Abstract
Background
The obesity epidemic, recognized in developed nations for decades, is now a worldwide phenomenon. All age groups are affected, including women of childbearing age, fueling concern that maternal obesity before and during pregnancy and lactation impairs developmental establishment of body weight regulatory mechanisms in the fetus or infant, causing transgenerational amplification of obesity prevalence and severity. The biological mechanisms underlying such processes remain unknown.
Methods
We used agouti viable yellow (Avy) mice to test the hypothesis that maternal obesity induces transgenerational amplification of obesity. We passed the Avy allele through three generations of Avy/a females and assessed cumulative effects on coat color and body weight. By studying two separate but contemporaneous populations of mice, one provided a standard diet and the other a methyl-supplemented diet that induces DNA hypermethylation during development, we tested whether potential transgenerational effects on body weight might be mediated by alterations in epigenetic mechanisms including DNA methylation.
Results
The genetic tendency for obesity in Avy mice was progressively exacerbated when the Avy allele was passed through successive generations of obese Avy females. This transgenerational amplification of body weight was prevented by a promethylation dietary supplement. Importantly, the effect of methyl supplementation on body weight was independent of epigenetic changes at the Avy locus, indicating this model may have direct relevance to human transgenerational obesity.
Conclusion
Our results show that in a population with a genetic tendency for obesity, effects of maternal obesity accumulate over successive generations to shift the population distribution toward increased adult body weight, and suggest that epigenetic mechanisms are involved in this process.
Keywords: epigenetic, body weight regulation, DNA methylation, methyl supplementation, agouti viable yellow
Introduction
As maternal body weight is correlated with offspring body weight in humans,1 it has been hypothesized that a mother’s obesity before and during pregnancy causes developmental adaptations that promote obesity in her children.2–4 Recent studies provide strong support for this hypothesis: children born after dramatic maternal weight loss (from bariatric surgery) are less prone to obesity compared with their siblings born before surgery,5 and maternal weight gain between two pregnancies is associated with increased body weight in the second child.6 Such observational studies indicate an interaction between genes and environment in the entrainment of obesity, but offer little insight into the biologic mechanisms by which maternal obesity might exert transgenerational influences on body weight regulation.
Appropriate animal models are necessary to advance our understanding of these processes. Rodent studies show that maternal obesity induced by a high-fat diet causes persistent7 and heritable8 alterations in offspring adiposity and metabolism, but it is unclear whether these effects are attributable to maternal obesity per se or to the high-fat diet. Unlike many mouse models of genetic obesity, in which the affected females are infertile,9,10 the agouti viable yellow (Avy) mouse provides an ideal model system in which to study transgenerational effects of maternal obesity. The Avy allele resulted from transposition of a murine intracisternal A particle retrotransposon upstream of the agouti gene. Although agouti is normally expressed only in hair follicles, a cryptic promoter in the Avy intracisternal A particle confers ectopic agouti expression.11 Further, the intracisternal A particle induces dramatic interindividual variation in Avy DNA methylation and agouti expression among isogenic Avy/a mice12 (‘a,’ the nonagouti allele, does not produce functional agouti protein). Because the agouti signaling molecule both induces yellow pigmentation in hair follicles and antagonizes satiety signaling at the melanocortin 4 receptor in the hypothalamus, Avy/a mice have variably yellow coats and are prone to hyperphagic obesity.13 Adult-onset obesity occurs in Avy/a mice provided ad libitum access to a standard mouse diet; they are thus an apt model for modern man who, having evolved to survive periods of food deprivation, now finds himself genetically predisposed to obesity in an environment of food surfeit.14
At the outset of a three-generation study of transgenerational epigenetic inheritance in Avy mice,15 we realized an excellent opportunity to test the hypothesis that maternal obesity induces transgenerational amplification of obesity. We passed the Avy allele through three generations of Avy/a females and assessed cumulative effects on coat color and body weight. Our study involved two separate but contemporaneous populations of mice, one provided a standard diet and the other a methyl-supplemented diet that induces DNA hypermethylation during development,16,17 enabling us to determine if potential transgenerational effects on body weight might be mediated by alterations in epigenetic mechanisms including DNA methylation. The results of this naturalistic experiment show that maternal obesity causes transgenerational amplification of body weight. The hypermethylating dietary supplement prevented this effect, suggesting that epigenetic mechanisms including DNA methylation may play a role in the transgenerational perpetuation of mammalian obesity.
