Abstract
BACKGROUND
The optimal intensity of renal-replacement therapy in critically ill patients with acute kidney injury is controversial.
METHODS
We randomly assigned critically ill patients with acute kidney injury and failure of at least one nonrenal organ or sepsis to receive intensive or less intensive renal-replacement therapy. The primary end point was death from any cause by day 60. In both study groups, hemodynamically stable patients underwent intermittent hemodialysis, and hemodynamically unstable patients underwent continuous venovenous hemodiafiltration or sustained low-efficiency dialysis. Patients receiving the intensive treatment strategy underwent intermittent hemodialysis and sustained low-efficiency dialysis six times per week and continuous venovenous hemodiafiltration at 35 ml per kilogram of body weight per hour; for patients receiving the less-intensive treatment strategy, the corresponding treatments were provided thrice weekly and at 20 ml per kilogram per hour.
RESULTS
Baseline characteristics of the 1124 patients in the two groups were similar. The rate of death from any cause by day 60 was 53.6% with intensive therapy and 51.5% with less-intensive therapy (odds ratio, 1.09; 95% confidence interval, 0.86 to 1.40; P = 0.47). There was no significant difference between the two groups in the duration of renalreplacement therapy or the rate of recovery of kidney function or nonrenal organ failure. Hypotension during intermittent dialysis occurred in more patients randomly assigned to receive intensive therapy, although the frequency of hemodialysis sessions complicated by hypotension was similar in the two groups.
CONCLUSIONS
Intensive renal support in critically ill patients with acute kidney injury did not decrease mortality, improve recovery of kidney function, or reduce the rate of nonrenal organ failure as compared with less-intensive therapy involving a defined dose of intermittent hemodialysis three times per week and continuous renal-replacement therapy at 20 ml per kilogram per hour. (ClinicalTrials.gov number, NCT00076219.)
Acute kidney injury is a common complication of acute illness, affecting approximately 2 to 7% of hospitalized patients1-4 and more than 35% of critically ill patients.5-8 Renal-replacement therapy is the mainstay of supportive treatment of patients with severe acute kidney injury; its use is required in 5 to 6% of critically ill patients and is associated with in-hospital mortality rates of 50 to 80%.5,9-12
The optimal timing for the initiation, method, and dosing of renal-replacement therapy remains uncertain more than 60 years after the first clinical use of hemodialysis in patients with acute kidney injury.13-15 Several single-center studies have suggested that more intensive renal-replacement therapy (i.e., dialysis at a higher dose) is associated with improved survival16-18; however, results have been inconsistent.19,20 Given this uncertainty, we tested the hypothesis that more intensive renal-replacement therapy decreases mortality among critically ill patients with acute kidney injury. Although previous studies of the intensity of therapy were limited to single methods of renal-replacement therapy,16-20 we compared two integrated therapeutic strategies that reflect typical clinical practice in that they allow for changing the method of renal-replacement therapy as the patient's hemodynamic status changes.
METHODS
STUDY SETTING
The Veterans Affairs/National Institutes of Health (VA/NIH) Acute Renal Failure Trial Network study (VA Cooperative Study number 530) was a multicenter, prospective, randomized, parallel-group trial of two strategies for renal-replacement therapy in critically ill patients with acute kidney injury conducted between November 2003 and July 2007 at 27 VA and university-affiliated medical centers (see the Appendix). The design of the study has been described previously,21 and the complete study protocol is available in the Supplementary Appendix (available with the full text of this article at www.nejm.org).
The study was approved by the Human Rights Committee at the West Haven VA Cooperative Studies Program (CSP) Coordinating Center and by the institutional review boards at each of the participating study sites. The integrity of data collection was audited by the VA CSP Site Monitoring, Auditing, and Resource Team. An independent data and safety monitoring committee reviewed the safety data and interim results. No manufacturer of dialysis machines or supplies was involved in study design, data accrual, data analysis, or manuscript preparation.
STUDY POPULATION
Patients were eligible for enrollment if they were critically ill adults (18 years or older) who had acute kidney injury clinically consistent with acute tubular necrosis and requiring renal-replacement therapy, as well as failure of one or more nonrenal organ systems (defined as a nonrenal Sequential Organ Failure Assessment22 [SOFA] organ system score ≥2 [range, 0 to 4, with a higher score indicating more severe organ dysfunction]) or sepsis. Eligible patients could not have undergone more than one session of intermittent hemodialysis or sustained low-efficiency dialysis or more than 24 hours of continuous renal-replacement therapy before randomization. Specific inclusion and exclusion criteria21 are listed in Table 1 in the Supplementary Appendix. Written informed consent was obtained from all patients or their health care surrogates, and if patients who required surrogates regained decision-making capacity, consent was then obtained directly from the patients.
RANDOMIZATION
Eligible patients were randomly assigned to one of the two treatment groups by means of a centralized, computer-generated adaptive randomization scheme. Randomization was stratified according to and within site on the basis of the SOFA cardiovascular score (0 to 2 vs. 3 to 4)22 and by the presence or absence of oliguria (defined as an average urine output of <20 ml per hour for >24 hours). Although treatment groups could not be masked, investigators were unaware of aggregate outcomes during the study.
INTERVENTIONS
Selection of and transition between methods of renal-replacement therapy were specified in the protocol (Fig. 1 in the Supplementary Appendix). In both treatment groups, patients underwent intermittent hemodialysis when they were hemodynamically stable (defined as having a SOFA cardiovascular score of 0 to 2) and underwent continuous venovenous hemodiafiltration or sustained low-efficiency dialysis when they were hemodynamically unstable (defined as having a SOFA cardiovascular score of 3 to 4). The selection of continuous venovenous hemodiafiltration or sustained low-efficiency dialysis was determined by site-specific practice. Patients were transitioned from intermittent hemodialysis to continuous venovenous hemodiafiltration or sustained low-efficiency dialysis if they became hemodynamically unstable and from continuous venovenous hemodiafiltration or sustained low-efficiency dialysis to intermittent hemodialysis when the hemodynamic instability resolved (i.e., when the SOFA cardiovascular score was 0 to 1 for >24 hours).
In the group receiving the intensive-therapy strategy, intermittent hemodialysis and sustained low-efficiency dialysis were provided six times per week (every day except Sunday), and continuous venovenous hemodiafiltration was prescribed to provide a flow rate of the total effluent (the sum of the dialysate and ultrafiltrate) of 35 ml per kilogram of body weight per hour, based on the weight before the onset of acute illness. In the less-intensive strategy, intermittent hemodialysis and sustained low-efficiency dialysis were provided three times per week (on alternate days except Sunday), and continuous venovenous hemodiafiltration was prescribed to provide a total effluent flow rate of 20 ml per kilogram per hour. In both treatment groups, intermittent hemodialysis and sustained low-efficiency hemodialysis were prescribed to provide a single-pool Kt/Vurea (a dimensionless index of the dialysis dose in which K is the urea clearance of the dialyzer, t is the duration of dialysis, and V is the volume of distribution of urea) of 1.2 to 1.4 per session. If required for the management of severe volume overload, patients receiving intermittent therapy could undergo isolated ultrafiltration on days when dialysis was not performed. Cellulose triacetate or synthetic membranes were used for all treatments (Table 2 in the Supplementary Appendix). Adherence to the treatment assignment and to the prescribed and delivered doses of renal-replacement therapy was regularly monitored at the coordinating center.
