Abstract
S100B is a calcium binding protein with both extracellular and intracellular regulatory activities in the mammalian brain. We have identified a novel interaction between S100B and the dopamine D2 receptor. Our results also suggest that the binding of S100B to the dopamine D2 receptor enhances receptor signaling. This conclusion is based on the following observations: 1) S100B and the third cytoplasmic loop of the dopamine D2 receptor interact in a bacterial two-hybrid system and in a polyHis pull-down assay; 2) immunoprecipitation of the D2 receptor also precipitates FLAG-S100B from human embryonic kidney 293 cell homogenates and endogenous S100B from rat neostriatal homogenates; 3) S100B immunoreactivity was detected in cultured neostriatal neurons expressing the D2 receptor; 4) a putative S100B binding motif is located at residues 233–240 of the D2 receptor, towards the amino terminus of the third cytoplasmic loop. D3-IC3, which does not bind S100B, does not contain this motif; 5) co-expression of S100B in D2 receptor-expressing 293 cells selectively increased D2 receptor stimulation of extracellular signal-regulated kinases and inhibition of adenylate cyclase.
Interest in dopamine receptor research has been fueled by studies of brain diseases such as Parkinson’s disease and schizophrenia, showing that dopamine has a role in either the pathogenesis or symptoms of the diseases and that substances acting at the receptors act as therapeutic agents (Strange, 1992). The dopamine receptor family is comprised of D1-like (D1 and D5) and D2-like (D2L, D2S, D3, and D4) receptors (Neve and Neve, 1997). The dopamine D2 receptor belongs to a subfamily of 7-transmembrane domain G protein-coupled receptors that interact with the G proteins Gαi and Gαo to modulate several effectors, including adenylate cyclase, potassium channels, and mitogen-activated protein kinases (MAPKs) (Neve et al., 2004).
Protein-protein interactions are central to most important cellular processes including DNA replication, transcription, translation, cell cycle control, and signal transduction. The yeast two-hybrid assay is a powerful method for identifying and characterizing protein-protein interactions (Fields and Song, 1989), but it is a tedious procedure, limited by the basic biology of the yeast. Yeast grows slowly, is difficult to transform efficiently, and requires unique reagents and techniques. The bacterial two-hybrid (B2H) system has the following advantages: fast growth rate, high transformation efficiency, and manipulations that are routine in most molecular biology laboratories (Joung et al., 2000). The purpose of this study was to use the B2H system to identify additional proteins that bind to and regulate the function of the D2 receptor.
S100 proteins comprise an extremely diverse and highly specialized family of about 21 Ca2+-binding proteins (Donato, 1999; Marenholz et al., 2004; Zimmer et al., 2003). An S100 protein is typically a low molecular weight protein (molecular weight between 9 and 13 kDa) characterized by the presence of two Ca2+-binding sites of the EF-hand type (Donato, 2003). S100 proteins have been implicated in the regulation of protein phosphorylation, Ca2+ homeostasis, enzyme activity, gene transcription, cell growth and differentiation, and the inflammatory response (Donato, 1999; Schafer and Heizmann, 1996;). Alterations of S100 function have been implicated in many diseases including cancer, Down’s syndrome, Alzheimer’s diseases, cardiomyopathy, psoriasis, cystic fibrosis, amyotrophic lateral sclerosis, and epilepsy (Heizmann, 2002; Heizmann et al., 2002). Thus, S100 proteins may be important diagnostic markers as well as therapeutic targets. The results of clinical studies on the S100 protein S100B in schizophrenia suggest that patients suffering from schizophrenia have increased S100B serum concentrations in the acutely psychotic stage of disease (Rothermundt et al., 2004). S100B is an acidic protein with a molecular mass of 21 kDa as a homodimer, and it is perhaps the best characterized of the S100 proteins (McClintock and Shaw, 2000). S100B has no known enzymatic function and exert its intracellular effects by interacting with and modulating the activity of other proteins. In vitro, S100B interacts with more than 20 substrates in a Ca2+ sensitive manner (Donato, 1999).
We now describe a novel interaction between S100B and the dopamine D2 receptor, identified using the B2H system. We confirmed the novel interaction using co-immunoprecipitation in human embryonic kidney (HEK) 293 cells and in rat neostriatum. We identified S100B immunoreactivity in D2 receptor-expressing neostriatal neurons. We determined that the third intracellular loop of the D2 receptor (D2-IC3) is a contact point for the interaction with S100B by a His-tagged pull-down assay. S100B bound to IC3 of both D2L and D2S, but not D3. We also proposed a putative binding motif for the interaction by sequence alignment. Finally, we found that co-expression of the D2 receptor and S100B significantly increased D2 receptor stimulation of ERKs and inhibition of adenylate cyclase in HEK293 cells.
Materials and Methods
Materials
The B2H System and BacterioMatch® II Rat Brain Library were purchase from Stratagene (La Jolla, CA). Quinpirole, 7-OH DPAT, (+)-butaclamol, 3-isobutyl-1-methylxanthine (IBMX), adenine HCl, n-dodecylmaltoside, ethylene-bis(oxyethylenenitrilo)tetraacetic acid, 3-amino-1,2,4-triazole (3-AT), and culture media were purchased from Sigma-Aldrich (St. Louis, MO). His dropout supplement (mixtures of amino acids and other nutrients) was purchased from BD Biosciences Clontech (Palo Alto, CA). [3H]Spiperone (95 Ci/mmol) was purchased from GE Healthcare Bio-Sciences Corp (Piscataway, NJ). Fetal and calf bovine sera for cell culture were purchased from HyClone (Logan, UT). The Lipofectamine 2000 cell transfection kit was purchased from Invitrogen (Carlsbad, CA). Pre-cast gels and rat neostriatal neurons were purchased from Lonza (Walkersville, MD). Protein G Plus agarose, normal rabbit IgG, and normal mouse IgG were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies used include: rabbit anti-dopamine D2L/S (1/500 dilution, from Upstate, Lake Placid, NY), mouse anti-S100B (1/1000 dilution, from GeneTex, San Antonio, TX), rabbit anti-His (1/500 dilution, from Santa Cruz Biotechnology), mouse anti-FLAG M2 (1/1000 dilution, from Sigma), rabbit anti-lambda-cI antibody (1/1000 dilution, from Stratagene), rabbit anti-myc (1/1000 dilution, from Bethyl, Montgomery, TX), mouse anti-myc (1/1000 dilution, from Upstate), rabbit anti-dually phosphorylated (i.e., activated) ERKs (1/1000 dilution, from Invitrogen), mouse anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1/50,000 dilution, from Upstate), and rabbit anti-microtubule-associated protein-2 (MAP2) (1/1000 dilution, from Abcam, Cambridge, MA). Alexa 568-labeled goat anti-mouse IgG antibody, Alexa Fluor-486 goat anti-rabbit IgG antibody, and Prolong™ anti-fade kit were obtained from Invitrogen. S100B was from US Biological. The cyclic AMP EIA kit was from Cayman Chemical (Ann Arbor, MI). The BCA protein assay kit, secondary antibodies for immunoblot analysis, and the SuperSignal™ West Pico chemiluminescent kit were from Pierce Biotechnology (Rockford, IL). Protease inhibitor cocktail (set III) was from EMD Biosciences (San Diego, CA). HEK293 cells, a transformed cell line from human embryonic kidney, were purchased from ATCC (Manassas, VA).
DNA Constructs for Bacterial Two-Hybrid Assay
The sequence encoding the IC3 of the dopamine D2 receptor (D2-IC3), amino acids 206–375 (Leu-Met), was amplified by PCR and subcloned in-frame with the lambda-cI DNA-binding domain into pBT (B2H System, Stratagene) to generate pBT-D2-IC3 as “bait”. The construct was verified by DNA sequencing, and the presence of lambda-cI tagged D2-IC3 with the expected molecular size was also verified by immunoblot using anti-lambda-cI antibody. A B2H rat brain cDNA library (as a “target”) was purchased from Stratagene. It contains pooled rat brain tissues (Sprague-Dawley, male, 10 weeks). The vector is pTRG, and the average insert size is about 1.8 kb.