Methods
Animals and body composition
Avy mice were originally obtained from the colony at the National Center for Toxicological Research,16 and have been maintained for hundreds of generations by mating Avy/a males with a/a sisters, resulting in an essentially invariant genetic background. Slightly mottled yellow Avy/a females born to a/a mothers were weaned on postnatal day 21 (P21) onto either the unsupplemented (NIH-31, TD95262, Harlan-Teklad, Madison, WI, USA) or methyl-supplemented diet (NIH-31 supplemented with extra folic acid, vitamin B12, betaine and choline, TD01308, Harlan-Teklad).16 At P56 these F0 females were mated with a/a males. Their (F1) offspring were genotyped, weighed, digitally photographed and classified for coat color17 at P21. Each Avy/a female was weaned onto the same diet as her mother; male Avy/a offspring were weaned onto a standard mouse diet (LabDiet 5053, St. Louis, MO, USA). Mice were housed by sex in groups of up to five per cage and provided ad libitum access to diet and water. F1 Avy/a females were mated with a/a males at P56, and their (F2) offspring were treated identically to the F1 generation. In the F3 generation, both females and males were weaned onto the standard mouse diet, and females were not mated. Body weight of all Avy/a mice (F0, F1, F2 and F3) was measured at P180. The digital photographs enabled Avy/a coat color phenotype to be rated by a single observer (RAW) blind to diet group. Coat color was rated as yellow (<5% brown), slightly mottled (⩾5% but less than half brown), mottled ( approximately half brown), heavily mottled (more than half but ⩽95% brown) or pseudoagouti (>95% brown).15 Body composition was measured by dual-energy X-ray absorptiometry (Piximus Series Densitometer, GE Medical Systems, Madison, WI, USA), as per the manufacturers’ instructions. We certify that all applicable institutional and governmental regulations concerning the ethical use of animals were followed during this research. The protocol was approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine.
Statistical approaches
All data were entered independently into two Excel spreadsheets, which were compared electronically to eliminate data entry errors. Statistical analyses were performed in a stepwise manner to reduce biases associated with unbalanced data. Full models were initially performed to identify statistically significant effects, which were then verified by reduced models on data subsets. Reported significance values are those for the full analyses, unless stated otherwise. Statistical significance levels for data subset analyses were always sufficient to meet conservative Bonferroni corrections for multiple comparisons. For generational effects on wean weight, initial differences among groups over time were determined by ANCOVA (analysis of covariance) and confirmed by regression of P21 weight over time on group subsets. Supplement versus maternal effects on P180 weight were analyzed by ANCOVA at each generation. Potential genetic impacts on adult body weight were assessed by both multiple and individual regression of F0, F1 or F2 body weight on F3 adult body weight.