The assigned renal-replacement therapy was provided for up to 28 days after randomization or until recovery of kidney function, discharge from acute care, withdrawal of life-sustaining therapy, or death. Recovery of kidney function was defined on the basis of creatinine clearance, measured with the use of 6-hour timed urine collections when urine flow increased to more than 30 ml per hour or when there was a spontaneous fall in the serum creatinine level. Renal-replacement therapy was continued if the creatinine clearance was less than 12 ml per minute (0.2 ml per second) and was discontinued if the creatinine clearance was greater than 20 ml per minute (0.3 ml per second); decisions regarding discontinuation of renal-replacement therapy for intermediate values of creatinine clearance were left to the clinician. Further management of renal-replacement therapy in patients with persistent kidney failure 28 days after randomization, or in those who were discharged from acute care before day 28, was at the discretion of the treating physicians.
END POINTS
The primary study end point was death from any cause by day 60. Secondary end points included in-hospital death and recovery of kidney function (defined as lack of need for continuing dialysis support, with a minimum creatinine clearance of 20 ml per minute). Recovery of kidney function was considered to be complete if the serum creatinine level was no more than 0.5 mg per deciliter (44 μmol per liter) above the baseline value or partial if the level remained at more than 0.5 mg per deciliter above the baseline value but the patient was not dialysis-dependent. Additional end points were the duration of renal-replacement therapy, lengths of stay in the intensive care unit (ICU) and hospital, days free of nonrenal organ failure (defined as days on which individual SOFA organ-system scores were 0 to 2), and whether the patients returned to their previous living situation (“home”), and did not require dialysis, by day 60.
STATISTICAL ANALYSIS
We calculated that 1164 patients would need to be enrolled to detect a decrease in the 60-day rate of death from any cause from 55% (with less-intensive therapy) to 45% (with intensive therapy), with a statistical power of 90% and a two-sided significance level of 0.05, assuming a 10% loss to follow-up. Two interim efficacy analyses were performed according to the method of Haybittle and Peto, after 600 and 900 patients had been enrolled and had been followed for 60 days.23 All analyses were performed according to the intention-to-treat principle. The 60-day mortality data were analyzed with the use of conditional logistic regression and adjusted according to the randomization strata (the SOFA cardiovascular score, presence or absence of oliguria, and site). Patients who were lost to follow-up were counted as alive in the analysis. Cumulative mortality rates were calculated according to the Kaplan-Meier method. Prespecified subgroup analyses were performed according to the presence or absence of oliguria and sepsis, the SOFA cardiovascular score, and sex. Subgroup effects were assessed by testing the interaction of treatment group and subgroup strata, with P values of less than 0.10 considered to indicate statistical significance. Complete and partial recovery of kidney function was analyzed with the use of the weighted least-squares method; analysis of variance was used for analysis of all other secondary end points. P values for all outcomes were two-sided; values less than 0.05 were considered to indicate statistical significance. Statistical analyses were performed with the use of SAS software, version 9.1 (SAS Institute).
RESULTS
ENROLLMENT
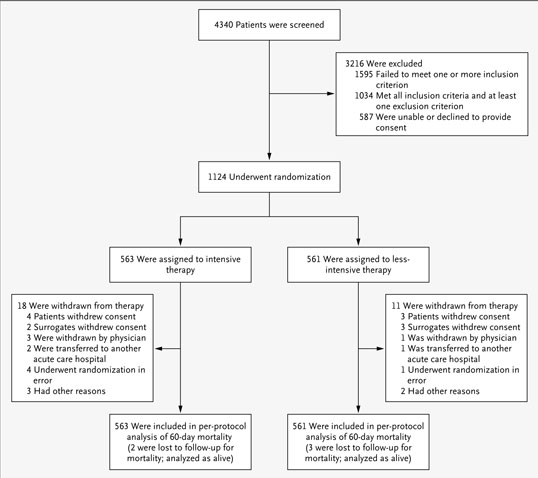
Between November 3, 2003, and July 2, 2007, 4340 patients were screened for eligibility; 1124 patients were randomly assigned to a treatment group: 563 to the intensive treatment strategy and 561 to the less-intensive treatment strategy (Fig. 1). Reasons for nonenrollment have been described previously.24 A total of 29 patients (18 receiving intensive therapy and 11 receiving less-intensive therapy) were withdrawn from study therapy after randomization. Vital status 60 days after randomization was ascertained for all but five patients (two receiving intensive therapy and three receiving less-intensive therapy).
Figure 1.
Enrollment, Randomization, and Follow-up of Study Patients.
BASELINE CHARACTERISTICS
As shown in Table 1, baseline characteristics were similar between the two groups. The mean (±SD) age was 59.7±15.3 years; 70.6% were male; 74.4% were white, 15.9% were black, and 6.9% were Hispanic; race or ethnic group was self-reported. The serum creatinine level before onset of acute kidney injury was 1.1±0.4 mg per deciliter (97±35 μmol per liter); 88.2% of patients had an estimated glomerular filtration rate of at least 45 ml per minute per 1.73 m2, and no patients had an estimated glomerular filtration rate of less than 30 ml per minute per 1.73 m2. Acute kidney injury was attributed to ischemia in 80.9% of patients, nephrotoxins in 28.0%, and sepsis in 54.9%; 59.0% of patients were considered to have multifactorial acute kidney injury. The mean Charlson comorbidity index score was 4.3±2.9, the mean Acute Physiology and Chronic Health Evaluation (APACHE) II score was 26.4±7.3, and the mean overall SOFA score was 14.5±3.7. (Possible scores range from 0 to 6 for each of 17 indicators on the Charlson index, 0 to 71 for APACHE II, and 0 to 4 for each of six organ systems for SOFA; higher scores indicate more severe organ dysfunction.) In all, 80.6% of patients required mechanical ventilation, 63.0% had sepsis, and 78.0% had oliguria. As permitted in the protocol, 64.5% of patients had received one session of intermittent hemodialysis or sustained low-efficiency dialysis or continuous renal-replacement therapy for up to 24 hours before randomization.
Table 1.