Bacterial Two-Hybrid Screening
The B2H System reporter strain competent cells (Stratagene) were transformed with pBT-D2-IC3 and the BacterioMatch® II Rat Brain Library (Stratagene) according to the manufacture’s protocol (Stratagene). Detection of protein-protein interaction is based on transcriptional activation of the HIS3 reporter gene, which allows growth in the presence of 3-AT (5–20 mM), a competitive inhibitor of the His3 enzyme. Positives are verified using the aadA gene, which confers streptomycin resistance, as a secondary reporter. All positive clones were analyzed by DNA sequencing. To validate the putative protein-protein interactions, we then re-transformed the reporter strain with the isolated target plasmid plus bait plasmid as described by the manufacturer (Stratagene).
Cell Culture, Transfection, and Selection
Cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 5% fetal bovine serum and 5% calf bovine serum, penicillin-streptomycin, appropriate selection antibiotics (G418 sulfate 600 μg/ml; puromycin 2 μg/ml), and grown in a humidified incubator at 37°C in the presence of 10% CO2.
The creation of a cell line stably expressing a c-myc-tagged D2L dopamine receptor (referred to hereinafter as the D2 receptor) was described in a previous report (Liu et al., 2007). Since there is no detectable endogenously expressed S100B via immunoblotting using anti-S100B antibody, a cell line stably co-expressing c-myc-tagged D2 dopamine receptor and FLAG-tagged S100B (myc-D2/FLAG-S100B-HEK293) was generated as follows: cDNA encoding rat brain S100B was amplified using the polymerase chain reaction, digested with EcoRI-BamHI, and subcloned into the pcDNA-DNA3 expression vector, placing the FLAG-tag at the NH2 terminus of S100B. The FLAG-S100B construct was transfected into myc-D2-HEK cells using Lipofectamine 2000 transfection reagent and selection with puromycin (2 μg/ml) and G418 (600 μg/ml). Cell lines expressing the myc-tagged D2 receptor and FLAG-tagged S100B were isolated by screening via radioligand binding using [3H]spiperone, and via immunoblot analysis using a mouse anti-myc and a mouse anti-FLAG antibody. The binding of [3H]spiperone was assessed as described previously (Liu et al., 2006), and the c-myc-tagged D2 receptor with co-expression of FLAG-tagged S100B had similar affinity for [3H]spiperone as the previous report for the c-myc-tagged D2 receptor (Liu et al., 2007). The molar ratio of D2: S100B in the myc-D2/FLAG-S100B-HEK293 cell line was approximately 1:0.9 (data not shown).
Neostriatal Neuronal Cultures
Rat striatal neurons were cultured as follows: the cells were removed from liquid nitrogen and placed in a 37°C water-bath for 2–3 minutes, then gently into a 15 ml centrifuge tube to which was added pre-warmed Primary Neuron Growth Medium (Lonza) drop-wise into the cells, while rotating the tube by hand. The cell suspension was mixed by inverting the tube twice. Cells were plated on 18 mm-diameter poly-D-lysine-coated glass coverslips at a density of 75,000 cells per coverslip and placed in a humidified 5% CO2 incubator at 37°C. After 4 hr, the medium was replaced with fresh, pre-warmed medium. After 4 days, the medium was changed, and cells were ready for use after 6 to 8 days in culture. Just under 90% of the cells in the cultures were neurons, as determined by the presence of MAP2 immunoreactivity.
Co-immunoprecipitation of the myc-tagged D2 Receptor and S100B, and Endogenous D2 receptor and S100B
myc-D2/FLAG-S100B-HEK293 cells from confluent 10 cm2 plates were washed and incubated twice for 3 minutes each time with calcium- and magnesium-free phosphate-buffered saline (58 mM Na2HPO4, 17 mM NaH2PO4, 68 mM NaCl, pH 7.4). Cells were released from the plate using trypsin, triturated, and centrifuged at 600 × g. The cells were resuspended in phosphate-buffered saline with 1.0% n-dodecylmaltoside and protease inhibitor cocktail and solubilized on ice for 2 hr with gentle shaking. Sprague-Dawley rats (12-week old, female) were killed by decapitation, and the heads of the animals were immediately immersed in liquid nitrogen for 6 s. The brains were then removed, and the striatum rapidly (20 s) dissected out on an ice-cold surface. The tissue was then triturated using fire-polished Pasteur pipettes in phosphate-buffered saline with 1.0% n-dodecylmaltoside and protease inhibitor cocktail and solubilized on ice for 8 hr with gentle shaking. The insoluble material from the two tissue preparations was removed by centrifugation at 25,000 × g for 30 min. The protein concentration of the supernatant was analyzed by BCA protein assay reagent. An aliquot with 1.5 mg protein was incubated with 2 μg rabbit anti-myc antibody (for co-IP with myc-D2/FLAG-S100B-HEK293 cells) or rabbit anti-D2 antibody (for co-IP with neostriatal lysates) at 4 °C for 2 hr and further incubated with 20 μl of a 50% slurry of Protein G Plus beads overnight at 4 °C. Beads were washed and samples were eluted according to the manufacturer’s instructions, separated by SDS-PAGE, and immunoblotted using mouse anti-FLAG antibody for myc-D2/FLAG-S100B-HEK293 cells or using mouse anti-S100B antibody for endogenous D2 receptor and S100B.
In vitro His-tagged Dopamine Receptor-IC3 Pulldown Assay
For construction of the His-tagged fusion proteins, the third cytoplasmic loop of the dopamine D2S receptor (D2S-IC3), amino acid 206–346, D2L receptor (D2L-IC3), amino acids 206–375, and D3 receptor (D3-IC3), amino acids 206–376, were PCR-amplified. The PCR products were cut as BamHI-SalI fragments and subcloned into pET-24a (+) (Novagen, Madison, WI), then transformed into BL21(DE3) competent cells (Novagen). Transformants were screened by induction with 0.5 mM IPTG and immunoblot analysis using a rabbit anti-His antibody. For larger-scale purification, the His-tagged dopamine receptor-IC3 clones were grown in LB broth containing kanamycin (50 μg/mL) at 37 °C to A600 = 0.5 and induced with 0.5 mM IPTG for 4 hr at 23 °C. Bacteria were pelleted and washed with phosphate-buffered saline. Pellets were resuspended in B-PER II bacterial protein extraction reagent (Pierce Biotechnology) with 0.5 mg/ml lysozyme (Fermentas, Hanover, MD) and protease inhibitor, and incubated for 20 min with gentle rotation at room temperature. The bacterial cell lysates containing the same amount of His-tagged dopamine receptor-IC3 fusion proteins or a hexa-His peptide without insert as control were clarified by centrifugation, and the supernatants were applied to Ni-NTA agarose (Qiagen, Valencia, CA). Pre-bound, washed beads were incubated with 500 ng of purified S100B overnight at 4 °C, followed by wash and elution steps. The eluates were separated by SDS-PAGE, and bound proteins were analyzed by immunoblotting with rabbit anti-S100B antibody.
Confocal Immunofluorescence Imaging
Neostriatal neurons grown on glass coverslips were fixed in 4% paraformaldehyde in phosphate-buffered saline (58 mM Na2HPO4, 17 mM NaH2PO4, 68 mM NaCl, pH 7.4) for 15 min, permeabilized with 0.5% Triton X-100 for 15 min, then blocked with 5% goat serum for 1 hr at room temperature. Neurons were incubated with rabbit anti-MAP2 or rabbit anti-D2L/S and mouse anti-S100B at 4° C overnight, then incubated for 1 hr with Alexa Fluor 486 goat anti-rabbit IgG (1/1000) and Alexa Fluor-568-tagged goat anti–mouse IgG (1/1000), and followed by five 10-min washes with phosphate-buffered saline. The coverslips were then mounted onto a slide with the ProLong™ antifade kit, dried in the dark, and scanned alternating between 486 and 568 nm using a Leica TCS SP confocal laser scanning microscope (Leica, Wetzlar, Germany). System settings were held constant for all imaging.
Immunoblotting
Proteins were separated by SDS-PAGE through a 4–20 % or 10% polyacrylamide gel and transferred to polyvinyl membranes (Millipore, Bedford, MA). The membranes were blocked for 1 hr at room temperature with 5 % non-fat milk with 0.05 % Tween 20 in Tris-buffered saline (TBS), pH 7.4 at 4°C, washed twice for 5 min, followed by 2 10-min washes with TBS, and incubated with primary antibody at room temperature for 2 hr or overnight at 4 °C. The membranes were washed twice for 5 min, followed by two 10-min washes with TBS, then incubated with secondary antibody (horseradish peroxidase-conjugated goat anti-mouse IgG or anti rabbit IgG) at room temperature for 1 hr. In addition to measuring protein concentrations to ensure equal loading, in many experiments in which phosphorylated forms of ERKs were detected, the membranes were then stripped with Restore western blot stripping buffer (Pierce) for 20 min at room temperature, followed by two 5-min washes with TBS, blocked for 1 hr at room temperature with 5 % non-fat milk in TBS, then incubated with mouse anti-GAPDH antibody, a house-keeping gene product, to further ensure that equal amounts of proteins were loaded, followed by goat anti-mouse IgG. Immunodetection was accomplished using a SuperSignal™ West Pico chemiluminescent kit. The intensity of bands was quantified using Gel Doc EQ System (Bio-Rad). A one-way ANOVA and Bonferroni post hoc comparison was used to analyze data.