Results
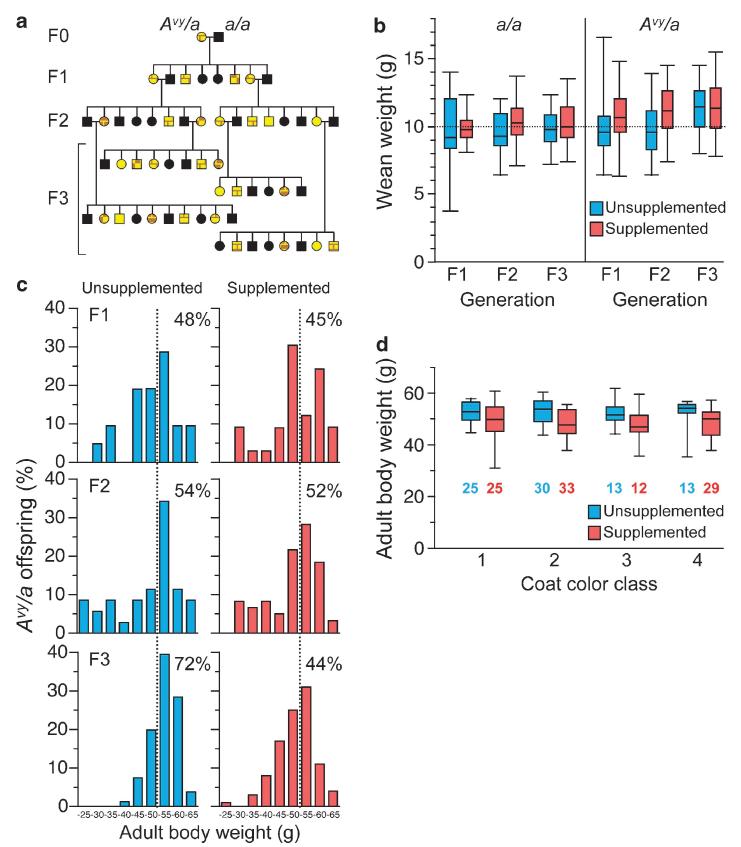
F0 dams were obese slightly mottled yellow Avy/a females who were born to a/a mothers and weaned onto either the unsupplemented or supplemented diet. At the age of 8 weeks, F0 dams were mated with lean a/a males. Each F1 and F2 Avy/a female was weaned onto the same diet as her mother and mated with an a/a male at the age of 8 weeks (Figure 1a). To avoid bias due to potential effects of methyl supplementation on fecundity, each Avy/a female in the F0, F1 and F2 generations was mated until she either produced two Avy/a female offspring or stopped producing litters. Further details of the study population have been reported.15
Figure 1.
Methyl supplementation prevents transgenerational increase in adult body weight. (a) Genogram illustrating study design; a/a mice are shown in black and Avy/a mice in various shades of yellow/brown. (b) Body weight at weaning (P21) in a/a and Avy/a offspring born to Avy/a females (ntotal=119 (F1), 214 (F2) and 379 (F3)). Box plots indicate median, 25th—75th percentiles (box), and 5th—95th percentiles (whiskers). In unsupplemented Avy/a offspring only, P21 weight increases with successive generations (P=0.007). (c) Distribution of adult (P180) body weight of Avy/a offspring by generation and group (nunsuppl=29 (F1), 36 (F2) and 88 (F3); nsuppl=33 (F1), 62 (F2) and 117 (F3)). The percentages in each panel indicate the proportion of offspring above 50 g (dotted line). Body weight is relatively constant in the supplemented group, but increases transgenerationally in the unsupplemented group (P=0.000006). (d) P180 body weight versus coat color class for Avy/a animals in the F3 generation (1 = yellow, 2 = slightly mottled, 3 = mottled, 4 = heavily mottled; number of mice in each class is indicated). Body coat color class. weight is not associated with Avy epigenotype. Supplemented mice tend to weigh less within every coat color class.
In a/a offspring, body weight at weaning (postnatal day 21, P21) was not affected by methyl supplementation or generation (Figure 1b). In congenic Avy/a offspring, however, P21 body weight increased with successive generations specifically in the unsupplemented group (P 0.007) (Figure 1b), suggesting that the Avy mutation confers susceptibility to the transgenerational effects of maternal obesity. We therefore measured body weight of Avy/a offspring in adulthood (P180). Within each generation, isogenic Avy/a offspring showed dramatic interindividual variation in adult body weight (Figure 1c). This variation was not explained by sex. In the unsupplemented group, the adult body weight distribution shifted to the right with successive generations, indicating a feed-forward effect of maternal obesity. Supplementation did not significantly affect adult body weight in the F1 or F2 generations. By the F3 generation, however, the cumulative transgenerational effect of methyl donor supplementation caused a highly significant decrease in P180 body weight relative to unsupplemented animals (P=0.000006). Among unsupplemented F3 mice, 72% weighed more than 50 g at P180, versus only 44% of those supplemented (Figure 1c). (The lines at 50 g in Figure 1c indicate an arbitrary reference value, which was not used in statistical tests.)
