Summary
Although it is widely known that dietary restriction (DR) not only extends the longevity of a wide range of species but also reduces their reproductive output, the interrelationship of DR, longevity-extension and reproduction is not well understood in any organism. Here we address the question: “Under what nutritional conditions do the longevity-enhancing effects resulting from food restriction either counteract, complement or reinforce the mortality costs of reproduction? To answer this question we designed a fine-grained DR study involving 4,800 individuals of the tephritid fruit fly Anastrepha ludens in which we measured sex-specific survival and daily reproduction in females in each of 20 different treatments (sugar:yeast ratios) plus 4 starvation controls. The database generated from this 3-year study consisted of approximately 100,000 life-days for each sex and 750,000 eggs distributed over the reproductive lives of 2,400 females. The fertility and longevity-extending responses were used to create contour maps (X-Y grid) that show the demographic responses (Z-axis) across dietary gradients that range from complete starvation to both ad libitum sugar-only and ad libitum standard diet (3:1 sugar-to-yeast). The topographic perspectives reveal demographic equivalencies along nutritional gradients, differences in the graded responses of males and females, egg production costs that are sensitive to the interaction of food amounts and constituents, and orthogonal contours (equivalencies in longevity or reproduction) representing demographic thresholds related to both caloric content and sugar-yeast ratios. If general, the finding that lifespan and reproductive maxima occur at much different nutritional coordinates poses a major challenge for the use of food restriction (or a mimetic) in humans to improve health and extend longevity in humans.
Keywords: cost of reproduction, longevity extension, food restriction, Anastrepha ludens, life tables, contour graphs
Introduction
The longevity-extending effects of dietary restriction (DR), known since the seminal work on rats by McCay (McCay et al. 1935), are widely documented in research on this laboratory rodent (Davis et al. 1984; Masoro 1988) as well as on a wide variety of other species. Excepting diet-restricted house flies (Musca domestica) which do not experience a longevity increase (Cooper et al. 2004), these species include conventional model organisms such the yeast Saccharomyces cerevisiae (Lin et al. 2002), the nematode Caenorhabditis elegans (Lakowski & Hekimi 1998; Houthoofd et al. 2005; Lee & al 2006), the fruit fly Drosophila melanogaster (Chippindale et al. 1997; Good & Tatar 2001; Clancy et al. 2002; Partridge et al. 2005b; Piper et al. 2005; Pletcher et al. 2005; Min & Tatar 2006b), the zebrafish Danio rerio (Keller et al. 2006), the mouse Mus musculus (Weindruch & Walford 1982; Weindruch & Walford 1988; Weindruch 1996; Austad & Kristan 2003; Harper et al. 2006), and the rhesus monkey Macaca mulatta (DeRousseau 1994; Bodkin et al. 2003; Mattison et al. 2003; Lane & al 2004), and several non-traditional species including the spider Frontinella pyramitela (Austad 1989) and the medfly Ceratitis capitata (Carey et al. 1998a; Carey et al. 2002; Carey et al. 2005b). The underlying assumption regarding why this longevity-extension response has been conserved across such a wide variety of species is that it serves as a mechanism for bridging dearth periods by (among other changes) reducing the metabolic demand of reproduction and thereby postponing reproductive senescence (Harrison & Archer 1989; Holliday 1989; Shanley & Kirkwood 2000).
Despite a near-universal agreement among biologists that the longevity-extending effects of DR are the evolutionary outcome of selection for individuals capable of surviving through and reproducing after unfavorable periods, until recently remarkably little research was conducted on the relationship between reproduction, longevity-extension, and DR (Partridge et al. 2005a; Lee & al 2006). One reason for the paucity of early work was that much of the DR research involving reproduction was on rodents (e.g. Visscher et al. 1952; Merry & Holehan 1979; Bronson & Marsteller 1985; Hamilton & Bronson 1985; Holehan & Merry 1985; Nelson et al. 1985; Krackow 1989) and therefore, both cost and time constraints precluded large-scale studies designed to assess the effect of multiple DR treatments on reproduction. With rare exceptions (e.g. Chippindale et al. 1997) the majority of DR research involving the joint effects on longevity extension and reproduction using D. melanogaster (Good & Tatar 2001; Mair et al. 2003; Tu & Tatar 2003; Mair et al. 2004; Mair et al. 2005; Min & Tatar 2006b; Min & Tatar 2006a; Bass et al. 2007; Burger et al. 2007) as well as using both the nematode C. elegans (Lee & al 2006), and the Mediterranean fruit fly C. capitata (Carey et al. 1998a; Carey et al. 2002; Carey et al. 2005b) has been conducted relatively recently.
The results of these and related (Bross et al. 2005; Bhandari et al. 2007; Burger et al. 2007; Ja & al 2007; Libert et al. 2007) DR studies in Drosophila and other invertebrates underscore the complexity of the relationship. On the one hand, the results of experiments on D. melanogaster reveal that lifespan extension via DR is not exclusively due to the reduction in reproductive costs (Mair et al. 2004). On the other hand, mortality costs are still inextricably linked to reproduction in that, regardless of how the level of reproduction is manipulated whether through sterilizing radiation (Carey et al. 2001; Mair et al. 2004) or through access to mates (Partridge et al. 1987; Rogina et al. 2007), oviposition hosts (Carey et al. 1986), or protein-rich diets (Good & Tatar 2001; Piper et al. 2005), cohorts that are capable of laying their full complements of eggs nearly always experience higher mortality rates than do cohorts in which egg laying is arrested.
Although these findings shed important light on the complexity of the relationship of DR, longevity extension, and the cost of reproduction, important questions still remain including the one we use to frame our study: “Under what nutritional conditions do the longevity-altering effects resulting from food restriction either counteract, complement or reinforce the mortality costs of reproduction?” Although the collective DR studies have examined the food restriction response of organisms across a large array of treatments, including measurement of survival in both sexes as well as female reproduction (Graves 1993; Chapman & Partridge 1996; Chapman et al. 1998; Good & Tatar 2001; Piper et al. 2005), heretofore there have been no previous investigations that have included all of these aspects in a single study. i.e. monitoring daily survival of large numbers of both sexes subjected to multiple DR treatments across both caloric and sugar-yeast gradients, with age-specific and lifetime reproduction measured in females.