Cell Stimulation for Immunodetection of ERKs
Cells expressing myc-D2 or myc-D2/FLAG-S100B were grown in 12-well plates to 80–85% confluence. The cells were starved in serum-free Dulbecco’s modified Eagle’s medium overnight, then incubated with the D2-like receptor agonist quinpirole or epidermal growth factor at the indicated concentrations for 5 min at 37°C. Incubation was terminated by placing the tissue culture cluster on ice and rapidly aspirating the medium, followed by the addition of ice-cold RIPA buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% NP-40, 0.5 % deoxycholate, 0.1% SDS, 1 mM NaVO3, protease inhibitors, and phosphatase inhibitor) and incubation for 20 min with shaking. After centrifugation (14,000 × g at 4°C for 15 min), the supernatant was collected and the protein concentration was measured and adjusted using RIPA buffer. The cell lysates (20 μl) with equal amounts of protein mixed with Laemmli loading buffer were denatured at 70°C for 10 min and separated by SDS-PAGE for immunodetection as described.
Cyclic AMP Accumulation Assay
The ability of the D2 receptor agonist 7-OH-DPAT to inhibit 30 μM forskolin-stimulated cylic AMP accumulation was measured in intact myc-D2-HEK293 cells and myc-D2/FLAG-S100B-HEK293 cells. Cells were plated at between 100,000 and 150,000 cells/well in 48-well tissue culture plates and used in experiments 2–3 days later. Prior to the assay, cells were pre-incubated with Earle’s balanced salt solution with 0.2% ascorbic acid, 500 μM IBMX (a phosphodiesterase inhibitor), and 2% fetal bovine serum, pH 7.4, for 20 min at 37°C. The cell were placed on ice for the addition of 7-OH DPAT and 30 μM forskolin, then incubated at 37°C. The assay was terminated after 20 minutes by decanting the medium, and the cells were lysed with 100 μl 3% trichloroacetic acid. Lysates were stored at 4°C at least 2 hr before quantification of cyclic AMP. The amount of cyclic AMP in each well was measured using a cyclic AMP EIA kit from Cayman Chemical.
RESULTS
Identification of D2 Receptor-binding Proteins by Bacterial Two-hybrid Library Screening
A cDNA library screening based on the B2H system was used to identify proteins that bind to the D2 receptor. For this purpose, the rat D2L-IC3 coding sequence was subcloned into plasmid pBT (pBT-D2-IC3) to serve as bait with which to screen a rat brain cDNA library in the pTRG plasmid. In screens of about 2.5 million clones, we identified 200 positive clones. To validate the detected protein-protein interactions, bacteria were re-transformed with each cloned cDNA and pBT-D2-IC3. About 40 of the 200 clones reproducibly grew on selective screening medium (3-AT) when co-transformed with the bait protein but failed to grow on selective screening medium when co-transformed with the empty pBT vectors, and thus were verified positives. The DNA sequence analysis revealed that clone number 28 matched the full-length sequence encoding rat S100B (GenBank™ accession number NM_01319).
Interaction between Dopamine Receptor–IC3 and S100B in Vitro
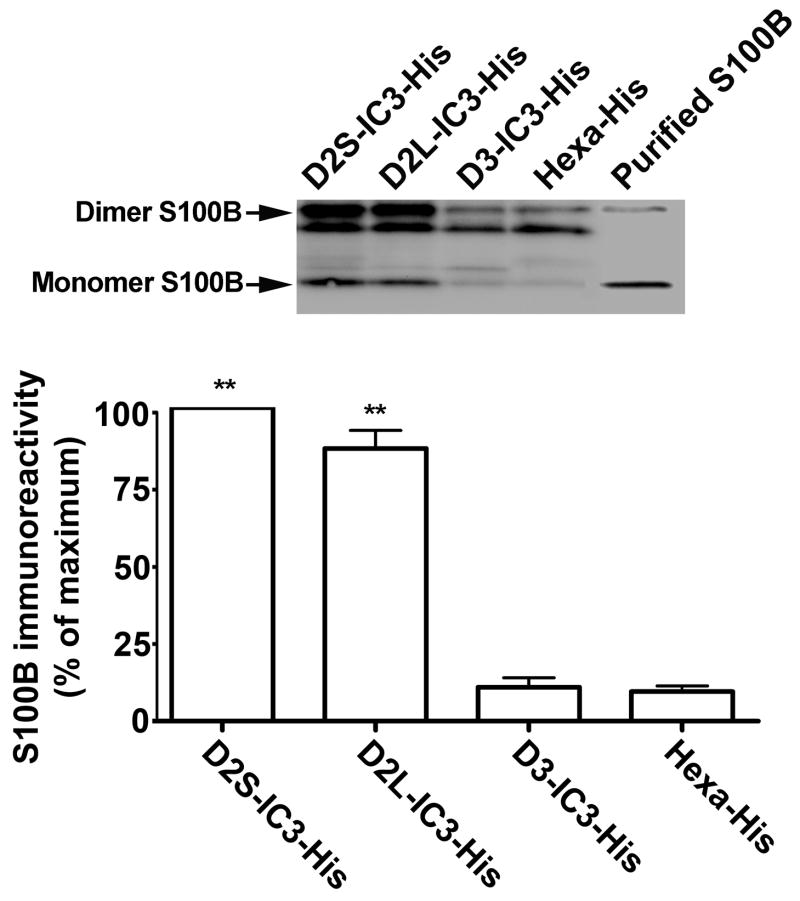
The in vitro interaction between S100B and the third cytoplasmic loops of D2S, D2L, and D3 was studied using a His-tagged dopamine receptor-IC3 pulldown assay. D2S, D2L, and D3-IC3-His fusion proteins were synthesized in bacteria, purified, immobilized on Ni-NTA beads, and incubated with purified recombinant S100B. The bound proteins eluted from the beads were separated by SDS-PAGE. S100B immunoreactivity was detected in the eluates from bacterial cell lysate expressing His-tagged D2S-IC3 and His-tagged D2L-IC3, but not His-tagged D3-IC3 or hexa-His peptide without insert, confirming that S100B binds to the third intracellular loop of both D2S and D2L, but not D3 (Fig. 1).
Fig. 1. S100B binds to D2-IC3 in an in vitro pull-down assay.
S100B immunoreactivity was present in the eluates from bacterial cell lysate expressing His-tagged D2S-IC3 and His-tagged D2L-IC3, but not His-tagged D3-IC3 or hexa-His peptide without insert, demonstrating a specific interaction between the third cytoplasmic loop of the dopamine D2 receptor and S100B. The construction of His-tagged receptor fragments and the in vitro pull down assay protocol were as described in Methods. In the upper panel, lanes D2S-IC3-His, D2L-IC3-His, and D3-IC3-His show the result of a representative pull-down assay, and lane Hexa-His is the no-insert control for that experiment. The right lane is a positive control for immunoblotting (50 ng purified S100B). The lower panel depicts the mean ± SE from three independent experiments. ** p < 0.01 compared to the no-insert control, paired t-test
Co-immunoprecipitation of the myc-tagged D2 Receptor and FLAG-S100B, and endogenous D2 receptor and S100B
To confirm a direct interaction between full-length D2 receptor and S100B, we expressed both FLAG epitope-tagged S100B and c-myc-D2L receptor in HEK293 cells. Immunoprecipitation of the D2 receptor with anti-myc resulted in the precipitation of FLAG-S100B, as indicated by immunoblotting with anti-FLAG antibody (Fig. 2A). FLAG-S100B immunoreactivity was not detected in control cells in which the immunoprecipitation was performed with an irrelevant antibody to a hemaglutinin epitope. The amount of S100B immunoreactivity in eluates from cells treated with the D2-like receptor agonist quinpirole (10 μM) for 5 minutes or 2 hours was similar to cells treated with vehicle. Immunoprecipitation of the endogenous D2 receptor from rat neostriatum also resulted in the precipitation of S100B immunoreactivity; S100B immunoreactivity was not detected in control samples in which the immunoprecipitation was performed with rabbit normal IgG (Fig. 2B). Therefore, the D2 dopamine receptor specifically and constitutively bound S100B in HEK293 cells and in rat neostriatum.