Given that the obesogenic Avy allele can be epigenetically silenced by DNA methylation, one might suppose that the transgenerational effect we found is explained by progressive Avy methylation in the methyl-supplemented group. There were, in fact, more heavily mottled yellow mice in the supplemented group,15 and one might expect them to have lower body weights. There was no association in either group, however, between P180 body weight and coat color phenotype (a proxy for Avy DNA methylation15) (Figure 1d). Further, statistical analyses of the transgenerational effect of methyl supplementation on adult body weight were not affected by including coat color phenotype in the models. Hence, rather than occurring at Avy, the transgenerational effect of methyl supplementation on body weight among isogenic Avy/a mice instead suggests the involvement of epigenetic mechanisms operating at other loci.
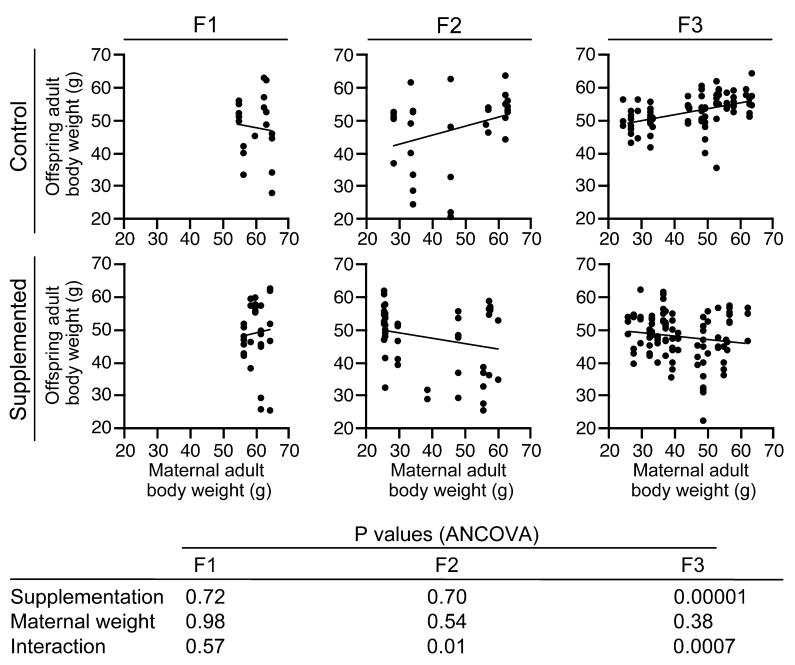
If maternal obesity begets obesity in her offspring, maternal body weight should predict offspring body weight. Indeed, in unsupplemented Avy/a mice, adult (P180) body weight was positively correlated with maternal P180 body weight in both the F2 and F3 generations (Figure 2). Methyl supplementation abrogated these associations (Figure 2). Hence, maternal obesity had an obesogenic effect on her offspring, and methyl supplementation eliminated this effect, explaining the marked difference in adult body weight distribution between unsupplemented and supplemented F3 animals (Figure 1c).
Figure 2.
Methyl supplementation changes the association between maternal and offspring adult body weight. Adult body weight was measured at P180 for all dams and Avy offspring in the study. In both the F2 and F3 generations, maternal adult body weight predicts offspring adult body weight in the unsupplemented group only. The P-values of the analysis of covariance are provided.