In light of this shortcoming in the literature, we initiated fine-grained DR studies on both sexes of the tephritid fruit fly, Anastrepha ludens, commonly known as the Mexican fruit fly (Carey et al. 2005a), and measured daily survival and female reproduction. The A. ludens system was chosen because large numbers of newly-eclosed flies of both sexes were continually available for use in conducting large-scale experiments, the magnitude of both lifetime reproduction and life expectancy varied substantially across restriction treatments, and the range of sugar-yeast combinations elicited changes in longevity and reproduction that were both positively- and negatively-correlated. An additional advantage of this system was that food could be dispensed in micro-quantities using a methodology that, on the one hand, used rodent restriction-like methods (Masoro 1988; Masoro 1995; Shanley & Kirkwood 2000) to limit the quantity of food and, on the other hand, used Drosophila restriction-like (dilution) methods to alter the quality of food (Carvalho et al. 2005; Partridge et al. 2005b). This allowed us to create a 2-dimensional, ‘fine-grained’ treatment grid consisting of yeast restriction on one axis and of sugar restriction on the other. We gathered data on a total of 4,800 flies (see Methods) including daily survival and female reproduction for approximately 100 individuals of each sex fed daily according to a 24-treatment design using 4 compositional levels (sugar-only and 24:1, 9:1 and 3:1 sugar:yeast (SY) ratios) and 6 concentrations relative to the base stock (100%, 75, 50, 25, 10 and 0). Contour maps based on the response surface data (i.e. response plotted on Z axis with respect to variables plotted on the X and Y axes) were constructed for male and female life expectancies and lifetime reproduction in females using local linear fitting techniques (Facer & Müller 2003). We use SY to denote sugar:yeast ratio, CR to denote calorie restriction, and DR to denote the general concept of dietary restriction (i.e. a reduction in food availability).
Results
Longevity extension due to food restriction was evident for both sexes with the highest life expectancy occurring at intermediate levels of both calorie and yeast restriction (Table 1). For example, the highest average life expectancy along the calorie restriction gradient occurred in the 9-to-1 SY treatment cohort where males and females lived 53.1 and 59.5 days, respectively, and along the yeast restriction gradient at the 25% dilution treatment where males and females lived 49.3 and 53.0 days, respectively. The range in female life expectancies varied by nearly 40 days (28.2 vs 67.2 days) and male life expectancy varied by over 32 days (26.4 to 58.8). With the exception of the female cohort subject to the 3:1 SY/10% CR treatment, life expectancy was significantly less in the sugar-only treatments than in the full diet (SY=3:1) treatments at all levels of CR. Indeed, life expectancy for females and males subject to all of the sugar-only (SY=1:0) treatments averaged 17 and 19 days respectively less than for cohorts subject to all of the full-diet treatments.
Table 1.
Expectation of life (days) for A. ludens females and males subject to various combinations of yeast and caloric restriction regimes. The column labels refer to percentage of ad libitum food flies were given access to daily and the row labels refer to the dietary composition of sugar-to-yeast. Both linear and quadratic effects of yeast fraction and percentage of ad libitum food are statistically significant (p <0.001, see supporting online material for details).a.
| 100% | 75% | 50% | 25% | 10% | Means | |
|---|---|---|---|---|---|---|
| FEMALES | ||||||
| Sugar (1:0) | 28.2 | 30.1 | 31.1 | 33.4 | 36.9 | 31.9 |
| 24-to-1 | 45.5 | 49.9 | 51.8 | 57.4 | 48.0 | 50.5 |
| 9-to-1 | 55.6 | 64.5 | 63.4 | 67.2 | 46.8 | 59.5 |
| 3-to-1 | 51.3 | 52.4 | 55.2 | 53.9 | 30.0 | 48.6 |
| Means | 45.2 | 49.2 | 50.4 | 53.0 | 40.4 | 47.6 |
| MALES | ||||||
| Sugar | 26.4 | 27.4 | 30.2 | 34.1 | 33.0 | 30.2 |
| 24-to-1 | 42.1 | 39.2 | 43.3 | 48.6 | 46.0 | 43.8 |
| 9-to-1 | 43.7 | 52.3 | 58.6 | 58.8 | 52.1 | 53.1 |
| 3-to-1 | 50.1 | 56.0 | 55.1 | 55.7 | 39.5 | 51.3 |
| Means | 40.6 | 43.7 | 46.8 | 49.3 | 42.6 | 44.6 |
life expectancies for female cohorts maintained on water-only were 4.0, 4.0, 4.1, and 3.9 days for sugar, 24-to-1, 9-to-1, and 3-to-1 diets, respectively and for male cohorts were 4.2, 4.3, 4.4, and 4.3, respectively.
Lifetime fecundity in females was dependent primarily on yeast composition and secondarily on caloric content (Table 2). For example, per capita egg laying in treatments involving different sugar-yeast levels ranged from 4 to 8 eggs in the sugar-only treatments, 25 to 50 eggs in 24:1 SY treatments, 180 to 280 eggs in the 9:1 treatments, and excepting the 10% CR level, over 850 eggs in the 3:1 SY treatments. In contrast, large differences in lifetime fecundity occurred between treatments in which caloric restriction levels were similar with cohorts maintained on sugar-only diets (across all caloric restriction levels) averaging 6 eggs/female and cohorts maintained on 3:1 SY diets averaging nearly 780 eggs/female. These differences reveal the profound importance of yeast content in the diet inasmuch as lifetime fecundity differed by a maximum of 2-fold across CR treatments but a remarkable 100-fold across the SY gradient.