Fig. 2. Co-immunoprecipitation of dopamine D2 receptor and S100B.
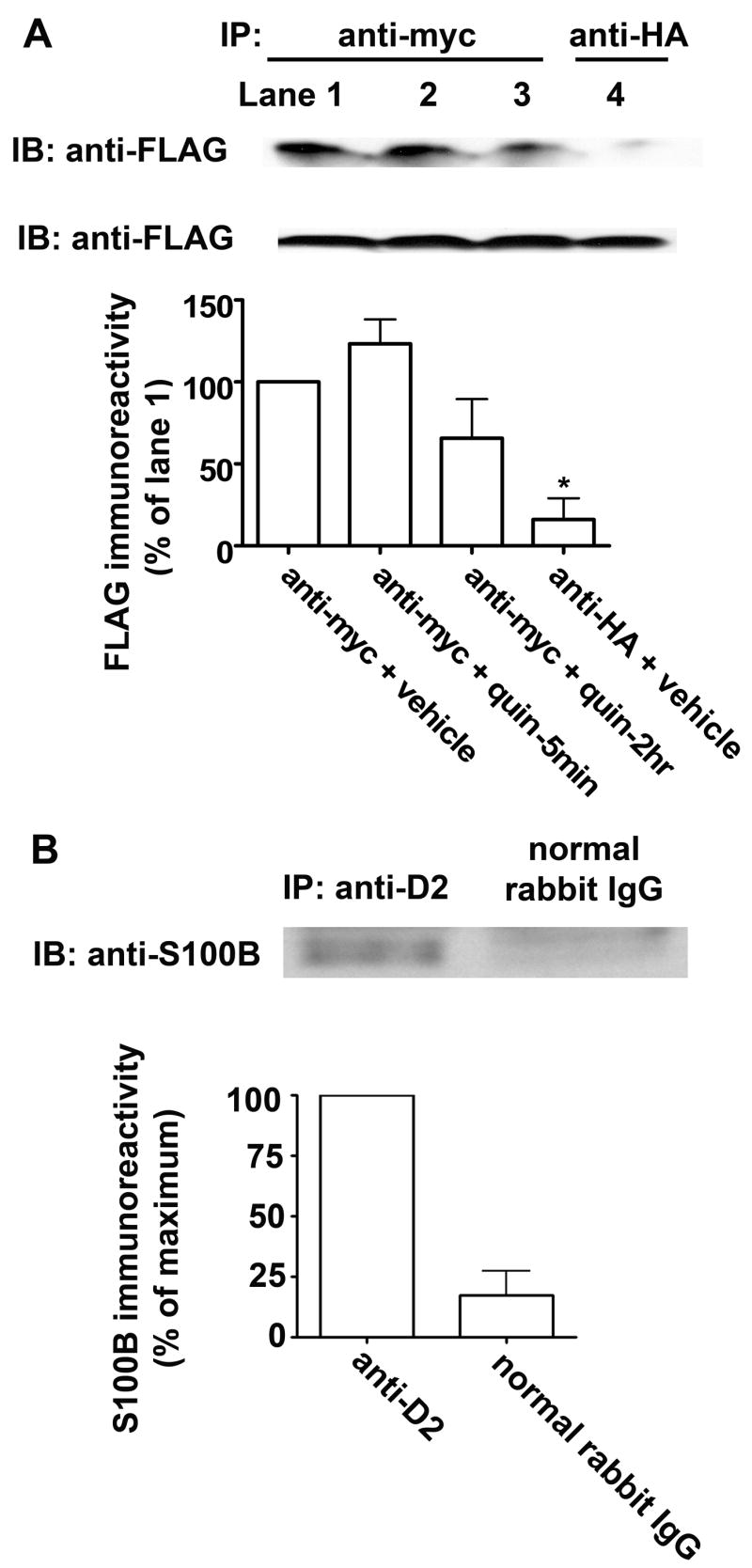
A, co-immunoprecipitation from HEK293 cells. Upper panel: Representative co-IP of myc-D2 and FLAG-S100B from myc-D2/FLAG-S100B-HEK293 cells is shown. FLAG immunoreactivity was present in the eluates when the immunoprecipitation was with anti-myc (lanes 1–3), but not when using an irrelevant antibody to a hemaglutinin epitope (anti-HA, lane 4). Additionally, FLAG-S100B immunoreactivity was similar in eluates from cells treated with vehicle (lane 1) or with the D2 receptor agonist quinpirole (1 μM) for 5 min or 2 hr (lanes 2&3). Middle panel: immunoblot analysis of the input material with anti-FLAG suggests that all immunoprecipitation samples had similar amounts of FLAG-S100B. Lower panel: the results shown are the mean ± SE from three independent experiments. There was no significant difference between samples prepared from vehicle- or quinpirole- (quin) treated cells ( p > 0.05). B, co-immunoprecipitation from rat striatal homogenate. Upper panel: S100B immunoreactivity was present in the eluates when anti-D2 was used to precipitate the D2 receptor (lane 1), with little present when normal rabbit IgG was used (lane 2). Lower panel: The results shown are the mean ± SE from three independent experiments, demonstrating a constitutive interaction between the endogenous dopamine D2 receptor and S100B in rat neostriatum. *p < 0.05 compared to anti-myc + vehicle, Bonferroni post hoc comparison
Detection of Endogenous S100B in Primary Neostriatal Neurons
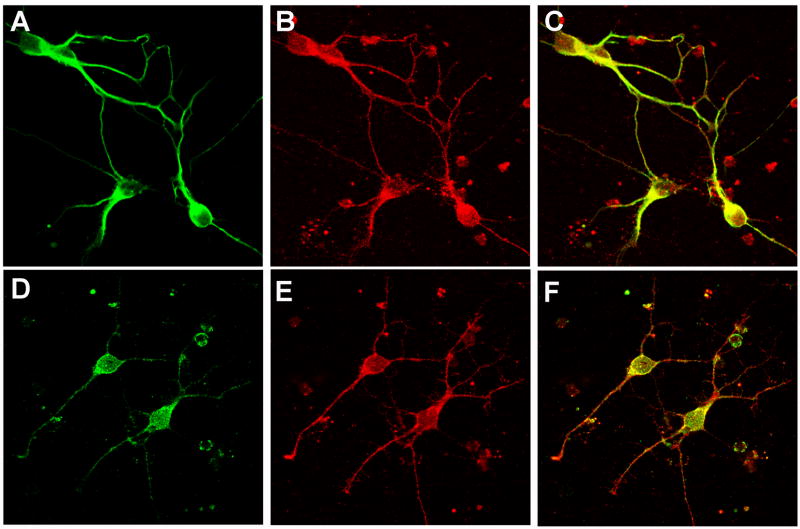
S100B is expressed most abundantly in astroglial cells (Suzuki et al., 1987). To determine if S100B is also expressed in neostriatal neurons, which express the D2 receptor, the colocalization of immunoreactivity for S100B and the neuronal marker MAP2 (Fig. 3A–C), and for S100B and D2 receptor (Fig. 3D–F), was determined in neostriatal neuronal cultures. In 12 visual fields from 3 independently prepared cultures, all cells that expressed immunoreactivity for MAP2 (green; 85 neurons) also expressed S100B (red). In two additional experiments, we also assessed the colocalization of immunoreactivity for S100B and the D2 receptor. In 8 visual fields with 83 cells exhibiting a neuronal morphology, ~75% of the cells expressed both S100B (red) and the D2 receptor (green) (Fig. 3).
Fig. 3. Confocal imaging of S100B.
Upper row, representative confocal fluorescence images depict immunoreactivity for MAP2 (A) and S100B (B) in neostriatal neurons. In the merged image (C) pixels containing immunoreactivity for both S100B and the neuronal marker MAP2 appear as yellow. Lower row, representative confocal fluorescence images depict immunoreactivity for D2 (D) and S100B (E) in neostriatal neurons. In the merged image (F) pixels containing immunoreactivity for both S100B and the D2 receptor appear as yellow.