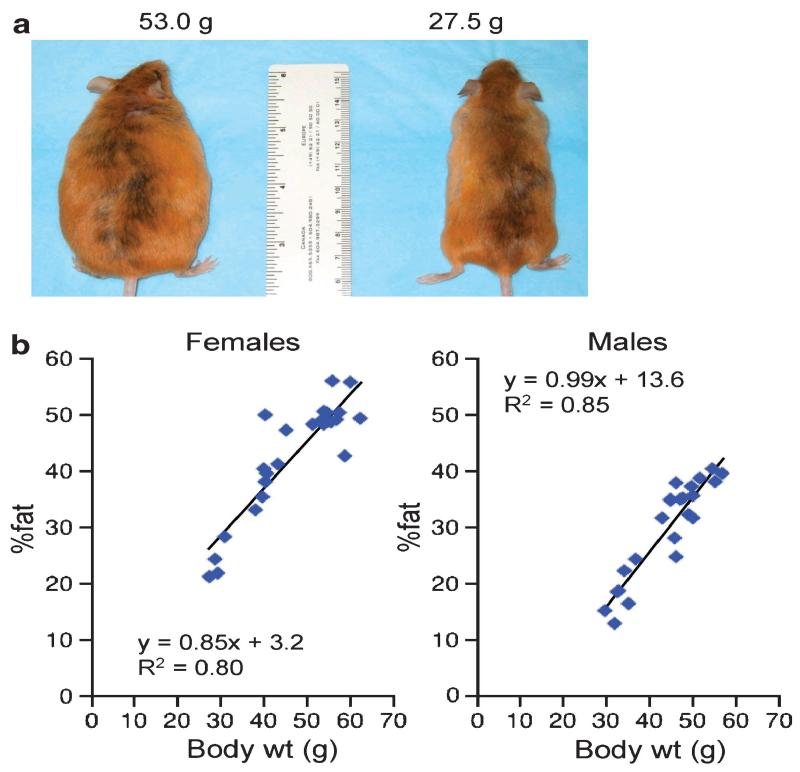
Whereas body weight variation could reflect individual differences in linear growth rather than adiposity, casual observation of P180 Avy/a mice discordant for body weight (Figure 3a) clearly suggests that the heavier mice are indeed obese. Body composition was measured by dual-energy X-ray absorptiometry in a subset of male and female P180 mice (Figure 3b), confirming that most of the variance in body weight is attributable to interindividual variation in adiposity, and indicating that the transgenerational body weight effects found here do indeed reflect differences in fatness.
Figure 3.
Adiposity is highly correlated with body weight in isogenic Avy/a mice. (a) P180 isogenic Avy/a female mice discordant for body weight. (b) Variation in body weight is mostly explained by variation in adiposity among P180 female (n=24) and male (n=28) Avy/a mice.
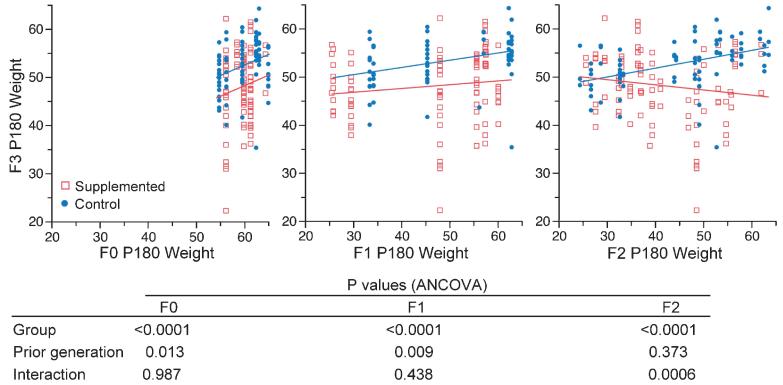
Although the Avy allele has been maintained on an inbred background by sibling mating for hundreds of generations,16 subtle genetic variation associated with de novo germline mutations cannot be excluded. To determine if group differences in F3 body weight might be attributable to genetic variation among F0 dams, we analyzed independently the effect of great-grandmaternal (F0), grandmaternal (F1) and maternal (F2) body weight on F3 adult body weight (Figure 4). F3 P180 body weight was positively correlated with great-grandmaternal and grandmaternal P180 body weight in both groups, consistent with a genetic effect. When F0 dam was included in a lineage-based multiple regression analysis, however, the effect of supplementation on F3 adult body weight remained highly significant (P=0.0002), showing that it is not due to confounding by genetic differences among F0 dams.