Table 2.
Lifetime egg production for A. ludens females subject to different combinations of dietary dilution levels and dietary ratios. i.e. 1-to-0 refers to sugar-only, 24-to-1, 9-to-1, and 3-to-1 refers to 24, 9, and 3 parts sugar to one part yeast, respectively. For log (lifetime egg production), both linear and quadratic effects of yeast fraction and percentage of ad libitum food are statistically significant (p-values <0.03, see supporting online material for details).
| Dietary Ratios |
Percent Dietary Contenta |
|||||
|---|---|---|---|---|---|---|
| 100% | 75% | 50% | 25% | 10% | Means | |
| Sugar (1-to-0) | 3.8 | 6.7 | 7.2 | 4.7 | 7.6 | 6.0 |
| 24-to-1 | 24.6 | 16.6 | 18.2 | 25.0 | 48.3 | 26.5 |
| 9-to-1 | 179.3 | 209.9 | 241.7 | 281.8 | 201.8 | 222.9 |
| 3-to-1 | 875.6 | 891.2 | 1071.4 | 861.8 | 191.4 | 778.3 |
| MEANS | 270.8 | 281.1 | 334.6 | 293.3 | 112.3 | 258.4 |
no eggs were produced in 0% (water-only) dietary content treatments
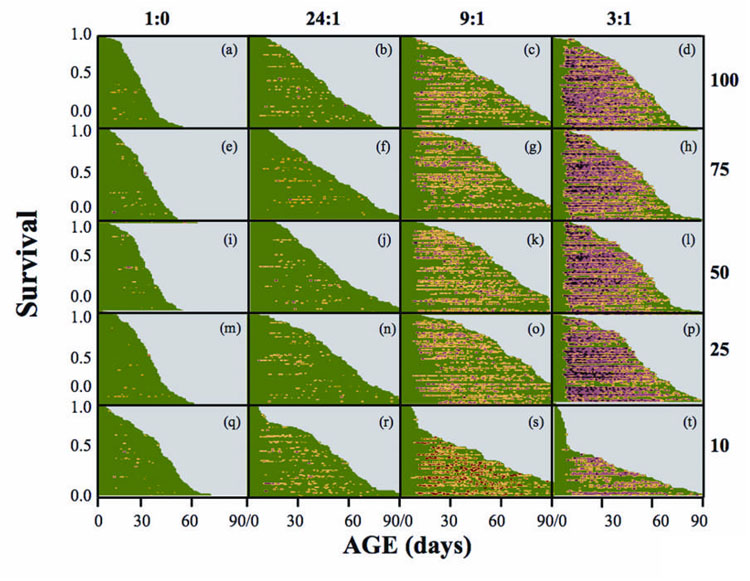
The event-history graphs (Carey et al. 1998b) contained in Fig. 1 arranged by columns (SY) and rows (CR) show the large increase in levels of age-specific reproduction from left-to-right (increasing yeast concentration) but little change from bottom-to-top (increasing caloric levels). Differences in survival across the CR-SY treatments were less pronounced than were differences in reproduction with high survival rates in sugar-only cohorts (left-most column of graphs) at young ages but near-zero survival by 60 days. Broad patterns of survival in the 24:1 yeast restriction treatments were similar except that the near-linear decline was less steep than in the sugar-only (1:0) cohorts. For the 9:1 and 3:1 treatments the survival curves were noticeably more concave due to low mortality at young ages followed by increasing mortality at middle and older ages (i.e. >30 days). The trajectories of the survival curves at the 10% caloric restriction treatments declined rapidly for the first 2 weeks and leveled off to a much slower rate of decrease thereafter. Cohorts that exhibited the highest life expectancies were similar in two respects: i) all were maintained on an intermediate (9:1) SY regime; and ii) for females, all exhibited moderate level lifetime fecundity (i.e. between 210 to 280 eggs/female).
Figure 1.
Event history charts (Carey et al. 1998b) for A. ludens females showing relationship of cohort survival and individual-level reproduction across treatments. The ratios across the top denote the sugar:yeast ratios and the numbers aligned vertically at the right denote the percent concentration of the original stock solution (see Methods). Each horizontal line within a graph denotes a life line for an individual female, the length of which is proportional to her lifespan and with color-coded segments corresponding to age classes. The age-specific egg laying intensity for each individual female corresponds to the shading: green=zero eggs; yellow=1–20 eggs; red=>20 eggs.
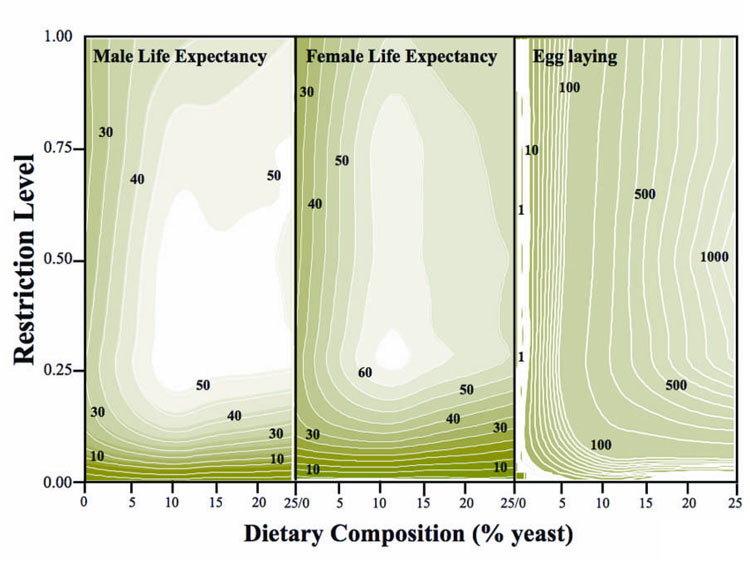
The contour graphs (Fig. 2) that were constructed from the response surface data (supporting online material) reveal the within- and between-sex equivalencies in both levels and rates of change for longevity of both sexes and for female reproduction across the entire nutritional domain. Two nutritional thresholds are also evident: i) a CR threshold (≈0.10) below which both female reproduction and survival of both sexes decline precipitously, and ii) a SY threshold (≈5%) below which reproduction declines precipitously but where survival decreases gradually. The square contours in the reproductive surface (Fig. 2) show that the yeast level sets a threshold in terms of the range of increasing reproduction due to increasing calories, and calories set a threshold in terms of the corresponding domain where yeast increase leads to reproduction increase.