Baseline Characteristics of the Study Patients.*
| Characteristic | Intensive Strategy (N = 563) |
Less-Intensive Strategy (N = 561) |
P Value† | ||
|---|---|---|---|---|---|
| Age — yr | 59.6±15.3 | 59.7±15.2 | 0.97 | ||
| Sex — no./no. with data (%) | |||||
| Male | 409/563 (72.6) | 384/560 (68.6) | 0.13 | ||
| Female | 154/563 (27.4) | 176/560 (31.4) | |||
| Race or ethnic group — no./no. with data (%)‡ | |||||
| White | 415/563 (73.7) | 420/560 (75.0) | 0.43 | ||
| Black | 91/563 (16.2) | 88/560 (15.7) | |||
| Hispanic | 44/563 (7.8) | 33/560 (5.9) | |||
| Other | 13/563 (2.3) | 19/560 (3.4) | |||
| Renal function before onset of acute kidney injury | |||||
| Serum creatinine — mg/dl | 1.1±0.4 | 1.1±0.3 | 0.71 | ||
| Estimated GFR — no./no. with data (%) | |||||
| ≥60 ml/min/1.73 m2 | 342/524 (65.3) | 344/523 (65.8) | 0.84 | ||
| 45–59 ml/min/1.73 m2 | 122/524 (23.3) | 115/523 (22.0) | |||
| 30–44 ml/min/1.73 m2 | 60/524 (11.5) | 64/523 (12.2) | |||
| Cause of acute kidney injury — no./no. with data (%) | |||||
| Ischemia | 440/536 (82.1) | 431/541 (79.7) | 0.31 | ||
| Nephrotoxins | 143/514 (27.8) | 143/509 (28.1) | 0.92 | ||
| Sepsis | 300/531 (56.5) | 279/524 (53.2) | 0.29 | ||
| Multifactorial causes | 317/534 (59.4) | 309/527 (58.6) | 0.81 | ||
| Primary treating service — no./no. with data (%) | |||||
| Medical | 272/563 (48.3) | 259/560 (46.2) | 0.76 | ||
| Surgical | 229/563 (40.7) | 234/560 (41.8) | |||
| Other | 62/563 (11.0) | 67/560 (12.0) | |||
| Weight before acute illness — kg | 84.1±19.6 | 84.1±18.9 | >0.99 | ||
| Length of stay before randomization — days | |||||
| Hospital | 11.1±13.6 | 10.3±14.7 | 0.36 | ||
| ICU | 6.9±10.1 | 6.4±7.8 | 0.38 | ||
| Charlson comorbidity index | 4.3±3.0 | 4.2±2.8 | 0.66 | ||
| Mechanical ventilation — no./no. with data (%) | 453/563 (80.5) | 452/560 (80.7) | 0.91 | ||
| Sepsis — no. (%) | 358 (63.6) | 350 (62.4) | 0.68 | ||
| APACHE II score | 26.6±7.2 | 26.1±7.5 | 0.29 | ||
| SOFA organ-system score | |||||
| Respiratory | 2.4±1.1 | 2.3±1.1 | 0.10 | ||
| Coagulation | 1.4±1.2 | 1.3±1.2 | 0.49 | ||
| Liver | 1.5±1.3 | 1.4±1.3 | 0.29 | ||
| Cardiovascular | 2.3±1.7 | 2.2±1.7 | 0.23 | ||
| Central nervous system | 2.5±1.4 | 2.5±1.4 | 0.69 | ||
| Total | 14.7±3.7 | 14.4±3.7 | 0.21 | ||
| Cleveland Clinic ICU Acute Renal Failure score | 12.3±3.3 | 12.0±3.4 | 0.11 | ||
| BUN at initiation of RRT — mg/dl | 65.9±30.2 | 66.7±35.2 | 0.68 | ||
| Cardiovascular SOFA score — no. (%) | |||||
| 0–2 | 255 (45.3) | 254 (45.3) | >0.99 | ||
| 3–4 | 308 (54.7) | 307 (54.7) | |||
| Oliguria — no. (%) | |||||
| No | 124 (22.0) | 123 (21.9) | 0.97 | ||
| Yes | 439 (78.0) | 438 (78.1) | |||
| One session of IHD or SLED or <24 hr of continuous RRT before randomization — no./no. with data (%) |
358/563 (63.6) | 366/560 (65.4) | 0.54 | ||
Plus–minus values are means ±SD. Percentages are based on the number of patients without missing data. Possible scores range from 0 to 6 for each of 17 indicators on the Charlson comorbidity index, 0 to 71 for the Acute Physiology and Chronic Health Evaluation (APACHE) II, and 0 to 4 for each of six organ systems for Sequential Organ Failure Assessment (SOFA) (with the renal-system score not reported separately but included in the calculation of the total SOFA score); higher scores indicate more severe organ dysfunction. The Cleveland Clinic Intensive Care Unit (ICU) Acute Renal Failure score can range from 1 to 20, with higher scores predictive of increased risk of death. To convert values for creatinine to micromoles per liter, multiply by 88.4. To convert values for blood urea nitrogen (BUN) to millimoles per liter, multiply by 0.357. GFR denotes glomerular filtration rate, IHD intermittent hemodialysis, RRT renalreplacement therapy, and SLED sustained low-efficiency dialysis.
P values were calculated with the use of the chi-square test for categorical variables and the t-test for continuous variables.
Race or ethnic group was self-reported.
MANAGEMENT OF STUDY THERAPY
Patients receiving intensive renal-replacement therapy required 13.4±9.6 days of therapy, and those receiving less-intensive therapy required 12.8±9.3 days of therapy. Table 2 lists the details of therapy in the two groups.
Table 2.
Management of Renal-Replacement Therapy (RRT) during the Therapy Phase.*
| Characteristic of Therapy | Intensive Strategy (N = 563) |
Less-Intensive Strategy (N = 561) |
|
|---|---|---|---|
| Any RRT | |||
| Days of therapy — no./patient | 13.4±9.6 | 12.8±9.3 | |
| Treatments provided — no. | 6681 | 4921 | |
| Intermittent hemodialysis | |||
| Treatments provided — no. | 3241 | 1836 | |
| Treatments/patient — no. | 9.3±7.1 | 5.5±3.7 | |
| Treatments/wk — no. (95% CI)† | 5.4 (5.2–5.6) | 3.0 (2.8–3.1) | |
| Days between treatments — no. (95% CI)† | 1.1 (1.1–1.2) | 2.1 (2.0–2.2) | |
| Duration of session — hr | |||
| Median | 4.0 | 4.0 | |
| Interquartile range | 3.3–4.5 | 3.5–4.5 | |
| Blood flow — ml/min | 360±59 | 360±62 | |
| Dialysate flow — ml/min | 730±123 | 710±135 | |
| Net ultrafiltration — liters/treatment | 1.7±1.2 | 2.1±1.4 | |
| Anticoagulant — no. of treatments (%) | |||
| None | 2148 (66.3) | 1184 (64.5) | |
| Heparin | 949 (29.3) | 585 (31.9) | |
| Other | 144 (4.4) | 67 (3.6) | |
| Blood urea nitrogen — mg/dl | |||
| Predialysis | 45±25 | 70±33 | |
| Postdialysis | 16±12 | 25±15 | |
| Kt/Vurea | |||
| First treatment | 1.13±0.31 | 1.13±0.32 | |
| Subsequent treatments | 1.32±0.37 | 1.31±0.33 | |
| Average value ≥1.2 — no./no. with data (%) | 199/297 (67.0) | 184/266 (69.2) | |