Figure 4.
Impact of prior generations on F3 Avy/a adult body weight. Shown are F3 P180 body weight versus great-grandmaternal (F0, left panel), grandmaternal (F1, center panel) and maternal (F2, right panel) P180 body weight. Heavier mice from F0 and F1 lead to heavier F3 mice, regardless of supplementation. Supplementation abrogates only the effect of maternal body weight on offspring body weight (right panel), rather than prior generational effects. The P-values of the analysis of covariance are provided.
One could suppose that the supplemented mice ended up leaner merely because of a toxic effect or that they simply did not like the taste of the supplement and therefore ate less. This is unlikely, however, for several reasons. Supplementation did not affect litter size or fecundity,15 and body weight at weaning (P21) actually tended to be higher in supplemented animals (Figure 1b). Also, supplementation did not significantly affect adult body weight until the F3 generation. Hence, our data suggest that rather than simply curbing food intake, methyl supplementation operated principally by altering the association between maternal and offspring adult body weight (Figure 2).
Discussion
The results of this naturalistic experiment show that in a population with a genetic tendency for obesity, maternal obesity has an obesogenic effect on her offspring, causing transgenerational amplification of body weight. Previous animal models have employed early dietary manipulations18 or high-energy diets19 to test the hypothesis that maternal obesity promotes obesity in her offspring. Our current data, however, provide the first demonstration that spontaneous interindividual variation in maternal adiposity induces body weight variation in the subsequent generation. As such, these results provide definitive evidence of non-genetic transgenerational transmission of obesity risk at the population level.
Providing a hypermethylating dietary supplement prevented the effect of maternal obesity on offspring body weight, suggesting a role for epigenetic mechanisms in this phenomenon. Dietary methyl supplementation of female mice before and during pregnancy induces persistent hypermethylation at the Avy locus.16,17 As transgenerational epigenetic inheritance occurs at Avy,12 it may seem intuitive that methyl supplementation over successive generations caused cumulative Avy hypermethylation, leading to progressive epigenetic silencing of the obesogenic Avy allele. Detailed analysis of Avy coat color variation in this study, however, showed no evidence of transgenerational epigenetic inheritance of diet-induced hypermethylation at Avy.15 Moreover, adult body weight of Avy/a mice was independent of coat color (Figure 1d), demonstrating that the body weight effects reported here are not due to epigenetic variation at Avy. We instead interpret our results as evidence that maternal obesity affects developmental establishment of epigenetic mechanisms at other genomic loci affecting body weight regulation (for example in offspring hypothalamus) and that methyl supplementation interacts with these processes.
Given that ectopic agouti expression causes hyperphagic obesity, and coat color variation among Avy/a mice correlates with ectopic agouti expression,13 it may seem surprising that we found no association between Avy/a coat color and adult body weight (Figure 1d). However, Wolff and colleagues13 reported years ago that among Avy/a mice, only pseudoagouti animals (hypermethylated at Avy) are protected from obesity. This was recently verified by Dolinoy et al.20 who showed that only pseudoagouti Avy/a mice remain lean as adults, and body weight does not differ among the other coat color classes. Therefore, as the current study included only one pseudoagouti mouse,15 the lack of an association between adult body weight and coat color is in fact consistent with previous studies.