Figure 2.
Contour graphs showing the response surfaces for restriction level (concentration relative to ad libitum mother stock) and percent yeast in A. ludens— left, middle and right panels shows male life expectancy (days), female life expectancy, and female lifetime egg reproduction, respectively. Local linear fitting techniques (Facer & Müller 2003) were used to produce the numerical values of the response surfaces from the data and DeltaGraph® graphics program was used to generate the contour maps.
Reproduction steadily increases in each of the graphs in Fig. 2 from left-to-right (increasing yeast composition) but not from top-to-bottom (or vice versa). More calories at a fixed yeast fraction do not increase reproduction even though absolute yeast intake increases with the increase in calories under these conditions. In contrast to the monotonic changes in reproduction with changes in dietary restriction levels, survival increases and then decreases (is bimodal) as DR levels change.
Contour graphs constructed from the female longevity and reproduction data yielded additional insights into the demographics of DR that were not apparent from the data contained in the tables or visualized in other figures. The intensity of egg laying (Fig. 2; right panel) is mainly regulated via yeast and not calories. Flies need to have access to high quality foods once they have the minimum calories needed to sustain minimum longevity without which reproduction would be low.
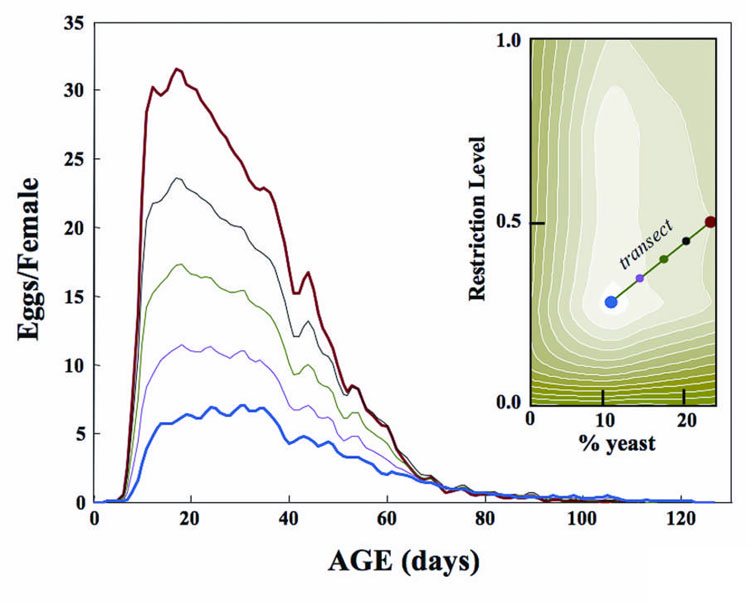
Age-specific reproductive schedules were estimated using local linear fitting techniques (Facer & Müller 2003) for points along the transect shown in the inset in Figure 3. These connect the nutritional coordinates corresponding to maximum longevity (blue circle) to the coordinates corresponding to maximum reproduction (red circle). The color-coded schedules corresponding to the points along the transect show that the changes in age-specific reproduction along this transect (max longevity-to-max reproduction) include an increase in both peak and total reproduction, the emergence of a distinct reproductive peak, a reduction in the mean age of reproduction, and a convergence to a rate of one or fewer eggs per day for females beyond around 70 days for all cohorts. The longevity decrease and reproductive increase along the transect (Figure 3 inset) reveals a mortality cost of reproduction. Conversely, the reproductive decrease with age reveals a reproductive cost of aging—total egg production by day 40 differs by 5-fold across cohorts yet all reproductive schedules decrease rapidly after this age independent of the level of egg production at younger ages.
Figure 3.
Age-specific (net) reproduction functions for the surface transect that connects the coordinates corresponding to the longevity (blue dot) and reproduction (red dot) maxima with three equidistant highlighted locations on the transect line in the female longevity contour graph shown in the inset (from Figure 2b). Colors for each transect location correspond to those for the reproductive schedules. Lifetime egg production for the respective schedules are 295, 463, 663, 872, and 1,086 eggs/female, respectively, and the mean age of reproduction are 37.4, 34.0, 32.4, 31.6 and 29.9 days, respectively.
Discussion
The current study involving fine-grained treatments and measurements of mortality in both sexes and of individual-level female reproduction produced results that shed new light on several aspects of the effects of food restriction on reproductive costs and longevity. One revelation was the difficulty of identifying the right DR combination that maximizes longevity when individuals are allowed to reproduce freely. The greatest longevity (especially for females) occurred in a relatively small nutritional parameter space where reproductive costs for the flies were minimized relative to the maintenance benefits. The longevity isoclines that emerged along the treatment gradients revealed that small changes in longevity may be observed even with large changes in restriction levels. This observation may partly explain why longevity increases are observed in many restriction studies (Harshman & Zera 2006) but not in all (LeBourg & Minois 1996; Carey et al. 2002). That similar patterns (isoclines) of longevity change over the same nutritional gradients were observed in both sexes despite the lack of an obvious counterpart in males to the cost of egg laying in females suggests that males also experience an energetically-derived cost of reproduction (Yuval et al. 1998). This general finding is similar to the results of a recent study on the effects of DR on sex differences in lifespan and mortality differences in D. melanogaster in which (Magwere et al. 2004): i) lifespan peaked at an intermediate food concentration; and ii) the relative magnitude of the longevity-extension response due to DR was greater in females. However, unlike their findings that the food concentrations that minimized adult mortality in D. melanogaster were different between males and female, they were approximately the same in the current study with A. ludens. This may be due either to species-specific differences in the behavior and ecology (Bateman 1972) that impacts sex-specific differences between the species or to differences in the experimental designs. Whereas the D. melanogaster study (Magwere et al. 2004) involved 8 types of food media (treatments) containing identical sugar-yeast ratios but different agar dilutions that altered the food concentration (i.e. DR dilution method), the protocol in the current study involved 20 treatments based on fixed amounts of food available daily to flies that differed in both sugar-yeast concentrations and ratios.