Putative S100B Binding Motif
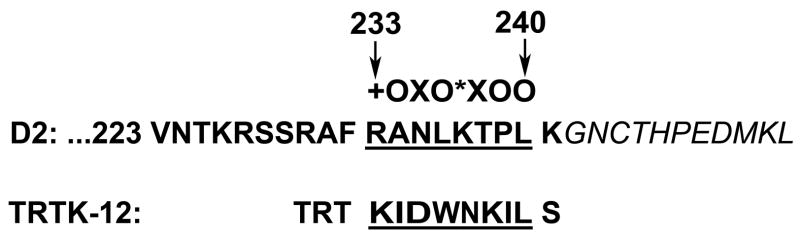
The identification of an S100B binding “epitope” from bacteriophage studies provides a useful probe to search for a binding motif. Ivanenkov et al. (1995) screened a bacteriophage random peptide display library and identified the consensus sequence +OXO*XOO (+ = basic, O– hydrophobic, * = hydrophilic, X = variable) as the “epitope” binding region for S100B. The peptide TRTKIDWNKILS (TRTK-12), a 12-residue peptide containing the consensus sequence, successfully competes with other S100B-binding proteins such as glial fibrillary acidic protein and CapZ for calcium-sensitive S100B binding (Bianchi et al., 1996; Ivanenkov et al., 1995). This consensus sequence for S100B binding has been identified in 25 proteins found to interact in vitro with S100B (McClintock and Shaw, 2000). We scanned the D2 receptor for this motif, and identified a potential S100B binding site at amino acid residues 233–240, close to the amino terminus of the third intracellular loop and adjacent to the alternatively spliced region of the receptor (Fig. 4). We did not find this motif in the third intracellular loop of the D3 receptor or in the D4 receptor.
Fig. 4. A putative S100B-binding motif is located near the N-terminus of the third intracellular loop of the D2 receptor.
The amino acid sequence for the rat D2 receptor was analyzed based on the previous reports (Bianchi et al., 1996; Ivanenkov et al., 1995; McClintock and Shaw, 2000). A putative S100B binding motif close to the N-terminus (amino acids: 233–240) of the third intracellular loop of the D2 receptor is aligned with TRTK-12 peptide characterized by Ivanenkov et al. (1995). (+, basic residue; O, hydrophobic residue; *, hydrophilic residue; X, any residue). Italics denote residues at the beginning of the alternatively spliced region that distinguishes D2S and D2L.
Expression of S100B with the Dopamine D2 receptor Modulates Receptor Activation of ERKs
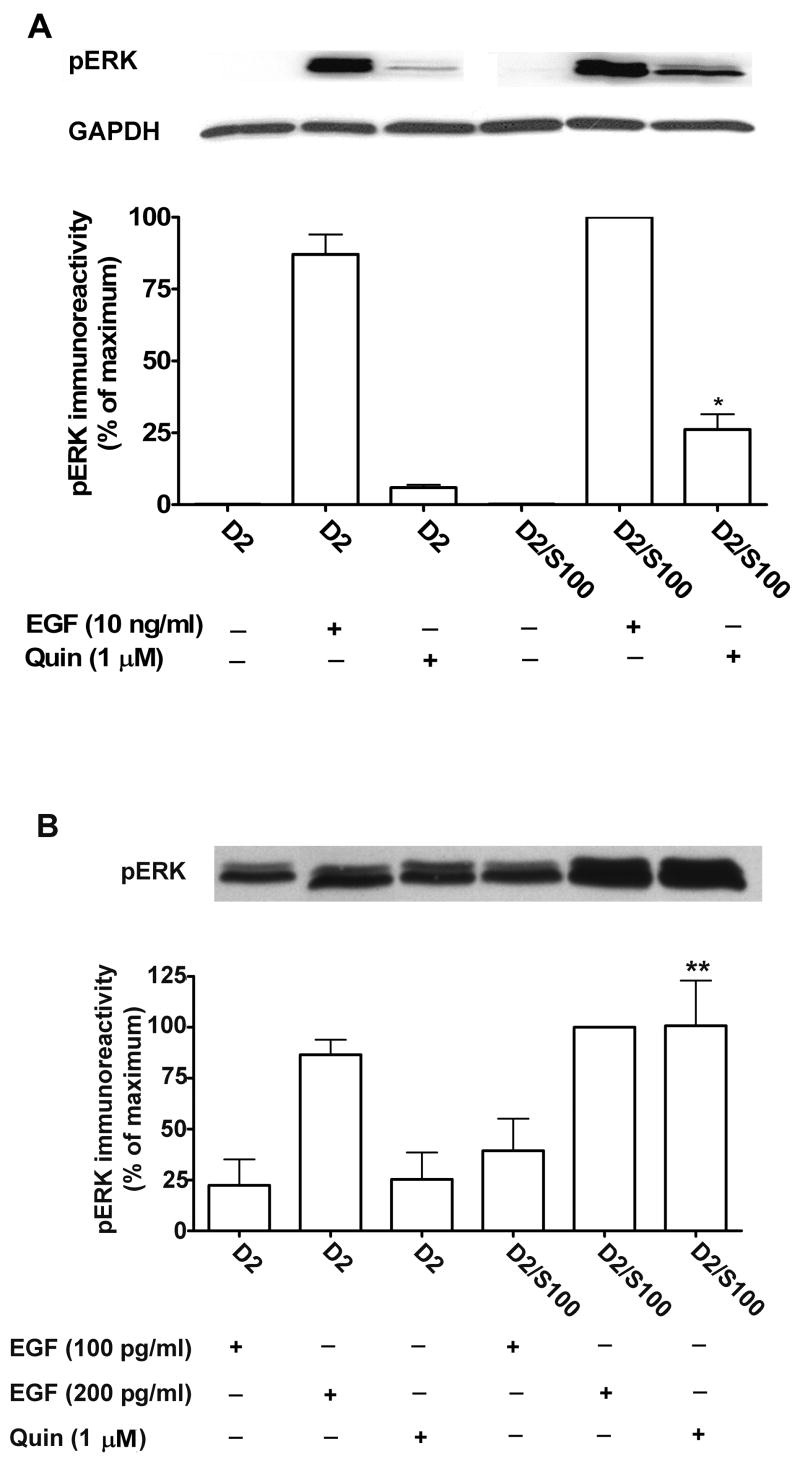
Receptor-stimulated activation of ERKs (ERK1, 44 kDa and ERK2, 42 kDa) was measured using an antibody for phospho-ERKs to quantify the abundance of dually phosphorylated ERKs. HEK293 cells expressing D2 receptor alone (myc-D2L-HEK293) or expressing D2 receptor and FLAG-S100B (myc-D2L/FLAG-S100B-HEK293) were selected to express similar D2 receptor densities (Bmax values for myc-D2L-HEK293 and myc-D2L/FLAG-S100B-HEK293 were 4660 ± 230 and 4250 ± 700 fmol/mg protein, respectively). Treatment with the D2 receptor agonist quinpirole (1 μM) induced rapid and robust activation of ERKs in HEK293 cells expressing the myc-D2 receptor (Fig. 5A). The stimulation of ERKs by quinpirole was significantly increased in cells co-expressing FLAG-S100B.
Fig. 5. Co-expression of S100B with the D2 receptor in HEK293 cells modulates receptor activation of ERKs.
A, compared to the non-treated samples, treatment with 1 μM of the D2 receptor agonist quinpirole (Quin) at 37°C for 5 min induced rapid and robust activation of ERKs in HEK293 cells expressing the D2 receptor (D2), and stimulation was increased in cell lines expressing both D2 and S100B (D2/S100). The upper panel depicts a representative immunoblot for activated ERK (pERK) and GAPDH as a loading control. The results shown in the lower panel are the mean ± SE from three (EGF) or four (Quin) independent experiments. B, the upper panel depicts a representative immunoblot for activated ERK (pERK) in cells expressing the D2 receptor alone (D2) or with S100B (D2/S100B) and treated with 1 μM quinpirole (Quin) or EGF at 100 or 200 pg/ml. There was no significant difference between the two cell lines for any concentration of EGF (p > 0.05). * p < 0.05, ** p < 0.01 compared to the same treatment in the cell line expressing D2 alone, Bonferroni post hoc comparison
To determine if S100B enhanced activation of ERKs at a step downstream from the D2 receptor, we assessed the effect of S100B expression on epidermal growth factor (EGF) induced activation of ERKs. Co-expression of S100B had no significant effect on the ERK response to EGF (10 ng/ml) (Fig. 5A). To confirm that the lack of effect of S100B on the response to EGF was not because ERK was already maximally activated, we also tested lower concentrations of EGF (100 pg/ml and 200 pg/ml). Although there was a tendency for EGF-induced activation of ERK to be enhanced in cells expressing S100B, the effect was not statistically significant, in contrast to the robust enhancement of quinpirole-induced activation of ERK in cells co-expressing S100B (Fig. 5B).