| Sustained low-efficiency dialysis‡ | |||
| Treatments provided — no. | 222 | 77 | |
| No. of treatments/patient | 6.2±4.7 | 2.9±2.7 | |
| Duration of sessions — hr | |||
| Median | 8.1 | 8.3 | |
| Interquartile range | 6.8–12.0 | 7.0–12.0 | |
| Blood flow — ml/min | 220±45 | 210±40 | |
| Dialysate flow — ml/min | 250±121 | 240±102 | |
| Net ultrafiltration — liters/treatment | 1.3±1.4 | 1.4±1.6 | |
| Anticoagulant — no. (%) | |||
| None | 131 (58.7) | 53 (68.8) | |
| Heparin | 72 (32.3) | 23 (29.9) | |
| Other | 20 (9.0) | 1 (1.3) | |
| Continuous venovenous hemodiafiltration | |||
| Treatments provided — no. | 3178 | 2789 | |
| Days of therapy — no./patient | 7.8±6.4 | 7.3±5.9 | |
| Daily duration of therapy/patient — hr | |||
| Median | 20.9 | 21.0 | |
| Interquartile range | 13.0–23.7 | 13.0–24.0 | |
| Blood flow — ml/min | 150±33 | 140±40 | |
| Dialysate flow — ml/hr | 1410±346 | 820±250 | |
| Replacement flow — ml/hr | 1390±316 | 830±249 | |
| Net ultrafiltration — ml/hr | 130±135 | 130±189 | |
| 24-hr effluent volume — liters | 49.6±22.4 | 30.5±14.3 | |
| Effluent flow — ml/kg/hr | |||
| Prescribed | 36.2±3.8 | 21.5±4.3 | |
| Delivered | 35.8±6.4 | 22.0±6.1 | |
| Mean daily BUN — mg/dl | 33±18 | 47±23 | |
| Anticoagulant — no. of treatments (%) | |||
| None | 1736 (54.6) | 1666 (59.7) | |
| Heparin | 645 (20.3) | 530 (19.0) | |
| Citrate | 649 (20.4) | 495 (17.7) | |
| Other | 148 (4.7) | 98 (3.5) | |
| Percent of prescribed dose of therapy delivered | 89±39 | 95±35 | |
| Isolated ultrafiltration | |||
| Treatments provided — no. | 40 | 219 | |
| Net ultrafiltration — liters/treatment | 2.1±1.3 | 2.7±1.0 | |
Plus–minus values are means ±SD. The therapy phase was defined as the time of randomization through the time of discontinuation of study therapy. For continuous venovenous hemodiafiltration, each day of therapy was counted as a treatment. Kt/Vurea is a dimensionless index of the dialysis dose in which K is the urea clearance of the dialyzer, t is the duration of dialysis, and V is the volume of distribution of urea. To convert values for blood urea nitrogen (BUN) to millimoles per liter, multiply by 0.357. CI denotes confidence interval.
For intermittent hemodialysis, the number of treatments per week and the number of days between treatments were calculated as Poisson rates.
For sustained low-efficiency dialysis, the number of treatments per week and the number of days between treatments were not calculated because the number of treatments provided was too small to be meaningful.
Intermittent Hemodialysis and Sustained Low-Efficiency Dialysis
The intensive-therapy group underwent an average of 5.4 sessions (95% confidence interval [CI], 5.3 to 5.6) of intermittent hemodialysis or sustained low-efficiency dialysis per week, with an average interval between treatments (excluding Sundays) of 1.1 days (95% CI, 1.1 to 1.2), as compared with patients receiving less-intensive therapy, who underwent 3.0 sessions (95% CI, 2.9 to 3.1) of intermittent hemodialysis or sustained low-efficiency dialysis treatments per week, with an average interval between treatments of 2.0 days (95% CI, 2.0 to 2.1) (P<0.001). Missed sessions (of any method of therapy) occurred on only 1.9% of study days among patients receiving intensive therapy and on 1.1% of study days among those receiving less-intensive therapy; extra sessions (beyond those specified in the protocol) of intermittent hemodialysis or sustained low-efficiency dialysis were provided only on 0.5% of study days of intensive therapy and on 1.5% of study days of less-intensive therapy, with medical indications (hyperkalemia, metabolic acidosis, inadequate metabolic control, and pericarditis) accounting for 14.3% and 27.3% of the extra treatments, respectively (Table 3 in the Supplementary Appendix). The overall mean delivered Kt/Vurea per session after the first intermittent-hemodialysis session was 1.32±0.36 (Fig. 2 in the Supplementary Appendix).
Continuous Venovenous Hemodiafiltration
The mean prescribed dose of continuous venovenous hemodiafiltration was 36.2±3.8 ml per kilogram per hour with intensive therapy and 21.5±4.3 ml per kilogram per hour with less-intensive therapy (P<0.001) (Fig. 3A in the Supplementary Appendix), with corresponding mean delivered doses of 35.8±6.4 ml per kilogram per hour and 22.0±6.1 ml per kilogram per hour, respectively (P<0.001) (Fig. 3B in the Supplementary Appendix). The most common reason for a decreased dose of continuous venovenous hemodiafiltration in the intensive-therapy group was prescription error (occurring on 1.6% of treatment days); the most common reasons for an increased dose among patients receiving less-intensive therapy were prescription error (on 3.6% of treatment days) and attempting to make up for time off of therapy (on 1.2% of treatment days) (Table 4 in the Supplementary Appendix). The median daily duration of continuous venovenous hemodiafiltration was 20.9 hours for the intensive strategy and 21.0 hours for the less-intensive strategy.
PRIMARY OUTCOMES
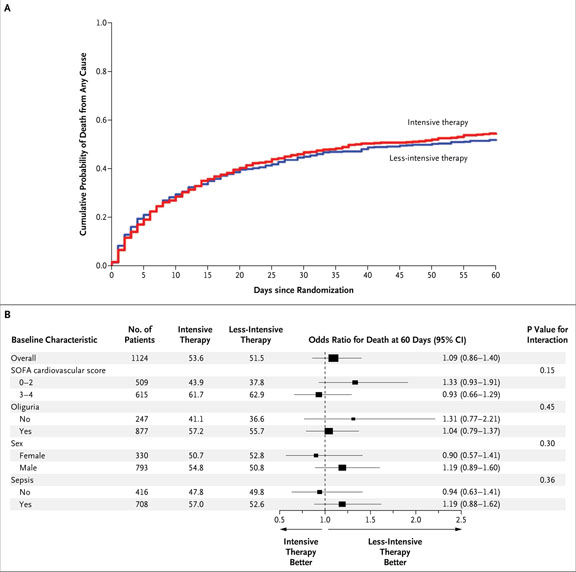
In all, 302 of the 563 patients (53.6%) in the intensive-therapy group died within 60 days after randomization, as compared with 289 of the 561 patients (51.5%) undergoing less-intensive therapy (odds ratio, 1.09; 95% CI, 0.86 to 1.40; P = 0.47) (Table 3 and Fig. 2A). Mortality was similar between the two treatment groups with regard to all prespecified subgroups (Fig. 2B).
Table 3.