Another important insight from the current study was that the location of maximum reproduction and maximum female longevity at different nutritional coordinates (i.e. diet amount and composition) suggests that maximum lifespan is not necessary for maximum reproduction, and that the nutritional conditions for maximum reproduction are not conducive to maximum lifespan. It is thus doubtful, even in principle, that a single diet exists in which both reproduction and longevity are maximized—the greatest longevity is achieved at intermediate nutritional levels whereas the highest reproduction is achieved at maximal nutritional levels.
A third important result was the discovery that the effect on longevity extension in A. ludens due to CR for the calories that are derived from yeast depended on the caloric content of the food available. This was evident from the contour graphs (Fig. 2) showing that the effect of increased yeast on longevity was greater when overall restriction levels were high. This result complements recent findings on D. melanogaster in which Mair and co-workers (Mair et al. 2005) noted that yeast had a much greater effect on longevity extension per calorie than did sugar. Whereas these researchers concluded that calorie intake is not the key factor in determining mortality rate in D. melanogaster, our result suggests that the exact impact is conditional on caloric content of the diet and not just calories independent of dietary composition.
A general perspective that was evident from our results is the notion that food restriction regimes that maximize longevity and those that maximize reproduction are fundamentally different. Although negative effects of food restriction on reproductive capacity or performance are well documented including menstrual irregularities in women (Dirks & Leeuwenburgh 2006) and loss of libido in men (Frisch 2002), the importance of the longevity-fertility nutritional conundrum is seldom addressed. Thus one major challenge for research on longevity extension using either food restriction or a mimetic (Ingram et al. 2006) is finding a diet (if indeed one single diet exists) and identifying mechanisms that extend longevity but have minimal negative effects on reproduction, health, and quality-of-life components (Heilbronn et al. 2006).
Methods
The design for this study was created using the 24 two-way combinations involving 6 caloric restriction (CR) levels on one axis (relative to content of ad libitum mother stock) by 4 ratios of sugar-yeast (SY) on the other. The 4 SY ratios were 3:1, 9:1, 24:1, and 1:0 (i.e. sugar-only; no yeast) and the 6 CR levels were 100, 75, 50, 25, 10 and 0% [even though the water-only (0% CR) treatments were redundant, all four were retained for operational simplicity]. The percent yeast contents for the 4 SY ratios were 25, 10, 4, and 0%, respectively. The caloric and yeast content of each of 24 food-restriction treatments used in the study are given in Table S-1.
A 1.5 µl droplet of the appropriate food treatment and a 6 µl droplet of water were supplied to individual flies each day on glass slides using separate Eppendorf® needles. Newly-emerged (virgin) individual flies were housed in 4 × 4 × 10 cm plexiglass cages, each of which was part of a 24-unit cage unit. Treatment distribution was made in a randomized block design, using different color codes to identify both the treatment and the food slides. Females and males were placed in alternate cages to eliminate the possibility of eggs from two females overlapping on the egg collection surface. Females laid eggs through organdy mesh fastened to the front of the cage and were counted daily using an electronic image analysis system that automatically records the number of eggs that fall in a 4.5 × 4.5 cm2 field. A total of 100 individuals of each sex were used per treatment. Daily mortality and female reproduction were monitored daily throughout the life of each fly. Environmental conditions were 12:12 LD cycle, 24.0° C (±2°) and 65% RH (±9%).
Supplementary Material
Table S-1. Sugar and yeast hydrolysate composition of treatments. Sugar and yeast hydrolysate composition of the 24 treatments used in the food restriction study. Tephritid adult diet consists of a 3:1 ratio of sugar to yeast hydrolysate (Vargas 1989). Ad libitum was estimated from pilot experiments to be 0.015 ml of the mother stock. This droplet size was used for all treatments adjusted either for caloric content by adding water or by yeast content by changing the sugar-yeast ratios. Treatments 6, 12, 18 and 24 are all redundant (i.e. water-only) controls.
Table S-2. Statistical modeling. Predictors for the models are sugar amount and yeast amount, these are obtained simply by multiplying the diet amounts by the proportions of sugar and yeast as specified by diet composition. The best fitting regression model was a quadratic regression with these two predictors and longevity or number of eggs as response variable. Variable selection is done by automatic backward selection. The results for female and male flies are reported in the following tables. Both main predictor effects are significant at p<0.0001 and square and interaction terms at level p<0.05.
Tables S-3 to S-5. Values for Response Surfaces (Contour Maps in Fig. 2)
Table S-6. Survival in female Anastrepha ludens by treatment (see treatment key at bottom). n=number of individuals in initial cohort (age 0).
Table S-7. Survival in male Anastrepha ludens by treatment (see treatment key at bottom). n=number of individuals in initial cohort (age 0).
Acknowledgments
Research supported by NIA/NIH grants PO1 AG022500-01 and PO1 AG08761-10, the Ellison Medical Foundation, and pilot funds from the Center for the Economics and Demography of Aging, UC Berkeley. We thank A. Oropeza, R. Bustamente, E. de Leon, S. Salgado, S. Rodriguez, R. Rincon and G. Rodas for technical assistance and the Moscamed-Moscafrut program in Mexico for their laboratory facilities at Metapa, Chiapas. We are also grateful to Sige Zou for comments on the manuscript and Leslie Sandberg for editorial assistance.