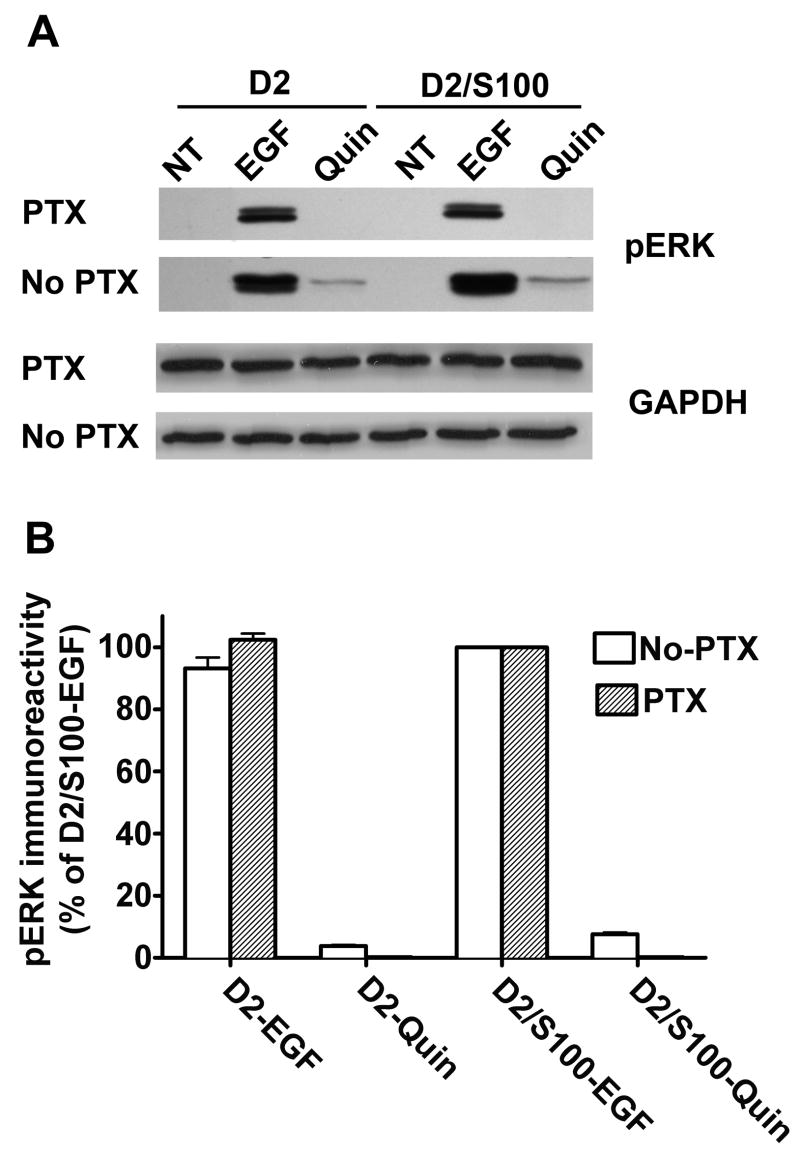
To verify that EGF-induced signaling was independent of D2 receptor activation of Gαi/o, we used pertussis toxin (PTX) to inactivate this class of G proteins. Treatment of HEK293 cells with PTX (50 ng/ml overnight) abolished D2 receptor activation of ERKs without altering EGF-induced activation (Fig 6). Overall, these results imply that the increased ERK activation with co-expression of S100B is due primarily to the interaction of D2 and S100B, rather than to binding of S100B to downstream elements that are shared between signaling pathways of the EGF and D2 receptors.
Fig. 6. EGF-induced signaling was independent of D2 receptor activation of Gαi/o.
Pretreatment with PTX completely prevented activation of ERK by quinpirole (Quin), but had no effect on EGF-induced activation of ERK. HEK293 cells expressing the D2 receptor alone (D2) or coexpressed with S100B (D2/S100B) were treated with vehicle (NT), EGF (10 ng/ml), or quinpirole (Quin, 1 μM) for 5 min. A, an immunoblot from a representative experiment shows EGF- and quinpirole-induced activation of ERK (pERK), with GAPDH as a loading control, in cells treated with PTX or vehicle (No PTX). B, the results shown are the mean ± SE from three independent experiments, expressed as a percentage of the optical density of bands from D2/S100B cells treated with EGF.
Increased Inhibition of Forskolin-stimulated Cyclic AMP Accumulation in myc-D2/FLAG-S100B Cell Line
We also characterized the effect of co-expression of S100B on D2L receptor inhibition of adenylate cyclase. The experiment in Fig. 7, representative of 3 independent experiments, demonstrates that the potency of the agonist is unchanged by the co-expression of S100B, but maximal inhibition of forskolin-stimulated cyclic AMP accumulation is significantly increased. The average EC50 values for the D2 and the D2/S100B cell lines were 21 nM and 15 nM, respectively. The maximal inhibition of forskolin-stimulated cyclic AMP accumulation for the D2 and D2/S100B cell lines was 57 ± 12 % and 66 ± 9 %, respectively (p < 0.05, by paired t-test, n = 3), respectively. This is qualitatively similar to the effect of S100B co-expression on quinpirole-induced activation of ERK.
Fig. 7. Co-expression of S100B enhances D2 receptor inhibition of cyclic AMP accumulation in HEK293 cells.
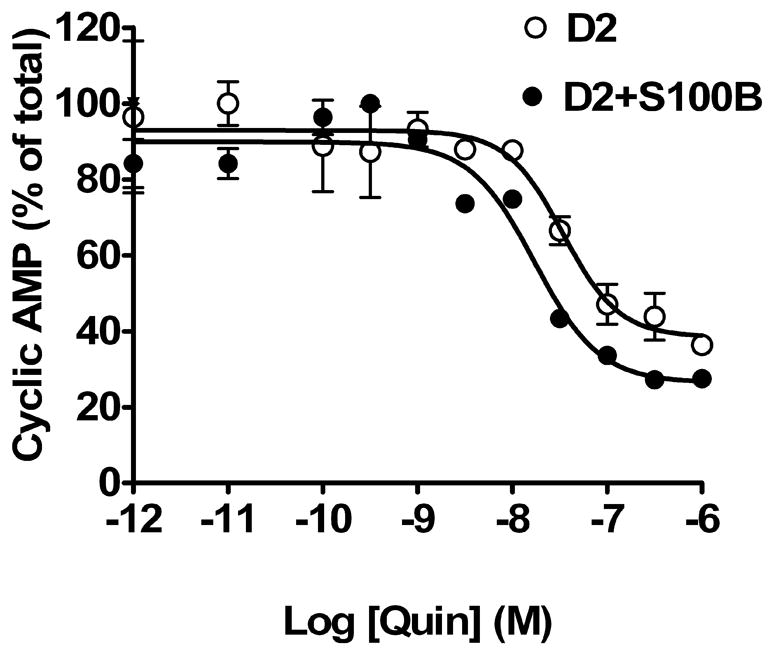
HEK293 cells stably expressing myc-D2 only or both myc-D2 and FLAG-S100B were incubated with 30 μM forskolin and increasing concentrations of quinpirole, and cyclic AMP accumulation was determined as described in Methods. The curve shown is representative of three independent experiments.
Discussion
Dopamine receptors are important molecules underlying neuropsychiatric disorders such as Parkinson’s disease, schizophrenia, and drug addiction. Among dopamine receptor subtypes, the D2 receptor has been extensively studied because most antipsychotics presently in use have high affinity for this receptor (Dixon et al., 1999). It has been postulated that modulation, rather than direct blockade, of the D2 receptor might offer the therapeutic benefit without the adverse effects of most antipsychotic drugs (Dixon et al., 1999). One way to achieve this would be to inhibit the binding of dopamine receptor-interacting proteins that enhance D2 receptor signaling.