Primary and Secondary Outcomes.*
| Outcome | Intensive Strategy (N = 563) |
Less-Intensive Strategy (N = 561) |
Odds Ratio or Mean Difference (95% CI)† |
P Value | |
|---|---|---|---|---|---|
| Death from any cause by day 60 — no. (%) |
302 (53.6) | 289 (51.5) | 1.09 (0.86 to 1.40) | 0.47 | |
| In-hospital death — no. (%) | 288 (51.2) | 269 (48.0) | 1.15 (0.90 to 1.47) | 0.27 | |
| Discharged to home, off dialysis, by day 60 — no./no. with data (%) |
88/560 (15.7) | 92/561 (16.4) | 0.95 (0.68 to 1.32) | 0.75 | |
| Recovery of kidney function by day 28 — no./no. with data (%)‡ |
0.03 (0.02 to 0.07) | 0.24 | |||
| Complete | 85/553 (15.4) | 102/555 (18.4) | |||
| Partial | 49/553 (8.9) | 50/555 (9.0) | |||
| None | 419/553 (75.8) | 403/555 (72.6) | |||
| RRT-free days through day 28 | 6.0±0.4 | 7.0±0.4 | −0.9 (−1.9 to 0.1) | 0.07 | |
| Hospital-free days through day 60 | 11.0±0.7 | 13.0±0.7 | −1.9 (−3.9 to 0.0) | 0.053 | |
| ICU-free days through day 60 | 18.7±0.9 | 20.1±0.9 | −1.5 (−4.0 to 1.0) | 0.25 | |
| Organ-failure–free days through day 14, according to SOFA organ- system score |
|||||
| Cardiovascular | 7.4±0.2 | 7.4±0.2 | 0.0 (−0.6 to 0.5) | 0.94 | |
| Respiratory | 7.1±0.2 | 7.2±0.2 | −0.1 (−0.8 to 0.5) | 0.71 | |
| Liver | 8.8±0.2 | 8.7±0.2 | 0.0 (−0.6 to 0.7) | 0.91 | |
| Coagulation | 9.0±0.2 | 9.1±0.2 | 0.0 (−0.6 to 0.6) | 0.95 | |
| Central nervous system | 7.1±0.2 | 7.2±0.2 | −0.1 (−0.7 to 0.6) | 0.89 | |
Plus–minus values are adjusted means ±SE. CI denotes confidence interval, ICU intensive care unit, and RRT renalreplacement therapy.
Odds ratios are reported for death by 60 days, in-hospital death, and discharge. Mean differences are reported for all other variables. The odds ratios were calculated with the use of conditional logistic-regression analysis adjusted for site and randomization strata: the Sequential Organ Failure Assessment (SOFA) cardiovascular organ-system score and the presence or absence of oliguria. Except for recovery of kidney function, the mean difference between the group receiving intensive therapy and that receiving less-intensive therapy was calculated with the use of analysis of variance adjusted for the following prespecified baseline covariates: age, sex, SOFA cardiovascular score, and presence or absence of oliguria; the reported mean is a least-squares mean.
Recovery of kidney function was coded as 2 for complete recovery, 1 for partial recovery, and 0 for no recovery; the mean difference between the group receiving intensive therapy and that receiving less-intensive therapy was calculated with the use of weighted least-squares analysis adjusted for the SOFA cardiovascular score and the presence or absence of oliguria.
Figure 2. Kaplan–Meier Plot of Cumulative Probabilities of Death (Panel A) and Odds Ratios for Death at 60 Days, According to Baseline Characteristics (Panel B).
Panel A shows the cumulative probability of death from any cause in the entire study cohort. Panel B shows odds ratios (and 95% confidence intervals [CI]) for death from any cause by 60 days in the group receiving the intensive treatment strategy as compared with the group receiving the less-intensive treatment strategy, as well as P values for the interaction between the treatment group and baseline characteristics. P values were calculated with the use of the Wald statistic. Higher Sequential Organ Failure Assessment (SOFA) scores indicate more severe organ dysfunction. There was no significant interaction between treatment and subgroup variables, as defined according to the prespecified threshold level of significance for interaction (P = 0.10). Sex was not recorded for one patient receiving lessintensive therapy.
SECONDARY OUTCOMES
In-hospital mortality through day 60 was 51.2% among patients undergoing intensive therapy and 48.0% among those undergoing less-intensive therapy (P = 0.27) (Table 3). A total of 85 of 553 patients (15.4%) in the intensive-therapy group had complete recovery of kidney function by day 28, and 49 patients (8.9%) had partial recovery, as compared with 102 (18.4%) and 50 (9.0%) of the 555 patients, respectively, receiving less-intensive therapy (P = 0.24). A total of 88 of 560 patients (15.7%) undergoing intensive therapy were discharged to their previous living situation (“home”), without requiring dialysis, by day 60, as compared with 92 of 561 patients (16.4%) undergoing less-intensive therapy (P = 0.75). There were no significant differences in the numbers of days free of organ failure in the two groups.
COMPLICATIONS OF THERAPY
Across all methods of renal-replacement therapy, 55 of the 563 patients (9.8%) receiving intensive therapy had hypotension that required discontinuation of at least one treatment (vs. 49 of 561 patients [8.7%] receiving less-intensive therapy, P = 0.55), 81 (14.4%) required initiation of vasopressor support (vs. 56 patients [10.0%], P = 0.02), and 212 (37.7%) required other interventions because of treatment-associated hypotension (vs. 168 patients [30.0%], P = 0.006) (Table 4). However, hypotension was reported as a complication of therapy during 18.5% of intermittent hemodialysis sessions in the intensive strategy as compared with 18.9% in the less-intensive strategy. Mean arterial blood pressure declined from 86±15 mm Hg before dialysis to a lowest value of 75±16 mm Hg during dialysis among patients receiving intensive therapy, as compared with 86±16 mm Hg to 74±16 mm Hg among those receiving less-intensive therapy (mean decline, 11±12 mm Hg vs. 12±13 mm Hg; P = 0.92) (Table 6 in the Supplementary Appendix). Hypophosphatemia developed in 17.6% of patients in the intensive-therapy group as compared with 10.9% undergoing less-intensive therapy (P = 0.001), and hypokalemia developed in 7.5% and 4.5%, respectively (P = 0.03). There were no significant differences between the two groups in the numbers of patients with reported serious adverse events, catheter-related complications, or other treatment-related complications (Table 4).
Table 4.
Summary of Complications Associated with Study Therapy.*
| Event | Intensive Strategy (N = 563) |
Less-Intensive Strategy (N = 561) |
P Value | ||
|---|---|---|---|---|---|
| no. of patients (%) | |||||
| Any serious adverse event† | 287 (51.0) | 280 (49.9) | 0.72 | ||
| Not related to study therapy | 207 (72.1) | 202 (72.1) | |||
| Possibly or probably related to study therapy | 48 (16.7) | 51 (18.2) | |||
| Definitely related to study therapy | 32 (11.1) | 27 (9.6) | |||
| Nonfatal only‡ | 137 (47.7) | 128 (45.7) | |||
| Catheter-related complications | |||||
| Insertion-related complications | 28 (5.0) | 31 (5.5) | 0.68 | ||
| Late catheter-related complications | 48 (8.5) | 38 (6.8) | 0.27 | ||
| Hypotension | |||||
| Requiring vasopressor support | 81 (14.4) | 56 (10.0) | 0.02 | ||
| Requiring discontinuation of treatment | 55 (9.8) | 49 (8.7) | 0.55 | ||
| Requiring other intervention | 212 (37.7) | 168 (29.9) | 0.006 | ||
| Other treatment-related complications | |||||
| Any nonhypotensive complication | 216 (38.4) | 194 (34.6) | 0.19 | ||
| Electrolyte disturbance | 144 (25.6) | 116 (20.7) | 0.05 | ||
| Hypokalemia | 42 (7.5) | 25 (4.5) | 0.03 | ||
| Hypophosphatemia | 99 (17.6) | 61 (10.9) | 0.001 | ||
| Other | 99 (17.6) | 85 (15.2) | 0.27 | ||
Table 5 in the Supplementary Appendix provides a detailed tabulation of complications associated with study therapy.