References
- Austad SN. Life extension by diet restriction in the bowl and doily spider. Frontinella pyramitela. Exper. Geront. 1989;24:83–92. doi: 10.1016/0531-5565(89)90037-5. [DOI] [PubMed] [Google Scholar]
- Austad SN, Kristan DM. Are mice calorically restricted in nature? Aging Cell. 2003;2:201–207. doi: 10.1046/j.1474-9728.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- Bass TM, Grandison RC, Wong R, Martinez P, Partridge L, Piper MDW. Optimization of dietary restriction protocols in Drosophila. J. of Geront.: Biol. Sci. 2007;62A:1071–1081. doi: 10.1093/gerona/62.10.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman MA. The ecology of fruit flies. Ann. Review of Entom. 1972;17:493–518. [Google Scholar]
- Bhandari P, Jones MA, Martin I, Grotewiel MS. Dietary restriction alters demographic but not behavioral aging in Drosophila. Aging Cell. 2007;6:631–637. doi: 10.1111/j.1474-9726.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- Bodkin NL, Alexander TM, Ortmeyer HK, Johnson E, Hansen BC. Mortality and morbidity in laboratory-maintained Rhesus monkeys and effects of long-term dietary restriction. J. of Geront.: Med. Scis. 2003;58A:212–219. doi: 10.1093/gerona/58.3.b212. [DOI] [PubMed] [Google Scholar]
- Bronson FH, Marsteller FA. Effect of short-term food deprivation on reproduction in female mice. Biol. of Reproduction. 1985;33:660–667. doi: 10.1095/biolreprod33.3.660. [DOI] [PubMed] [Google Scholar]
- Bross TG, Rogina B, Helfand SL. Behavioral, physical, and demographic changes in Drosophila populations through dietary restriction. Aging Cell. 2005;4:309–317. doi: 10.1111/j.1474-9726.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- Burger JMS, Hwangbo DS, Corby-Harris V, Promislow DEL. The functional costs and benefits of dietary restriction in Drosophila. Aging Cell. 2007;6:63–71. doi: 10.1111/j.1474-9726.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- Carey JR, Krainacker D, Vargas R. Life history response of Mediterranean fruit fly females to periods of host deprivation. Entomologia experimentalis et applicata. 1986;42:159–167. [Google Scholar]
- Carey JR, Liedo P, Harshman L, Müller H-G, Partridge L, Wang J-L, Zhang Y. Life history response of Mediterranean fruit flies to dietary restriction. Aging Cell. 2002;1:140–148. doi: 10.1046/j.1474-9728.2002.00019.x. [DOI] [PubMed] [Google Scholar]
- Carey JR, Liedo P, Müller H-G, Wang J-L, Love B, Harshman L, Partridge L. Female sensitivity to diet and irradiation treatments underlies sex-mortality differentials in the Mediterranean fruit fly. J. of Geront.: Biol. Sci. 2001;56A:B89–B93. doi: 10.1093/gerona/56.2.b89. [DOI] [PubMed] [Google Scholar]
- Carey JR, Liedo P, Müller H-G, Wang J-L, Senturk D, Harshman L. Biodemography of a long-lived tephritid: Reproduction and longevity in a large cohort of Mexican fruit flies, Anastrepha ludens. Exper. Geront. 2005a;40:793–800. doi: 10.1016/j.exger.2005.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR, Liedo P, Müller H-G, Wang J-L, Vaupel JW. Dual modes of aging in Mediterranean fruit fly females. Science. 1998a;281:996–998. doi: 10.1126/science.281.5379.996. [DOI] [PubMed] [Google Scholar]
- Carey JR, Liedo P, Müller H-G, Wang J-L, Vaupel JW. A simple graphical technique for displaying individual fertility data and cohort survival: case study of 1000 Mediterranean fruit fly females. Funct.Ecol. 1998b;12:359–363. [Google Scholar]
- Carey JR, Liedo P, Müller H-G, Wang J-L, Zhang Y, Harshman L. Stochastic dietary restriction using a Markov-chain feeding protocol elicits complex, life history response in medflies. Aging Cell. 2005b;4:31–39. doi: 10.1111/j.1474-9728.2004.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho GB, Kapahi P, Benzer S. Compensatory ingestion upon dietary restriction in Drosophila melanogaster. Nature Methods. 2005;2:1–3. doi: 10.1038/nmeth798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T, Miyatake T, Smith HK, Partridge L. Interactions of mating, egg production and death rates in females of the Mediterranean fruit fly, Ceratitis capitata. Proc. of the Royal Soc. of London, B. 1998;265:1879–1894. doi: 10.1098/rspb.1998.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T, Partridge L. Female fitness in Drosophila melanogaster: an interaction between the effect of nutrition and of encounter rate of males. Proc. of the Royal Soc. of London; Series B. 1996;263:755–759. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- Chippindale AK, Leroi AM, Saing H, Borash DJ, Rose MR. Phenotypic plasticity and selection in Drosophila life history evolution. 2. Diet, mates and the cost of reproduction. J. of Evol. Biol. 1997;10:269–293. [Google Scholar]
- Clancy DJ, Gems D, Hafen E, Leevers SJ, Partridge L. Dietary restriction in long-lived dwarf flies. Science. 2002;269:319. doi: 10.1126/science.1069366. [DOI] [PubMed] [Google Scholar]
- Cooper TM, Mockett RJ, Sohal BH, Sohal RS, Orr WC. Effect of calorie restriction on life span of the housefly, Musca domestica. The FASEB Journal. 2004;18:1591–1593. doi: 10.1096/fj.03-1464fje. [DOI] [PubMed] [Google Scholar]
- Davis TA, Bales CW, Beauchene RE. Differential effects on dietary caloric and protein restriction in the aging rat. Experimental Gerontology. 1984;18:427–435. doi: 10.1016/0531-5565(83)90021-9. [DOI] [PubMed] [Google Scholar]
- DeRousseau JC. Primate gerontology: an emerging discipline. In: Crews DE, Garruto RM, editors. Biological Anthropology and Aging. New York: Oxford University Press; 1994. pp. 127–153. [Google Scholar]