Because the third cytoplasmic loop is the primary contact site between G protein-coupled receptors and G proteins, interactions that have been identified between the third cytoplasmic loop of D2-like receptors and a number of other proteins are likely to influence D2-like receptor signaling. For example, D2 and D3 receptors, but not D1 or D4 receptors, bind the actin-binding protein filamin A, or ABP-280, at a segment in the carboxyl terminus of the third cytoplasmic loop, where both D2 and D3 receptors have a potential site of phosphorylation by protein kinase C. D2 and D3 receptors expressed in cells that lack ABP-280 have diminished ability to inhibit adenylate cyclase (Li et al., 2000; Li et al., 2002). Calmodulin (CaM) modulates D2 receptor signaling by binding to the amino terminal end of the D2 receptor third cytoplasmic loop (Bofill-Cardona et al., 2000; Liu et al., 2007). Clearly, understanding fully how the D2 receptor functions will require determining the full complement of binding partners for the receptor.
The purpose of this study was to identify and characterize novel binding partners of the dopamine D2 receptor that might modulate receptor signaling. The B2H system is an efficient E. coli-based method for detecting protein-protein interactions in vivo. In this system, detection of protein-protein interactions is based on transcriptional activation of the HIS3 reporter gene, which allows growth in the presence of 3-AT, a competitive inhibitor of His3 enzyme. Positives are verified using the aadA gene, which confers streptomycin resistance, as a secondary reporter. The B2H system offers the ability to screen for binding partners with little background, and using E. coli for two-hybrid screening instead of a eukaryotic cell reduces the chance that the host harbors a homologue of one of the interacting protein partners (Joung et al., 2000).
Using the B2H system to screen a rat brain cDNA library, we identified a novel interaction between S100B and the D2 receptor. The S100 protein family is a highly conserved group of Ca2+-binding proteins with molecular masses from 9 to 13 kDa. S100B, a particularly well-characterized member of the S100 family, was first discovered as a major constituent of glia (Moore, 1965); however, it is now known to be expressed in tissues and cell lines including C6 glioma cells, cardiomyocytes, renal tumors, and melanomas (Donato, 1991; Suzushima et al., 1994; Takashi et al., 1994; Zimmer et al., 1997). S100B is a homodimer of 21 kDa, and each S100B subunit contains two EF-hand calcium-binding domains (Zimmer et al., 1997). While the precise mechanisms for intra- and extracellular functions of S100B are not well understood, processes such as neurite extension, Ca2+-flux, cell growth, apoptosis, energy metabolism, and protein phosphorylation are all thought to be modulated in some manner by S100B (Donato, 1991; Kligman and Hilt, 1988; Schafer and Heizmann, 1996). Most significantly for the proposed interaction with the D2 receptor, S100B has also been identified in neurons (Ellis et al., 2007). The general model for S100-target protein interactions is similar to that of other Ca2+-binding proteins such as CaM and troponin C; S100B undergoes a conformational change upon binding Ca2+ that promotes its interaction with a variety of target proteins (Chaudhuri et al., 1997; Drohat et al., 1997; Kligman and Hilt, 1988).
For further evaluation of the interaction between the D2 receptor and S100B, we used confocal microscopy to assess the colocalization of endogenous S100B with the neuronal marker MAP2 or with endogenous D2 receptor in neostriatal neurons. We observed extensive co-expression of S100B and MAP2 as well as S100B and D2 receptor in our neuronal cultures. We verified the interaction by co-immunoprecipitation of the D2 receptor with FLAG-S100B from HEK 293 cell homogenates and with endogenous S100B from rat neostriatal homogenates. We demonstrated that the third intracellular loop of the D2L and D2S receptors but not D3 is a contact point for the interaction with S100B using an in-vitro His-tag pull-down assay. We also identified an S100B binding motif located at residues 233–240 of the D2 receptor, towards the amino terminus of D2–IC3 and immediately upstream of the alternatively spliced region, a motif that is not found in the D3 receptor.
The first signaling pathway identified for D2-like receptors was inhibition of cyclic AMP accumulation. Another important effector in the D2 receptor signaling pathway that is potentially regulated by the interaction between the D2 receptor and S100B is extracellular signal-regulated kinase (ERK). ERKs belong to the family of MAPKs, components of parallel protein kinase cascades that transmit signals from a variety of extracellular stimuli to the cell nucleus, thus participating in cell proliferation, differentiation, and survival (Gutkind, 1998). Although the pathway from D2-like receptors to ERK has not been thoroughly elucidated and may differ depending on cell type and receptor subtype, D2-like receptor activation of ERK is frequently mediated by pertussis toxin-sensitive G proteins (Choi et al., 1999; Wang et al., 2005; Welsh et al., 1998). Both of these signaling responses to D2 receptor stimulation were enhanced in cells co-expressing S100B. The mechanism by which S100B enhanced D2 receptor signaling in unknown. Receptor-interacting proteins frequently alter the trafficking of receptors to the membrane, but preliminary results using cell-surface fluorescence and inhibition of radioligand binding by the membrane-impermeant ligand sulpiride suggest that the abundance of cell-surface receptors was similar in myc-D2L-HEK293 cells and myc-D2L/S100B-HEK293 cells (data not shown). Because S100B is a homodimer, another possibility is that it binds to both the D2 receptor and to another protein involved in signaling, bringing the two proteins together, but it is not known what other protein might be involved.
Another Ca2+ binding protein, CaM, is important for the serotonin 5-hydroxytryptamine receptor to activate ERK, in a process involving agonist-induced receptor internationalization (Della Rocca et al., 1999; Melien et al., 2002). CaM mediates activation of ERK by the μ-opioid receptor through a pathway involving the transactivation of the epidermal growth factor (EGF) receptor (Belcheva et al., 2001). Our previous work suggests that binding of CaM to the D2 receptor enhances receptor signaling (Liu et al., 2007). The data presented here suggest that binding of S100B, too, enhances D2 receptor signaling to both ERKs and cyclic AMP. To our knowledge, this is the first report regarding the interaction of S100B and the D2 receptor and its role in dopamine D2 receptor signaling.
ABBREVIATIONS
- 3-AT
3-amino-1,2,4-triazole
- B2H
Bacteriomatch™ Two-Hybrid
- CaM
calmodulin
- EGF
epidermal growth factor
- ERK
extracellular signal-regulated kinase
- G protein
heterotrimeric GTP-binding protein
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HEK293
human embryonic kidney 293 cells
- D2-IC3
the third intracellular loop of the D2 receptor
- MAP2
microtubule-associated protein-2
- MAPKs
mitogen-activated protein kinases
- PTX
pertussis toxin
- TBS
Tris-buffered saline
Footnotes
This work was supported by United States Public Health Service grant MH045372 (K.A.N.), the VA Merit Review and Career Scientist programs (K.A.N.), and the N. L. Tartar Trust (Y.L.).