Patients with multiple serious adverse events were categorized in a hierarchical fashion and are reported as definitely related if any event was considered definitely related, possibly or probably related if no events were considered definitely related and at least one event was considered possibly or probably related, and not related if all events were considered not related to study therapy. Percentages are based on the number of patients with serious adverse events.
Nonfatal serious adverse events were defined as those other than death (i.e., an adverse event identified by the investigator as life-threatening, causing disability or incapacity, or prolonging hospitalization or any other event believed to be serious by the investigator).
DISCUSSION
In this large, multicenter, randomized, controlled trial of the intensity of renal-replacement therapy in critically ill patients with acute kidney injury, we found no added benefit from an intensive (high-dose) treatment strategy as compared with the more conventional, less-intensive treatment strategy. There were no significant differences in mortality, rate of recovery of kidney function, duration of renal-replacement therapy, or evolution of nonrenal organ failure. Hypotension occurred at a similar rate during intermittent-hemodialysis sessions in the two groups, albeit in a greater percentage of patients in the intensive-therapy group. Hypophosphatemia and hypokalemia were more frequent complications in the intensive-therapy group.
Our results contrast with those from three smaller, single-center trials that showed improved survival with more-intensive renal-replacement therapy in patients with acute kidney injury.16-18 Among patients whose care involved hemodialysis, Schiffl et al.17 reported a reduction in 28-day mortality from 46% with alternate-day dialysis to 28% with daily dialysis; however, the delivered dialysis dose per session was substantially lower than that used in the present study. Ronco et al.16 observed a reduction in mortality from 59% to 43% when ultrafiltration was increased from 20 to 35 ml per kilogram per hour during continuous venovenous hemofiltration but observed no further benefit with an increase to 45 ml per kilogram per hour. Similarly, Saudan et al.18 reported a reduction in mortality from 61% to 41% with the addition of dialysate at a dose of 18 ml per kilogram per hour to continuous venovenous hemofiltration at an ultrafiltration rate of approximately 25 ml per kilogram per hour.
In contrast, our results are similar to those reported by Bouman et al.,19 who observed no improvement in survival with early high-volume continuous venovenous hemofiltration (48 ml per kilogram per hour) as compared with either early or late low-volume continuous venovenous hemofiltration (19 to 20 ml per kilogram per hour). More recently, Tolwani et al.20 reported no significant difference in survival associated with continuous venovenous hemodiafiltration at 20 and 35 ml per kilogram per hour.
Our less-intensive strategy of management of renal-replacement therapy was similar to usual-care practices, as assessed by means of a survey before initiation of the study.25 Although our delivered hemodialysis dose was substantially higher than that in the study by Schiffl et al.,17 we did achieve a mean difference of 2.4 treatments per week between the two study groups and a difference in the mean predialysis blood urea nitrogen level of 25 mg per deciliter (8.9 mmol per liter). The doses of continuous venovenous hemodiafiltration in the two groups were similar to those in other studies,16,18-20 and the daily duration of therapy was greater than that reported in an observational series.26 Our predominant use of predilution replacement fluids resulted in a reduction of approximately 8 to 14% in effective clearance, as compared with postdilution infusion, although predilution fluid administration is associated with improved circuit patency and no net overall reduction in solute clearance.27
In our study, intensity of therapy was defined in terms of removal of small solutes (e.g., urea), and did not take into consideration issues of volume management. Patients receiving intermittent therapy were permitted to have additional isolated ultrafiltration if required for volume management. However, only 219 such treatment sessions were required with less-intensive therapy; this was fewer than 0.5 treatment per patient. There was no difference in net ultrafiltration between the two groups during continuous venovenous hemodiafiltration. Net weekly volume removal was greater in the intensive-therapy group, by approximately 2 to 3 liters. In contrast to the experience of Schiffl et al.,17 the frequency of hypotension during intermittent-hemodialysis sessions was similar in the two groups, although, because they had more such sessions and more exposure to dialysis, a greater proportion of patients in the intensive-therapy group had at least one treatment-associated episode of hypotension.
Our study has several limitations. The timing of the initiation of renal-replacement therapy was not strictly standardized in the study protocol, although we excluded patients in whom the initiation of therapy was excessively delayed. No consensus currently exists regarding the optimal timing of initiation of renal support in critically ill patients,15,28 and wide variation was observed in practice among and within participating institutions. Men were overrepresented in the study population, in part because 25% of patients were enrolled at VA medical centers, where the patient population is predominantly male. However, epidemiologic studies have shown an increased incidence of acute kidney injury in men as compared with women; men constitute 59 to 64% of cases of severe acute kidney injury among critically ill patients.9,29
The exclusion of patients with advanced chronic kidney disease may also limit the generalizability of the study results. Although patients with chronic kidney disease in whom acute kidney injury develops constitute a substantial proportion of those with acute kidney injury requiring renal-replacement therapy, we excluded such patients because epidemiologic studies suggest that they have a distinct natural history, characterized by lower mortality and less frequent recovery of kidney function as compared with patients without underlying chronic kidney disease.25 Given their exclusion from our study, it may be inappropriate to extrapolate our results to persons in whom acute kidney injury develops in the context of moderate-to-severe chronic kidney disease.
In summary, our study indicates that a strategy of intensive renal support in critically ill patients with acute kidney injury does not decrease mortality, accelerate recovery of kidney function, or alter the rate of nonrenal organ failure as compared with a less-intensive regimen similar to usual-care practices.25 These results do not imply that the dose of renal-replacement therapy is not important in critically ill patients with acute kidney injury. The less-intensive treatment strategy in our study provided a dose of renal-replacement therapy that exceeded that in usual care, particularly for intermittent hemodialysis.30 However, our results suggest that outcomes are not improved by providing intermittent hemodialysis to hemodynamically stable patients more frequently than three times per week, with a target achieved Kt/Vurea value of 1.2 to 1.4 per treatment, or providing continuous renal-replacement therapy to hemodynamically unstable patients at an effluent flow rate of more than 20 ml per kilogram per hour. Other treatment strategies will be necessary to decrease mortality in critically ill patients with acute kidney injury.
Supplementary Material
Acknowledgments
Supported by the Cooperative Studies Program of the Department of Veterans Affairs Office of Research and Development and by the National Institute of Diabetes and Digestive and Kidney Diseases (interagency agreement Y1-DK-3508-01).
Drs. Finkel, Paganini, and Vijayan report receiving consulting fees from NxStage; Drs. Finkel, Paganini, and Vijayan, grant support from Nephros Therapeutics; Dr. Kellum, lecture fees and grant support from Gambro and consulting fees from Gambro, Baxter, and Fresenius; Dr. Paganini, grant support from NxStage and Amgen, consulting fees from Quark Pharmaceuticals, and lecture fees from Watson Pharmaceuticals; and Dr. Vijayan, lecture fees from GE Healthcare and grant support from Genentech and Biosite. No other potential conflict of interest relevant to this article was reported.