- Dirks AJ, Leeuwenburgh C. Calorie restriction in humans: Potential pitfalls and health concerns. Mech. of Ageing and Devel. 2006;127:1–7. doi: 10.1016/j.mad.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Facer M, Müller HG. Nonparametric estimation of the location of a maximum in a response surface. J. of Multivariate Analysis. 2003;87:191–217. [Google Scholar]
- Frisch RE. Female Fertility and the Body Fat Connection. Chicago: The Univesity of Chicago Press; 2002. [Google Scholar]
- Good TP, Tatar M. Age-specific mortality and reproduction respond to adult dietary restriction in Drosophila melanogaster. J. of Insect Physiol. 2001;47:1467–1473. doi: 10.1016/s0022-1910(01)00138-x. [DOI] [PubMed] [Google Scholar]
- Graves JL. The costs of reproduction and dietary restriction: parallels between insects and mammals. Growth, Development and Aging. 1993;57:233–249. [PubMed] [Google Scholar]
- Hamilton GD, Bronson FH. Food restriction and reproductive development in wild house mice. Biology of Reproduction. 1985;32:773–778. doi: 10.1095/biolreprod32.4.773. [DOI] [PubMed] [Google Scholar]
- Harper JM, Leathers CW, Austad SN. Does caloric restriction extend life in wild mice? Aging Cell. 2006;5:441–449. doi: 10.1111/j.1474-9726.2006.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Archer JR. Natural selection for extended longevity from food restriction. Growth, Development and Aging. 1989;53:3–6. [PubMed] [Google Scholar]
- Harshman LG, Zera AJ. The cost of reproduction: The devil in the details. Trends in Ecol. and Evol. 2006;22:80–86. doi: 10.1016/j.tree.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, deJonge L, Frisard MI, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals. J. of the Amer. Med. Assoc. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holehan AM, Merry BJ. Lifetime breeding studies in fully fed and dietary restricted female CFY Sprague-Dawley rates. 1. effect of age, housing conditions and diet on fecundity. Mech. of Ageing and Devel. 1985;33:19–28. doi: 10.1016/0047-6374(85)90106-x. [DOI] [PubMed] [Google Scholar]
- Holliday R. Food, reproduction and longevity: is the extended lifespan of calorie-restricted animals an evolutionary adaptation? Bioassays. 1989;10:125–127. doi: 10.1002/bies.950100408. [DOI] [PubMed] [Google Scholar]
- Houthoofd K, Johnson TE, Vanfleteren JR. Dietary restriction in the nematode Caenorhabditis elegans. J. of Geron.: Biol. Sci. 2005;60A:1125–1131. doi: 10.1093/gerona/60.9.1125. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Zhu M, Mamczarz J, Zou S, Lane MA, Roth GS, deCabol R. Calorie restriction mimetics: an emerging research field. Aging Cell. 2006;5:97–108. doi: 10.1111/j.1474-9726.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- Ja WW, et al. Prandiology of Drosophila and the CAFE assay. Proc. of the National Academy of Sci. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller ET, Keller JM, Gillespie G. The use of mature zebrafish (Danio rerio) as a model for human aging. In: Conn PM, editor. Handbook of Models for Human Aging. Amsterdam: Elsevier; 2006. pp. 299–314. [Google Scholar]
- Krackow S. Effect of food restriction on reproduction and lactation in house mice mated post partum. J. of Reproduction and Fertility. 1989;86:341–347. doi: 10.1530/jrf.0.0860341. [DOI] [PubMed] [Google Scholar]
- Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proc. of the National Academy of Sciences. 1998;95:13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, et al. Effects of long-term diet restriction on aging and longevity in primates remains uncertain. J. of Geront.: Biol. Sci. 2004;59A:405–407. doi: 10.1093/gerona/59.5.b405. [DOI] [PubMed] [Google Scholar]
- LeBourg E, Minois N. Failure to confirm increased longevity in Drosophila melanogaster submitted to a food restriction procedure. J. of Geron.: Biol. Sci. 1996;51A:B280–B283. doi: 10.1093/gerona/51a.4.b280. [DOI] [PubMed] [Google Scholar]
- Lee GD, et al. Dietary deprivation extends lifespan in Caenorhabditis elegans. Aging Cell. 2006;5:515–524. doi: 10.1111/j.1474-9726.2006.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert S, Zwiner J, Chu X, Voorhies WV, Roman G, Pletcher SD. Regulation of Drosophila life span by olfaction and food-derived odors. Science. 2007;315:1133–1137. doi: 10.1126/science.1136610. [DOI] [PubMed] [Google Scholar]
- Lin S-J, Kaeberlein M, Andalis AA, Sturtz LA, Defossez P-A, Culottas VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- Magwere T, Chapman T, Partridge L. Sex differences in the effect of dietary restriction on life span and mortality rates in female and male Drosophila melanogaster. J. of Geront: Biol. Sci. 2004;59A:3–9. doi: 10.1093/gerona/59.1.b3. [DOI] [PubMed] [Google Scholar]
- Mair W, Goymer P, Pletcher SC, Partridge L. Demography of dietary restriction and death in Drosophila. Science. 2003;301:1731–1733. doi: 10.1126/science.1086016. [DOI] [PubMed] [Google Scholar]
- Mair W, Piper MDW, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. Public Library of Science. 2005;3:1–7. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Sgro CM, Johnson AP, Chapman T, Partridge L. Lifespan extension by dietary restriction in female Drosophila melanogaster is not caused by a reduction in vitellogenesis or ovarian activity. Exper. Geront. 2004;39:1011–1019. doi: 10.1016/j.exger.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Masoro E. Design issues in dietary restriction. In: Hart RW, Neumann DA, Robertxon RT, editors. Dietary Restriction: Implications for the Design and Interpretation of Toxidity and Carninogenicity Studies. Washington, D.D: ILSI Press; 1995. pp. 341–350. [Google Scholar]
