. doi: 10.1167/iovs.07-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]