As the Avy mouse has been studied for decades,21 one may wonder why transgenerational increases in body weight among Avy/a mice have not been reported previously. It should be noted, however, that Avy colonies are customarily propagated by mating a/a females with Avy/a males. This approach maintains Avy in forced heterozygosity with the a allele and obviates the need for genotyping; offspring genotype (a/a or Avy/a) can be inferred from coat color. Passing Avy through the male rather than the female avoids both accumulation of epigenetic inheritance at Avy12 and the potential for maternal obesity to interfere with reproductive efficiency. Hence, although it has not been customary to pass Avy through obese Avy/a females, our data indicate that doing so provides a very useful model in which to study the effects of maternal obesity on her offspring.
Future studies will be required to determine exactly why Avy/a mice progressively become more obese as the Avy allele is passed through successive generations of obese Avy/a females. Measurements of food intake and energy expenditure, both of the pregnant dams and their growing offspring, would establish whether the transgenerational increases in adiposity are due to increases in energy intake, decreased energy expenditure or both. Distinguishing among these possibilities will be a critical first step toward determining the organ system(s) in which fetal and/or early postnatal development is affected by maternal obesity and methyl supplementation. Another limitation of this study is that only Avy/a mice were studied. Although our data indicate that epigenetic variation at Avy does not mediate the reported dietary and transgenerational effects on body weight, the complex epigenetic characteristics of the obesogenic Avy allele underscore the need to determine if our multigenerational approach yields similar cumulative effects on body weight in other rodent models of obesity. Also, due to practical considerations, we did not measure adult body weight of a/a mice. It will be interesting to know if transgenerational increases in body weight and effects of methyl supplementation also occur in mice without a genetic tendency for obesity.
Twenty years ago, Holliday22 proposed that just as genetic mutations can cause cancer, so too might epimutations such as aberrant DNA methylation. This prediction has proved correct; the role of epigenetic dysregulation in cancer is now firmly established.23 Analogously, just as genetic variation can contribute to human obesity,24 so too can interindividual epigenetic variation. Data from animal models and human developmental syndromes demonstrate that epigenetic dysregulation causes obesity.25 Remarkably, however, we know virtually nothing about the epigenetic regulation of genes that play a central role in food intake regulation. Given the escalating worldwide prevalence of obesity among women of childbearing age, it is of crucial importance to understand the biologic mechanisms by which maternal obesity affects development of mammalian body weight regulation.
Acknowledgements
We thank Vincent Dion, Hannah Landecker and Lanlan Shen for comments on the manuscript, and Adam Gillum for help with the figures. This study was supported by the NIH Grant 5K01DK070007, research Grant no. 5-FY05-47 from the March of Dimes Birth Defects Foundation, and USDA CRIS no. 6250-51000-049 (RAW) and NSF DEB-021306 and NSF DEB-0445351 (MT).
References
- 1.Lawlor DA, Smith GD, O’Callaghan M, Alati R, Mamun AA, Williams GM, et al. Epidemiologic evidence for the fetal over-nutrition hypothesis: findings from the mater-university study of pregnancy and its outcomes. Am J Epidemiol. 2007;165:418–424. doi: 10.1093/aje/kwk030. [DOI] [PubMed] [Google Scholar]
- 2.Levin BE. The obesity epidemic: metabolic imprinting on genetically susceptible neural circuits. Obes Res. 2000;8:342–347. doi: 10.1038/oby.2000.41. [DOI] [PubMed] [Google Scholar]