We thank Gambro for providing discounted pricing for PrismaSate, Dialysis Solutions (DSI), for providing discounted pricing for Normocarb, and the nurses in our dialysis units and intensive care units, our physician colleagues, and our patients and their families for their support of this trial.
APPENDIX
The members of the VA/NIH Acute Renal Failure Trial Network Study are as follows: Executive Committee: VA Pittsburgh Healthcare System, Pittsburgh: P.M. Palevsky (chair); Stanford University, Palo Alto, CA: G.M. Chertow; VA Connecticut Healthcare System, West Haven: S.T. Crowley, T.Z. O'Connor; VA North Texas Healthcare System, Dallas: D. Choudhury; University of Pittsburgh, Pittsburgh: J.A. Kellum; Cleveland Clinic Foundation, Cleveland: E. Paganini; Miami VA Healthcare System, Miami: R.M.H. Schein; Health Economics Resource Center, VA Palo Alto Health Care System, Menlo Park, CA: M.W. Smith; Cooperative Studies Program (CSP) Clinical Research Pharmacy Coordinating Center, Albuquerque, NM: K.M. Swanson; Massachusetts General Hospital, Boston: B.T. Thompson; CSP Coordinating Center, VA Connecticut Healthcare System, West Haven: J.H. Zhang; CSP Coordinating Center, VA Connecticut Healthcare System, West Haven: P. Peduzzi (ex officio); National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD: R. Star (ex officio). Site Investigators and Study Coordinators: VA Ann Arbor Healthcare System, Ann Arbor, MI: E. Young, R. Fissel, W. Fissel, U. Patel, K. Belanger, A. Raine, N. Ricci; VA Western New York Healthcare System, Buffalo: J. Lohr, P. Arora, D. Cloen, D. Wassel, L. Yohe; VA North Texas Health Care System, Dallas: D. Choudhury, J. Amanzadeh, J. Penfield, M. Hussain, R. Katneni, A. Sajgure, A. Swann; Michael E. DeBakey VA Medical Center, Houston: G. Dolson, V. Ramanathan, G. Tasby; Richard L. Roudebush VA Medical Center, Indianapolis: R. Bacallao, M. Jaradat, K. Graves, Q. Li; John L. McClellan Memorial Veterans Hospital, Little Rock, AR: M. Krause, M. Shaver, M. Alam, K. Morris, T. Bland, E. Satter; West Los Angeles VA Healthcare Center, Los Angeles: J. Kraut, A. Felsenfeld, B. Levine, G. Nagami, B. Vaghaiwalla, J. Duffney, J. Moore; Miami VA Healthcare System, Miami: R.M.H. Schein, C. Cely, E. Jaimes, D. Kett, A. Quartin, M. Arcia, A. Barchi-Chung; New Orleans VA Medical Center, New Orleans: V. Batuman, A. Alper, A. Dreisbach, E. Simon, C. Kulivan; VA Pittsburgh Healthcare System, Pittsburgh: N. Aslam, M. Ramkumar, E. Grum, P. Rogers, S. Weisbord, C. Geffel; Portland VA Medical Center, Portland, OR: S. Watnick, I. Wahba, D. Kelly, J. Walczyk; Hunter Holmes McGuire VA Medical Center, Richmond, VA: G. Feldman, A. Mogyorosi, G. Viol, M. Halverson, S. Schmid, H. Totten; VA San Diego Healthcare System, San Diego, CA: F. Gabbai, S. Mullaney, R. Smith, J. Dingsdale, S. Woods; San Francisco VA Medical Center, San Francisco: K. Johansen, D. Lovett, A. O'Hare, J. McCarthy; VA Caribbean Healthcare System, San Juan, Puerto Rico: C. Rosado-Rodriguez, A. Galera, G. Rodriguez-Vega, W. Rodriguez, C. Vilchez; VA Puget Sound Health Care System, Seattle: B. Young, D. Andress, A. Lindner, G. Galvin; VA Connecticut Healthcare System, West Haven: S. Crowley, N. Gourley, A. Peixoto, M. Perkal, C. Joncas; Cleveland Clinic Foundation, Cleveland: E. Paganini, S. Demirjian, J. Yared, R. Brienza, M. Garcia, T. Seifert, L. Sweeney; Johns Hopkins Hospital, Baltimore: H. Rabb, M. Atta, R. Brower, M. Choi, J. Eustace, P. Scheel, E. Heck, H. Rahman; Massachusetts General Hospital, Boston: J. Niles, H. Bazari, K. Smirnakis, D. Steele, R. Thadhani, K. Laliberte, B. Leeman, C. McCarthy, M. Pescatore; Medical College of Georgia, Augusta: H. Szerlip, P. Fall, M. Jagadeesan, L. Mulloy, W. Paulson, J. White, N. Sickafoose; University of California, San Francisco, San Francisco: G. Chertow, K. Cho, M. Gropper, K. Liu, M. Matthay, K. Borovitz, M. Koenigsberg, S. Rodriguez; University of Miami–Jackson Memorial Hospital, Miami: G. Contreras, R.M.H. Schein, S. Cohn, J. Diego, D. Kett, A. Quartin, C. Carvalho, D. Carvalho, M. Castro, C. de la Cuesta, I. Espinal, A. Hurtado, P. Oyuela; University of Pittsburgh Medical Center, Pittsburgh: N. Aslam, M. Unruh, J. Kellum, R. Burr, M. Donahoe, J. Marszalek, M. Shields, R. Venkataraman, S. Weisbord, J. Aubrecht, H. Sterling, L. Mandich; University of Texas Medical School at Houston (Memorial–Hermann & MD Anderson Hospitals), Houston: K. Finkel, A. Shaw, J. Foringer, J. Samuels, B. Efron; Wake Forest University, Winston-Salem, NC: M. Rocco, E. Deterding, S. Moossavi, C. Bethea, D. McBride, S. Warren; Washington University in St. Louis, St. Louis: A. Vijayan, M. Kollef, K. Sambandam, E. Hammer, M. Hoffman. Data Monitoring Committee: University of Iowa College of Medicine, Iowa City: J. Stokes (chair); Case Western Reserve University at MetroHealth Medical Center, Cleveland: A.F. Connors, Jr.; University of Pennsylvania, Philadelphia: H. Feldman; Centers for Medicare and Medicaid Services, Baltimore: J. Greer; University of North Carolina, Chapel Hill: G.G. Koch; University of Toronto, Toronto: T. Stewart; Statistics Collaborative, Washington, DC: J. Wittes; Study Chair's Office, VA Pittsburgh Healthcare System, Pittsburgh: P.M. Palevsky (chair), P. Overberger (national study coordinator), S. Michler; CSP Coordinating Center, VA Connecticut Healthcare System, West Haven: P. Peduzzi (director), M. Antonelli (assistant director), T.Z. O'Connor, J.H. Zhang, K. Dellert, L. Durant, R. Franchini, A. Kossack, V. McBride, S. O'Neil, T. Roy, J. Russo, J. Vitale; CSP Central Research Pharmacy Coordinating Center, Albuquerque, NM: M. Sather (director), C. Fye, C.M. Haakenson, D.H. Krueger, K. Swanson, J. Thornton; Interactive Touch-Tone Randomization System, CSP Coordinating Center, VA Maryland Healthcare System, Perry Point: C. Dalzell, R. Horney; Health Economics Resource Center, VA Palo Alto Health Care System, Menlo Park, CA: M.W. Smith (associate director), A. Siroka, P. Su; Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC), VA Boston Healthcare System, Boston: M. Brophy (Director, MAVERIC Core Laboratory), D. Humphries, D. Govan; VA Office of Research and Development, Clinical Science Research and Development, Washington, DC: T.J. O'Leary (director), G.D. Huang (deputy director, CSP), J.R. Feussner (chief research and development officer and director, CSP, 1996–2002); National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD: R.A. Star (director, Division of Kidney, Urology, and Hematology Diseases), P. Eggers.