- Masoro EJ. Minireview: food restriction in rodents: an evaluation of its role in the study of aging. J. of Geront. 1988;43:B59–B64. doi: 10.1093/geronj/43.3.b59. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Lane MA, Roth GS, Ingram DI. Calorie restriction in rhesus monkeys. Exper. Geront. 2003;38:35–46. doi: 10.1016/s0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. J. of Nutrition. 1935;10:63–79. [PubMed] [Google Scholar]
- Merry BJ, Holehan AM. Onset of puberty and duration of fertility in rats fed a restricted diet. J. of Reproduction and Fertility. 1979;57:253–259. doi: 10.1530/jrf.0.0570253. [DOI] [PubMed] [Google Scholar]
- Min K-J, Tatar M. Drosophila diet restriction in practice: Do flies consume fewer nutrients? Mech.of Ageing and Develop. 2006a;127:93–96. doi: 10.1016/j.mad.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Min K-J, Tatar M. Restriction of amino acids extends lifespan in Drosophila melanogaster. Mech. of Ageing and Develop. 2006b;127:643–646. doi: 10.1016/j.mad.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Gosden RG, Felicio LS. Effect of dietary restriction on estrous cyclicity and follicular reserves in aging C57BL/6J mice. Biology of Reproduction. 1985;32:515–522. doi: 10.1095/biolreprod32.3.515. [DOI] [PubMed] [Google Scholar]
- Partridge L, Gems D, Withers DJ. Sex and death: What is the connection? Cell. 2005a;120:461–472. doi: 10.1016/j.cell.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Partridge L, Green A, Fowler K. Effects of egg-production and of exposure to males on female survival in Drosophila melanogaster. J. of Insect Physiology. 1987;33:745–749. [Google Scholar]
- Partridge L, Piper MDW, Mair W. Dietary restriction in Drosophila. Mech. of Ageing and Develop. 2005b;126:938–950. doi: 10.1016/j.mad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Piper MDW, Mair W, Partridge L. Counting the calories: The role of specific nutrients in extension of life span by food restriction. J.of Geront: Biol. Sci. 2005;60A:549–555. doi: 10.1093/gerona/60.5.549. [DOI] [PubMed] [Google Scholar]
- Pletcher SD, Libert S, Skorupa D. Flies and their Golden Apples: The effect of dietary restriction on Drosophila aging and age-dependent gene expression. Ageing Research Reviews. 2005;4:451–480. doi: 10.1016/j.arr.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Rogina B, Wolverton T, Bross TG, Chen K, Müller H-G, Carey JR. Distinct biological epochs in the reproductive life of female Drosophila melanogaster. Mech. of Aging and Develop. 2007;128:477–485. doi: 10.1016/j.mad.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley DP, Kirkwood TBL. Calorie restriction and aging: A life-history analysis. Evolution. 2000;54:740–750. doi: 10.1111/j.0014-3820.2000.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Tu M-P, Tatar M. Juvenile diet restriction and the aging and reproduction of adult Drosophila melanogaster. Aging Cell. 2003;2:327–333. doi: 10.1046/j.1474-9728.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- Vargas R. Mass production of tephritid fruit flies. In: Robinson AS, Hooper G, editors. World Crop Pests. Fruit Flies: Their Biology and Control. Amsterdam: Elsevier; 1989. pp. 141–151. [Google Scholar]
- Visscher MB, King JT, Lee YCP. Further studies on influence of age and diet upon reproductive senescence in Strain A female mice. Amer. J. of Physiol. 1952;170:72–76. doi: 10.1152/ajplegacy.1952.170.1.72. [DOI] [PubMed] [Google Scholar]
- Weindruch R. Caloric restriction and aging. Scientific American. 1996;274:46–52. doi: 10.1038/scientificamerican0196-46. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: Effect on life-span and spontaneous cancer incidence. Science. 1982;215:1415–1418. doi: 10.1126/science.7063854. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Springfield: Charles C. Thomas, Publisher; 1988. [Google Scholar]
- Yuval B, Kaspi R, Shloush S, Warburg MS. Nutritional reserves regulate male participation in Mediterranean fruit fly leks. Ecol. Entom. 1998;23:211–215. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S-1. Sugar and yeast hydrolysate composition of treatments. Sugar and yeast hydrolysate composition of the 24 treatments used in the food restriction study. Tephritid adult diet consists of a 3:1 ratio of sugar to yeast hydrolysate (Vargas 1989). Ad libitum was estimated from pilot experiments to be 0.015 ml of the mother stock. This droplet size was used for all treatments adjusted either for caloric content by adding water or by yeast content by changing the sugar-yeast ratios. Treatments 6, 12, 18 and 24 are all redundant (i.e. water-only) controls.
Table S-2. Statistical modeling. Predictors for the models are sugar amount and yeast amount, these are obtained simply by multiplying the diet amounts by the proportions of sugar and yeast as specified by diet composition. The best fitting regression model was a quadratic regression with these two predictors and longevity or number of eggs as response variable. Variable selection is done by automatic backward selection. The results for female and male flies are reported in the following tables. Both main predictor effects are significant at p<0.0001 and square and interaction terms at level p<0.05.
Tables S-3 to S-5. Values for Response Surfaces (Contour Maps in Fig. 2)
Table S-6. Survival in female Anastrepha ludens by treatment (see treatment key at bottom). n=number of individuals in initial cohort (age 0).
Table S-7. Survival in male Anastrepha ludens by treatment (see treatment key at bottom). n=number of individuals in initial cohort (age 0).