References
- Belcheva MM, Szucs M, Wang DX, Sadee W, Coscia CJ. μ-Opioid receptor-mediated ERK activation involves calmodulin-dependent epidermal growth factor receptor transactivation. J Biol Chem. 2001;276:33847–33853. doi: 10.1074/jbc.M101535200. [DOI] [PubMed] [Google Scholar]
- Bianchi R, Garbuglia M, Verzini M, Giambanco I, Ivanenkov VV, Dimlich RV, Jamieson GA, Jr, Donato R. S-100 (alpha and beta) binding peptide (TRTK-12) blocks S-100/GFAP interaction: identification of a putative S-100 target epitope within the head domain of GFAP. Biochim Biophys Acta. 1996;1313:258–267. doi: 10.1016/0167-4889(96)00098-5. [DOI] [PubMed] [Google Scholar]
- Bofill-Cardona E, Kudlacek O, Yang Q, Ahorn H, Freissmuth M, Nanoff C. Binding of calmodulin to the D2-dopamine receptor reduces receptor signaling by arresting the G protein activation switch. J Biol Chem. 2000;275:32672–32680. doi: 10.1074/jbc.M002780200. [DOI] [PubMed] [Google Scholar]
- Chaudhuri D, Horrocks WD, Jr, Amburgey JC, Weber DJ. Characterization of lanthanide ion binding to the EF-hand protein S100 beta by luminescence spectroscopy. Biochemistry. 1997;36:9674–9680. doi: 10.1021/bi9704358. [DOI] [PubMed] [Google Scholar]
- Choi EY, Jeong D, Park KW, Baik JH. G protein-mediated mitogen-activated protein kinase activation by two dopamine D2 receptors. Biochem Biophys Res Commun. 1999;256:33–40. doi: 10.1006/bbrc.1999.0286. [DOI] [PubMed] [Google Scholar]
- Della Rocca GJ, Mukhin YV, Garnovskaya MN, Daaka Y, Clark GJ, Luttrell LM, Lefkowitz RJ, Raymond JR. Serotonin 5-HT1A receptor-mediated Erk activation requires calcium/calmodulin-dependent receptor endocytosis. J Biol Chem. 1999;274:4749–4753. doi: 10.1074/jbc.274.8.4749. [DOI] [PubMed] [Google Scholar]
- Dixon DA, Fenix LA, Kim DM, Raffa RB. Indirect modulation of dopamine D2 receptors as potential pharmacotherapy for schizophrenia: I. Adenosine agonists. Ann Pharmacother. 1999;33:480–488. doi: 10.1345/aph.18215. [DOI] [PubMed] [Google Scholar]
- Donato R. Perspectives in S-100 protein biology. Review article. Cell Calcium. 1991;12:713–726. doi: 10.1016/0143-4160(91)90040-l. [DOI] [PubMed] [Google Scholar]
- Donato R. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim Biophys Acta. 1999;1450:191–231. doi: 10.1016/s0167-4889(99)00058-0. [DOI] [PubMed] [Google Scholar]
- Donato R. Intracellular and extracellular roles of S100 proteins. Microsc Res Tech. 2003;60:540–551. doi: 10.1002/jemt.10296. [DOI] [PubMed] [Google Scholar]
- Drohat AC, Nenortas E, Beckett D, Weber DJ. Oligomerization state of S100B at nanomolar concentration determined by large-zone analytical gel filtration chromatography. Protein Sci. 1997;6:1577–1582. doi: 10.1002/pro.5560060721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis EF, Willoughby KA, Sparks SA, Chen T. S100B protein is released from rat neonatal neurons, astrocytes, and microglia by in vitro trauma and anti-S100 increases trauma-induced delayed neuronal injury and negates the protective effect of exogenous S100B on neurons. J Neurochem. 2007;101:1463–1470. doi: 10.1111/j.1471-4159.2007.04515.x. [DOI] [PubMed] [Google Scholar]
- Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Gutkind JS. The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogen-activated protein kinase cascades. J Biol Chem. 1998;273:1839–1842. doi: 10.1074/jbc.273.4.1839. [DOI] [PubMed] [Google Scholar]
- Heizmann CW. The multifunctional S100 protein family. Methods Mol Biol. 2002;172:69–80. doi: 10.1385/1-59259-183-3:069. [DOI] [PubMed] [Google Scholar]
- Heizmann CW, Fritz G, Schafer BW. S100 proteins: structure, functions and pathology. Front Biosci. 2002;7:d1356–d1368. doi: 10.2741/A846. [DOI] [PubMed] [Google Scholar]
- Ivanenkov VV, Jamieson GA, Jr, Gruenstein E, Dimlich RV. Characterization of S-100b binding epitopes. Identification of a novel target, the actin capping protein, CapZ. J Biol Chem. 1995;270:14651–14658. doi: 10.1074/jbc.270.24.14651. [DOI] [PubMed] [Google Scholar]
- Joung JK, Ramm EI, Pabo CO. A bacterial two-hybrid selection system for studying protein-DNA and protein-protein interactions. Proc Natl Acad Sci U S A. 2000;97:7382–7387. doi: 10.1073/pnas.110149297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kligman D, Hilt DC. The S100 protein family. Trends Biochem Sci. 1988;13:437–443. doi: 10.1016/0968-0004(88)90218-6. [DOI] [PubMed] [Google Scholar]
- Li M, Bermak JC, Wang ZW, Zhou QY. Modulation of dopamine D2 receptor signaling by actin-binding protein (ABP-280) Mol Pharmacol. 2000;57:446–452. doi: 10.1124/mol.57.3.446. [DOI] [PubMed] [Google Scholar]
- Li M, Li C, Weingarten P, Bunzow JR, Grandy DK, Zhou QY. Association of dopamine D3 receptors with actin-binding protein 280 (ABP-280) Biochem Pharmacol. 2002;63:859–863. doi: 10.1016/s0006-2952(01)00932-7. [DOI] [PubMed] [Google Scholar]
- Liu Y, Buck DC, Macey TA, Lan H, Neve KA. Evidence that calmodulin binding to the dopamine D2 receptor enhances receptor signaling. J Recept Signal Transduct Res. 2007;27:47–65. doi: 10.1080/10799890601094152. [DOI] [PubMed] [Google Scholar]
- Liu Y, Teeter MM, DuRand CJ, Neve KA. Identification of a Zn2+-binding site on the dopamine D2 receptor. Biochem Biophys Res Commun. 2006;339:73–879. doi: 10.1016/j.bbrc.2005.11.110. [DOI] [PubMed] [Google Scholar]
- Marenholz I, Heizmann CW, Fritz G. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature) Biochem Biophys Res Commun. 2004;322:1111–1122. doi: 10.1016/j.bbrc.2004.07.096. [DOI] [PubMed] [Google Scholar]
- McClintock KA, Shaw GS. A logical sequence search for S100B target proteins. Protein Sci. 2000;9:2043–2046. doi: 10.1110/ps.9.10.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melien O, Nilssen LS, Dajani OF, Sand KL, Iversen JG, Sandnes DL, Christoffersen T. Ca2+-mediated activation of ERK in hepatocytes by norepinephrine and prostaglandin F2 alpha: role of calmodulin and Src kinases. BMC Cell Biol. 2002;3:5. doi: 10.1186/1471-2121-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BW. A soluble protein characteristic of the nervous system. Biochem Biophys Res Commun. 1965;19:739–744. doi: 10.1016/0006-291x(65)90320-7. [DOI] [PubMed] [Google Scholar]
- Neve KA, Neve RL. Molecular biology of dopamine receptors. In: Neve KA, Neve RL, editors. The Dopamine Receptors. Humana Press; Totawa, NJ: 1997. pp. 27–76. [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Rothermundt M, Ponath G, Arolt V. S100B in schizophrenic psychosis. Int Rev Neurobiol. 2004;59:445–470. doi: 10.1016/S0074-7742(04)59017-7. [DOI] [PubMed] [Google Scholar]
- Schafer BW, Heizmann CW. The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem Sci. 1996;21:134–140. doi: 10.1016/s0968-0004(96)80167-8. [DOI] [PubMed] [Google Scholar]
- Strange PG. Brain Biochemistry and Brain Disorders. Oxford University Press; Oxford, UK: 1992. [Google Scholar]
- Suzuki F, Kato K, Kato T, Ogasawara N. S-100 protein in clonal astroglioma cells is released by adrenocorticotropic hormone and corticotropin-like intermediate-lobe peptide. J Neurochem. 1987;49:1557–1563. doi: 10.1111/j.1471-4159.1987.tb01027.x. [DOI] [PubMed] [Google Scholar]
- Suzushima H, Asou N, Hattori T, Takatsuki K. Adult T-cell leukemia derived from S100 beta positive double-negative (CD4- CD8-) T cells. Leuk Lymphoma. 1994;13:257–262. doi: 10.3109/10428199409056289. [DOI] [PubMed] [Google Scholar]
- Takashi M, Sakata T, Nakano Y, Yamada Y, Miyake K, Kato K. Elevated concentrations of the beta-subunit of S100 protein in renal cell tumors in rats. Urol Res. 1994;22:251–255. doi: 10.1007/BF00541902. [DOI] [PubMed] [Google Scholar]
- Wang C, Buck DC, Yang R, Macey TA, Neve KA. Dopamine D2 receptor stimulation of mitogen-activated protein kinases mediated by cell type-dependent transactivation of receptor tyrosine kinases. J Neurochem. 2005;93:899–909. doi: 10.1111/j.1471-4159.2005.03055.x. [DOI] [PubMed] [Google Scholar]
- Welsh GI, Hall DA, Warnes A, Strange PG, Proud CG. Activation of microtubule-associated protein kinase (Erk) and p70 S6 kinase by D2 dopamine receptors. J Neurochem. 1998;70:2139–2146. doi: 10.1046/j.1471-4159.1998.70052139.x. [DOI] [PubMed] [Google Scholar]
- Zimmer DB, Chessher J, Wilson GL, Zimmer WE. S100A1 and S100B expression and target proteins in type I diabetes. Endocrinology. 1997;138:5176–5183. doi: 10.1210/endo.138.12.5579. [DOI] [PubMed] [Google Scholar]
- Zimmer DB, Wright SP, Weber DJ. Molecular mechanisms of S100-target protein interactions. Microsc Res Tech. 2003;60:552–559. doi: 10.1002/jemt.10297. [DOI] [PubMed] [Google Scholar]