References
- 1.Nash K, Hafeez A, Hou S. Hospitalacquired renal insufficiency. Am J Kidney Dis. 2002;39:930–6. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 2.Liangos O, Wald R, O'Bell JW, Price L, Pereira BJ, Jaber BL. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1:43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 3.Xue JL, Daniels F, Star RA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17:1135–42. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 4.Waikar SS, Curhan GC, Wald R, Mc-Carthy EP, Chertow GM. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006;17:1143–50. doi: 10.1681/ASN.2005091017. [DOI] [PubMed] [Google Scholar]
- 5.Liaño F, Junco E, Pascual J, Madero R, Verde E. The spectrum of acute renal failure in the intensive care unit compared with that seen in other settings. Kidney Int Suppl. 1998;66:S16–S24. [PubMed] [Google Scholar]
- 6.Ostermann M, Chang RW. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med. 2007;35:1837–43. doi: 10.1097/01.CCM.0000277041.13090.0A. 1852. [DOI] [PubMed] [Google Scholar]
- 7.Bagshaw SM, George C, Dinu I, Bellomo R. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1203–10. doi: 10.1093/ndt/gfm744. [DOI] [PubMed] [Google Scholar]
- 8.de Mendonça A, Vincent JL, Suter PM, et al. Acute renal failure in the ICU: risk factors and outcome evaluated by the SOFA score. Intensive Care Med. 2000;26:915–21. doi: 10.1007/s001340051281. [DOI] [PubMed] [Google Scholar]
- 9.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–8. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 10.Metnitz PG, Krenn CG, Steltzer H, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30:2051–8. doi: 10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy JT. Prognosis of patients with acute renal failure in the intensivecare unit: a tale of two eras. Mayo Clin Proc. 1996;71:117–26. doi: 10.4065/71.2.117. [DOI] [PubMed] [Google Scholar]
- 12.Star RA. Treatment of acute renal failure. Kidney Int. 1998;54:1817–31. doi: 10.1046/j.1523-1755.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- 13.Kolff WJ. First clinical experience with the artificial kidney. Ann Intern Med. 1965;62:608–19. doi: 10.7326/0003-4819-62-3-608. [DOI] [PubMed] [Google Scholar]
- 14.Rondon-Berrios H, Palevsky PM. Treatment of acute kidney injury: an update on the management of renal replacement therapy. Curr Opin Nephrol Hypertens. 2007;16:64–70. doi: 10.1097/MNH.0b013e32802ef4a5. [DOI] [PubMed] [Google Scholar]
- 15.Pannu N, Klarenbach S, Wiebe N, Manns B, Tonelli M. Renal replacement therapy in patients with acute renal failure: a systematic review. JAMA. 2008;299:793–805. doi: 10.1001/jama.299.7.793. [DOI] [PubMed] [Google Scholar]
- 16.Ronco C, Bellomo R, Homel P, et al. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet. 2000;356:26–30. doi: 10.1016/S0140-6736(00)02430-2. [DOI] [PubMed] [Google Scholar]
- 17.Schiffl H, Lang SM, Fischer R. Daily hemodialysis and the outcome of acute renal failure. N Engl J Med. 2002;346:305–10. doi: 10.1056/NEJMoa010877. [DOI] [PubMed] [Google Scholar]
- 18.Saudan P, Niederberger M, De Seigneux S, et al. Adding a dialysis dose to continuous hemofiltration increases survival in patients with acute renal failure. Kidney Int. 2006;70:1312–7. doi: 10.1038/sj.ki.5001705. [DOI] [PubMed] [Google Scholar]
- 19.Bouman CS, Oudemans-Van Straaten HM, Tijssen JG, Zandstra DF, Kesecioglu J. Effects of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: a prospective, randomized trial. Crit Care Med. 2002;30:2205–11. doi: 10.1097/00003246-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Tolwani AJ, Campbell RC, Stofan BS, Lai KR, Oster RA, Wille KM. Standard versus high-dose CVVHDF for ICU-related acute renal failure. J Am Soc Nephrol. in press. [DOI] [PMC free article] [PubMed]
- 21.Palevsky PM, O'Connor T, Zhang JH, Star RA, Smith MW. Design of the VA/ NIH Acute Renal Failure Trial Network (ATN) Study: intensive versus conventional renal support in acute renal failure. Clin Trials. 2005;2:423–35. doi: 10.1191/1740774505cn116oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/ failure. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 23.Lakatos E. Sample sizes based on the log-rank statistic in complex clinical trials. Biometrics. 1988;44:229–41. [PubMed] [Google Scholar]; Erratum, Biometrics. 1988;44:923. [Google Scholar]
- 24.Crowley S, Chertow G, Vitale J, et al. Lessons for successful study enrollment from the Veterans Administration/National Institutes of Health Acute Renal Failure Trial Network (ATN) Study. Clin J Am Soc Nephrol. in press. [DOI] [PMC free article] [PubMed]
- 25.Overberger P, Pesacreta M, Palevsky PM. Management of renal replacement therapy in acute kidney injury: a survey of practitioner prescribing practices. Clin J Am Soc Nephrol. 2007;2:623–30. doi: 10.2215/CJN.00780207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venkataraman R, Kellum JA, Palevsky P. Dosing patterns for continuous renal replacement therapy at a large academic medical center in the United States. J Crit Care. 2002;17:246–50. doi: 10.1053/jcrc.2002.36757. [DOI] [PubMed] [Google Scholar]
- 27.Uchino S, Fealy N, Baldwin I, Morimatsu H, Bellomo R. Pre-dilution vs. postdilution during continuous veno-venous hemofiltration: impact on filter life and azotemic control. Nephron Clin Pract. 2003;94:c94–c98. doi: 10.1159/000072492. [DOI] [PubMed] [Google Scholar]
- 28.Gibney N, Hoste E, Burdmann EA, et al. Timing of initiation and discontinuation of renal replacement therapy in AKI: unanswered key questions. Clin J Am Soc Nephrol. 2008;3:876–80. doi: 10.2215/CJN.04871107. [DOI] [PubMed] [Google Scholar]
- 29.Mehta RL, Pascual MT, Soroko S, et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 2004;66:1613–21. doi: 10.1111/j.1523-1755.2004.00927.x. [DOI] [PubMed] [Google Scholar]
- 30.Palevsky PM, O'Connor T, Zhang J. Management of renal replacement therapy in acute renal failure: an observational study. J Am Soc Nephrol. 2005;16:910A. abstract. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.