- 3.Darnton-Hill I, Nishida C, James WP. A life course approach to diet, nutrition and the prevention of chronic diseases. Public Health Nutr. 2004;7:101–121. doi: 10.1079/phn2003584. [DOI] [PubMed] [Google Scholar]
- 4.Keith SW, Redden DT, Katzmarzyk PT, Boggiano MM, Hanlon EC, Benca RM, et al. Putative contributors to the secular increase in obesity: exploring the roads less traveled. Int J Obes (Lond) 2006;30:1585–1594. doi: 10.1038/sj.ijo.0803326. [DOI] [PubMed] [Google Scholar]
- 5.Kral JG, Biron S, Simard S, Hould FS, Lebel S, Marceau S, et al. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years. Pediatrics. 2006;118:e1644–e1649. doi: 10.1542/peds.2006-1379. [DOI] [PubMed] [Google Scholar]
- 6.Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet. 2006;368:1164–1170. doi: 10.1016/S0140-6736(06)69473-7. [DOI] [PubMed] [Google Scholar]
- 7.Guo F, Jen KL. High-fat feeding during pregnancy and lactation affects offspring metabolism in rats. Physiol Behav. 1995;57:681–686. doi: 10.1016/0031-9384(94)00342-4. [DOI] [PubMed] [Google Scholar]
- 8.Wu Q, Suzuki M. Parental obesity and overweight affect the body-fat accumulation in the offspring: the possible effect of a high-fat diet through epigenetic inheritance. Obes Rev. 2006;7:201–208. doi: 10.1111/j.1467-789X.2006.00232.x. [DOI] [PubMed] [Google Scholar]
- 9.Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 10.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Duhl DM, Barsh GS. Opposite orientations of an inverted duplication and allelic variation at the mouse agouti locus. Genetics. 1996;144:265–277. doi: 10.1093/genetics/144.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan HD, Sutherland HG, Martin DI, Whitelaw E.Epigenetic inheritance at the agouti locus in the mouse (see comments).Nat Genet 199923314-318 [DOI] [PubMed] [Google Scholar]
- 13.Yen TT, Gill AM, Frigeri LG, Barsh GS, Wolff GL. Obesity, diabetes, and neoplasia in yellow A(vy)/- mice: ectopic expression of the agouti gene. FASEB J. 1994;8:479–488. doi: 10.1096/fasebj.8.8.8181666. [DOI] [PubMed] [Google Scholar]
- 14.Bjorntorp P. Thrifty genes and human obesity. Are we chasing ghosts? Lancet. 2001;358:1006–1008. doi: 10.1016/S0140-6736(01)06110-4. [DOI] [PubMed] [Google Scholar]
- 15.Waterland RA, Travisano M, Tahiliani KG. Diet-induced hyper-methylation at agouti viable yellow is not inherited transgenerationally through the female. FASEB J. 2007;21:3380–3385. doi: 10.1096/fj.07-8229com. [DOI] [PubMed] [Google Scholar]
- 16.Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998;12:949–957. [PubMed] [Google Scholar]
- 17.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vadlamudi S, Kalhan SC, Patel MS. Persistence of metabolic consequences in the progeny of rats fed a HC formula in their early postnatal life. Am J Physiol. 1995;269:E731–E738. doi: 10.1152/ajpendo.1995.269.4.E731. [DOI] [PubMed] [Google Scholar]
- 19.Levin BE, Govek E. Gestational obesity accentuates obesity in obesity-prone progeny. Am J Physiol. 1998;275:R1374–R1379. doi: 10.1152/ajpregu.1998.275.4.R1374. [DOI] [PubMed] [Google Scholar]
- 20.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114:567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolff GL. Body composition and coat color correlation in different phenotypes of ‘Viable Yellow’ mice. Science. 1965;147:1145–1147. doi: 10.1126/science.147.3662.1145. [DOI] [PubMed] [Google Scholar]
- 22.Holliday R. The inheritance of epigenetic defects. Science. 1987;238:163–170. doi: 10.1126/science.3310230. [DOI] [PubMed] [Google Scholar]
- 23.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 24.Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, et al. The human obesity gene map: the 2005 update. Obesity (Silver Spring) 2006;14:529–644. doi: 10.1038/oby.2006.71. [DOI] [PubMed] [Google Scholar]
- 25.Waterland RA. Does nutrition during infancy and early childhood contribute to later obesity via metabolic imprinting of epigenetic gene regulatory mechanisms? In: Hernell OSJ, editor. Feeding During Late Infancy and Early Childhood: Impact on Health. Nestec Ltd.; Vevey: 2005. pp. 157–174. [DOI] [PubMed] [Google Scholar]